Actin Up: An Overview of the Rac GEF Dock1/Dock180 and Its Role in Cytoskeleton Rearrangement
Abstract
1. Introduction
2. Early Discovery
3. Identification of Rac Activating Activity
3.1. Dock180 in the Rac Signaling Pathway
3.2. Dock180 GEF Activity
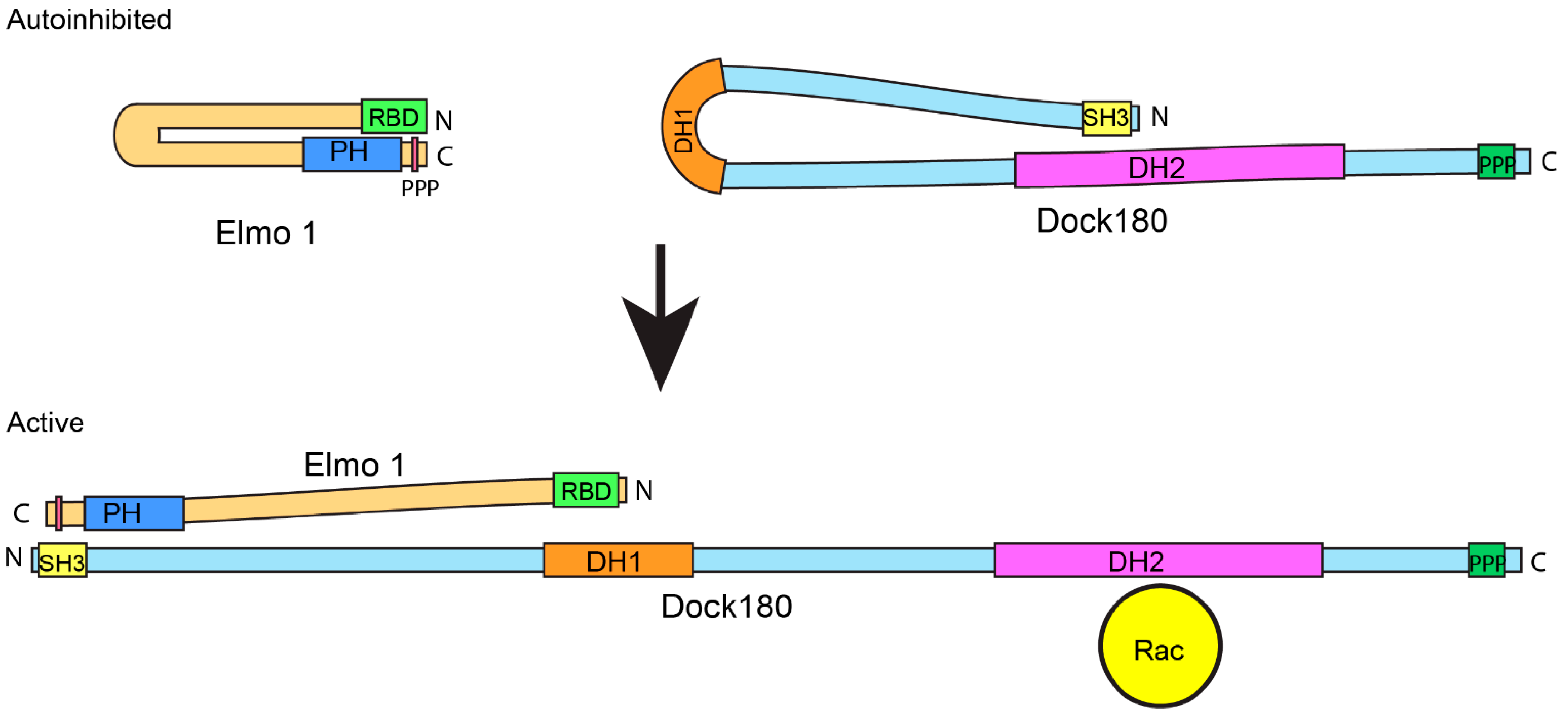
4. Dock180 and Elmo: Better Together
4.1. Early Discovery of Elmo
4.2. Elmo and Dock180 GEF Activity: A Complicated Relationship
5. Dock180 Signaling
5.1. Signal Transduction Leading to Dock180 Activation
5.2. Impacts of Dock180 Activity
6. Dock180 in Pathological Processes
6.1. Dock180 in Cancer
6.2. Dock180 in Infectious Disease
7. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Bos, J.L.; Rehmann, H.; Wittinghofer, A. GEFs and GAPs: Critical Elements in the Control of Small G proteins. Cell 2007, 129, 865–877. [Google Scholar] [CrossRef]
- Hasegawa, H.; Kiyokawa, E.; Tanaka, S.; Nagashima, K.; Gotoh, N.; Shibuya, M.; Kurata, T.; Matsuda, M. DOCK180, a major CRK-binding protein, alters cell morphology upon translocation to the cell membrane. Mol. Cell Biol. 1996, 16, 1770–1776. [Google Scholar] [CrossRef]
- Matsuda, M.; Ota, S.; Tanimura, R.; Nakamura, H.; Matuoka, K.; Takenawa, T.; Nagashima, K.; Kurata, T. Interaction between the Amino-terminal SH3 Domain of CRK and Its Natural Target Proteins. J. Biol. Chem. 1996, 271, 14468–14472. [Google Scholar] [CrossRef] [PubMed]
- Rushton, E.; Drysdale, R.; Abmayr, S.M.; Michelson, A.M.; Bate, M. Mutations in a novel gene, myoblast city, provide evidence in support of the founder cell hypothesis for Drosophila muscle development. Development 1995, 121, 1979–1988. [Google Scholar] [CrossRef]
- Erickson, M.R.; Galletta, B.J.; Abmayr, S.M. Drosophila myoblast city encodes a conserved protein that is essential for myoblast fusion, dorsal closure, and cytoskeletal organization. J. Cell Biol. 1997, 138, 589–603. [Google Scholar] [CrossRef]
- Ellis, R.E.; Jacobson, D.M.; Horvitz, H.R. Genes required for the engulfment of cell corpses during programmed cell death in Caenorhabditis elegans. Genetics 1991, 129, 79–94. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Horvitz, H.R.C. elegans phagocytosis and cell-migration protein CED-5 is similar to human DOCK180. Nature 1998, 392, 501–504. [Google Scholar] [CrossRef]
- Duchek, P.; Somogyi, K.; Jékely, G.; Beccari, S.; Rørth, P. Guidance of cell migration by the drosophila PDGF/VEGF receptor. Cell 2001, 107, 17–26. [Google Scholar] [CrossRef]
- Nolan, K.M.; Barrett, K.; Lu, Y.; Hu, K.-Q.; Vincent, S.; Settleman, J. Myoblast city, the Drosophila homolog of DOCK180/CED-5, is required in a Rac signaling pathway utilized for multiple developmental processes. Genes Dev. 1998, 12, 3337–3342. [Google Scholar] [CrossRef] [PubMed]
