Buffering Adaptive Immunity by Hydrogen Sulfide
Abstract
1. Introduction
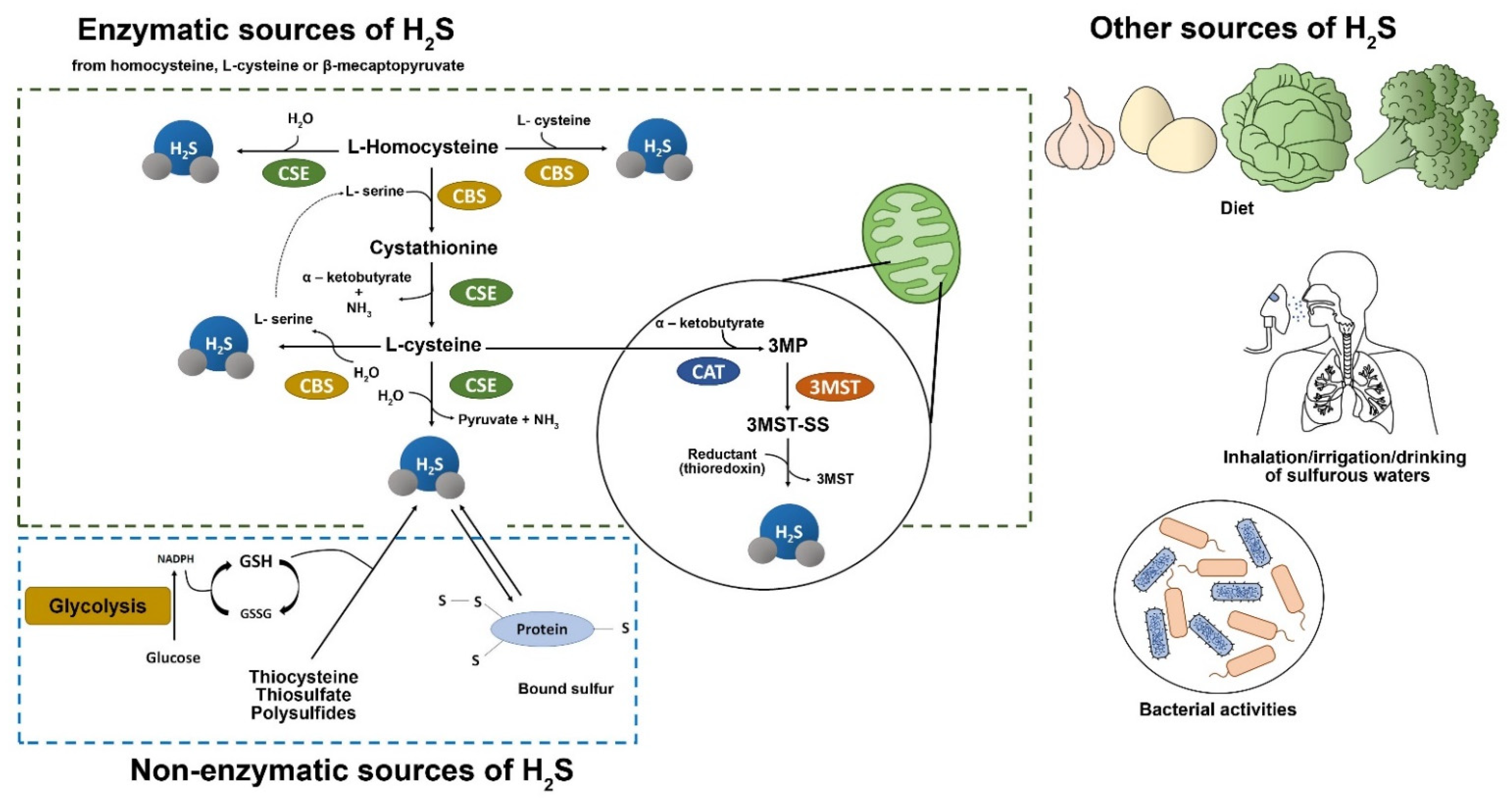
2. Hydrogen Sulfide Biology, Intracellular Signal Transduction and Potential Targets
2.1. Hydrogen Sulfide Biology
2.2. Hydrogen Sulfide Intracellular Signal Transduction Pathways
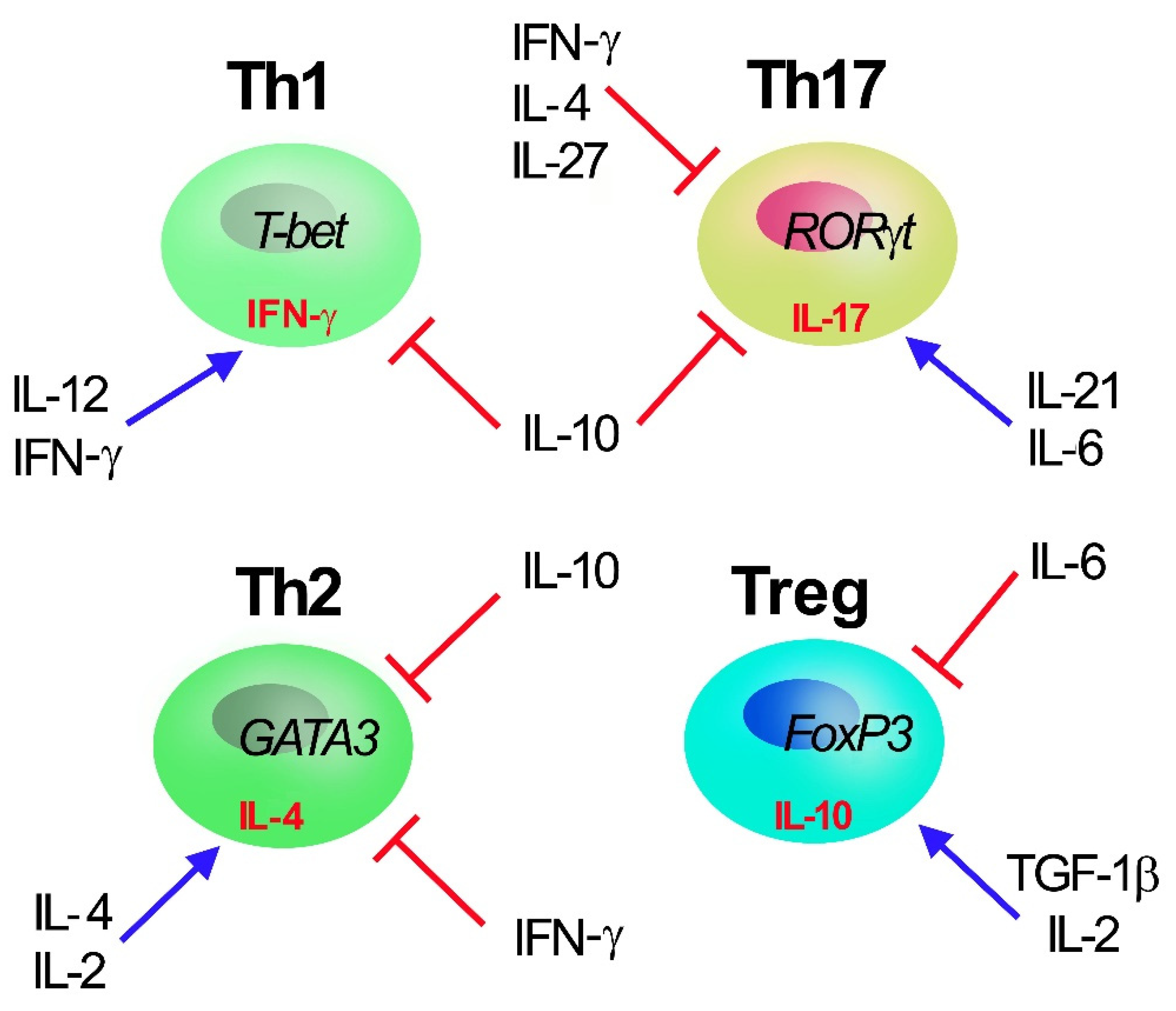
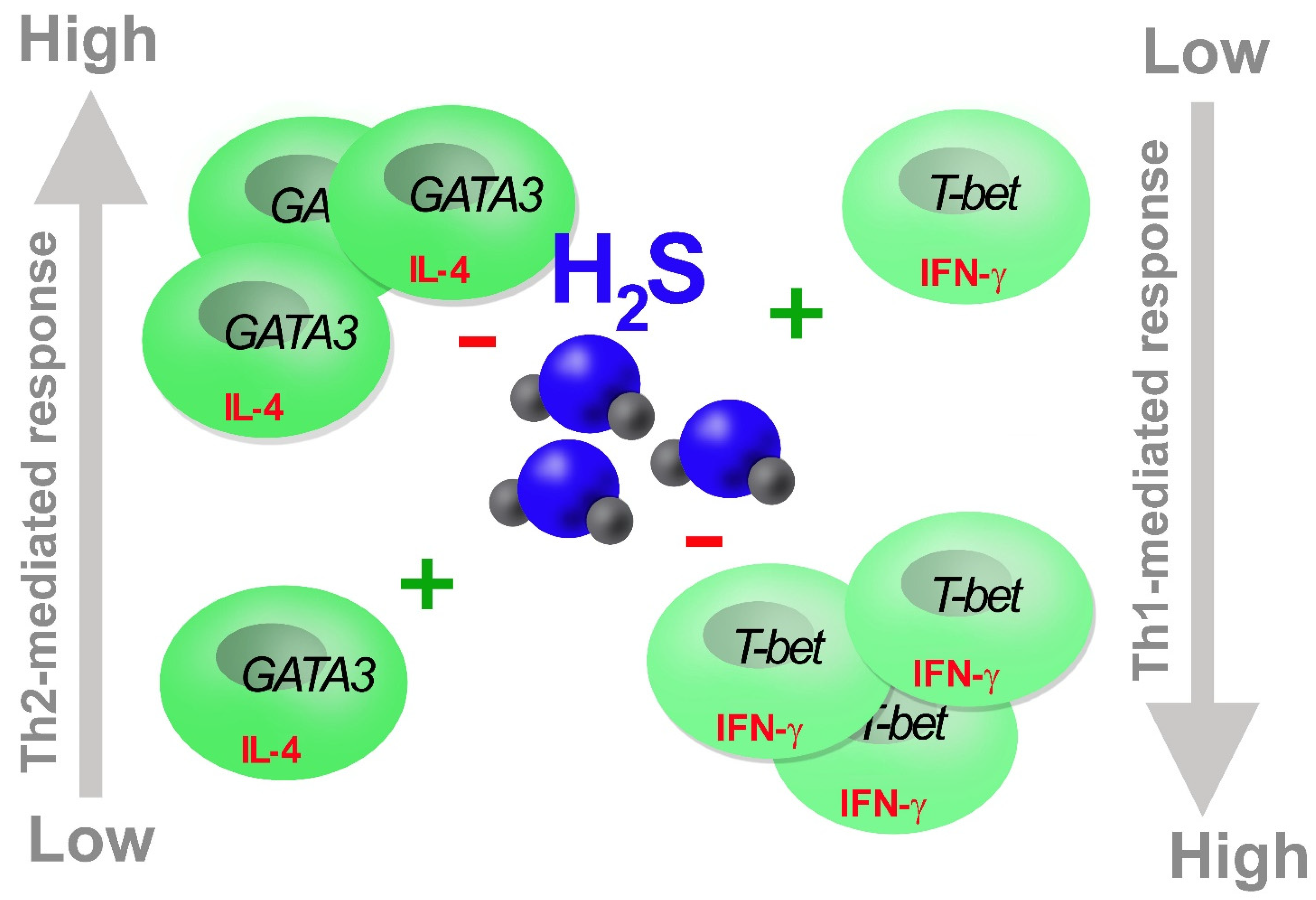
3. H2S in T Cells
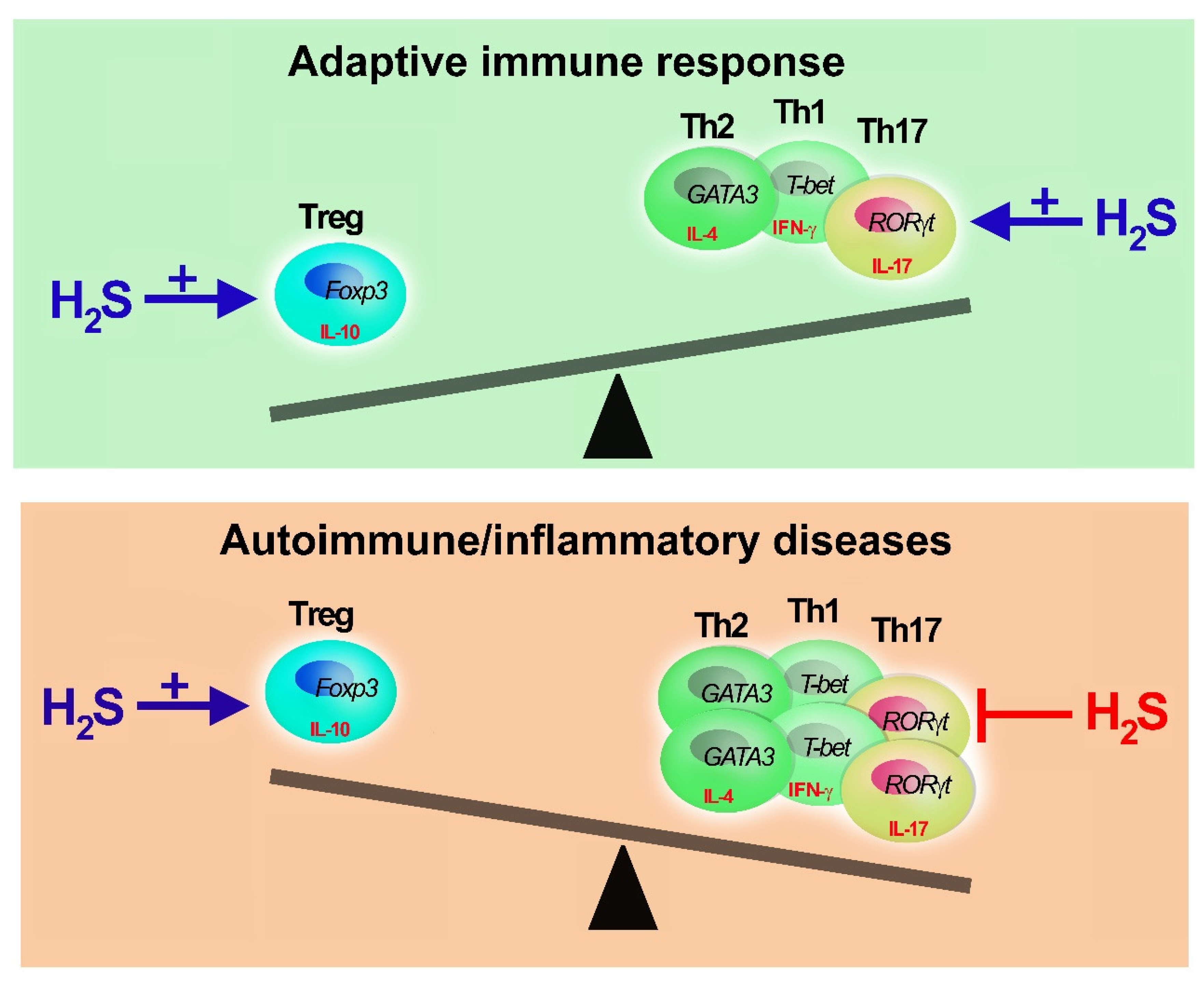
4. Role of H2S in Th17 Cells
5. Role of H2S in Treg
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Medzhitov, R. Recognition of microorganisms and activation of the immune response. Nature 2007, 449, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yamane, H.; Paul, W.E. Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol. 2010, 28, 445–489. [Google Scholar] [CrossRef]
- Dilek, N.; Papapetropoulos, A.; Toliver-Kinsky, T.; Szabo, C. Hydrogen sulfide: An endogenous regulator of the immune system. Pharmacol. Res. 2020, 161, 105119. [Google Scholar] [CrossRef]
- Dong, C. Cytokine Regulation and Function in T Cells. Annu. Rev. Immunol. 2021, 39, 51–76. [Google Scholar] [CrossRef] [PubMed]
- Martini, S.; Pozzi, G.; Carubbi, C.; Masselli, E.; Galli, D.; Di Nuzzo, S.; Banchini, A.; Gobbi, G.; Vitale, M.; Mirandola, P. PKCε promotes human Th17 differentiation: Implications in the pathophysiology of psoriasis. Eur. J. Immunol. 2018, 48, 644–654. [Google Scholar] [CrossRef]
- Bassini, A.; Zauli, G.; Migliaccio, G.; Migliaccio, A.R.; Pascuccio, M.; Pierpaoli, S.; Guidotti, L.; Capitani, S.; Vitale, M. Lineage-restricted expression of protein kinase C isoforms in hematopoiesis. Blood 1999, 93, 1178–1188. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, G.; Mirandola, P.; Carubbi, C.; Galli, D.; Vitale, M. Protein kinase C ε in hematopoiesis: Conductor or selector? Semin. Thromb. Hemost. 2013, 39, 59–65. [Google Scholar] [CrossRef]
- Mosmann, T.R.; Cherwinski, H.; Bond, M.W.; Giedlin, M.A.; Coffman, R.L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986, 136, 2348–2357. [Google Scholar] [PubMed]
- Harrington, L.E.; Hatton, R.D.; Mangan, P.R.; Turner, H.; Murphy, T.L.; Murphy, K.M.; Weaver, C.T. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005, 6, 1123–1132. [Google Scholar] [CrossRef]
- Park, H.; Li, Z.; Yang, X.O.; Chang, S.H.; Nurieva, R.; Wang, Y.H.; Wang, Y.; Hood, L.; Zhu, Z.; Tian, Q.; et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005, 6, 1133–1141. [Google Scholar] [CrossRef]
- Tesmer, L.A.; Lundy, S.K.; Sarkar, S.; Fox, D.A. Th17 cells in human disease. Immunol. Rev. 2008, 223, 87–113. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T cells and immune tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef]
- Ohkura, N.; Hamaguchi, M.; Morikawa, H.; Sugimura, K.; Tanaka, A.; Ito, Y.; Osaki, M.; Tanaka, Y.; Yamashita, R.; Nakano, N.; et al. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity 2012, 37, 785–799. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Q.; Jin, H.F.; Zhang, H.; Tang, C.S.; Du, J.B. Interaction among Hydrogen Sulfide and Other Gasotransmitters in Mammalian Physiology and Pathophysiology. Adv. Exp. Med. Biol. 2021, 1315, 205–236. [Google Scholar] [CrossRef]
- Predmore, B.L.; Lefer, D.J.; Gojon, G. Hydrogen sulfide in biochemistry and medicine. Antioxid. Redox Signal. 2012, 17, 119–140. [Google Scholar] [CrossRef]
- Wang, R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, L.; Song, S.; Pan, L.; Arslan, M.I.; Chen, Y.; Yang, S. Hydrogen sulfide: Recent progress and perspectives for the treatment of dermatological diseases. J. Adv. Res. 2020, 27, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C. Hydrogen sulfide, an endogenous stimulator of mitochondrial function in cancer cells. Cells 2021, 10, 220. [Google Scholar] [CrossRef]
- Wang, R. Two’s company, three’s a crowd: Can H2S be the third endogenous gaseous transmitter? FASEB J. 2002, 16, 1792–1798. [Google Scholar] [CrossRef] [PubMed]
- Braga, P.C.; Sasso, M.D.; Culici, M.; Falchi, M.; Spallino, A.; Nappi, G. Free radical–scavenging activity of sulfurous water investigated by electron paramagnetic resonance (EPR) spectroscopy. Exp. Lung Res. 2012, 38, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Carbajo, J.; Maraver, F. Sulphurous mineral waters: New applications for health. Evid.-Based Complement. Alternat. Med. 2017, 2017, 8034084. [Google Scholar] [CrossRef]
