Effects of Heat Stress on Bovine Oocytes and Early Embryonic Development—An Update
Abstract
1. Introduction
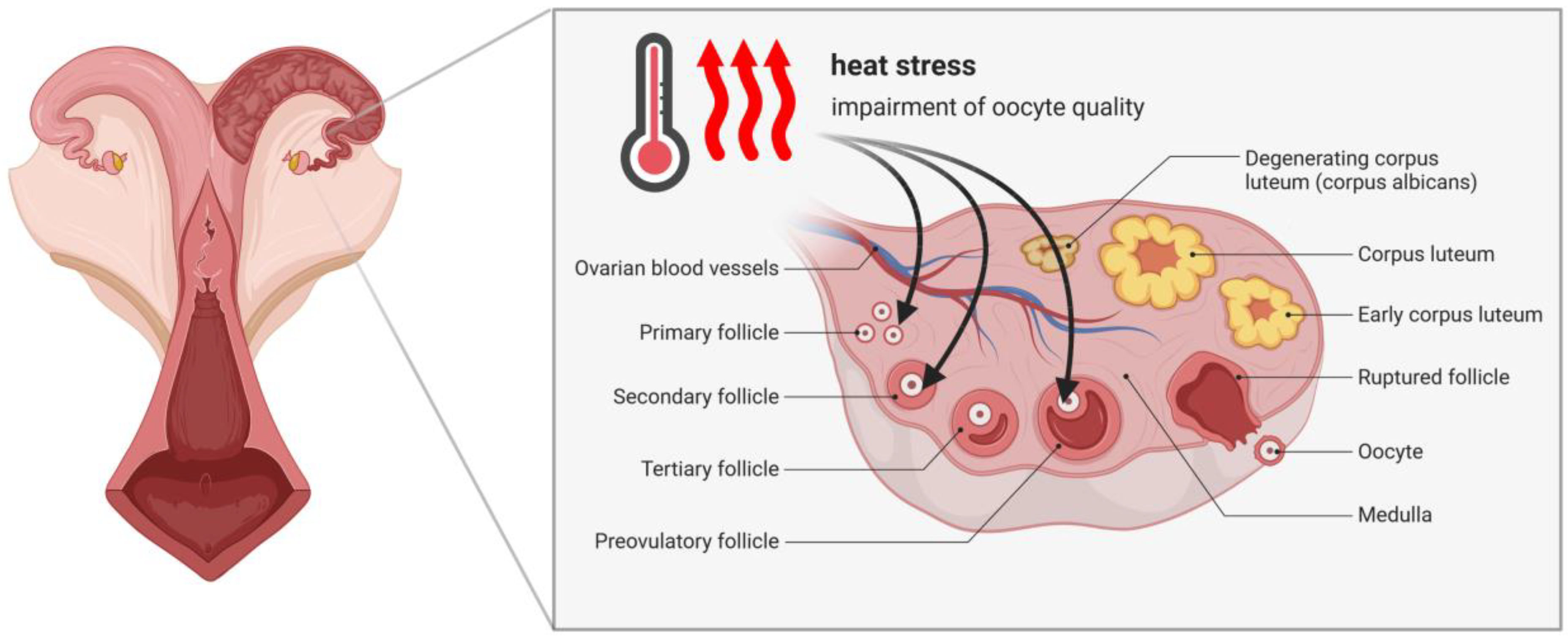
2. Effect of Heat Stress on Bovine Oocytes
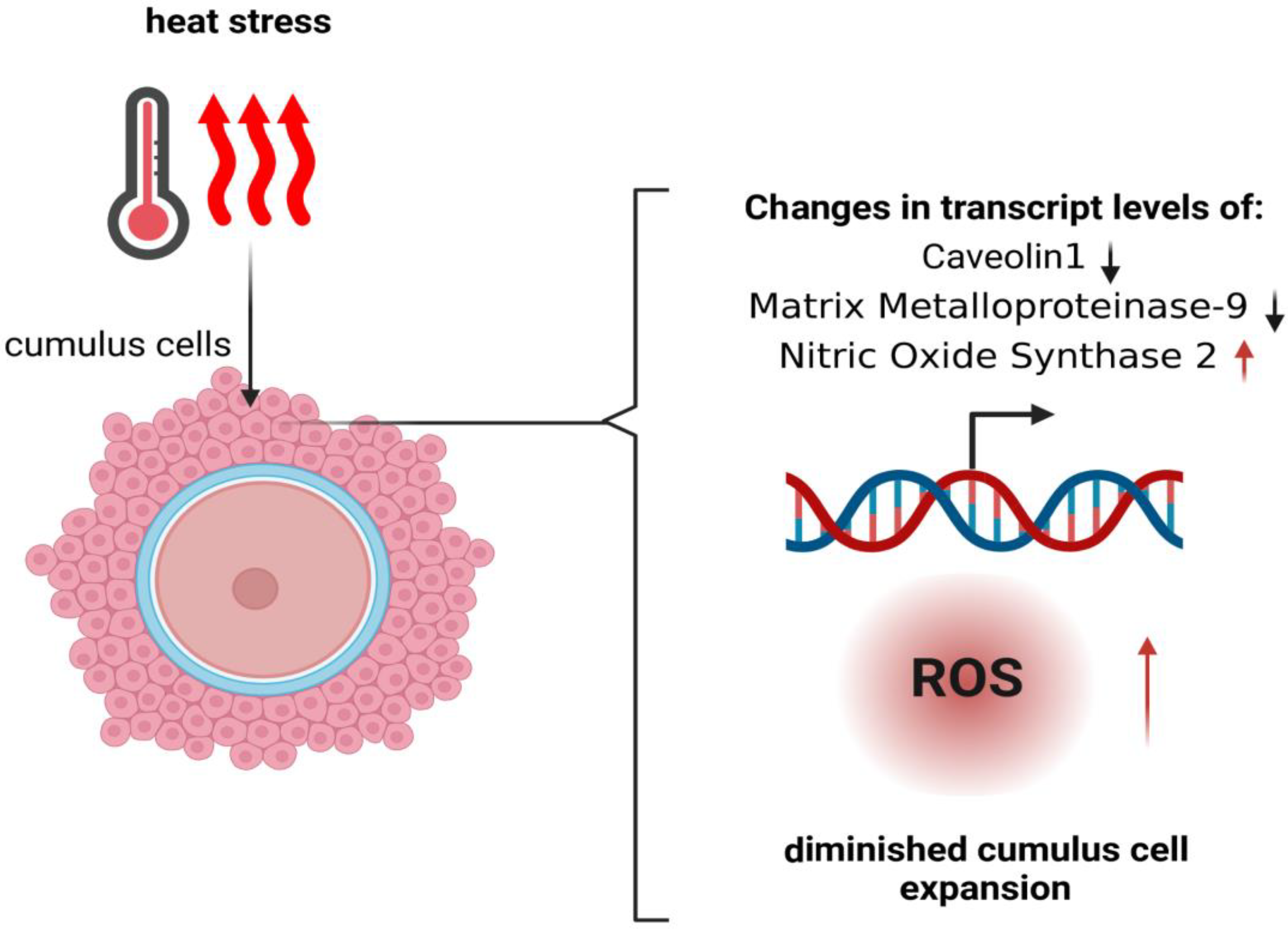
3. Influence of Heat Stress on Granulosa Cells
4. Influence of Heat Stress on the Development of Bovine Embryos
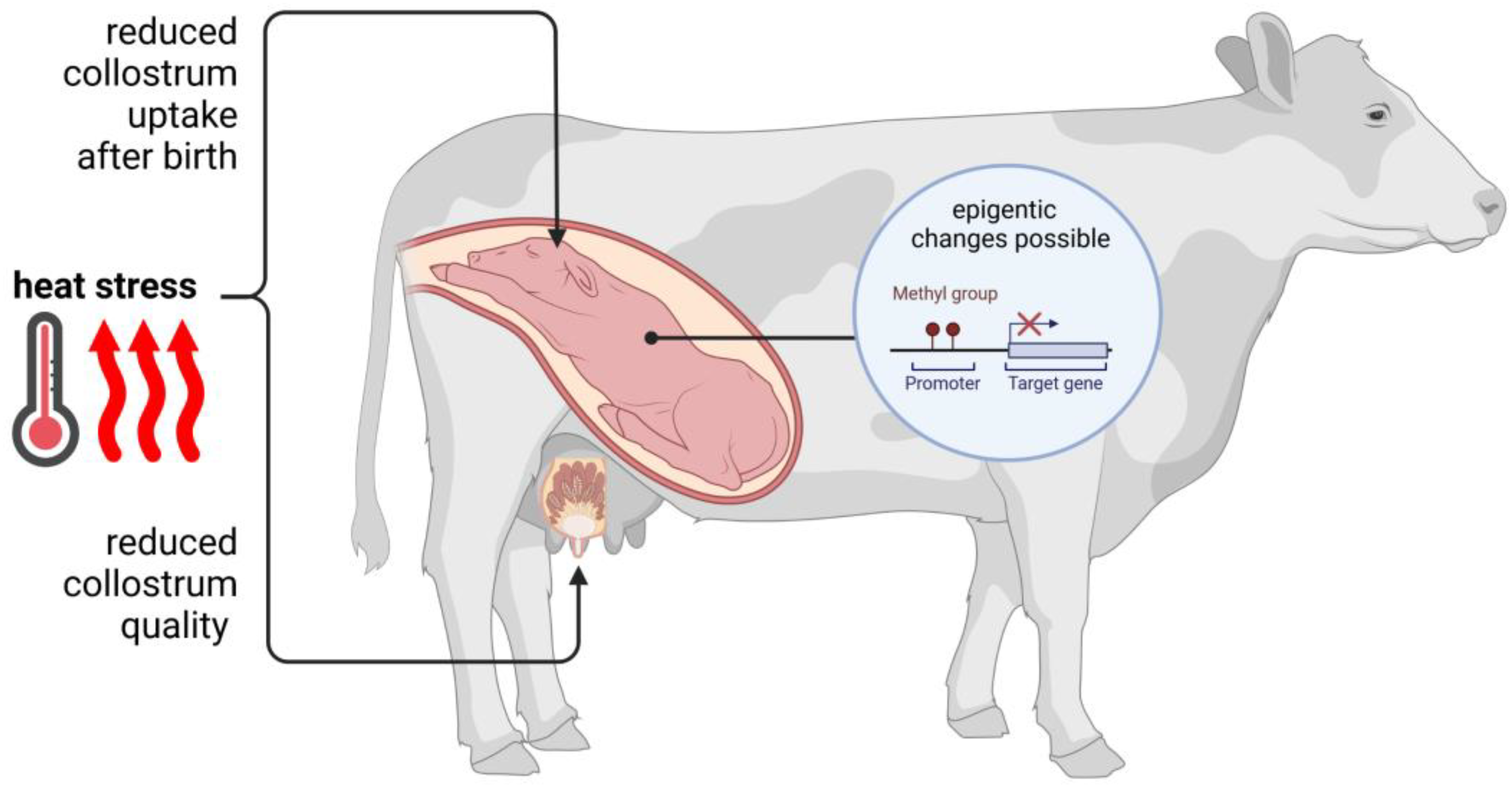
5. Thermal Stress and Its Influence on Pregnancy and Fetal Development
6. Heat Stress and Mammary Gland Function and Milk Quality
7. How to Counteract the Development of Heat Stress?
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Souza-Cácares, M.B.; Fialho, A.L.L.; Silva, W.A.L.; Cardoso, C.J.T.; Pöhland, R.; Martins, M.I.M.; Melo-Sterza, F.A. Oocyte quality and heat shock proteins in oocytes from bovine breeds adapted to the tropics under different conditions of environmental thermal stress. Theriogenology 2019, 130, 103–110. [Google Scholar] [CrossRef]
- Stamperna, K.; Dovolou, E.; Giannoulis, T.; Kalemkeridou, M.; Nanas, I.; Dadouli, K.; Moutou, K.; Mamuris, Z.; Amiridis, G.S. Developmental competence of heat stressed oocytes from Holstein and Limousine cows matured in vitro. Reprod. Domest. Anim. 2021, 56, 1302–1314. [Google Scholar] [CrossRef]
- Satrapa, R.A.; Razza, E.M.; Castilho, A.C.; Simões, R.A.; Silva, C.F.; Nabhan, T.; Pegorer, M.F.; Barros, C.M. Differential expression of IGF family members in heat-stressed embryos produced in vitro from OPU-derived oocytes of Nelore (Bos indicus) and Holstein (Bos taurus) cows. Reprod. Domest. Anim. 2013, 48, 1043–1048. [Google Scholar] [CrossRef]
- De Barros, F.R.O.; Paula-Lopes, F.F. Cellular and epigenetic changes induced by heat stress in bovine preimplantation embryos. Mol. Reprod. Dev. 2018, 85, 810–820. [Google Scholar] [CrossRef]
- Tao, S.; Dahl, G.E. Invited review: Heat stress effects during late gestation on dry cows and their calves. J. Dairy Sci. 2013, 96, 4079–4093. [Google Scholar] [CrossRef]
- Camargo, L.S.A.; Aguirre-Lavin, T.; Adenot, P.; Araujo, T.D.; Mendes, V.R.A.; Louro, I.D.; Beaujean, N.; Souza, E.D. Heat shock during in vitro maturation induces chromatin modifications in the bovine embryo. Reproduction 2019, 158, 313–322. [Google Scholar] [CrossRef]
- Trout, J.P.; McDowell, L.R.; Hansen, P.J. Characteristics of the estrous cycle and antioxidant status of lactating Holstein cows exposed to heat stress. J. Dairy Sci. 1998, 81, 1244–1250. [Google Scholar] [CrossRef]
- Gunnar, M.; Quevedo, K. The neurobiology of stress and development. Annu. Rev. Psychol. 2007, 58, 145–173. [Google Scholar] [CrossRef]
- Valentino, R.J.; Foote, S.L.; Page, M.E. The locus coeruleus as a site for integrating corticotropin-releasing factor and noradrenergic mediation of stress responses. Ann. N. Y. Acad. Sci. 1993, 697, 173–188. [Google Scholar] [CrossRef]
- Kovács, L.; Kézér, F.L.; Ruff, F.; Szenci, O.; Bakony, M.; Jurkovich, V. Effect of artificial shade on saliva cortisol concentrations of heat-stressed dairy calves. Domest. Anim. Endocrinol. 2019, 66, 43–47. [Google Scholar] [CrossRef]
