Evaluation of Tissue Ischemia/Reperfusion Injury in Lung Recipients Supported by Intraoperative Extracorporeal Membrane Oxygenation: A Single-Center Pilot Study
Abstract
1. Introduction
2. Materials and Methods
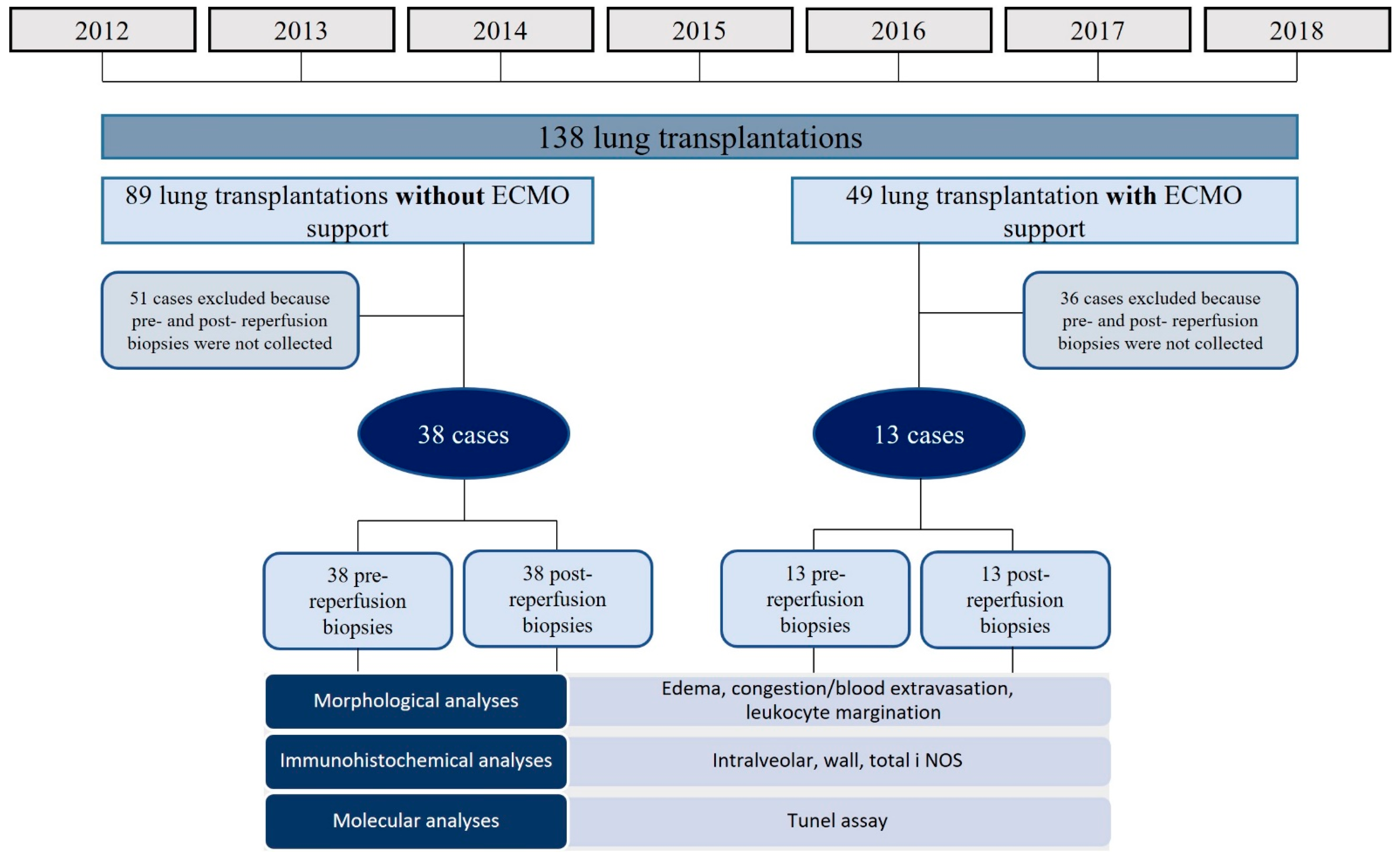
2.1. Study Design and Population
2.2. Morphological Analyses, Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Assay, and Immunohistochemistry
2.3. Real-Time PCR for iNOS Expression
2.4. Statistical Analyses
3. Results
Morphological and Molecular Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen-Yoshikawa, T.F. Ischemia–Reperfusion Injury in Lung Transplantation. Cells 2021, 10, 1333. [Google Scholar] [CrossRef] [PubMed]
- Gelman, A.E.; Fisher, A.J.; Huang, H.J.; Baz, M.A.; Shaver, C.M.; Egan, T.M.; Mulligan, M.S. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction Part III: Mechanisms: A 2016 Consensus Group Statement of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2017, 36, 1114–1120. [Google Scholar] [CrossRef]
- de Perrot, M.; Liu, M.; Waddell, T.K.; Keshavjee, S. Ischemia–Reperfusion–Induced Lung Injury. Am. J. Respir. Crit. Care Med. 2003, 167, 490–511. [Google Scholar] [CrossRef] [PubMed]
- Cypel, M.; Rubacha, M.; Yeung, J.; Hirayama, S.; Torbicki, K.; Madonik, M.; Fischer, S.; Hwang, D.; Pierre, A.; Waddell, T.K.; et al. Normothermic Ex Vivo Perfusion Prevents Lung Injury Compared to Extended Cold Preservation for Transplantation. Am. J. Transplant. 2009, 9, 2262–2269. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.C.; Loor, G.; Carrott, P.; Shafii, A. Review of donor and recipient surgical procedures in lung transplantation. J. Thorac. Dis. 2019, 11 (Suppl. S14), S1810–S1816. [Google Scholar] [CrossRef] [PubMed]
- Machuca, T.N.; Collaud, S.; Mercier, O.; Cheung, M.; Cunningham, V.; Kim, S.J.; Azad, S.; Singer, L.; Yasufuku, K.; de Perrot, M.; et al. Outcomes of intraoperative extracorporeal membrane oxygenation versus cardiopulmonary bypass for lung transplantation. J. Thorac. Cardiovasc. Surg. 2015, 149, 1152–1157. [Google Scholar] [CrossRef]