- Kolluru, G.K.; Shen, X.; Bir, S.C.; Kevil, C.G. Hydrogen sulfide chemical biology: Pathophysiological roles and detection. Nitric Oxide 2013, 35, 5–20. [Google Scholar] [CrossRef]
- Bazhanov, N.; Ansar, M.; Ivanciuc, T.; Garofalo, R.; Casola, A. Hydrogen sulfide: A novel player in airway development, pathophysiology of respiratory diseases, and antiviral defenses. Am. J. Respir. Cell Mol. Biol. 2017, 57, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Benavides, G.; Squadrito, G.; Mills, R.; Patel, H.; Isbell, T.; Patel, R.; Darley-Usmar, V.M.; Doeller, J.E.; Kraus, D.W. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. USA 2007, 104, 17977–17982. [Google Scholar] [CrossRef] [PubMed]
- Perry, M.; Hui, C.; Whiteman, M.; Wood, M.; Adcock, I.; Kirkham, P.; Michaeloudes, C.; Chung, K.F. Hydrogen sulfide inhibits proliferation and release of IL-8 from human airway smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 2011, 45, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Pouokam, E.; Althaus, M. Epithelial electrolyte transport physiology and the gasotransmitter hydrogen sulfide. Oxidative Med. Cell. Longev. 2016, 2016, 4723416. [Google Scholar] [CrossRef]
- Pozzi, G.; Masselli, E.; Gobbi, G.; Mirandola, P.; Taborda-Barata, L.; Ampollini, L.; Carbognani, P.; Micheloni, C.; Corazza, F.; Galli, D.; et al. Hydrogen sulfide inhibits TMPRSS2 in human airway epithelial cells: Implications for SARS-CoV-2 infection. Biomedicines 2021, 9, 1273. [Google Scholar] [CrossRef]
- Wallace, J.; Wang, R. Hydrogen sulfide-based therapeutics: Exploiting a unique but ubiquitous gasotransmitter. Nat. Rev. Drug Discov. 2015, 14, 329. [Google Scholar] [CrossRef]
- Yuan, S.; Patel, R.; Kevil, C. Working with nitric oxide and hydrogen sulfide in biological systems. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 308, L403–L415. [Google Scholar] [CrossRef]
- Benedetti, F.; Davinelli, S.; Krishnan, S.; Gallo, R.; Scapagnini, G.; Zella, D.; Curreli, S. Sulfur compounds block MCP-1 production by Mycoplasma fermentans-infected macrophages through NF-kB inhibition. J. Transl. Med. 2014, 12, 145. [Google Scholar] [CrossRef]
- Benedetti, F.; Curreli, S.; Krishnan, S.; Davinelli, S.; Cocchi, F.; Scapagnini, G.; Gallo, R.C.; Zella, D. Anti-inflammatory effects of H2S during acute bacterial infection: A review. J. Transl. Med. 2017, 15, 100. [Google Scholar] [CrossRef] [PubMed]
- Viegas, J.; Esteves, A.F.; Cardoso, E.M.; Arosa, F.A.; Vitale, M.; Taborda-Barata, L. Biological Effects of thermal water-associated hydrogen sulfide on human airways and associated immune cells: Implications for respiratory diseases. Front. Public Health 2019, 7, 128. [Google Scholar] [CrossRef]
- Carubbi, C.; Masselli, E.; Calabrò, E.; Bonati, E.; Galeone, C.; Andreoli, R.; Goldoni, M.; Corradi, M.; Sverzellati, N.; Pozzi, G.; et al. Sulphurous thermal water inhalation impacts respiratory metabolic parameters in heavy smokers. Int. J. Biometeorol. 2019, 63, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Pattillo, C.B.; Pardue, S.; Bir, S.C.; Wang, R.; Kevil, C.G. Measurement of plasma hydrogen sulfide in vivo and in vitro. Free Radic. Biol. Med. 2011, 50, 1021–1031. [Google Scholar] [CrossRef]
- Klingerman, C.M.; Trushin, N.; Prokopczyk, B.; Haouzi, P. H2S concentrations in the arterial blood during H2S administration in relation to its toxicity and effects on breathing. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2013, 305, R630–R638. [Google Scholar] [CrossRef]
- Szabó, C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007, 6, 917–935. [Google Scholar] [CrossRef]
- Olson, K.R. Is hydrogen sulfide a circulating “gasotransmitter” in vertebrate blood? Biochim. Biophys. Acta (BBA)-Bioenerg. 2009, 1787, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.L. Transport of H2S and HS(-) across the human red blood cell membrane: Rapid H2S diffusion and AE1-mediated Cl(-)/HS(-) exchange. Am. J. Physiol. Cell Physiol. 2013, 305, C941–C950. [Google Scholar] [CrossRef] [PubMed]
- Vitvitsky, V.; Yadav, P.K.; Kurthen, A.; Banerjee, R. Sulfide oxidation by a noncanonical pathway in red blood cells generates thiosulfate and polysulfides. J. Biol. Chem. 2015, 290, 8310–8320. [Google Scholar] [CrossRef] [PubMed]
- Pietri, R.; Román-Morales, E.; López-Garriga, J. Hydrogen sulfide and hemeproteins: Knowledge and mysteries. Antioxid. Redox Signal. 2011, 15, 393–404. [Google Scholar] [CrossRef]