- Ronchi, B.; Stradaioli, G.; Verini Supplizi, A.; Bernabucci, U.; Lacetera, N.; Accorsi, P.A.; Nardone, A.; Seren, E. Influence of heat stress or feed restriction on plasma progesterone, oestradiol-17β, LH, FSH, prolactin and cortisol in Holstein heifers. Livest. Prod. Sci. 2001, 68, 231–241. [Google Scholar] [CrossRef]
- Van Gool, J.; van Vugt, H.; Helle, M.; i Aarden, L.A. The relation among stress, adrenalin, interleukin-6 and acute phase proteins in the rat. Clin. Immunol. Immunopathol. 1990, 57, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Elenkov, I.J.; Papanicolaou, D.A.; Wilder, R.L.; Chrousos, G.P. Modulatory effects of glucocorticoids and catecholamines on human interleukin-12 and interleukin-10 production: Clinical implications. Proc. Assoc. Am. Physicians 1996, 108, 374–381. [Google Scholar] [PubMed]
- Bagath, M.; Krishnan, G.; Devaraj, C.; Rashamol, V.P.; Pragna, P.; Lees, A.M.; Sejian, V. The impact of heat stress on the immune system in dairy cattle: A review. Res. Vet. Sci. 2019, 126, 94–102. [Google Scholar] [CrossRef]
- Min, L.; Zhao, S.; Tian, H.; Zhou, X.; Zhang, Y.; Li, S.; Yang, H.; Zheng, N.; Wang, J. Metabolic responses and “omics” technologies for elucidating the effects of heat stress in dairy cows. Int. J. Biometeorol. 2017, 61, 1149–1158. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Rhoads, R.P., Jr. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef]
- Khan, A.; Khan, M.Z.; Umer, S.; Khan, I.M.; Xu, H.; Zhu, H.; Wang, Y. Cellular and Molecular Adaptation of Bovine Granulosa Cells and Oocytes under Heat Stress. Animals 2020, 9, 110. [Google Scholar] [CrossRef]
- Rispoli, L.A.; Payton, R.R.; Gondro, C.; Saxton, A.M.; Nagle, K.A.; Jenkins, B.W.; Schrick, F.N.; Edwards, J.L. Heat stress effects on the cumulus cells surrounding the bovine oocyte during maturation: Altered matrix metallopeptidase 9 and progesterone production. Reproduction 2013, 146, 193–207. [Google Scholar] [CrossRef]
- Sakatani, M.; Bonilla, L.; Dobbs, K.; Block, J.; Ozawa, M.; Shanker, S.; Yao, J.; Hansen, P. Changes in the transcriptome of morula-stage bovine embryos caused by heat shock: Relationship to developmental acquisition of thermotolerance. Reprod. Biol. Endocrinol. 2013, 11, 3. [Google Scholar] [CrossRef]
- Monty, D.E., Jr.; Wolff, L.K. Summer heat stress and reduced fertility in Holstein- Friesian cows in Arizona. Am. J. Vet. Res. 1974, 35, 1495–1500. [Google Scholar]
- West, J.W. Effects of heat-stress on production in dairy cattle. J. Dairy Sci. 2003, 86, 2131–2144. [Google Scholar] [CrossRef]
- De Rensis, F.; Saleri, R.; Garcia-Ispierto, I.; Scaramuzzi, R.; López-Gatius, F. Effects of Heat Stress on Follicular Physiology in Dairy Cows. Animals 2021, 11, 3406. [Google Scholar] [CrossRef]