- Ius, F.; Aburahma, K.; Boethig, D.; Salman, J.; Sommer, W.; Draeger, H.; Poyanmehr, R.; Avsar, M.; Siemeni, T.; Bobylev, D.; et al. Long-term outcomes after intraoperative extracorporeal membrane oxygenation during lung transplantation. J. Heart Lung Transplant. 2020, 39, 915–925. [Google Scholar] [CrossRef]
- Salman, J.; Bernhard, B.-A.; Ius, F.; Poyanmehr, R.; Sommer, W.; Aburahma, K.; Alhadidi, H.; Siemeni, T.; Kuehn, C.; Avsar, M.; et al. Intraoperative Extracorporeal Circulatory Support in Lung Transplantation for Pulmonary Fibrosis. Ann. Thorac. Surg. 2021, 111, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Bittner, H.B.; Binner, C.; Lehmann, S.; Kuntze, T.; Rastan, A.; Mohr, F.W. Replacing cardiopulmonary bypass with extracorporeal membrane oxygenation in lung transplantation operations. Eur. J. Cardio-Thorac. Surg. 2007, 31, 462–467. [Google Scholar] [CrossRef]
- Ruszel, N.; Kiełbowski, K.; Piotrowska, M.; Kubisa, M.; Grodzki, T.; Wójcik, J.; Kubisa, B. Central, peripheral ECMO or CPB? Comparsion between circulatory support methods used during lung transplantation. J. Cardiothorac. Surg. 2021, 16, 341. [Google Scholar] [CrossRef]
- Hoetzenecker, K.; Benazzo, A.; Stork, T.; Sinn, K.; Schwarz, S.; Schweiger, T.; Klepetko, W.; Kifjak, D.; Baron, D.; Hager, H.; et al. Bilateral lung transplantation on intraoperative extracorporeal membrane oxygenator: An observational study. J. Thorac. Cardiovasc. Surg. 2020, 160, 320–327.e1. [Google Scholar] [CrossRef] [PubMed]
- Pereszlenyi, A.; Lang, G.; Steltzer, H.; Hetz, H.; Kocher, A.; Neuhauser, P.; Wisser, W.; Klepetko, W. Bilateral lung transplantation with intra- and postoperatively prolonged ECMO support in patients with pulmonary hypertension. Eur. J. Cardio-Thorac. Surg. 2002, 21, 858–863. [Google Scholar] [CrossRef]