- Kiyokawa, E.; Hashimoto, Y.; Kobayashi, S.; Sugimura, H.; Kurata, T.; Matsuda, M. Activation of Rac1 by a Crk SH3-binding protein, DOCK180. Genes Dev. 1998, 12, 3331–3336. [Google Scholar] [CrossRef]
- Albert, M.L.; Kim, J.I.; Birge, R.B. Alphavbeta5 Integrin Recruits the Crkii-Dock180-Rac1 complex for phagocytosis of apoptotic cells. Nat. Cell Biol. 2000, 2, 899–905. [Google Scholar] [CrossRef]
- Gu, J.; Sumida, Y.; Sanzen, N.; Sekiguchi, K. Laminin-10/11 and fibronectin differentially regulate integrin-dependent rho and rac activation via P130(Cas)-Crkii-Dock180 pathway. J. Biol. Chem. 2001, 276, 27090–27097. [Google Scholar] [CrossRef]
- Tosello-Trampont, A.-C.; Brugnera, E.; Ravichandran, K.S. Evidence for a conserved role for CrkII and Rac in engulfment of apoptotic cells. J. Biol. Chem. 2001, 276, 13797–13802. [Google Scholar] [CrossRef] [PubMed]
- Reddien, P.W.; Horvitz, H.R. Ced-2/Crkii and Ced-10/Rac control phagocytosis and cell migration in Caenorhabditis elegans. Nat. Cell Biol. 2000, 2, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Rossman, K.L.; Der, C.J.; Sondek, J. Gef means go: Turning on Rho Gtpases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 2005, 6, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Côté, J.-F.; Vuori, K. Identification of an evolutionarily conserved superfamily of DOCK180-related proteins with guanine nucleotide exchange activity. J. Cell Sci. 2002, 115 Pt 24, 4901–4913. [Google Scholar] [CrossRef] [PubMed]
- Cote, J.F.; Motoyama, A.B.; Bush, J.A.; Vuori, K. A novel and evolutionarily conserved Ptdins(3,4,5)P3-Binding domain is necessary for Dock180 signalling. Nat. Cell Biol. 2005, 7, 797–807. [Google Scholar] [CrossRef]
- Balagopalan, L.; Chen, M.H.; Geisbrecht, E.R.; Abmayr, S.M. The Cdm superfamily protein Mbc directs Myoblast Fusion through a mechanism that requires Phosphatidylinositol 3,4,5-Triphosphate binding but is independent of direct interaction with Dcrk. Mol. Cell Biol. 2006, 26, 9442–9455. [Google Scholar] [CrossRef][Green Version]
- Brugnera, E.; Haney, L.; Grimsley, G.; Lu, M.; Walk, S.F.; Tosello-Trampont, A.C.; Macara, I.G.; Madhani, H.; Fink, G.R.; Ravichandran, K.S. Unconventional Rac-Gef activity is mediated through the Dock180-Elmo complex. Nat. Cell Biol. 2002, 4, 574–582. [Google Scholar] [CrossRef]
- Wu, X.; Ramachandran, S.; Lin, M.C.; Cerione, R.A.; Erickson, J.W. A minimal Rac activation domain in the unconventional guanine nucleotide exchange factor Dock180. Biochemistry 2011, 50, 1070–1080. [Google Scholar] [CrossRef]