- Payton, R.R.; Rispoli, L.A.; Nagle, K.A.; Gondro, C.; Saxton, A.M.; Voy, B.H.; Edwards, J.L. Mitochondrial-related consequences of heat stress exposure during bovine oocyte maturation persist in early embryo development. J. Reprod. Dev. 2018, 64, 243–251. [Google Scholar] [CrossRef]
- Arav, A.; Zvi, R. Do chilling injury and heat stress share the same mechanism of injury in oocytes? Mol. Cell Endocrinol. 2008, 282, 150–152. [Google Scholar] [CrossRef]
- Zeron, Y.; Ocheretny, A.; Kedar, O.; Borochov, A.; Sklan, D.; Arav, A. Seasonal changes in bovine fertility: Relation to developmental competence of oocytes, membrane properties and fatty acid composition of follicles. Reproduction 2001, 121, 447–454. [Google Scholar] [CrossRef]
- Roth, Z.; Hansen, P.J. Involvement of apoptosis in disruption of oocyte competence by heat shock in cattle. Biol. Reprod. 2004, 71, 1898–1906. [Google Scholar] [CrossRef]
- Aroyo, A.; Yavin, S.; Arav, A.; Roth, Z. Maternal hyperthermia disrupts developmental competence of follicle-enclosed oocytes: In vivo and ex vivo studies in mice. Theriogenology 2007, 15, 1013–1021. [Google Scholar] [CrossRef]
- Loureiro, B.; Bonilla, L.; Block, J.; Fear, J.M.; Bonilla, A.Q.; Hansen, P.J. Colonystimulating factor 2 (CSF-2) improves development and posttransfer survival of bovine embryos produced in vitro. Endocrinology 2009, 150, 5046–5054. [Google Scholar] [CrossRef]
- Laporta, J.; Ferreira, F.C.; Ouellet, V.; Dado-Senn, B.; Almeida, A.K.; De Vries, A.; Dahl, G.E. Late-gestation heat stress impairs daughter and granddaughter lifetime performance. J. Dairy Sci. 2020, 103, 7555–7568. [Google Scholar] [CrossRef]
- Sakatani, M. Effects of heat stress on bovine preimplantation embryos produced in vitro. J. Reprod. Dev. 2017, 63, 347–352. [Google Scholar] [CrossRef]
- Riezman, H. Why do cells require heat shock proteins to survive heat stress? Cell Cycle 2004, 3, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Pavani, K.C.; Baron, E.; Correia, P.; Lourenço, J.; Bettencourt, B.F.; Sousa, M.; da Silva, F.M. Gene expression, oocyte nuclear maturation and developmental competence of bovine oocytes and embryos produced after in vivo and in vitro heat shock. Zygote 2016, 24, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.L.; Saxton, A.M.; Lawrence, J.L.; Payton, R.R.; Dunlap, J.R. Exposure to a physiologically relevant elevated temperature hastens in vitro maturation in bovine oocytes. J. Dairy Sci. 2005, 88, 4326–4333. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumaran, B.; Yoshikuni, M.; Nagahama, Y. A shift in steroidogenesis occurring in ovarian follicles prior to oocyte maturation. Mol. Cell Endocrinol. 2004, 215, 11–18. [Google Scholar] [CrossRef]
- Sammad, A.; Luo, H.; Hu, L.; Zhu, H.; Wang, Y. Transcriptome Reveals Granulosa Cells Coping through Redox, Inflammatory and Metabolic Mechanisms under Acute Heat Stress. Cells 2022, 11, 1443. [Google Scholar] [CrossRef]
- Campen, K.A.; Abbott, C.R.; Rispoli, L.A.; Payton, R.R.; Saxton, A.M.; Edwards, J.L. Heat stress impairs gap junction communication and cumulus function of bovine oocytes. J. Reprod. Dev. 2018, 64, 385–392. [Google Scholar] [CrossRef]