- Moest, R.R. Hydrogen sulfide determination by the methylene blue method. Anal. Chem. 1975, 47, 1204–1205. [Google Scholar] [CrossRef]
- Ahn, Y.J.; Lee, Y.J.; Lee, J.; Lee, D.; Park, H.K.; Lee, G.J. Colorimetric detection of endogenous hydrogen sulfide production in living cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 177, 118–124. [Google Scholar] [CrossRef]
- Karunya, R.; Jayaprakash, K.S.; Gaikwad, R.; Sajeesh, P.; Ramshad, K.; Muraleedharan, K.M.; Dixit, M.; Thangaraj, P.R.; Sen, A.K. Rapid measurement of hydrogen sulphide in human blood plasma using a microfluidic method. Sci. Rep. 2019, 9, 3258. [Google Scholar] [CrossRef] [PubMed]
- Heshka, N.E.; Hager, D.B. A multidimensional gas chromatography method for the analysis of hydrogen sulfide in crude oil and crude oil headspace. J. Sep. Sci. 2014, 37, 3649–3655. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Jin, S.; Sun, J.; Gu, Z.; Sun, X.; Zhu, Y.; Huo, K.; Cao, Z.; Yang, P.; Xin, X.; et al. New method for quantification of gasotransmitter hydrogen sul-fide in biological matrices by LC-MS/MS. Sci. Rep. 2017, 7, 46278. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.D.; Hall, J.R.; Schoenfisch, M.H. A direct and selective electrochemical hydrogen sulfide sensor. Anal. Chim. Acta 2019, 1045, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Lin, V.S.; Chen, W.; Xian, M.; Chang, C.J. Chemical probes for molecular imaging and detection of hydrogen sulfide and reactive sulfur species in biological systems. Chem. Soc. Rev. 2015, 44, 4596–4618. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.; DeLeon, E.; Liu, F. Controversies and conundrums in hydrogen sulfide biology. Nitric Oxide 2014, 41, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Calvert, J.; Coetzee, W.; Lefer, D. Novel insights into hydrogen sulfide–mediated cytoprotection. Antioxid. Redox Signal. 2010, 12, 1203–1217. [Google Scholar] [CrossRef]
- Yang, G.; Sun, X.; Wang, R. Hydrogen sulfide-induced apoptosis of human aorta smooth muscle cells via the activation of mitogen-activated protein kinases and caspase-3. FASEB J. 2004, 18, 1782–1784. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, G.; Tang, G.; Wu, L.; Wang, R. Rat pancreatic level of cystathionine γ-lyase is regulated by glucose level via specificity protein 1 (SP1) phosphorylation. Diabetologia 2011, 54, 2615–2625. [Google Scholar] [CrossRef]
- Rinaldi, L.; Gobbi, G.; Pambianco, M.; Micheloni, C.; Mirandola, P.; Vitale, M. Hydrogen sulfide prevents apoptosis of human PMN via inhibition of p38 and caspase 3. Lab. Investig. 2006, 86, 391–397. [Google Scholar] [CrossRef]
- Gobbi, G.; Ricci, F.; Malinverno, C.; Carubbi, C.; Pambianco, M.; Panfilis, G.; Vitale, M.; Mirandola, P. Hydrogen sulfide impairs keratinocyte cell growth and adhesion inhibiting mitogen-activated protein kinase signaling. Lab. Investig. 2009, 89, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- Mirandola, P.; Gobbi, G.; Micheloni, C.; Vaccarezza, M.; Di Marcantonio, D.; Ruscitti, F.; de Panfilis, G.; Vitale, M. Hydrogen sulfide inhibits IL-8 expression in human keratinocytes via MAP kinase signaling. Lab. Investig. 2011, 91, 1188–1194. [Google Scholar] [CrossRef]
- Cai, W.J.; Wang, M.J.; Moore, P.K.; Jin, H.M.; Yao, T.; Zhu, Y.C. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc. Res. 2007, 76, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.W.; Wang, E.A.; Gould, S.; Stein, E.V.; Kaur, S.; Lim, L.; Amarnath, S.; Fowler, D.H.; Roberts, D.D. Hydrogen sulfide is an endogenous potentiator of T cell activation. J. Biol. Chem. 2012, 287, 4211–4221. [Google Scholar] [CrossRef]
- Li, L.; Salto-Tellez, M.; Tan, C.H.; Whiteman, M.; Moore, P.K. GYY4137, a novel hydrogen sulfide-releasing molecule, protects against endotoxic shock in the rat. Free Radic. Biol. Med. 2009, 47, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Calvert, J.W.; Jha, S.; Gundewar, S.; Elrod, J.W.; Ramachandran, A.; Pattillo, C.B.; Kevil, C.G.; Lefer, D.J. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ. Res. 2009, 105, 365–374. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, R.; Liu, X.; Zhou, Y.; Qu, C.; Kikuiri, T.; Wang, S.; Zandi, E.; Du, J.; Ambudkar, I.S.; et al. Hydrogen sulfide maintains mesenchymal stem cell function and bone homeostasis via regulation of Ca2+ channel sulfhydration. Cell Stem Cell 2014, 15, 66–78. [Google Scholar] [CrossRef]