- Hoetzenecker, K.; Schwarz, S.; Muckenhuber, M.; Benazzo, A.; Frommlet, F.; Schweiger, T.; Bata, O.; Jaksch, P.; Ahmadi, N.; Muraközy, G.; et al. Intraoperative extracorporeal membrane oxygenation and the possibility of postoperative prolongation improve survival in bilateral lung transplantation. J. Thorac. Cardiovasc. Surg. 2018, 155, 2193–2206.e3. [Google Scholar] [CrossRef] [PubMed]
- Dell’Amore, A.; Campisi, A.; Congiu, S.; Mazzarra, S.; Pastore, S.; Dolci, G.; Baiocchi, M.; Frascaroli, G. Extracorporeal life support during and after bilateral sequential lung transplantation in patients with pulmonary artery hypertension. Artif. Organs 2020, 44, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.K.; Hong, S.-B.; Shim, T.S.; Kim, D.K.; Choi, S.; Lee, G.D.; Kim, W.; Park, S.-I. Effects of the duration of bridge to lung transplantation with extracorporeal membrane oxygenation. PLoS ONE 2021, 16, e0253520. [Google Scholar] [CrossRef] [PubMed]
- Fessler, J.; Sage, E.; Roux, A.; Feliot, E.; Gayat, E.; Pirracchio, R.; Parquin, F.; Cerf, C.; Fischler, M.; Le Guen, M. Is Extracorporeal Membrane Oxygenation Withdrawal a Safe Option After Double-Lung Transplantation? Ann. Thorac. Surg. 2020, 110, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Snell, G.I.; Yusen, R.D.; Weill, D.; Strueber, M.; Garrity, E.; Reed, A.; Pelaez, A.; Whelan, T.P.; Perch, M.; Bag, R.; et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, part I: Definition and grading—A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2017, 36, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, F.; Schiavon, M.; Perissinotto, E.; Lunardi, F.; Marulli, G.; Di Gregorio, G.; Pezzuto, F.; Vuljan, S.E.; Forin, E.; Wiegmann, B.; et al. Organ Care System Lung resulted in lower apoptosis and iNOS expression in donor lungs. Am. J. Transplant. 2020, 20, 3639–3648. [Google Scholar] [CrossRef] [PubMed]