- Regassa, A.; Rings, F.; Hoelker, M.; Cinar, U.; Tholen, E.; Looft, C.; Schellander, K.; Tesfaye, D. Transcriptome dynamics and molecular cross-talk between bovine oocyte and its companion cumulus cells. BMC Genom. 2011, 12, 57. [Google Scholar] [CrossRef]
- Wolfenson, D.; Lew, B.J.; Thatcher, W.W.; Graber, Y.; Meidan, R. Seasonal and acute heat stress effects on steroid production by dominant follicles in cows. Anim. Reprod. Sci. 1997, 47, 9–19. [Google Scholar] [CrossRef]
- Matta, S.G.; Caldas-Bussiere, M.C.; Viana, K.S.; Faes, M.R.; Paes de Carvalho, C.S.; Dias, B.L.; Quirino, C.R. Effect of inhibition of synthesis of inducible nitric oxide synthase-derived nitric oxide by aminoguanidine on the in vitro maturation of oocyte–cumulus complexes of cattle. Anim. Reprod. Sci. 2009, 111, 189–201. [Google Scholar] [CrossRef]
- Ticianelli, J.S.; Emanuelli, I.P.; Satrapa, R.A.; Castilho, A.C.S.; Loureiro, B.; Sudano, M.J.; Fontes, P.K.; Pinto, R.F.P.; Razza, E.M.; Surjus, R.S.; et al. Gene expression profile in heat-shocked Holstein and Nelore oocytes and cumulus cells. Reprod. Fertil. Dev. 2017, 29, 1787–1802. [Google Scholar] [CrossRef]
- Latorraca, L.B.; Feitosa, W.B.; Mariano, C.; Moura, M.T.; Fontes, P.K.; Nogueira, M.F.G.; Paula-Lopes, F.F. Autophagy is a pro-survival adaptive response to heat shock in bovine cumulus-oocyte complexes. Sci. Rep. 2020, 10, 13711. [Google Scholar] [CrossRef]
- Gebremedhn, S.; Gad, A.; Aglan, H.S.; Laurincik, J.; Prochazka, R.; Salilew-Wondim, D.; Hoelker, M.; Schellander, K.; Tesfaye, D. Extracellular vesicles shuttle protective messages against heat stress in bovine granulosa cells. Sci. Rep. 2020, 10, 15824. [Google Scholar] [CrossRef]
- Maya-Soriano, M.J.; Taberner, E.; Lopez-Bejar, M. Retinol improves in vitro oocyte nuclear maturation under heat stress in heifers. Zygote 2013, 21, 377–384. [Google Scholar] [CrossRef]
- Saha, S.; Sparks, A.B.; Rago, C.; Akmaev, V.; Wang, C.J.; Vogelstein, B.; Kinzler, K.W.; Velculescu, V.E. Using the transcriptome to annotate the genome. Nat. Biotech. 2002, 19, 508–512. [Google Scholar] [CrossRef]
- Kidder, G.M.; Vanderhyden, B.C. Bidirectional communication between oocytes and follicle cells: Ensuring oocyte developmental competence. Can. J. Physiol. Pharmacol. 2010, 88, 399–413. [Google Scholar] [CrossRef]
- Umer, S.; Sammad, A.; Zou, H.; Khan, A.; Weldegebriall Sahlu, B.; Hao, H.; Zhao, X.; Wang, Y.; Zhao, S.; Zhu, H. Regulation of AMH, AMHR-II, and BMPs (2,6) Genes of Bovine Granulosa Cells Treated with Exogenous FSH and Their Association with Protein Hormones. Genes 2019, 10, 1038. [Google Scholar] [CrossRef]
- Hansen, P.J. Possible roles for heat shock protein 70 and glutathione in protection of the mammalian preimplantation embryo from heat shock. Ann. Rev. Biomed. Sci. 1999, 1, 5–29. [Google Scholar] [CrossRef]