- Grassi, F.; Tyagi, A.M.; Calvert, J.W.; Gambari, L.; Walker, L.D.; Yu, M.; Robinson, J.; Li, J.Y.; Lisignoli, G.; Vaccaro, C.; et al. Hydrogen sulfide is a novel regulator of bone formation implicated in the bone loss induced by estrogen deficiency. J. Bone Miner. Res. 2015, 31, 949–963. [Google Scholar] [CrossRef]
- Fox, B.; Schantz, J.T.; Haigh, R.; Wood, M.E.; Moore, P.K.; Viner, N.; Spencer, J.P.; Winyard, P.G.; Whiteman, M. Inducible hydrogen sulfide synthesis in chondrocytes and mesenchymal progenitor cells: Is H2S a novel cytoprotective mediator in the inflamed joint? J. Cell. Mol. Med. 2012, 16, 896–910. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Sun, A.; Zhu, W.; Huang, Z.; Hu, X.; Jia, J.; Zou, Y.; Ge, J. Transplantation of mesenchymal stem cells preconditioned with hydrogen sulfide enhances repair of myocardial infarction in rats. Tohoku J. Exp. Med. 2012, 226, 29–36. [Google Scholar] [CrossRef]
- Li, C.; Guo, Z.; Guo, B.; Xie, Y.; Yang, J.; Wang, A. Inhibition of the endogenous CSE/H2S system contributes to hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells. Mol. Med. Rep. 2014, 9, 2467–2472. [Google Scholar] [CrossRef][Green Version]
- Guo, Z.; Li, C.S.; Wang, C.M.; Xie, Y.J.; Wang, A.L. CSE/H2S system protects mesenchymal stem cells from hypoxia and serum deprivation induced apoptosis via mitochondrial injury, endoplasmic reticulum stress and PI3K/Akt activation pathways. Mol. Med. Rep. 2015, 12, 2128–2134. [Google Scholar] [CrossRef]
- Mustafa, A.K.; Gadalla, M.M.; Sen, N.; Kim, S.; Mu, W.; Gazi, S.K.; Barrow, R.K.; Yang, G.; Wang, R.; Snyder, S.H. H2S signals through protein S-sulfhydration. Sci. Signal. 2009, 2, ra72. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Snyder, S.H. H2S signalling through protein sulfhydration and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 499–507. [Google Scholar] [CrossRef]
- Tossounian, M.A.; Zhang, B.; Gout, I. The writers, readers, and erasers in redox regulation of GAPDH. Antioxidants 2020, 9, 1288. [Google Scholar] [CrossRef]
- Sen, N.; Paul, B.D.; Gadalla, M.M.; Mustafa, A.K.; Sen, T.; Xu, R.; Kim, S.; Snyder, S.H. Hydrogen sulfide-linked sulfhydration of NF-kappaB mediates its antiapoptotic actions. Mol. Cell 2012, 45, 13–24. [Google Scholar] [CrossRef]
- Yang, R.; Qu, C.; Zhou, Y.; Konkel, J.E.; Shi, S.; Liu, Y.; Chen, C.; Liu, S.; Liu, D.; Chen, Y.; et al. Hydrogen sulfide promotes Tet1- and Tet2-mediated Foxp3 demethylation to drive regulatory T cell differentiation and maintain immune homeostasis. Immunity 2015, 43, 251–263. [Google Scholar] [CrossRef]
- Tang, G.; Wu, L.; Wang, R. Interaction of hydrogen sulfide with ion channels. Clin. Exp. Pharmacol. Physiol. 2010, 37, 753–763. [Google Scholar] [CrossRef]
- Kimura, Y.; Dargusch, R.; Schubert, D.; Kimura, H. Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid. Redox Signal. 2006, 8, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Munaron, L.; Avanzato, D.; Moccia, F.; Mancardi, D. Hydrogen sulfide as a regulator of calcium channels. Cell Calcium 2013, 53, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.B.; Li, Y.S.; Miyano, K.; Nakata, Y. Phosphorylation of TRPV1 by neurokinin-1 receptor agonist exaggerates the capsaicin-mediated substance P release from cultured rat dorsal root ganglion neurons. Neuropharmacology 2008, 55, 1405–1411. [Google Scholar] [CrossRef]
- Jimenez, M. Hydrogen sulfide as a signaling molecule in the enteric nervous system. Neurogastroenterol. Motil. 2010, 22, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Qin, M.; Jia, W.; Huang, Z.; Li, Z.; Yang, D.; Huang, M.; Xiao, C.; Long, F.; Mao, J.; et al. Cystathionine-gamma-lyase ameliorates the histone demethylase JMJD3-mediated autoimmune response in rheumatoid arthritis. Cell. Mol. Immunol. 2019, 16, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Rios, C.S.; Szczesny, B.; Soriano, F.G.; Olah, G.; Szabo, C. Hydrogen sulfide attenuates cytokine production through the modulation of chromatin remodeling. Int. J. Mol. Med. 2015, 35, 1741–1746. [Google Scholar] [CrossRef] [PubMed]
- Behera, J.; Tyagi, S.C.; Tyagi, N. Role of hydrogen sulfide in the musculoskeletal system. Bone 2019, 124, 33–39. [Google Scholar] [CrossRef]
- Avni, O.; Lee, D.; Macian, F.; Szabo, S.J.; Glimcher, L.H.; Rao, A. T(H) cell differentiation is accompanied by dynamic changes in histone acetylation of cytokine genes. Nat. Immunol. 2002, 3, 643–651. [Google Scholar] [CrossRef]