- Gumienny, T.L.; Brugnera, E.; Tosello-Trampont, A.C.; Kinchen, J.M.; Haney, L.B.; Nishiwaki, N.; Walk, S.F.; Nemergut, M.E.; Macara, I.G.; Francis, R.; et al. Ced-12/Elmo, a novel member of the Crkii/Dock180/Rac pathway, is required for phagocytosis and cell migration. Cell 2001, 107, 27–41. [Google Scholar] [CrossRef]
- Wu, Y.C.; Tsai, M.C.; Cheng, L.C.; Chou, C.J.; Weng, N.Y.C. Elegans Ced-12 acts in the conserved Crkii/Dock180/Rac pathway to control cell migration and cell corpse engulfment. Dev. Cell 2001, 1, 491–502. [Google Scholar] [CrossRef]
- Zhou, Z.; Caron, E.; Hartwieg, E.; Hall, A.; Horvitz, H.R. The C. Elegans Ph domain protein Ced-12 Regulates Cytoskeletal Reorganization Via a Rho/Rac Gtpase signaling pathway. Dev. Cell 2001, 1, 477–489. [Google Scholar] [CrossRef]
- Geisbrecht, E.R.; Haralalka, S.; Swanson, S.K.; Florens, L.; Washburn, M.; Abmayr, S.M. Drosophila ELMO/CED-12 interacts with Myoblast city to direct myoblast fusion and ommatidial organization. Dev. Biol. 2008, 314, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Grimsley, C.M.; Kinchen, J.M.; Tosello-Trampont, A.C.; Brugnera, E.; Haney, L.B.; Lu, M.; Chen, Q.; Klingele, D.; Hengartner, M.O.; Ravichandran, K.S. Dock180 and Elmo1 proteins cooperate to promote evolutionarily conserved Rac-dependent cell migration. J. Biol. Chem. 2004, 279, 6087–6097. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Kinchen, J.; Rossman, K.L.; Grimsley, C.; Debakker, C.; Brugnera, E.; Tosello-Trampont, A.-C.; Haney, L.B.; Klingele, D.; Sondek, J.; et al. PH domain of ELMO functions in trans to regulate Rac activation via Dock180. Nat. Struct. Mol. Biol. 2004, 11, 756–762. [Google Scholar] [CrossRef]
- Lu, M.; Kinchen, J.M.; Rossman, K.L.; Grimsley, C.; Hall, M.; Sondek, J.; Hengartner, M.O.; Yajnik, V.; Ravichandran, K.S. A steric-inhibition model for regulation of nucleotide exchange via the Dock180 family of Gefs. Curr. Biol. 2005, 15, 371–377. [Google Scholar] [CrossRef]
- Côté, J.; Vuori, K. In vitro guanine nucleotide exchange activity of DHR-2/DOCKER/CZH2 domains. Methods Enzymol. 2006, 406, 41–57. [Google Scholar]
- Komander, D.; Patel, M.; Laurin, M.; Fradet, N.; Pelletier, A.; Barford, D.; Cote, J.T. An alpha-helical extension of the Elmo1 pleckstrin homology domain mediates direct interaction to Dock180 and is critical in Rac signaling. Mol. Biol. Cell 2008, 19, 4837–4851. [Google Scholar] [CrossRef]
- Patel, M.; Margaron, Y.; Fradet, N.; Yang, Q.; Wilkes, B.; Bouvier, M.; Hofmann, K.; Côté, J.-F. An evolutionarily conserved autoinhibitory molecular switch in ELMO proteins regulates Rac signaling. Curr. Biol. 2010, 20, 2021–2027. [Google Scholar] [CrossRef]