- Ealy, A.D.; Drost, M.; Hansen, P.J. Developmental Changes in Embryonic Resistance to Adverse Effects of Maternal Heat Stress in Cows1. J. Dairy Sci. 1993, 76, 2899–2905. [Google Scholar] [CrossRef]
- Putney, D.J.; Mullins, S.; Thatcher, W.W.; Drost, M.; Gross, T.S. Embryonic development in superovulated dairy cattle exposed to elevated ambient temperatures between the onset of estrus and insemination. Anim. Reprod. Sci. 1989, 19, 37–51. [Google Scholar] [CrossRef]
- Park, J.S.; Jeong, Y.S.; Shin, S.T.; Lee, K.-K.; Kang, Y.-K. Dynamic DNA methylation reprogramming: Active demethylation and immediate remethylation in the male pronucleus of bovine zygotes. Dev. Dyn. 2007, 236, 2523–2533. [Google Scholar] [CrossRef]
- DeChiara, T.M.; Robertson, E.J.; Efstratiadis, A. Parental imprinting of the mouse insulin-like growth factor II gene. Cell 1991, 64, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Dobbs, K.B.; Rodriguez, M.; Sudano, M.J.; Ortega, M.S.; Hansen, P.J. Dynamics of DNA Methylation during Early Development of the Preimplantation Bovine Embryo. PLoS ONE 2013, 8, e66230-10. [Google Scholar] [CrossRef] [PubMed]
- Rivera, R.M.; Hansen, P.J. Development of cultured bovine embryos after exposure to high temperatures in the physiological range. Reproduction 2001, 121, 107–115. [Google Scholar] [CrossRef]
- Paula-Lopes, F.F.; Hansen, P.J. Heat shock-induced apoptosis in preimplantation bovine embryos is a developmentally regulated phenomenon. Biol. Reprod. 2002, 66, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Nover, L.; Scharf, K.D. Heat shock proteins. In Heat Shock Response; Nover, L., Ed.; CRC Press, Inc.: Boca Raton, FL, USA, 1991; pp. 41–128. [Google Scholar]
- Chandolia, R.; Peltier, M.R.; Tian, W.; Hansen, P.J. Transcriptional control of development, protein synthesis, and heat-induced heat shock protein 70 synthesis in 2-cell bovine embryos. Biol. Reprod. 1999, 61, 1644–1648. [Google Scholar] [CrossRef]
- van der Reest, J.; Nardini Cecchino, G.; Haigis, M.C.; Kordowitzki, P. Mitochondria: Their relevance during oocyte ageing. Ageing Res. Rev. 2021, 70, 101378. [Google Scholar] [CrossRef]
- Zhu, J.-Q.; Liu, J.-H.; Liang, X.-W.; Xu, B.-Z.; Hou, Y.; Zhao, X.-X.; Sun, Q.-Y. Heat stress causes aberrant DNA methylation of H19 and Igf-2r in mouse blastocysts. Mol. Cells 2008, 25, 211–215. [Google Scholar]
- Ouellet, V.; Laporta, J.; Dahl, G.E. Late gestation heat stress in dairy cows: Effects on dam and daughter. Theriogenology 2020, 150, 471–479. [Google Scholar] [CrossRef]
- Bakony, M.; Jurkovich, V. Heat stress in dairy calves from birth to weaning. J. Dairy Res. 2020, 87, 53–59. [Google Scholar] [CrossRef]
- Dahl, G.E.; Skibiel, A.L.; Laporta, J. In Utero Heat Stress Programs Reduced Performance and Health in Calves. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 343–353. [Google Scholar] [CrossRef]
- Thompson, I.M.; Dahl, G.E. Dry period seasonal effects on the subsequent lactation. Prof. Anim. Sci. 2012, 28, 628–631. [Google Scholar] [CrossRef]
