Transcriptome Analysis Revealed the Mechanism of Inhibition of Saprophytic Growth of Sparassis latifolia by Excessive Oxalic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain, Sample Collection, and Preparation
2.2. RNA Isolation
2.3. Library Construction and Illumina Sequencing
2.4. DEG Analysis and Functional Annotation
2.5. Quantitative Real-Time PCR (qRT-PCR)-Based Confirmation of Expression Values of Selected Candidate Genes
2.6. Statistical Analysis
3. Results
3.1. Mycelial Growth of S. latifolia under Different Oxalic Acid Concentrations
3.2. Transcriptome Sequencing and Assembly
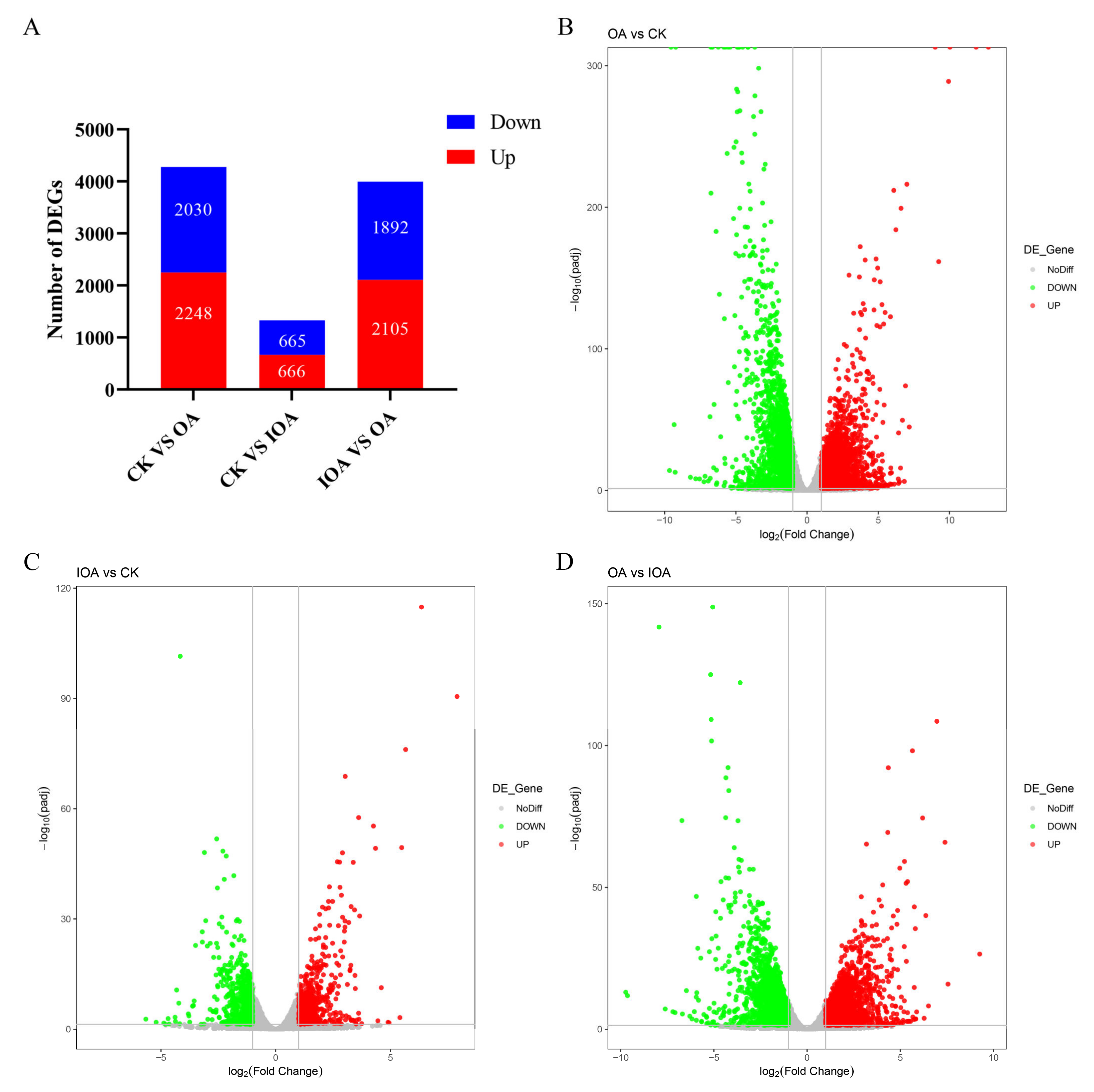
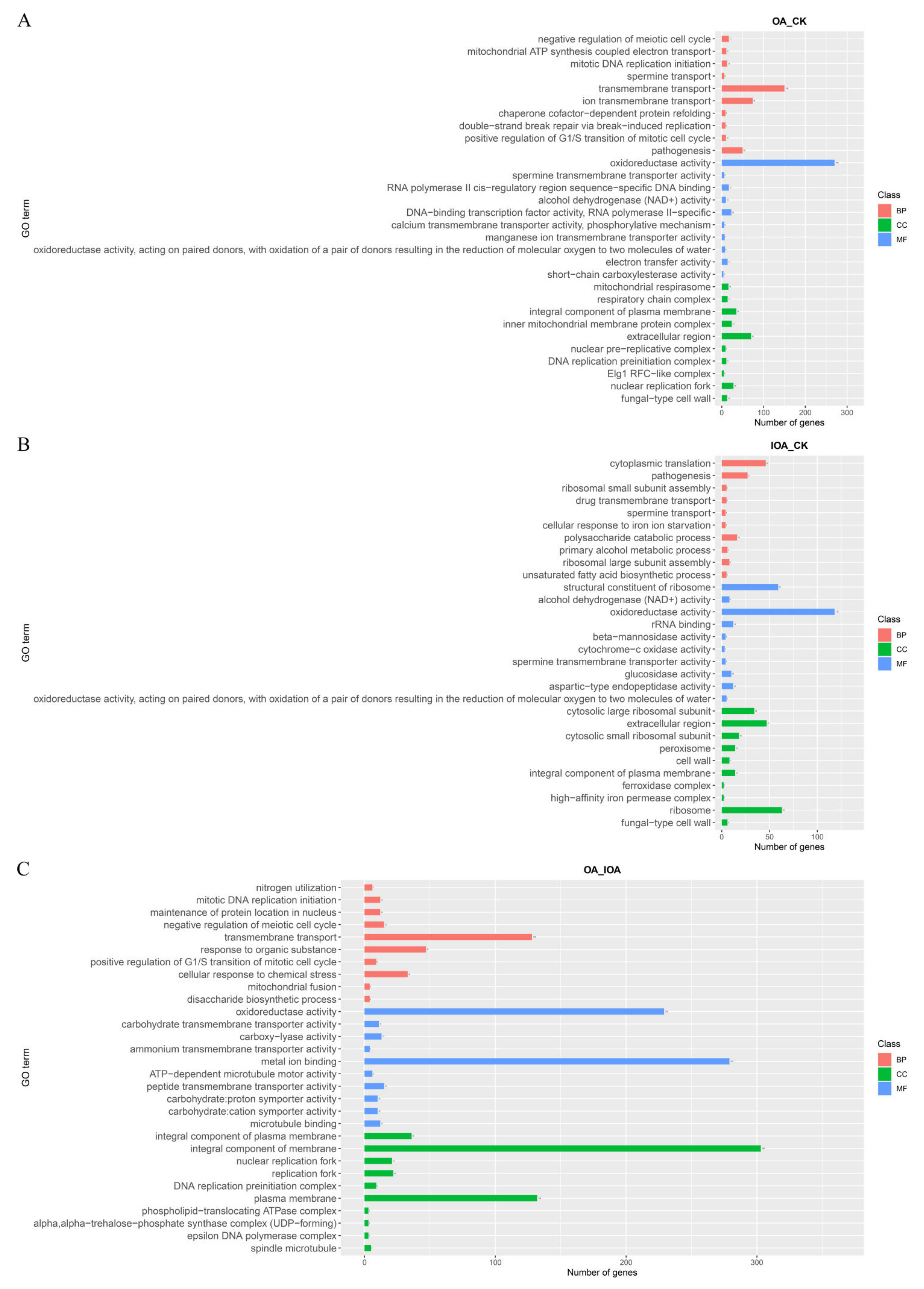
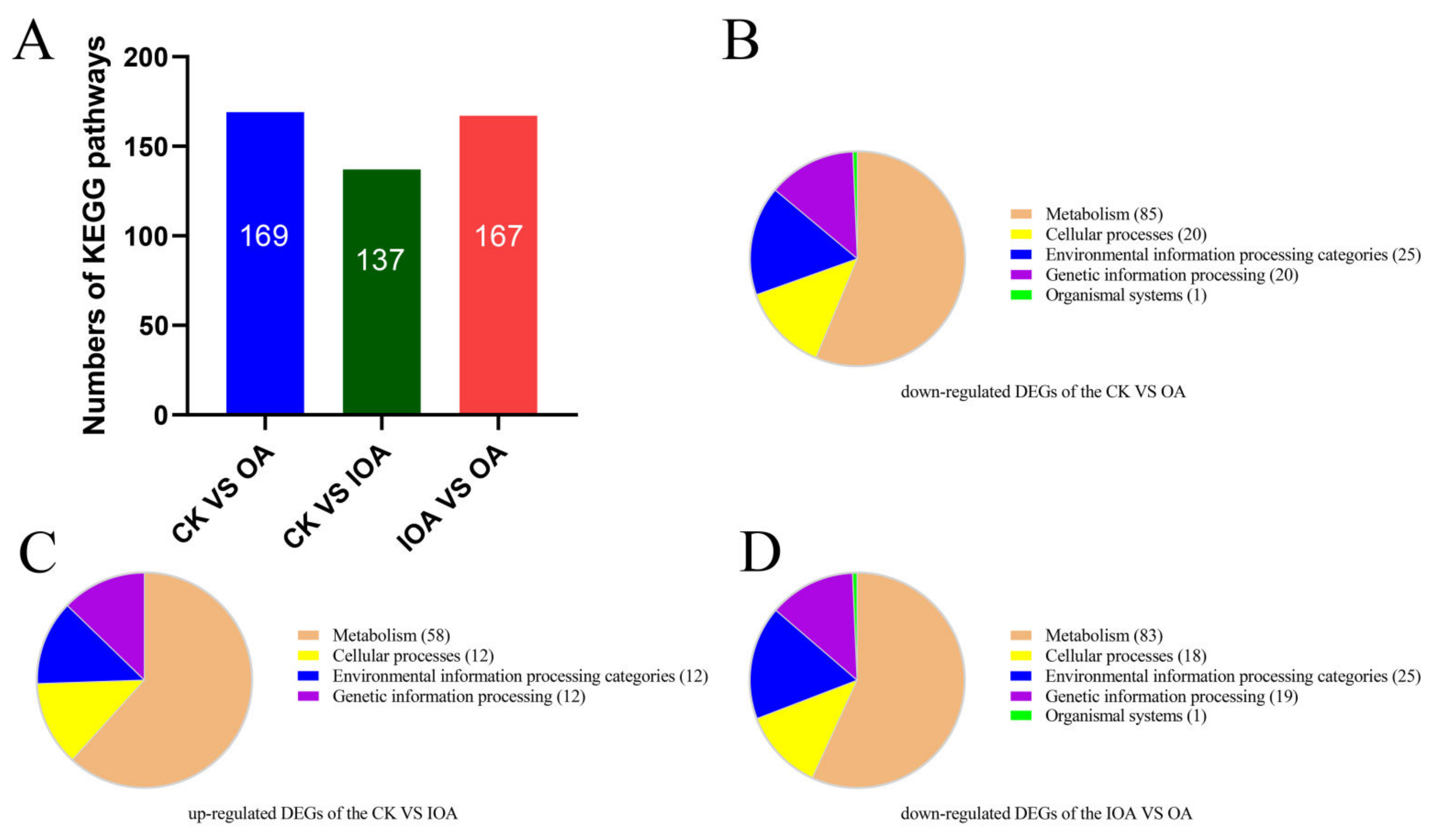
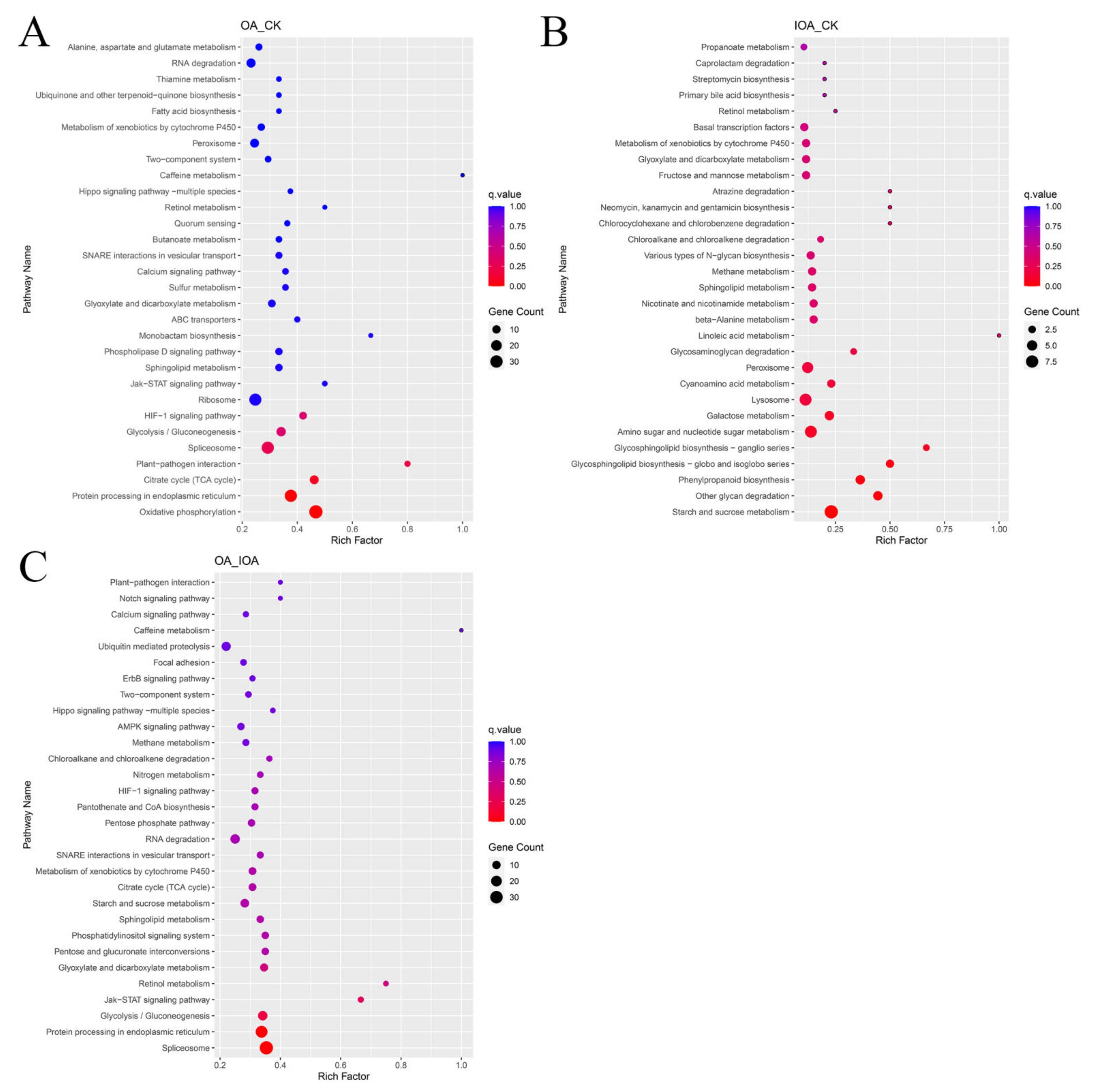
3.3. DEGs and Functional Analysis
3.4. DEGs Related to Lignocellulose Degradation Metabolism
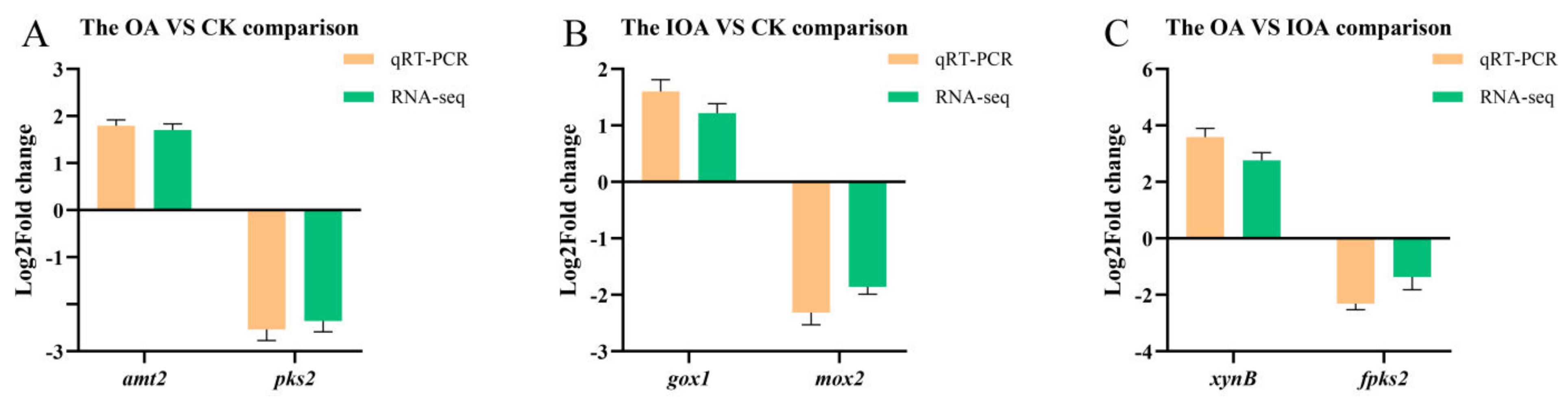
3.5. Validation of Transcriptome Data by qRT-PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Liu, J.; Zhang, X.; Zhang, J.; Yan, M.; Li, D.; Zhou, S.; Feng, J.; Liu, Y. Research on extraction, structure characterization and immunostimulatory activity of cell wall polysaccharides from Sparassis latifolia. Polymers 2022, 14, 549. [Google Scholar] [CrossRef] [PubMed]
- Duan, G.-L.; Yu, X.-B. Isolation, purification, characterization, and antioxidant activity of low-molecular-weight polysaccharides from Sparassis latifolia. Int. J. Biol. Macromol. 2019, 137, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Lin, Y.; Yang, C.; Ying, Z.; Jiang, X. Production of liquid spawn of an edible mushroom, Sparassis latifolia by submerged fermentation and mycelial growth on pine wood sawdust. Sci. Hortic. 2016, 209, 22–30. [Google Scholar] [CrossRef]
- Lili, S.; Li, Y. First Report of Sparassis latifolia causing basal rot of Pinus koraiensis in China. Plant Dis. 2017, 101, 833. [Google Scholar]