- Bhabra, M.S.; Hopkinson, D.N.; Shaw, T.E.; Onwu, N.; Hooper, T.L. Controlled Reperfusion Protects Lung Grafts During a Transient Early Increase in Permeability. Ann. Thorac. Surg. 1998, 65, 187–192. [Google Scholar] [CrossRef]
- Hopkinson, D.N.; Bhabra, M.S.; Odom, N.J.; Bridgewater, B.J.M.; Van Doorn, C.; Hooper, T.L. Controlled pressure reperfusion of rat pulmonary grafts yields improved function after twenty-four-hours’ cold storage in University of Wisconsin solution. J. Hear. Lung Transplant. 1996, 15, 283–290. [Google Scholar]
- Bhabra, M.S.; Hopkinson, D.N.; Shaw, T.E.; Hooper, T.L. Critical importance of the first 10 minutes of lung graft reperfusion after hypothermic storage. Ann. Thorac. Surg. 1996, 61, 1631–1635. [Google Scholar] [CrossRef]
- Guth, S.; Prüfer, D.; Kramm, T.; Mayer, E. Length of pressure-controlled reperfusion is critical for reducing ischaemia-reperfusion injury in an isolated rabbit lung model. J. Cardiothorac. Surg. 2007, 2, 54. [Google Scholar] [CrossRef]
- Ichimura, H.; Parthasarathi, K.; Issekutz, A.C.; Bhattacharya, J. Pressure-induced leukocyte margination in lung postcapillary venules. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2005, 289, L407–L412. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Maclean, A.A.; Liu, M.; Cardella, J.A.; Slutsky, A.S.; Suga, M.; Moreira, J.F.M.; Keshavjee, S. Dynamic Changes in Apoptotic and Necrotic Cell Death Correlate with Severity of Ischemia–Reperfusion Injury in Lung Transplantation. Am. J. Respir. Crit. Care Med. 2000, 162, 1932–1939. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.S.H.; Wan, S.; Yim, A.P.C. Pulmonary ischaemia-reperfusion injury: Role of apoptosis. Eur. Respir. J. 2005, 25, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.O.S.; Losada, D.M.; Jordani, M.C.; Évora, P.; Castro-E-Silva, O. Ischemia/Reperfusion Injury Revisited: An Overview of the Latest Pharmacological Strategies. Int. J. Mol. Sci. 2019, 20, 5034. [Google Scholar] [CrossRef]