- Attar, M.A.; Santy, L.C. The scaffolding protein GRASP/Tamalin directly binds to Dock180 as well as to cytohesins facilitating GTPase crosstalk in epithelial cell migration. BMC Cell Biol. 2013, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Poukkula, M.; Cliffe, A.; Mathieu, J.; Luque, C.M.; Fulga, T.A.; Rørth, P. Two distinct modes of guidance signalling during collective migration of border cells. Nature 2007, 448, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Hu, B.; Jarzynka, M.J.; Li, Y.; Keezer, S.; Johns, T.G.; Tang, C.K.; Hamilton, R.L.; Vuori, K.; Nishikawa, R.; et al. Phosphorylation of dedicator of Cytokinesis 1 (Dock180) at Tyrosine Residue Y722 by Src family kinases mediates egfrviii-driven glioblastoma tumorigenesis. Proc. Natl. Acad. Sci. USA 2012, 109, 3018–3023. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Xinghua, L.; Vuori, K.; Sarkaria, J.N.; Furnari, F.B.; Cavenee, W.K.; Cheng, S.-Y. EGFRvIII stimulates glioma growth and invasion through PKA-dependent serine phosphorylation of Dock180. Oncogene 2014, 33, 2504–2512. [Google Scholar] [CrossRef]
- Misek, S.A.; Chen, J.; Schroeder, L.; Rattanasinchai, C.; Sample, A.; Sarkaria, J.N.; Gallo, K.A. EGFR signals through a DOCK180-MLK3 axis to drive glioblastoma cell invasion. Mol. Cancer Res. 2017, 15, 1085–1095. [Google Scholar] [CrossRef]
- Feng, H.; Hu, B.; Liu, K.W.; Li, Y.; Lu, X.; Cheng, T.; Yiin, J.J.; Lu, S.; Keezer, S.; Fenton, T.; et al. Activation of Rac1 by Src-Dependent Phosphorylation of Dock180(Y1811) Mediates Pdgfralpha-Stimulated Glioma Tumorigenesis in mice and humans. J. Clin. Investig. 2011, 121, 4670–4684. [Google Scholar] [CrossRef]
- Koubek, E.J.; Santy, L.C. ARF1 and ARF6 regulate recycling of GRASP/Tamalin and the Rac1-GEF Dock180 during HGF-induced Rac1 activation. Small GTPases 2018, 9, 242–259. [Google Scholar] [CrossRef]
- Li, H.; Yang, L.; Fu, H.; Yan, J.; Wang, Y.; Guo, H.; Hao, X.; Xu, X.; Jin, T.; Zhang, N. Association between Galphai2 and Elmo1/Dock180 Connects Chemokine Signalling with Rac Activation and Metastasis. Nat. Commun. 2013, 4, 1706. [Google Scholar] [CrossRef]
- Sanematsu, F.; Hirashima, M.; Laurin, M.; Takii, R.; Nishikimi, A.; Kitajima, K.; Ding, G.; Noda, M.; Murata, Y.; Tanaka, Y.; et al. Dock180 is a Rac activator that regulates cardiovascular development by acting downstream of Cxcr4. Circ. Res. 2010, 107, 1102–1105. [Google Scholar] [CrossRef]
- Cheresh, D.A.; Leng, J.; Klemke, R.L. Regulation of cell contraction and membrane ruffling by distinct signals in migratory cells. J. Cell Biol. 1999, 146, 1107–1116. [Google Scholar] [CrossRef]