- Shu, L.; Wang, M.; Wang, S.; Li, Y.; Xu, H.; Qiu, Z.; Li, T. Excessive oxalic acid secreted by Sparassis latifolia inhibits the growth of mycelia during its saprophytic process. Cells 2022, 11, 2423. [Google Scholar] [CrossRef]
- Kirkland, B.; Eisa, A.; Keyhani, N. Oxalic acid as a fungal acaracidal virulence factor. J. Med. Entomol. 2005, 42, 346–351. [Google Scholar] [CrossRef]
- Rana, K.; Yuan, J.; Liao, H.; Banga, S.; Kumar, R.; Ding, Y.; Qian, W. Host-induced gene silencing reveals the role of Sclerotinia sclerotiorum oxaloacetate acetylhydrolase gene in fungal oxalic acid accumulation and virulence. Microbiol. Res. 2022, 258, 126981. [Google Scholar] [CrossRef]
- Munir, E.; Yoon, J.; Tokimatsu, T.; Hattori, T.; Shimada, M. A physiological role for oxalic acid biosynthesis in the wood-rotting basidiomycete Fomitopsis palutris. Proc. Natl. Acad. Sci. USA 2001, 98, 11126–11130. [Google Scholar] [CrossRef]
- Gonzalez, J.; Costa, M.D.; Silva, I.; César, J.; Barros, N.; Borges, A. Accumulation of oxalic acid and calcium crystals in eucalypt ectomycorrhizas.: I-oxalic acid production and nutrient concentration in fine lateral roots colonized with ectomicorrhizal fungi. Rev. Bras. Cienc. Solo 2009, 33, 541–553. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhuang, L.; Goodell, B.; Cao, J.; Mahaney, J. Iron sequestration in brown-rot fungi by oxalate and the production of reactive oxygen species (ROS). Int. Biodeterior. Biodegrad. 2016, 109, 185–190. [Google Scholar] [CrossRef]
- Shah, F.; Mali, T.; Lundell, T. Polyporales brown rot species Fomitopsis pinicola: Enzyme activity profiles, oxalic acid production, and Fe3+-reducing metabolite secretion. Appl. Environ. Microbiol. 2018, 84, e02662-17. [Google Scholar] [CrossRef]
- Ye, D.; Du, F.; Hu, Q.; Zou, Y.; Bai, X. Transcriptome analysis reveals candidate genes involved in light-induced primordium differentiation in Pleurotus eryngii. Int. J. Mol. Sci. 2021, 23, 435. [Google Scholar] [CrossRef]