- Sugimoto, R.; Okamoto, T.; Nakao, A.; Zhan, J.; Wang, Y.; Kohmoto, J.; Tokita, D.; Farver, C.F.; Tarpey, M.M.; Billiar, T.R.; et al. Nitrite Reduces Acute Lung Injury and Improves Survival in a Rat Lung Transplantation Model. Am. J. Transplant. 2012, 12, 2938–2948. [Google Scholar] [CrossRef]
- Diamond, J.M.; Lee, J.C.; Kawut, S.M.; Shah, R.J.; Localio, A.R.; Bellamy, S.L.; Lederer, D.J.; Cantu, E.; Kohl, B.A.; Lama, V.N.; et al. Clinical Risk Factors for Primary Graft Dysfunction after Lung Transplantation. Am. J. Respir. Crit. Care Med. 2013, 187, 527–534. [Google Scholar] [CrossRef]


| n | Non ECMO (n = 38) | ECMO (n = 13) | Combined (n = 51) | p-Value | |
|---|---|---|---|---|---|
| Age | 51 | 36.00/49.00/57.75 | 45.00/56.00/60.00 | 36.00/52.00/58.00 | 0.264 |
| Gender: M | 51 | 37% (14) | 23% (3) | 33% (17) | 0.363 |
| F | 63% (24) | 77% (10) | 67% (34) | ||
| Native disease: cystic fibrosis | 51 | 11% (4) | 8% (1) | 10% (5) | 0.577 |
| COPD | 47% (18) | 54% (7) | 49% (25) | ||
| ILD | 37% (14) | 23% (3) | 33% (17) | ||
| Other | 5% (2) | 15% (2) | 8% (4) | ||
| Weight | 51 | 50.5/64.0/77.5 | 65.0/78.0/86.0 | 54.5/65.0/81.5 | 0.058 |
| Height | 51 | 160.0/170.0/177.0 | 165.0/169.0/176.0 | 160.5/170.0/177.0 | 0.881 |
| BMI | 51 | 19.50781/21.77679/25.11628 | 21.97134/26.19619/29.93827 | 19.97871/23.09541/27.65886 | 0.016 * |
| Pulmonary arterial pression (mmHg) | 49 | 17.00/22.00/25.25 | 25.00/31.00/35.00 | 18.00/22.00/27.00 | 0.004 * |
| Total lung ischemia time (min) | 45 | 219.50/270.00/317.50 | 206.25/251.00/299.75 | 215.00/267.00/315.00 | 0.388 |
| Primary graft dysfunction h 0: 0–1 | 30 | 24 62% (15) | 6 20% (1) | 53% (16) | 0.08 |
| 2–3 | 38% (9) | 80% (5) | 47% (14) | ||
| Primary graft dysfunction h 24: 0–1 | 32 | 25 70% (17) | 7 30% (2) | 59% (19) | 0.09 |
| 2–3 | 30% (8) | 70% (5) | 41% (13) | ||