- He, X.; Liu, J.; Qi, Y.; Brakebusch, C.; Chrostek-Grashoff, A.; Edgar, D.; Yurchenco, P.D.; Corbett, S.A.; Lowry, S.F.; Graham, A.M.; et al. Rac1 is essential for basement membrane-dependent epiblast survival. Mol. Cell Biol. 2010, 30, 3569–3581. [Google Scholar] [CrossRef]
- Hsia, D.A.; Mitra, S.K.; Hauck, C.R.; Streblow, D.N.; Nelson, J.A.; Ilic, D.; Huang, S.; Li, E.; Nemerow, G.R.; Leng, J.; et al. Differential regulation of cell motility and invasion by FAK. J. Cell Biol. 2003, 160, 753–767. [Google Scholar] [CrossRef]
- Lamorte, L.; Rodrigues, S.; Naujokas, M.; Park, M. Crk synergizes with epidermal growth factor for epithelial invasion and morphogenesis and is required for the met morphogenic program. J. Biol. Chem. 2002, 277, 37904–37911. [Google Scholar] [CrossRef]
- Makino, Y.; Tsuda, M.; Ohba, Y.; Nishihara, H.; Sawa, H.; Nagashima, K.; Tanaka, S. Tyr724 phosphorylation of ELMO1 by Src is involved in cell spreading and migration via Rac1 activation. Cell Commun. Signal. 2015, 13, 35. [Google Scholar] [CrossRef]
- Smith, H.W.; Marra, P.; Marshall, C.J. uPAR promotes formation of the p130Cas–Crk complex to activate Rac through DOCK180. J. Cell Biol. 2008, 182, 777–790. [Google Scholar] [CrossRef]
- Katoh, H.; Negishi, M. RhoG activates Rac1 by direct interaction with the Dock180-binding protein Elmo. Nature 2003, 424, 461–464. [Google Scholar] [CrossRef]
- de Bakker, C.D.; Haney, L.B.; Kinchen, J.M.; Grimsley, C.; Lu, M.; Klingele, D.; Hsu, P.K.; Chou, B.K.; Cheng, L.C.; Blangy, A.; et al. Phagocytosis of apoptotic cells is regulated by a Unc-73/Trio-Mig-2/Rhog signaling module and armadillo repeats of Ced-12/Elmo. Curr. Biol. 2004, 14, 2208–2216. [Google Scholar] [CrossRef] [PubMed]
- Katoh, H.; Hiramoto, K.; Negishi, M. Activation of Rac1 by Rhog regulates cell migration. J. Cell Sci. 2006, 119 Pt 1, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Kucik, D.F.; Wu, C. Identification and kinetic analysis of the interaction between Nck-2 and DOCK180. FEBS Lett. 2001, 491, 193–199. [Google Scholar] [CrossRef]
- White, D.T.; McShea, K.M.; Attar, M.A.; Santy, L.C. GRASP and IPCEF promote ARF-to-Rac signaling and cell migration by coordinating the association of ARNO/cytohesin 2 with Dock180. Mol. Biol. Cell 2010, 21, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, M.; Kiyokawa, E.; Hara, S.; Iemura, S.-I.; Natsume, T.; Manabe, T.; Matsuda, M. Ankyrin repeat domain 28 (ANKRD28), a novel binding partner of DOCK180, promotes cell migration by regulating focal adhesion formation. Exp. Cell Res. 2009, 315, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.W.R.; Li, W.; Chang, R.C.C.; Lau, K. ARF6-Rac1 signaling-mediated neurite outgrowth is potentiated by the neuronal adaptor FE65 through orchestrating ARF6 and ELMO1. FASEB J. 2020, 34, 16397–16413. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Shirai, T.; Kiyokawa, E.; Mochizuki, N.; Matsuda, M.; Fukui, Y. Membrane recruitment of Dock180 by binding to Ptdins(3,4,5)P3. Biochem. J. 2001, 354 Pt 1, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, L.; Bobkov, A.A.; Patel, M.; Jaroszewski, L.; Bankston, L.A.; Stec, B.; Vuori, K.; Côté, J.-F.; Liddington, R.C. Structural Basis of Membrane Targeting by the Dock180 Family of Rho Family Guanine Exchange Factors (Rho-GEFs). J. Biol. Chem. 2010, 285, 13211–13222. [Google Scholar] [CrossRef] [PubMed]
- Santy, L.C.; Casanova, J.E. Activation of Arf6 by Arno Stimulates Epithelial cell migration through downstream activation of both Rac1 and Phospholipase D. J. Cell. Biol. 2001, 154, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Santy, L.C.; Ravichandran, K.S.; Casanova, J.E. The Dock180/Elmo complex couples Arno-Mediated Arf6 activation to the downstream activation of Rac1. Curr. Biol. 2005, 15, 1749–1754. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, O.; Desai, J.; Karbassi, F.; Sylvain, M.A.; Shi, A.; Zhou, Z.; Rocheleau, C.E.; Grant, B.D. Ced-10/Rac1 regulates endocytic recycling through the Rab-5 Gap Tbc-2. PLoS Genet. 2012, 8, e1002785. [Google Scholar] [CrossRef]
- Hara, S.; Kiyokawa, E.; Iemura, S.; Natsume, T.; Wassmer, T.; Cullen, P.J.; Hiai, H.; Matsuda, M. The Dhr1 Domain of Dock180 Binds to Snx5 and regulates cation-independent Mannose 6-Phosphate receptor transport. Mol. Biol. Cell. 2008, 19, 3823–3835. [Google Scholar] [CrossRef]
- Laurin, M.; Fradet, N.; Blangy, A.; Hall, A.; Vuori, K.; Côté, J.-F. The atypical Rac activator Dock180 (Dock1) regulates myoblast fusion in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 15446–15451. [Google Scholar] [CrossRef]
- Moore, C.A.; Parkin, C.A.; Bidet, Y.; Ingham, P.W. A role for the Myoblast city homologues Dock1 and Dock5 and the adaptor proteins Crk and Crk-like in zebrafish myoblast fusion. Development 2007, 134, 3145–3153. [Google Scholar] [CrossRef]
- Chung, S.; Gumienny, T.; Hengartner, M.; Driscoll, M. A common set of engulfment genes mediates removal of both apoptotic and necrotic cell corpses in C. elegans. Nat. Cell Biol. 2000, 2, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Panneerdoss, S.; Viswanadhapalli, S.; Abdelfattah, N.; Onyeagucha, B.C.; Timilsina, S.; Mohammad, T.; Chen, Y.; Drake, M.; Vuori, K.; Kumar, T.R.; et al. Cross-talk between miR-471-5p and autophagy component proteins regulates LC3-associated phagocytosis (LAP) of apoptotic germ cells. Nat. Commun. 2017, 8, 598. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tam, K.M.V.; Chan, W.W.; Koon, A.C.; Ngo, J.C.K.; Chan, H.Y.E.; Lau, K.