- Graz, M.; Jarosz-Wilkolazka, A.; Janusz, G.; Mazur, A.; Wielbo, J.; Koper, P.; Żebracki, K.; Kubik-Komar, A. Transcriptome-based analysis of the saprophytic fungus Abortiporus biennis—Response to oxalic acid. Microbiol. Res. 2017, 199, 79–88. [Google Scholar] [CrossRef]
- Xing, Y.; Li, B.; Zeng, X.; Li-Si, Z.; Lee, T.-S.; Lee, M.; Chen, X.-M.; Guo, S.-X. Use of transcriptomic profiling to identify candidate genes involved in Polyporus umbellatus sclerotial formation affected by oxalic acid. Sci. Rep. 2021, 11, 17326. [Google Scholar] [CrossRef]
- Zhang, Z.-f.; Song, T.; Cai, W.; Wang, F.; Lv, G. Effects of different depolymerisation methods on the physicochemical and antioxidant properties of polysaccharides derived from Sparassis latifolia. Process Biochem. 2021, 110, 110–117. [Google Scholar] [CrossRef]
- Han, Y.; Joosten, H.-J.; Niu, W.; Zhao, Z.; Mariano, P.; McCalman, M.; Kan, J.; Schaap, P. Oxaloacetate hydrolase, the C-C bond lyase of oxalate secreting fungi. J. Biol. Chem. 2007, 282, 9581–9590. [Google Scholar] [CrossRef]
- Ozsolak, F.; Milos, P. RNA sequencing: Advances, challenges and opportunities. Nat. Rev. 2011, 12, 87–98. [Google Scholar] [CrossRef]
- Bolger, A.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Trapnell, C.; Pachter, L.; Salzberg, S. TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Harris, M.; Deegan, J.; Ireland, A.; Lomax, J.; Ashburner, M.; Foulger, R.; Eilbeck, K.; Lewis, S.; Marshall, B.; Mungall, C.; et al. The Gene Oncology (GO) database and informatics resource. Nucleic Acids Res. 2004, 32, 258–261. [Google Scholar]
- Kanehisa, M.; Goto, S.; Hattori, M.; Aoki-Kinoshita, K.; Itoh, M.; Kawashima, S.; Katayama, T.; Araki, M.; Hirakawa, M. From genomics to chemical genomics: New developments in KEGG. Nucleic Acids Res. 2006, 34, D354–D357. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Hastrup, A.; Green, F.; Lebow, P.; Jensen, B. Enzymatic oxalic acid regulation correlated with wood degradation in four brown-rot fungi. Int. Biodeterior. Biodegrad. 2012, 75, 109–114. [Google Scholar] [CrossRef]