| Primary graft dysfunction h 48: 0–1 | 30 | 25 70% (18) | 5 40% (2) | 67% (20) | 0.3 |
| 2–3 | 30% (7) | 60% (3) | 33% (10) | ||
| Primary graft dysfunction h 72: 0–1 | 33 | 25 80% (19) | 8 50% (4) | 70% (23) | 0.2 |
| 2–3 | 20% (6) | 50% (4) | 30% (10) | ||
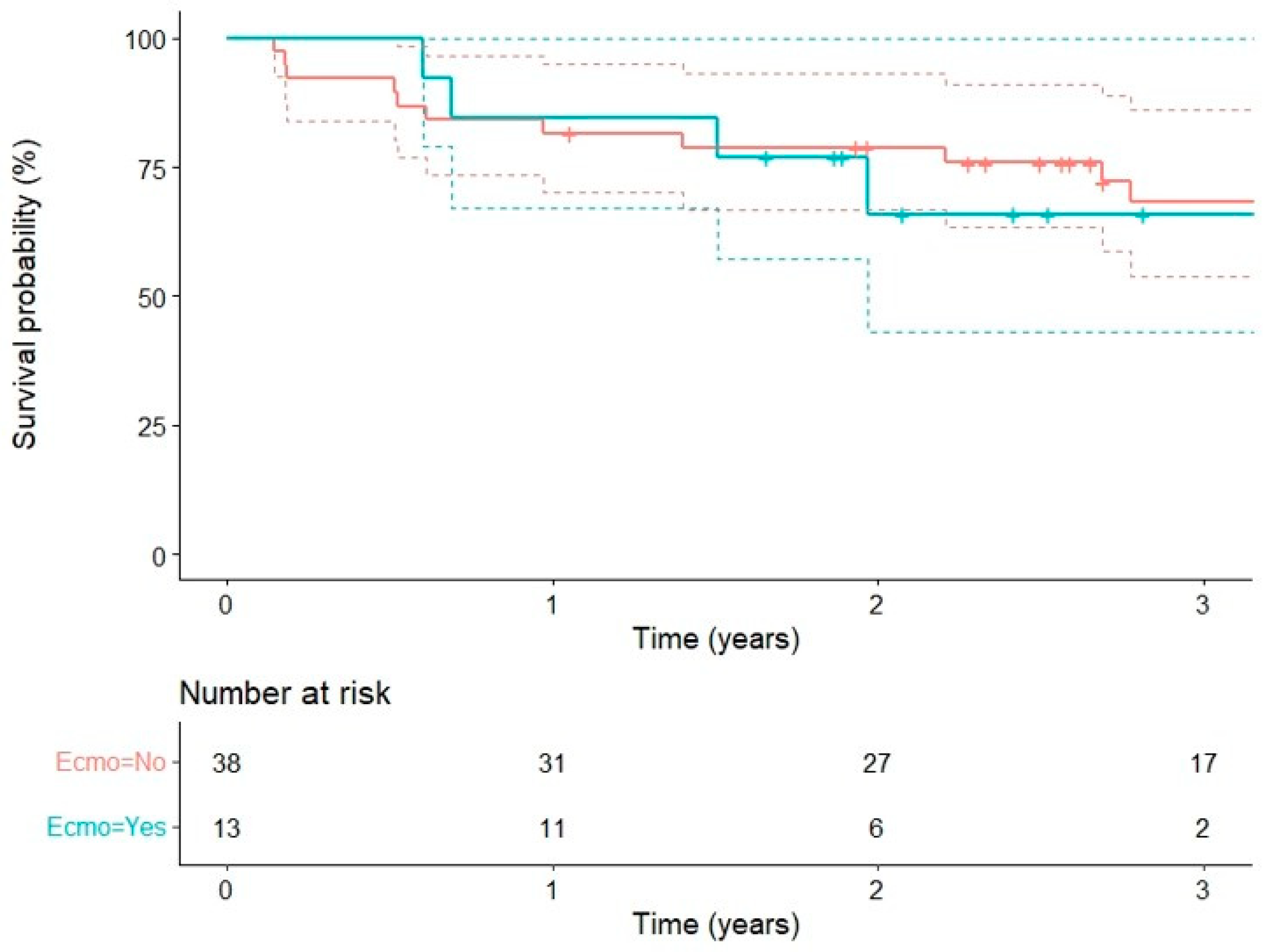
| Early mortality (<30 days) | 51 | 5.3% (2) | 0 | 3.9% (2) | - |
| n | Non-ECMO (n = 38) | ECMO (n = 13) | Combined (n = 51) | p-Value | |
|---|---|---|---|---|---|
| Age | 51 | 28.00/43.00/52.75 | 33.00/47.00/54.00 | 29.50/44.00/53.00 | 0.461 |
| Gender: M | 51 | 55% (21) | 77% (10) | 61% (31) | 0.167 |
| F | 45% (17) | 23% (3) | 39% (20) | ||
| Cause of death: Trauma | 48 | 34% (12) | 38% (5) | 35% (17) | 0.824 |
| Cardiovascular events | 51% (18) | 54% (7) | 52% (25) | ||
| Other | 14% (5) | 8% (1) | 12% (6) | ||
| Alcohol: No | 50 | 95% (36) | 83% (10) | 92% (46) | 0.204 |
| Yes | 5% (2) | 17% (2) | 8% (4) | ||
| Smoking: No | 50 | 82% (31) | 58% (7) | 76% (38) | 0.1 |
| Yes | 18% (7) | 42% (5) | 24% (12) | ||
| Oto score | 51 | 1/2/4 | 1/3/4 | 1/3/4 | 0.779 |
| Pre-Reperfusion | Post-Reperfusion | |||||
|---|---|---|---|---|---|---|
| Variables | ECMO Group (n = 13) | Non-ECMO Group (n = 38) | p-Value | ECMO Group (n = 13) | Non-ECMO Group (n = 38) | p-Value |
| Edema (median; IQR) | 0 (0–0) | 0 (0–0) | 0.30 0.57 | 0 (0–0) | 0 (0–0) | 0.86 0.62 |
| 0 (n, %) | 13 (100%) | 37 (97%) | 12 (92%) | 33 (87%) | ||
| 1 | 0 | 1 (3%) | 0 | 4 (10%) | ||
| 2 | 0 | 0 | 0 | 1 (3%) | ||
| 3 | 0 | 0 | 1 (8%) | 0 | ||
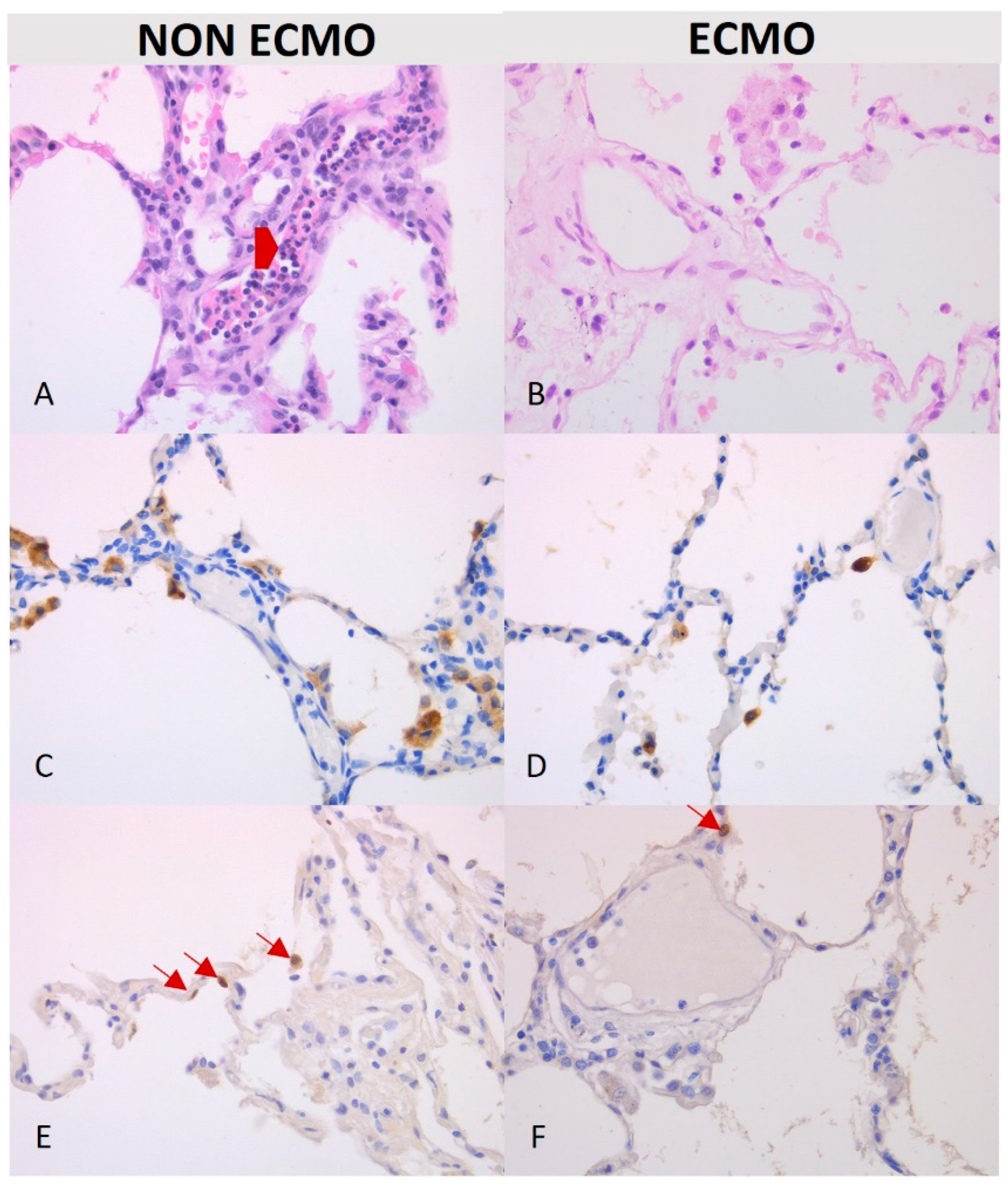
| Congestion/blood extravasation (median; IQR) | 0 (0–0) | 1 (0–1) | 0.39 0.42 | 0 (0.75–2) | 1 (0–2) | 0.95 0.98 |
| 0 (n, %) | 10 (77%) | 25 (66%) | 3 (23%) | 13 (34%) | ||
| 1 | 2 (15%) | 10 (26%) | 6 (46%) | 10 (26%) | ||
| 2 | 1 (8%) | 3 (8%) | 3 (23%) | 11 (29%) | ||
| 3 | 0 | 0 | 1 (8%) | 4 (11%) | ||
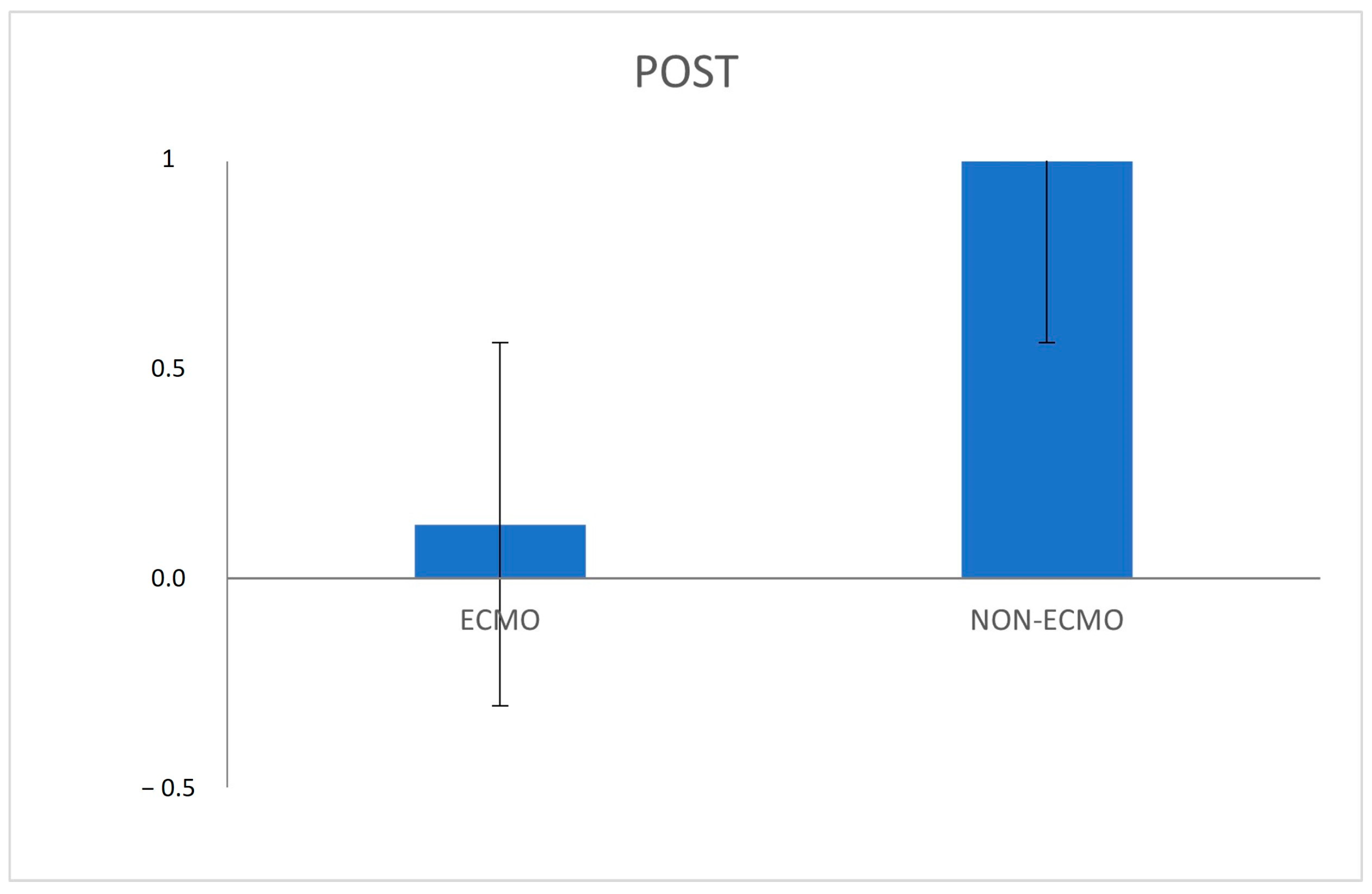