- Vitale, M. Sulphur balneotherapy and patient’s immunity: H2S effects on human CD4+ T cell polarization to Th17 and Treg phenotype. Bol. Soc. Esp. Hidrol. Méd. 2018, 33, 68–69. [Google Scholar] [CrossRef]
- Garg, S.K.; Yan, Z.; Vitvitsky, V.; Banerjee, R. Differential dependence on cysteine from transsulfuration versus transport during T cell activation. Antioxid. Redox Signal. 2011, 15, 39–47. [Google Scholar] [CrossRef]
- Lund, R.; Aittokallio, T.; Nevalainen, O.; Lahesmaa, R. Identification of novel genes regulated by IL-12, IL-4, or TGF-beta during the early polarization of CD4+ lymphocytes. J. Immunol. 2003, 171, 5328–5336. [Google Scholar] [CrossRef] [PubMed]
- Mirandola, P.; Gobbi, G.; Sponzilli, I.; Pambianco, M.; Malinverno, C.; Cacchioli, A.; De Panfilis, G.; Vitale, M. Exogenous hydrogen sulfide induces functional inhibition and cell death of cytotoxic lymphocytes subsets. J. Cell Physiol. 2007, 213, 826–833. [Google Scholar] [CrossRef]
- Han, Y.; Zeng, F.; Tan, G.; Yang, C.; Tang, H.; Luo, Y.; Feng, J.; Xiong, H.; Guo, Q. Hydrogen sulfide inhibits abnormal proliferation of lymphocytes via AKT/GSK3beta signal pathway in systemic lupus erythematosus patients. Cell Physiol. Biochem. 2013, 31, 795–804. [Google Scholar] [CrossRef]
- Bhoj, V.G.; Chen, Z.J. Ubiquitylation in innate and adaptive immunity. Nature 2009, 26, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhi, L.; Moochhala, S.; Moore, P.K.; Bhatia, M. Hydrogen sulfide acts as an inflammatory mediator in cecal ligation and puncture-induced sepsis in mice by upregulating the production of cytokines and chemokines via NF-kappaB. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, 960–971. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhao, K.; Ju, Y.; Mani, S.; Cao, Q.; Puukila, S.; Khaper, N.; Wu, L.; Wang, R. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid. Redox Signal. 2013, 18, 1906–1919. [Google Scholar] [CrossRef]
- Xie, L.; Gu, Y.; Wen, M.; Zhao, S.; Wang, W.; Ma, Y.; Meng, G.; Han, Y.; Wang, Y.; Liu, G.; et al. Hydrogen sulfide induces Keap1 S-sulfhydration and suppresses diabetes-accelerated atherosclerosis via Nrf2 activation. Diabetes 2016, 65, 3171–3184. [Google Scholar] [CrossRef] [PubMed]
- Bruzzese, L.; Fromonot, J.; By, Y.; Durand-Gorde, J.M.; Condo, J.; Kipson, N.; Guieu, R.; Fenouillet, E.; Ruf, J. NF-kappaB enhances hypoxia-driven T-cell immunosuppression via upregulation of adenosine A(2A) receptors. Cell. Signal. 2014, 26, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Merighi, S.; Battistello, E.; Giacomelli, L.; Varani, K.; Vincenzi, F.; Borea, P.A.; Gessi, S. Targeting A3 and A2A adenosine receptors in the fight against cancer. Expert Opin. Ther. Targets 2019, 23, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of adenosine receptors: The state of the art. Physiol. Rev. 2018, 98, 1591–1625. [Google Scholar] [CrossRef] [PubMed]
- Sitkovsky, M.V. Sufficient numbers of anti-tumor T cells is a condition of maximal efficacy of anti-hypoxia-A2-adenosinergic drugs during cancer immunotherapy. Curr. Opin. Pharmacol. 2020, 53, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, P.; Merrick, A.E.; West, E.; O’Donnell, D.; Selby, P.; Vile, R.; Melcher, A.A. Optimization of dendritic cell loading with tumor cell lysates for cancer immunotherapy. J. Immunother. 2008, 31, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, S.M.; Sitkovsky, M. A2A adenosine receptor antagonists to weaken the hypoxia-HIF-1alpha driven immunosuppression and improve immunotherapies of cancer. Curr. Opin. Pharmacol. 2016, 29, 90–96. [Google Scholar] [CrossRef]
- Westendorf, A.M.; Skibbe, K.; Adamczyk, A.; Buer, J.; Geffers, R.; Hansen, W.; Pastille, E.; Jendrossek, V. Hypoxia enhances immunosuppression by inhibiting CD4+ effector t cell function and promoting treg activity. Cell. Physiol. Biochem. 2017, 41, 1271–1284. [Google Scholar] [CrossRef] [PubMed]
- De Cicco, P.; Ercolano, G.; Rubino, V.; Terrazzano, G.; Ruggiero, G.; Cirino, G.; Ianaro, A. Modulation of the functions of myeloid-derived suppressor cells: A new strategy of hydrogen sulfide anti-cancer effects. Br. J. Pharmacol. 2020, 177, 884–897. [Google Scholar] [CrossRef] [PubMed]