- Nagy, N.; Kvaalen, H.; Fongen, M.; Fossdal, C.G.; Clarke, N.; Solheim, H.; Hietala, A. The pathogenic white-rot fungus Heterobasidion parviporum responds to spruce xylem defense by enhanced production of oxalic acid. Mol. Plant Microbe Interact. 2012, 25, 1450–1458. [Google Scholar] [CrossRef]
- Csarman, F.; Obermann, T.; Zanjko, M.; Man, P.; Halada, P.; Seiboth, B.; Ludwig, R. Functional expression and characterization of two laccases from the brown rot Fomitopsis pinicola. Enzym. Microb. Technol. 2021, 148, 109801. [Google Scholar] [CrossRef]
- Punja, Z.; Huang, J.S.; Jenkins, S.F. Relationship of mycelial growth and production of oxalic acid and cell wall degrading enzymes to virulence in Sclerotium rolfsii. Can. J. Plant Pathol. 2009, 1985, 109–117. [Google Scholar]
- Ahlawat, O.P.; Gupta, P.; Kamal, S.; Dhar, B. Development of molecular and biochemical markers for selecting a potential high yielding strain of paddy straw mushroom (Volvariella volvacea). J. Plant Biochem. Biotechnol. 2008, 17, 57–63. [Google Scholar] [CrossRef]
- Znameroski, E.; Coradetti, S.; Roche, C.; Tsai, J.; Iavarone, A.; Cate, J.; Glass, N. Induction of lignocellulose-degrading enzymes in Neurospora crassa by cellodextrins. Proc. Natl. Acad. Sci. USA 2012, 109, 6012–6017. [Google Scholar] [CrossRef]
- Gong, W.; Wang, Y.; Xie, C.; Zhou, Y.; Zhu, Z.; Peng, Y. Whole genome sequence of an edible and medicinal mushroom, Hericium erinaceus (Basidiomycota, fungi). Genomics 2020, 112, 2393–2399. [Google Scholar] [CrossRef]
- Derks, T.; Lubout, C.; Woidy, M.; Santer, R. Disorders of Carbohydrate Absorption, Transmembrane Transport and Metabolism. In Physician’s Guide to the Diagnosis, Treatment, and Follow-Up of Inherited Metabolic Diseases; Blau, N., Dionisi Vici, C., Ferreira, C.R., Vianey-Saban, C., van Karnebeek, C.D.M., Eds.; Springer: Cham, Switzerland, 2022; pp. 649–700. [Google Scholar]