-F. Neuronal adaptor FE65 stimulates Rac1-mediated neurite outgrowth by recruiting and activating ELMO1. J. Biol. Chem. 2018, 293, 7674–7688. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Oh, M.O.; Bernard, L.P.; Macara, I.G.; Zhang, H. The Rhog/Elmo1/Dock180 signaling module is required for spine morphogenesis in hippocampal neurons. J. Biol. Chem. 2011, 286, 37615–37624. [Google Scholar] [CrossRef]
- Pastuhov, S.I.; Fujiki, K.; Tsuge, A.; Asai, K.; Ishikawa, S.; Hirose, K.; Matsumoto, K.; Hisamoto, N. The core molecular machinery used for engulfment of apoptotic cells regulates the JNK pathway mediating axon regeneration in Caenorhabditis elegans. J. Neurosci. 2016, 36, 9710–9721. [Google Scholar] [CrossRef]
- Epting, D.; Wendik, B.; Bennewitz, K.; Dietz, C.T.; Driever, W.; Kroll, J. The Rac1 regulator ELMO1 controls vascular morphogenesis in Zebrafish. Circ. Res. 2010, 107, 45–55. [Google Scholar] [CrossRef]
- Schäker, K.; Bartsch, S.; Patry, C.; Stoll, S.J.; Hillebrands, J.L.; Wieland, T.; Kroll, J. The Bipartite Rac1 Guanine Nucleotide Exchange Factor Engulfment and Cell Motility 1/Dedicator of Cytokinesis 180 (Elmo1/Dock180) Protects Endothelial Cells from Apoptosis in Blood Vessel Development. J. Biol. Chem. 2015, 290, 6408–6418. [Google Scholar] [CrossRef]
- Jarzynka, M.J.; Hu, B.; Hui, K.-M.; Bar-Joseph, I.; Gu, W.; Hirose, T.; Haney, L.B.; Ravichandran, K.S.; Nishikawa, R.; Cheng, S.-Y. ELMO1 and Dock180, a Bipartite Rac1 Guanine Nucleotide exchange factor, promote human glioma cell invasion. Cancer Res. 2007, 67, 7203–7211. [Google Scholar] [CrossRef]
- Gomez, D.; Cardama, G.; Gonzalez, N.; Ciarlantini, M.; Comin, M.J.; Alonso, D.; Menna, P.L.; Donadio, L.G. Proapoptotic and antiinvasive activity of Rac1 small molecule inhibitors on malignant glioma cells. OncoTargets Ther. 2014, 7, 2021–2033. [Google Scholar] [CrossRef]
- Zhao, F.; Siu, M.K.Y.; Jiang, L.; Tam, K.F.; Ngan, H.Y.S.; Le, X.-F.; Wong, O.G.W.; Wong, E.S.Y.; Chan, H.Y.; Cheung, A.N.Y. Overexpression of dedicator of cytokinesis I (Dock180) in ovarian cancer correlated with aggressive phenotype and poor patient survival. Histopathology 2011, 59, 1163–1172. [Google Scholar] [CrossRef]
- Handa, Y.; Suzuki, M.; Ohya, K.; Iwai, H.; Ishijima, N.; Koleske, A.J.; Fukui, Y.; Sasakawa, C. Shigella IpgB1 promotes bacterial entry through the ELMO–Dock180 machinery. Nat. Cell Biol. 2007, 9, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Boehm, M.; Krause-Gruszczynska, M.; Rohde, M.; Tegtmeyer, N.; Takahashi, S.; Oyarzabal, S.A.; Backert, S. Major host factors Involved in Epithelial Cell Invasion of Campylobacter Jejuni: Role of Fibronectin, Integrin Beta1, Fak, Tiam-1, and Dock180 in activating Rho Gtpase Rac1. Front. Cell. Infect. Microbiol. 2011, 1, 17. [Google Scholar] [CrossRef] [PubMed]