- Figliuolo, V.R.; Dos Santos, L.M.; Abalo, A.; Nanini, H.; Santos, A.; Brittes, N.M.; Bernardazzi, C.; de Souza, H.S.P.; Vieira, L.Q.; Coutinho-Silva, R.; et al. Sulfate-reducing bacteria stimulate gut immune responses and contribute to inflammation in experimental colitis. Life Sci. 2017, 189, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Liu, L.; Ji, X.; Gao, Y.; Chen, X.; Liu, Y.; Liu, Y.; Zhao, X.; Li, Y.; Li, Y.; et al. The phosphatase DUSP2 controls the activity of the transcription activator STAT3 and regulates TH17 differentiation. Nat. Immunol. 2015, 16, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Bertin, S.; Lozano-Ruiz, B.; Bachiller, V.; Garcia-Martinez, I.; Herdman, S.; Zapater, P.; Francés, R.; Such, J.; Lee, J.; Raz, E.; et al. Dual-specificity phosphatase 6 regulates CD4+ T-cell functions and restrains spontaneous colitis in IL-10-deficient mice. Mucosal Immunol. 2015, 8, 505–515. [Google Scholar] [CrossRef]
- Brownlie, R.J.; Miosge, L.A.; Vassilakos, D.; Svensson, L.M.; Cope, A.; Zamoyska, R. Lack of the phosphatase PTPN22 increases adhesion of murine regulatory T cells to improve their immunosuppressive function. Sci. Signal. 2012, 5, ra87. [Google Scholar] [CrossRef] [PubMed]
- Pike, K.A.; Tremblay, M.L. Protein tyrosine phosphatases: Regulators of CD4 T cells in inflammatory bowel disease. Front. Immunol. 2018, 9, 2504. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.; Fu, C.; Pappin, D.J.; Tonks, N.K. H2S-Induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Sci. Signal. 2011, 4, ra86. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, P.; Yang, G.; Cao, Q.; Wang, R. The inhibitory role of hydrogen sulfide in airway hyperresponsiveness and inflammation in a mouse model of asthma. Am. J. Pathol. 2013, 182, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Cumming, B.M.; Addicott, K.W.; Pacl, H.T.; Russell, S.L.; Nargan, K.; Naidoo, T.; Ramdial, P.K.; Adamson, J.H.; Wang, R.; et al. Hydrogen sulfide dysregulates the immune response by suppressing central carbon metabolism to promote tuberculosis. Proc. Natl. Acad. Sci. USA 2020, 117, 6663–6674. [Google Scholar] [CrossRef] [PubMed]
- Vuillefroy de Silly, R.; Coulon, F.; Poirier, N.; Jovanovic, V.; Brouard, S.; Ferchaud-Roucher, V.; Blancho, G.; Vanhove, B. Transplant tolerance is associated with reduced expression of cystathionine-γ-lyase that controls IL-12 production by dendritic cells and TH-1 immune responses. Blood 2012, 119, 2633–2643. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Pan, L.L.; Long, F.; Wu, W.J.; Yan, D.; Xu, P.; Liu, S.Y.; Qin, M.; Jia, W.W.; Liu, X.H.; et al. Endogenous hydrogen sulfide ameliorates NOX4 induced oxidative stress in LPS-stimulated macrophages and mice. Cell. Physiol. Biochem. 2018, 47, 458–474. [Google Scholar] [CrossRef]
- Jing, H.; Gao, X.; Xu, L.; Lin, H.; Zhang, Z. H2S promotes a glycometabolism disorder by disturbing the Th1/Th2 balance during LPS-induced inflammation in the skeletal muscles of chickens. Chemosphere 2019, 222, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Zhou, X.; Zhang, J.; Huang, X.; Zhai, Y.; Zhang, X.; Chu, L. Hydrogen sulfide protects against bleomycin-induced pulmonary fibrosis in rats by inhibiting NF-kappaB expression and regulating Th1/Th2 balance. Toxicol. Lett. 2014, 224, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Poggi, A.; Musso, A.; Dapino, I.; Zocchi, M.R. Mechanisms of tumor escape from immune system: Role of mesenchymal stromal cells. Immunol. Lett. 2014, 159, 55–72. [Google Scholar] [CrossRef]
- Dong, Q.; Yang, B.; Han, J.G.; Zhang, M.M.; Liu, W.; Zhang, X.; Yu, H.L.; Liu, Z.G.; Zhang, S.H.; Li, T.; et al. A novel hydrogen sulfide-releasing donor, HA-ADT, suppresses the growth of human breast cancer cells through inhibiting the PI3K/AKT/mTOR and Ras/Raf/MEK/ERK signaling pathways. Cancer Lett. 2019, 455, 60–72. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Shi, X.; Liang, H.; Ye, J.; Wang, L.; Han, H.; Fang, H.; Kang, W.; Wang, T. Cystathionine- γ-lyase promotes process of breast cancer in association with STAT3 signaling pathway. Oncotarget 2017, 8, 65677–65686. [Google Scholar] [CrossRef]
- Youness, R.A.; Gad, A.Z.; Sanber, K.; Ahn, Y.J.; Lee, G.J.; Khallaf, E.; Hafez, H.M.; Motaal, A.A.; Ahmed, N.; Gad, M.Z. Targeting hydrogen sulphide signaling in breast cancer. J. Adv. Res. 2020, 27, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fu, Q.; Tang, S.; Xie, Y.; Meng, Q.; Tang, X.; Zhang, S.; Zhang, H.; Schroyen, M. Proteomics analysis of lung reveals inflammation and cell death induced by atmospheric H2S exposure in pig. Environ. Res. 2020, 191, 110204. [Google Scholar] [CrossRef] [PubMed]