- Braun, V.; Endriss, F. Energy-coupled outer membrane transport proteins and regulatory proteins. Biometals 2007, 20, 219–231. [Google Scholar] [CrossRef]
- Ke, R.; Xu, Q.; Li, C.; Luo, L.; Huang, D. Mechanisms of AMPK in the maintenance of ATP balance during energy metabolism. Cell Biol. Int. 2017, 42, 384–392. [Google Scholar] [CrossRef]
- Papa, S.; Martino, P.; Capitanio, G.; Gaballo, A.; De Rasmo, D.; Signorile, A.; Petruzzella, V. The oxidative phosphorylation system in mammalian mitochondria. Adv. Exp. Med. Biol. 2012, 942, 3–37. [Google Scholar]
- Ľupták, M.; Hroudová, J. Important role of mitochondria and the effect of mood stabilizers on mitochondrial function. Physiol. Res. 2019, 68, S3–S15. [Google Scholar] [CrossRef]
- Miettinen, M. Succinate dehydrogenase-deficient tumors—A novel mechanism of tumor formation. Duodecim 2015, 131, 2149–2156. [Google Scholar]
- Pedra-Rezende, Y.; Fernandes, M.; Mesquita-Rodrigues, C.; Stiebler, R.; Bombaça, A.; Pinho, N.; Cuervo, P.; Castro, S.; Menna-Barreto, R. Starvation and pH stress conditions induced mitochondrial dysfunction, ROS production and autophagy in Trypanosoma cruzi epimastigotes. Biochem. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166028. [Google Scholar] [CrossRef]
- Akhtar, M.; Ahamed, M.; Alhadlaq, H. Mechanism of ROS scavenging and antioxidant signalling by redox metallic and fullerene nanomaterials: Potential implications in ROS associated degenerative disorders. Biochem. Biophys. Acta Gen. Subj. 2017, 1861, 802–813. [Google Scholar] [CrossRef]
- Oka, T.; Futagami, T.; Goto, M. Cell wall biosynthesis in filamentous fungi. In Stress Biology of Yeasts and Fungi: Applications for Industrial Brewing and Fermentation; Springer: Tokyo, Japan, 2015; pp. 151–168. [Google Scholar]