- Merlot, E.; Couret, D.; Otten, W. Prenatal stress, fetal imprinting and immunity. Brain Behav. Immun. 2008, 22, 42–51. [Google Scholar] [CrossRef]
- Bate, L.A.; Hacker, R.R. Influence of environmental temperature during late gestation and soon after birth on IgG absorption by newborn piglets. Can. J. Anim. Sci. 1985, 65, 87–93. [Google Scholar] [CrossRef]
- Capuco, A.V.; Akers, R.M.; Smith, J.J. Mammary growth in Holstein cows during the dry period: Quantification of nucleic acids and histology. J. Dairy Sci. 1997, 80, 477–487. [Google Scholar] [CrossRef]
- Collier, R.J.; Stiening, C.M.; Pollard, B.C.; VanBaale, M.J.; Baumgard, L.H.; Gentry, P.C.; Coussens, P.M. Use of gene expression microarrays for evaluating environmental stress tolerance at the cellular level in cattle. J. Anim. Sci. 2006, 84 (Suppl. 13), E1–E13. [Google Scholar] [CrossRef]
- Vernon, R.G.; Pond, G.M. Adaptations of maternal adipose tissue to lactation. J. Mammary Gland Biol. Neoplasia 1997, 2, 231–241. [Google Scholar] [CrossRef]
- Donovan, G.A.; Dohoo, I.R.; Montgomery, D.M.; Bennett, F.L. Calf and disease factors affecting growth in female Holstein calves in Florida, USA. Prev. Vet. Med. 1998, 33, 1–10. [Google Scholar] [CrossRef]
- Wheelock, J.B.; Rhoads, R.P.; VanBaale, M.J.; Sanders, S.R.; Baumgard, L.H. Effects of heat stress on energetic metabolism in lactating Holstein cows. J. Dairy Sci. 2010, 93, 644–655. [Google Scholar] [CrossRef]
- Liu, Z.V.; Ezernieks, J.; Wang, N.W.; Arachchillage, J.B.; Garner, W.J.; Wales, B.; Cocks, G.; Rochfort, S. Heat stress in dairy cattle alters lipid composition of milk. Sci. Rep. 2017, 7, 961. [Google Scholar] [CrossRef]
- Heck, J.M.L.; van Valenberg, H.J.F.; Dijkstra, J.; van Hooijdonk, A.C.M. Seasonal variation in the Dutch bovine raw milk composition. J. Dairy Sci. 2009, 92, 4745–4755. [Google Scholar] [CrossRef]
- Sntana, M.L.; Bignardi, A.B.; Pereira, R.J.; Stefani, G.; El Faro, L. Genetics of heat tolerance for milk yield and quality in Holsteins. Animal 2017, 11, 4–14. [Google Scholar] [CrossRef]
- Cavallari, F.C.; Leal, C.L.V.; Zvi, R.; Hansen, P.J. Effects of melatonin on production of reactive oxygen species and developmental competence of bovine oocytes exposed to heat shock and oxidative stress during in vitro maturation. Zygote 2019, 27, 180–186, Erratum in Zygote 2019, 27, 262. [Google Scholar] [CrossRef]
- Nabenishi, H.; Takagi, S.; Kamata, H.; Nishimoto, T.; Morita, T.; Ashizawa, K.; Tsuzuki, Y. The role of mitochondrial transition pores on bovine oocyte competence after heat stress, as determined by effects of cyclosporin A. Mol. Reprod. Dev. 2012, 79, 31–40. [Google Scholar] [CrossRef]
- Roth, Z.; Hansen, P.J. Sphingosine 1-phosphate protects bovine oocytes from heat shock during maturation. Biol. Reprod. 2004, 71, 2072–2078. [Google Scholar] [CrossRef]