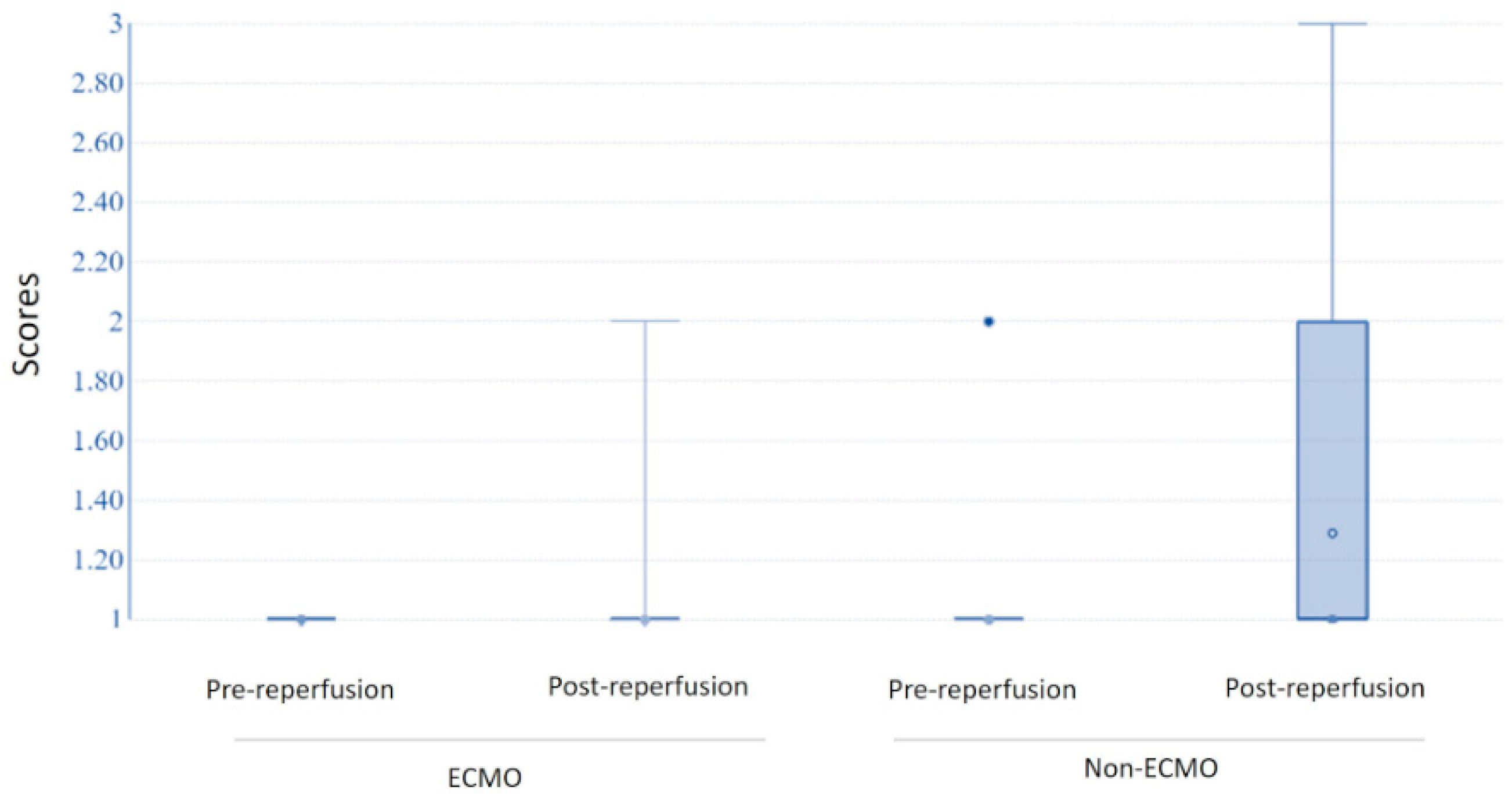
| Leucocyte margination (median; IQR) | 0 (0–0) | 0 (0–0) | 0.5 0.29 | 0 (0–1) | 1 (1–2) | 0.036 * 0.03 * |
| 0 (n, %) | 11 (85%) | 30 (79%) | 7 (70%) | 9 (24%) | ||
| 1 | 2 (15%) | 6 (16%) | 1 (10%) | 14 (37%) | ||
| 2 | 0 | 2 (5%) | 2 (20%) | 10 (26%) | ||
| 3 | 0 | 0 | 0 | 5 (13%) | ||
| Apoptotic index (median; IQR) | 0.67 (0–3.55) | 0.67 (0.33–1.33) | 0.89 | 0 (0–0.415) | 0.415 (0–0.67) | 0.48 |
| iNOS (median; IQR) | ||||||
| Alveolar | 10.5 (5.5–14.75) | 4.15 (2–19) | 0.07 | 7.5 (1.75–12.75) | 6.6 (1.5–12) | 0.71 |
| Wall | 7.5 (4–16.5) | 15.5 (8.55–33.75) | 0.12 | 8.5 (8–27.25) | 19 (13.25–32.5) | 0.2 |
| Total | 16.5 (9–34.25) | 25.5 (15.5–38.75) | 0.68 | 13 (6.5–42) | 24 (18.25–43.5) | 0.6 |
| Group of Comparison | Parameter | p-Boot |
|---|---|---|
| Pre- and post-reperfusion in ECMO | Apoptotic index | 0.308 |
| Intra-alveolar INOS | 0.0345 * | |
| Wall INOS | 0.0064 * | |
| Total INOS | 0.0061 * | |
| Pre- and post-reperfusion in non-ECMO | Apoptotic index | 0.790 |
| Intra-alveolar INOS | 0.1361 | |
| Wall INOS | 0.0501 * | |
| Total INOS | 0.0424 * | |
| ECMO vs. non-ECMO | Apoptotic index | 0.143 |
| Intra-alveolar INOS | 0.0484 * | |
| Wall INOS | 0.0154 * | |
| Total INOS | 0.0069 * |
| Parameter | Time Points | Coefficient | CI.95 | p-Value |
|---|---|---|---|---|
| Edema | Pre-reperfusion | Not estimable | - | - |
| Post-reperfusion | 0.44 | [0.05;4.09] | 0.4737 | |
| Congestion | Pre-reperfusion | 0.58 | [0.13;2.47] | 0.4584 |
| Post-reperfusion | 1.73 | [0.41;7.42] | 0.4584 | |
| Leukocyte margination | Pre-reperfusion | −0.11 | [−0.43;0.22] | 0.513006 |
| Post-reperfusion | −0.67 | [−1.26;−0.08] | 0.0295 * | |
| Apoptotic index | Pre-reperfusion | 0.79 | [−0.47;2.05] | 0.2258226 |
| Post-reperfusion | −0.51 | [−1.51;0.49] | 0.322918 | |
| Alveolar iNOS | Pre-reperfusion | −0.08 | [−9.61;9.44] | 0.9865585 |