- Gow, N.; Latge, J.-P.; Munro, C. The fungal cell wall: Structure, biosynthesis, and function. Microbiol. Spectr. 2017, 5, 3. [Google Scholar] [CrossRef]
- Xue, S.; Barna, M. Specialized ribosomes: A new frontier in gene regulation and organismal biology. Nat. Rev. Mol. Cell Biol. 2012, 13, 355–369. [Google Scholar] [CrossRef]
- Huter, P.; Graf, M.; Wilson, D. The Ribosome and Protein Synthesis. In Ribozymes; Sabine, M., Benoît, M., Wade, W., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2021; pp. 193–226. [Google Scholar]
- Fernandez-Pol, J.A. Conservation of multifunctional ribosomal protein metallopanstimulin-1 (RPS27) through complex evolution demonstrates its key role in growth regulation in Archaea, eukaryotic cells, DNA repair, translation and viral replication. Cancer Genom. Proteom. 2011, 8, 105–126. [Google Scholar]
- Byrne, E. A role of the ribosome in development. Trends Plant Sci. 2009, 14, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-P.; Yi, Y.; Ma, B.-G. Amino acid flux from metabolic network benefits protein translation: The role of resource availability. Sci. Rep. 2015, 5, 11113. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Ma, L.; Xiao, D.; Ying, Z.; Jiang, X.; Lin, Y. Integration of ATAC-Seq and RNA-seq identifies key genes in light-induced primordia formation of Sparassis latifolia. Int. J. Mol. Sci. 2019, 21, 185. [Google Scholar] [CrossRef] [PubMed]
- Jiejing, T.; Wu, M.; Zhang, J.; Li, G.; Yang, L. Botrytis cinerea G protein β subunit Bcgb1 controls growth, development and virulence by regulating cAMP signaling and MAPK signaling. J. Fungi 2021, 7, 431. [Google Scholar] [CrossRef]
- Jiang, L.; Situ, J.; Deng, Y.; Wan, L.; Xu, D.; Chen, Y.; Xi, P.; Jiang, Z. PlMAPK10, a mitogen-activated protein kinase (MAPK) in Peronophythora litchii, is required for mycelial growth, sporulation, laccase activity, and plant infection. Front. Microbiol. 2018, 9, 426. [Google Scholar] [CrossRef]