- Lawrence, J.L.; Payton, R.R.; Godkin, J.D.; Saxton, A.M.; Schrick, F.N.; Edwards, J.L. Retinol improves development of bovine oocytes compromised by heat stress during maturation. J. Dairy Sci. 2004, 87, 2449–2454. [Google Scholar] [CrossRef]
- Cebrian-Serrano, A.; Salvador, I.; García-Roselló, E.; Pericuesta, E.; Pérez-Cerezales, S.; Gutierrez-Adán, A.; Coy, P.; Silvestre, M.A. Effect of the bovine oviductal fluid on in vitro fertilization, development and gene expression of in vitro-produced bovine blastocysts. Reprod. Domest. Anim. 2013, 48, 331–338. [Google Scholar] [CrossRef]
- Eberhardt, D.M.; Will, W.A.; Godkin, J.D. Retinol administration to superovulated ewes improves in vitro embryonic viability. Biol. Reprod. 1999, 60, 1483–1487. [Google Scholar] [CrossRef]
- Bonilla, A.Q.; Oliveira, L.J.; Ozawa, M.; Newsom, E.M.; Lucy, M.C.; Hansen, P.J. Developmental changes in thermoprotective actions of insulin-like growth factor-1 on the preimplantation bovine embryo. Mol. Cell Endocrinol. 2011, 332, 170–179, Erratum in Mol. Cell Endocrinol. 2015, 413, 248. [Google Scholar] [CrossRef]
- Lima, P.F.; Oliveira, M.A.; Santos, M.H.; Reichenbach, H.D.; Weppert, M.; Paula-Lopes, F.F.; Neto, C.C.; Goncalves, P.B. Effect of retinoids and growth factor on in vitro bovine embryos produced under chemically defined conditions. Anim. Reprod. Sci. 2006, 95, 184–192. [Google Scholar] [CrossRef]
- Block, J.; Hansen, P.J. Interaction between season and culture with insulin-like growth factor-1 on survival of in vitro produced embryos following transfer to lactating dairy cows. Theriogenology 2007, 67, 1518–1529. [Google Scholar] [CrossRef]
- Sudano, M.J.; Caixeta, E.S.; Paschoal, D.M.; Martins, A., Jr.; Machado, R.; Buratini, J.; Landim-Alvarenga, F.D. Cryotolerance and global gene-expression patterns of Bos taurus indicus and Bos taurus taurus in vitro- and in vivo-produced blastocysts. Reprod. Fertil. Dev. 2014, 26, 1129–1141. [Google Scholar] [CrossRef] [PubMed]
- Ispada, J.; Rodrigues, T.A.; Risolia, P.H.B.; Lima, R.S.; Gonçalves, D.R.; Rettori, D.; Nichi, M.; Feitosa, W.B.; Paula-Lopes, F.F. Astaxanthin counteracts the effects of heat shock on the maturation of bovine oocytes. Reprod. Fertil. Dev. 2018, 30, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Wegele, H.; Müller, L.; Buchner, J. Hsp70 and Hsp90–a relay team for protein folding. Rev. Physiol. Biochem. Pharmacol. 2004, 151, 1–44. [Google Scholar] [PubMed]
- Al-Katanani, Y.; Hansen, P. Induced thermotolerance in bovine two-cell embryos and the role of heat shock protein 70 in embryonic development. Mol. Reprod. Dev. 2002, 62, 174–180. [Google Scholar] [CrossRef]
- Roth, Z. Cooling is the predominant strategy to alleviate the effects of heat stress on dairy cows. Reprod. Domest. Anim. 2022, 57 (Suppl. 1), 16–22. [Google Scholar] [CrossRef]
- Armstrong, D.V. Heat stress interaction with shade and cooling. J. Dairy Sci. 1994, 77, 2044–2050. [Google Scholar] [CrossRef]
- St-Pierre, N.R.; Cobanov, B.; Schnitkey, G. Economic losses from heat stress by us livestock industries. J. Dairy Sci. 2003, 86, E52–E77. [Google Scholar] [CrossRef]