- Eucker, T.P.; Konkel, M.E. The cooperative action of bacterial fibronectin-binding proteins and secreted proteins promote maximal Campylobacter jejuni invasion of host cells by stimulating membrane ruffling. Cell. Microbiol. 2012, 14, 226–238. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.-L.N.; Won, S.; Mattheisen, M.; Castaldi, P.J.; Cho, M.H.; Rutten, E.; Hardin, M.; Yip, W.-K.; Rennard, S.I.; Lomas, D.A.; et al. Body mass index change in gastrointestinal cancer and chronic obstructive pulmonary disease is associated with Dedicator of Cytokinesis 1. J. Cachex Sarcopenia Muscle 2017, 8, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Samani, A.; English, K.G.; Lopez, M.A.; Birch, C.L.; Brown, D.M.; Kaur, G.; Worthey, E.A.; Alexander, M.S. DOCKopathies: A systematic review of the clinical pathologies associated with human DOCK pathogenic variants. Hum. Mutat. 2022, 43, 1149–1161. [Google Scholar] [CrossRef]
- Yang, N.-I.; Yeh, C.-H.; Tsai, T.-H.; Chou, Y.-J.; Hsu, P.W.-C.; Li, C.-H.; Chan, Y.-H.; Kuo, L.-T.; Mao, C.-T.; Shyu, Y.-C.; et al. Artificial Intelligence-Assisted Identification of Genetic Factors Predisposing High-Risk Individuals to Asymptomatic Heart Failure. Cells 2021, 10, 2430. [Google Scholar] [CrossRef]
- Reviriego-Mendoza, M.M.; Santy, L.C. The Cytohesin Guanosine Exchange Factors (Gefs) are required to promote Hgf-Mediated renal recovery after Acute Kidney Injury (Aki) in Mice. Physiol. Rep. 2015, 3, e12442. [Google Scholar] [CrossRef]
- Mungunsukh, O.; McCart, E.A.; Day, R.M. Hepatocyte growth factor isoforms in tissue repair, cancer, and fibrotic remodeling. Biomedicines 2014, 2, 301–326. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koubek, E.J.; Santy, L.C. Actin Up: An Overview of the Rac GEF Dock1/Dock180 and Its Role in Cytoskeleton Rearrangement. Cells 2022, 11, 3565. https://doi.org/10.3390/cells11223565
Koubek EJ, Santy LC. Actin Up: An Overview of the Rac GEF Dock1/Dock180 and Its Role in Cytoskeleton Rearrangement. Cells. 2022; 11(22):3565. https://doi.org/10.3390/cells11223565
Chicago/Turabian StyleKoubek, Emily J., and Lorraine C. Santy. 2022. "Actin Up: An Overview of the Rac GEF Dock1/Dock180 and Its Role in Cytoskeleton Rearrangement" Cells 11, no. 22: 3565. https://doi.org/10.3390/cells11223565
APA StyleKoubek, E. J., & Santy, L. C. (2022). Actin Up: An Overview of the Rac GEF Dock1/Dock180 and Its Role in Cytoskeleton Rearrangement. Cells, 11(22), 3565. https://doi.org/10.3390/cells11223565