- Negrão, D.; Mischan, M.; Pinho, S.; Carvalho, L.; Gomes, R.; Passos, J. How temperature variation affects white-rot fungi mycelial growth dynamics: A nonlinear mixed models approach. Fungal Biol. 2021, 125, 860–868. [Google Scholar] [CrossRef]
- Yu, F.; Gu, Q.; Yingzi, Y.; Yin, Y.; Xu, J.R.; Shim, W.B.; Ma, Z. The TOR signaling pathway regulates vegetative development and virulence in Fusarium graminearum. New Phytol. 2014, 203, 219–232. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, X.; Ren, B.; Cheng, L. The regulation of hyphae growth in Candida albicans. Virulence 2020, 11, 337–348. [Google Scholar] [CrossRef]
- Shu, L.; Wang, M.; Xu, H.; Qiu, Z.; Li, T. De novo transcriptome assembly and comprehensive assessment provide insight into fruiting body formation of Sparassis latifolia. Sci. Rep. 2022, 12, 11075. [Google Scholar] [CrossRef]
| Gene | Description | Primer Sequences (5′-3′) |
|---|---|---|
| 18S rbs | 18S rRNA gene | Forward: GCGCTACACTGACAGAGCCA |
| Reverse: GCGGTGTGTACAAAGGGCAG | ||
| mRNA_12253 | pks2; Polyketide synthase | Forward: GTTCTTGATTGATCGCGGGC |
| Reverse: TGGATTTCTGCGAGGCCATT | ||
| mRNA_9796 | amt2; Aminotransferase | Forward: CTTCTTTGGCAACCTGCACC |
| Reverse: CGTATCGAAATTCCGCGAGC | ||
| mRNA_9500 | mox2; Monooxygenase | Forward: GCTTCACATCGAGTTCCCCA |
| Reverse: AAGCAACTCCTTCCCAACCC | ||
| mRNA_5483 | gox1; Glycolate oxidase | Forward: GACGATCACATCCTCGGCTT |
| Reverse: ATATCGTCTCCGTCCCGTCT | ||
| mRNA_5970 | fks2; 1,3-beta-Glucan synthase | Forward: TGTCGCACTCCATCTCATCG |
| Reverse: AAGCGATCGTTTGGACACCT | ||
| mRNA_7010 | xynB; Glycoside hydrolase | Forward: GACCGTACAGTCTGGCTCTG |
| Reverse: ATGGTACGGGTCTGTAGGCT |
| Gene ID | Log2 Fold Change | p Value | Function Description | Gene Name | GO ID | GO Term |
|---|---|---|---|---|---|---|
| mRNA_2483 | 1.52 × 10−34 | 1.93 × 10−33 | Endo-β-1,4-xylanase | xyl1 | GO:0005576 | Endo-1,4-β-xylanase activity |