- Ueno, A.; Jeffery, L.; Kobayashi, T.; Hibi, T.; Ghosh, S.; Jijon, H. Th17 plasticity and its relevance to inflammatory bowel disease. J. Autoimmun. 2018, 87, 38–49. [Google Scholar] [CrossRef]
- Hurtado, C.G.; Wan, F.; Housseau, F.; Sears, C.L. Roles for interleukin 17 and adaptive immunity in pathogenesis of colorectal cancer. Gastroenterology 2018, 155, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Lubberts, E. The IL-23-IL-17 axis in inflammatory arthritis. Nat. Rev. Rheumatol. 2015, 7, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Tong, Z.; Xu, Q.; Wu, X.; Wang, X.; Jin, X.; Ma, W.; Cheng, X.; Zhou, Q.; Shi, H. Interplay of Th1 and Th17 cells in murine models of malignant pleural effusion. Am. J. Respir. Crit. Care Med. 2014, 189, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, J.; Wang, W.; Qi, X.; Wang, Y.; Tian, B.; Dai, H.; Wang, J.; Ning, W.; Yang, T.; et al. Targeting IL-17 attenuates hypoxia-induced pulmonary hypertension through downregulation of β-catenin. Thorax 2019, 74, 564–578. [Google Scholar] [CrossRef] [PubMed]
- Stockinger, B.; Omenetti, S. The dichotomous nature of T helper 17 cells. Nat. Rev. Immunol. 2017, 17, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Farkas, A.M.; Panea, C.; Goto, Y.; Nakato, G.; Galan-Diez, M.; Narushima, S.; Honda, K.; Ivanov, I.I. Induction of Th17 cells by segmented filamentous bacteria in the murine intestine. J. Immunol. Methods 2015, 421, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Tanoue, T.; Umesaki, Y.; Honda, K. Regulation of Th17 cell differentiation by intestinal commensal bacteria. Benef. Microbes 2010, 1, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Tanoue, T.; Ando, M.; Kamada, N.; Nagano, Y.; Narushima, S.; Suda, W.; Imaoka, A.; Setoyama, H.; Nagamori, T.; et al. Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell 2015, 163, 367–380. [Google Scholar] [CrossRef]
- Jiang, W.; Su, J.; Zhang, X.; Cheng, X.; Zhou, J.; Shi, R.; Zhang, H. Elevated levels of Th17 cells and Th17-related cytokines are associated with disease activity in patients with inflammatory bowel disease. Inflamm. Res. 2014, 63, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Ohnmacht, C.; Park, J.H.; Cording, S.; Wing, J.B.; Atarashi, K.; Obata, Y.; Gaboriau-Routhiau, V.; Marques, R.; Dulauroy, S.; Fedoseeva, M.; et al. Mucosal Immunology. The microbiota regulates type 2 immunity through RORgammat+ T cells. Science 2015, 349, 989–993. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.A.; Penny, T.R.; Paton, M.C.B.; Sutherland, A.E.; Nekkanti, L.; Yawno, T.; Castillo-Melendez, M.; Fahey, M.C.; Jones, N.M.; Jenkin, G.; et al. Effects of umbilical cord blood cells, and subtypes, to reduce neuroinflammation following perinatal hypoxic-ischemic brain injury. J. Neuroinflammation 2018, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xin, D.; Wang, L.; Zhang, T.; Bai, X.; Li, T.; Xie, Y.; Xue, H.; Bo, S.; Liu, D.; et al. Therapeutic effects of L-Cysteine in newborn mice subjected to hypoxia-ischemia brain injury via the CBS/H2S system: Role of oxidative stress and endoplasmic reticulum stress. Redox Biol. 2017, 13, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Xin, D.; Chu, X.; Bai, X.; Ma, W.; Yuan, H.; Qiu, J.; Liu, C.; Li, T.; Zhou, X.; Chen, W.; et al. l-Cysteine suppresses hypoxia-ischemia injury in neonatal mice by reducing glial activation, promoting autophagic flux and mediating synaptic modification via H2S formation. Brain Behav. Immun. 2018, 73, 222–234. [Google Scholar] [CrossRef]
- Li, T.; Chu, X.; Xin, D.; Ke, H.; Wang, S.; Liu, D.; Chen, W.; Wang, Z. H2S prevents peripheral immune cell invasion, increasing [Ca2+]i and excessive phagocytosis following hypoxia-ischemia injury in neonatal mice. Biomed. Pharmacother. 2021, 135, 111207. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. Hydrogen sulfide (H2S) and polysulfide (H2Sn) signaling: The first 25 years. Biomolecules 2021, 11, 896. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Fleming, C.; Yan, J. New insights of T cells in the pathogenesis of psoriasis. Cell. Mol. Immunol. 2012, 9, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Diani, M.; Altomare, G.; Reali, E. T Helper cell subsets in clinical manifestations of psoriasis. J. Immunol. Res. 2016, 2016, 7692024. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.Y.; Yen, H.; Huang, Y.C. Serum homocysteine, folate and vitamin B12 levels in patients with psoriasis: A systematic review and meta-analysis. Br. J. Dermatol. 2019, 180, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Yakovleva, O.V.; Ziganshina, A.R.; Dmitrieva, S.A.; Arslanova, A.N.; Yakovlev, A.V.; Minibayeva, F.V.; Khaertdinov, N.N.; Ziyatdinova, G.K.; Giniatullin, R.A.; Sitdikova, G.F. Hydrogen sulfide ameliorates developmental impairments of rat offspring with prenatal hyperhomocysteinemia. Oxidative Med. Cell. Longev. 2018, 2018, 2746873. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Meng, X.; Song, Z. Homocysteine and psoriasis. Biosci. Rep. 2019, 39, BSR20190867. [Google Scholar] [CrossRef]