| Post-reperfusion | 7.77 | [5.21;10.34] | <1 × 10−4 * | |
| Wall iNOS | Pre-reperfusion | −10.63 | [−24.00;2.74] | 0.1264 |
| Post-reperfusion | −9.74 | [−23.06;3.58] | 0.1587 | |
| Total iNOS | Pre-reperfusion | −9.14 | [−25.10;6.83] | 0.268 |
| Post-reperfusion | −8.70 | [−24.13;6.73] | 0.2751 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabrese, F.; Pezzuto, F.; Fortarezza, F.; Lunardi, F.; Faccioli, E.; Lorenzoni, G.; Boscolo, A.; Sella, N.; Gregori, D.; Schiavon, M.; et al. Evaluation of Tissue Ischemia/Reperfusion Injury in Lung Recipients Supported by Intraoperative Extracorporeal Membrane Oxygenation: A Single-Center Pilot Study. Cells 2022, 11, 3681. https://doi.org/10.3390/cells11223681
Calabrese F, Pezzuto F, Fortarezza F, Lunardi F, Faccioli E, Lorenzoni G, Boscolo A, Sella N, Gregori D, Schiavon M, et al. Evaluation of Tissue Ischemia/Reperfusion Injury in Lung Recipients Supported by Intraoperative Extracorporeal Membrane Oxygenation: A Single-Center Pilot Study. Cells. 2022; 11(22):3681. https://doi.org/10.3390/cells11223681
Chicago/Turabian StyleCalabrese, Fiorella, Federica Pezzuto, Francesco Fortarezza, Francesca Lunardi, Eleonora Faccioli, Giulia Lorenzoni, Annalisa Boscolo, Nicolò Sella, Dario Gregori, Marco Schiavon, and et al. 2022. "Evaluation of Tissue Ischemia/Reperfusion Injury in Lung Recipients Supported by Intraoperative Extracorporeal Membrane Oxygenation: A Single-Center Pilot Study" Cells 11, no. 22: 3681. https://doi.org/10.3390/cells11223681
APA StyleCalabrese, F., Pezzuto, F., Fortarezza, F., Lunardi, F., Faccioli, E., Lorenzoni, G., Boscolo, A., Sella, N., Gregori, D., Schiavon, M., Navalesi, P., Dell’Amore, A., & Rea, F. (2022). Evaluation of Tissue Ischemia/Reperfusion Injury in Lung Recipients Supported by Intraoperative Extracorporeal Membrane Oxygenation: A Single-Center Pilot Study. Cells, 11(22), 3681. https://doi.org/10.3390/cells11223681