| mRNA_8946 | −1.637572757 | 7.47 × 10−5 | Exo-glucanase | GH12 | GO:0008810 | Cellulase activity |
| mRNA_449 | −2.254476744 | 1.58 × 10−2 | Glycoside hydrolase | GH12 | GO:0008810 | Cellulase activity |
| mRNA_581 | 2.118043022 | 2.87 × 10−10 | Glycoside hydrolase | GH5 | GO:0008810 | Cellulase activity |
| mRNA_12685 | −1.591430674 | 8.83 × 10−42 | Glycoside hydrolase | GH16 | GO:0015926 | Glucosidase activity |
| mRNA_6811 | −1.034962051 | 2.07 × 10−6 | Glycoside hydrolase | GH71 | GO:0051118 | Glucan endo-1,3-alpha-glucosidase activity |
| mRNA_6544 | −1.398229479 | 2.08 × 10−54 | Exo-β-1,3-glucosidase | EXG | GO:0004338 | Glucan exo-1,3-beta-glucosidase activity |
| mRNA_3922 | −1.297733133 | 1.18 × 10−6 | Laccase | Lac | GO:0046274 | Lignin catabolic process |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Z.; Wang, X.; Wang, S.; Cai, N.; Huang, J.; Wang, M.; Shu, L.; Li, T. Transcriptome Analysis Revealed the Mechanism of Inhibition of Saprophytic Growth of Sparassis latifolia by Excessive Oxalic Acid. Cells 2022, 11, 3636. https://doi.org/10.3390/cells11223636
Qiu Z, Wang X, Wang S, Cai N, Huang J, Wang M, Shu L, Li T. Transcriptome Analysis Revealed the Mechanism of Inhibition of Saprophytic Growth of Sparassis latifolia by Excessive Oxalic Acid. Cells. 2022; 11(22):3636. https://doi.org/10.3390/cells11223636
Chicago/Turabian StyleQiu, Zhiheng, Xinyi Wang, Shuang Wang, Nuo Cai, Jing Huang, Miaoyue Wang, Lili Shu, and Tianlai Li. 2022. "Transcriptome Analysis Revealed the Mechanism of Inhibition of Saprophytic Growth of Sparassis latifolia by Excessive Oxalic Acid" Cells 11, no. 22: 3636. https://doi.org/10.3390/cells11223636
APA StyleQiu, Z., Wang, X., Wang, S., Cai, N., Huang, J., Wang, M., Shu, L., & Li, T. (2022). Transcriptome Analysis Revealed the Mechanism of Inhibition of Saprophytic Growth of Sparassis latifolia by Excessive Oxalic Acid. Cells, 11(22), 3636. https://doi.org/10.3390/cells11223636