- Levit, H.; Pinto, S.; Amon, T.; Gershon, E.; Kleinjan-Elazary, A.; Bloch, V.; Ben Meir, Y.A.; Portnik, Y.; Jacoby, S.; Arnin, A.; et al. Dynamic cooling strategy based on individual animal response mitigated heat stress in dairy cows. Animal 2021, 15, 100093. [Google Scholar] [CrossRef]
- Van Os, J.M.C. Considerations for Cooling Dairy Cows with Water. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 157–173. [Google Scholar] [CrossRef]
- Sprícigo, J.F.W.; Guimarães, A.L.S.; Cunha, A.T.M.; Leme, L.O.; Carneiro, M.C.; Franco, M.M.; Dode, M.A.N. Using Cumulus Cell Biopsy as a Non-Invasive Tool to Access the Quality of Bovine Oocytes: How Informative Are They? Animals 2022, 12, 3113. [Google Scholar] [CrossRef]
- Anzalone, D.A.; Palazzese, L.; Czernik, M.; Sabatucci, A.; Valbonetti, L.; Capra, E.; Loi, P. Controlled spermatozoa-oocyte interaction improves embryo quality in sheep. Sci. Rep. 2021, 11, 22629. [Google Scholar] [CrossRef] [PubMed]
- Gorczyca, G.; Wartalski, K.; Romek, M.; Samiec, M.; Duda, M. The Molecular Quality and Mitochondrial Activity of Porcine Cumulus-Oocyte Complexes Are Affected by Their Exposure to Three Endocrine-Active Compounds under 3D In Vitro Maturation Conditions. Int. J. Mol. Sci. 2022, 23, 4572. [Google Scholar] [CrossRef] [PubMed]
- Hochi, S.; Ide, M.; Ueno, S.; Hirabayashi, M. High survival of bovine mature oocytes after nylon mesh vitrification, as assessed by intracytoplasmic sperm injection. J. Reprod. Dev. 2022, 68, 335–339. [Google Scholar] [CrossRef]
- Samiec, M.; Skrzyszowska, M. Extranuclear Inheritance of Mitochondrial Genome and Epigenetic Reprogrammability of Chromosomal Telomeres in Somatic Cell Cloning of Mammals. Int. J. Mol. Sci. 2021, 22, 3099. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Meng, R.; Song, W.; Sun, H.; An, Q.; Zhang, C.; Zhang, Y.; Su, J. ZFP57 regulates DNA methylation of imprinted genes to facilitate embryonic development of somatic cell nuclear transfer embryos in Holstein cows. J. Dairy Sci. 2022, in press. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miętkiewska, K.; Kordowitzki, P.; Pareek, C.S. Effects of Heat Stress on Bovine Oocytes and Early Embryonic Development—An Update. Cells 2022, 11, 4073. https://doi.org/10.3390/cells11244073
Miętkiewska K, Kordowitzki P, Pareek CS. Effects of Heat Stress on Bovine Oocytes and Early Embryonic Development—An Update. Cells. 2022; 11(24):4073. https://doi.org/10.3390/cells11244073
Chicago/Turabian StyleMiętkiewska, Klaudia, Pawel Kordowitzki, and Chandra S. Pareek. 2022. "Effects of Heat Stress on Bovine Oocytes and Early Embryonic Development—An Update" Cells 11, no. 24: 4073. https://doi.org/10.3390/cells11244073
APA StyleMiętkiewska, K., Kordowitzki, P., & Pareek, C. S. (2022). Effects of Heat Stress on Bovine Oocytes and Early Embryonic Development—An Update. Cells, 11(24), 4073. https://doi.org/10.3390/cells11244073