- Rose, P.; Moore, P.K.; Whiteman, M.; Kirk, C.; Zhu, Y.Z. Diet and hydrogen sulfide production in mammals. Antioxid. Redox Signal. 2021, 34, 1378–1393. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Percival, S.S. Immunomodulatory effects of glutathione, garlic derivatives, and hydrogen sulfide. Nutrients 2019, 11, 295. [Google Scholar] [CrossRef]
- Rose, P.; Moore, P.K.; Zhu, Y.Z. Garlic and gaseous mediators. Trends Pharmacol. Sci. 2018, 39, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Münchberg, U.; Anwar, A.; Mecklenburg, S.; Jacob, C. Polysulfides as biologically active ingredients of garlic. Org. Biomol. Chem. 2007, 5, 1505–1518. [Google Scholar] [CrossRef]
- Arsenijevic, D.; Stojanovic, B.; Milovanovic, J.; Arsenijevic, A.; Simic, M.; Pergal, M.; Kodranov, I.; Cvetkovic, O.; Vojvodic, D.; Ristanovic, E.; et al. Hepatoprotective Effect of Mixture of Dipropyl Polysulfides in Concanavalin A-Induced Hepatitis. Nutrients 2021, 13, 1022. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.J.; Li, H.R.; Chen, Y.; Zhang, C.; Chen, D.G.; Liang, Z.C.; Shi, Y.Q.; Zhang, L.L.; Xin, L.; Zhao, D.B. Diallyl Trisulfide can induce fibroblast-like synovial apoptosis and has a therapeutic effect on collagen-induced arthritis in mice via blocking NF-kappaB and Wnt pathways. Int. Immunopharmacol. 2019, 71, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L.; Schuler, P.; Schilling, B. Induced and natural regulatory T cells in human cancer. Expert Opin. Biol. Ther. 2012, 12, 1383–1397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Gu, W.; He, L.; Sun, B. Th1/Th2 cell’s function in immune system. Adv. Exp. Med. Biol. 2014, 841, 45–65. [Google Scholar] [CrossRef]
- Karimi, S.; Chattopadhyay, S.; Chakraborty, N.G. Manipulation of regulatory T cells and antigen-specific cytotoxic T lymphocyte-based tumour immunotherapy. Immunology 2015, 144, 186–196. [Google Scholar] [CrossRef]
- Yang, R.; Yu, T.; Liu, D.; Shi, S.; Zhou, Y. Hydrogen sulfide promotes immunomodulation of gingiva-derived mesenchymal stem cells via the Fas/FasL coupling pathway. Stem Cell Res. Ther. 2018, 9, 62. [Google Scholar] [CrossRef]
- Whiteman, M.; Li, L.; Rose, P.; Tan, C.H.; Parkinson, D.B.; Moore, P.K. The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. Antioxid. Redox Signal. 2010, 12, 1147–1154. [Google Scholar] [CrossRef]
- Castelblanco, M.; Lugrin, J.; Ehirchiou, D.; Nasi, S.; Ishii, I.; So, A.; Martinon, F.; Busso, N. Hydrogen sulfide inhibits NLRP3 inflammasome activation and reduces cytokine production both in vitro and in a mouse model of inflammation. J. Biol. Chem. 2018, 293, 2546–2557. [Google Scholar] [CrossRef]
- Liu, F.; Liu, G.J.; Liu, N.; Zhang, G.; Zhang, J.X.; Li, L.F. Effect of hydrogen sulfide on inflammatory cytokines in acute myocardial ischemia injury in rats. Exp. Ther. Med. 2015, 9, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozzi, G.; Gobbi, G.; Masselli, E.; Carubbi, C.; Presta, V.; Ambrosini, L.; Vitale, M.; Mirandola, P. Buffering Adaptive Immunity by Hydrogen Sulfide. Cells 2022, 11, 325. https://doi.org/10.3390/cells11030325
Pozzi G, Gobbi G, Masselli E, Carubbi C, Presta V, Ambrosini L, Vitale M, Mirandola P. Buffering Adaptive Immunity by Hydrogen Sulfide. Cells. 2022; 11(3):325. https://doi.org/10.3390/cells11030325
Chicago/Turabian StylePozzi, Giulia, Giuliana Gobbi, Elena Masselli, Cecilia Carubbi, Valentina Presta, Luca Ambrosini, Marco Vitale, and Prisco Mirandola. 2022. "Buffering Adaptive Immunity by Hydrogen Sulfide" Cells 11, no. 3: 325. https://doi.org/10.3390/cells11030325
APA StylePozzi, G., Gobbi, G., Masselli, E., Carubbi, C., Presta, V., Ambrosini, L., Vitale, M., & Mirandola, P. (2022). Buffering Adaptive Immunity by Hydrogen Sulfide. Cells, 11(3), 325. https://doi.org/10.3390/cells11030325






