RNA-Sequencing Reveals Upregulation and a Beneficial Role of Autophagy in Myoblast Differentiation and Fusion
Abstract
1. Introduction
2. Materials and Methods
2.1. Major Reagents Used in this Study
2.2. Cell Culture
2.3. RNA Extraction and RNA-Seq Library Construction and Sequencing
2.4. Gene Expression Analysis and Gene Ontology Analysis of RNA-Seq Data
2.5. Reverse Transcription Quantitative PCR (RT-qPCR)
2.6. Immunocytochemistry
2.7. Determination of Fusion Index, Myotube Length, and Myotube Area
2.8. Total Cellular Protein Extraction and Western Blot Analysis
2.9. Statistical Analyses
3. Results
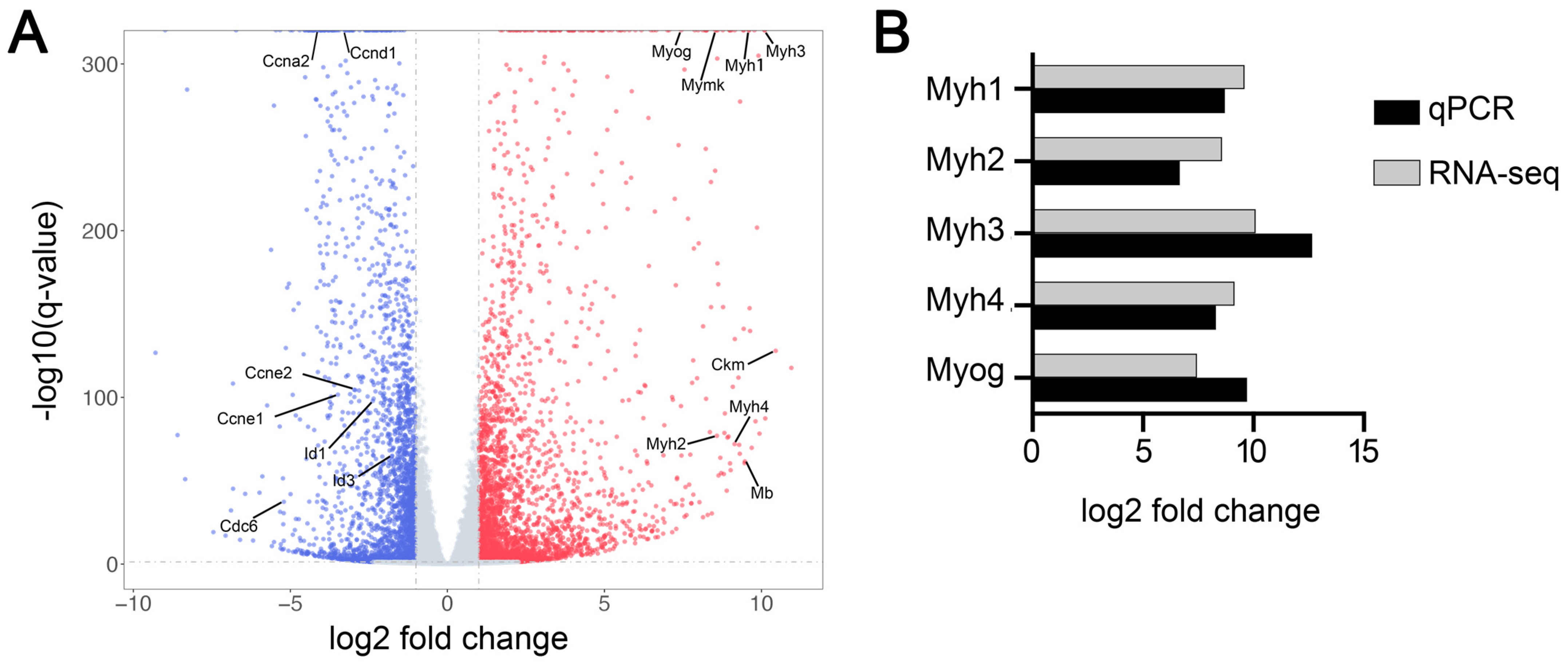
3.1. More Than Ten Thousand Genes Were Differentially Expressed during Myoblast Differentiation
3.2. Autophagy Was Upregulated during Myoblast Differentiation
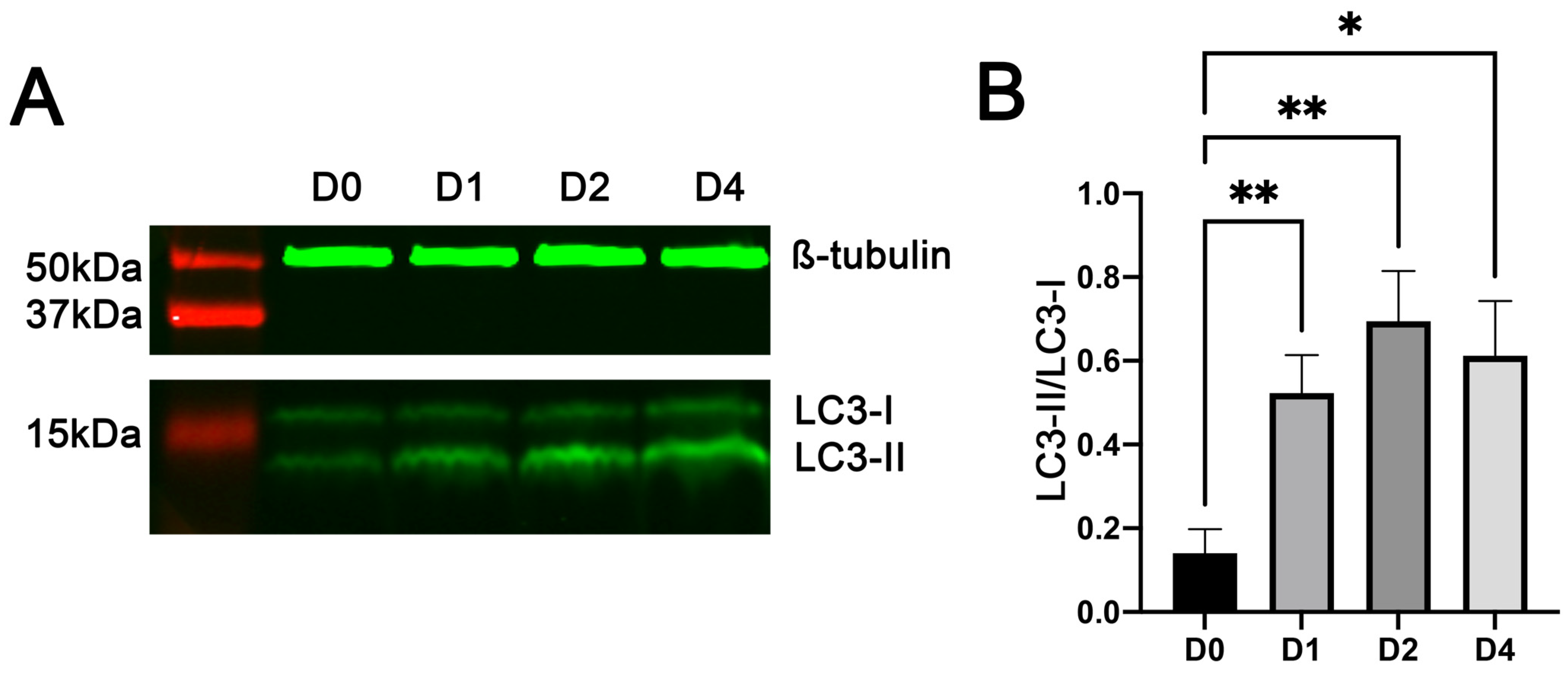
3.3. Myoblast Differentiation Was Associated with Increased Conversion of LC3-I to LC3-II
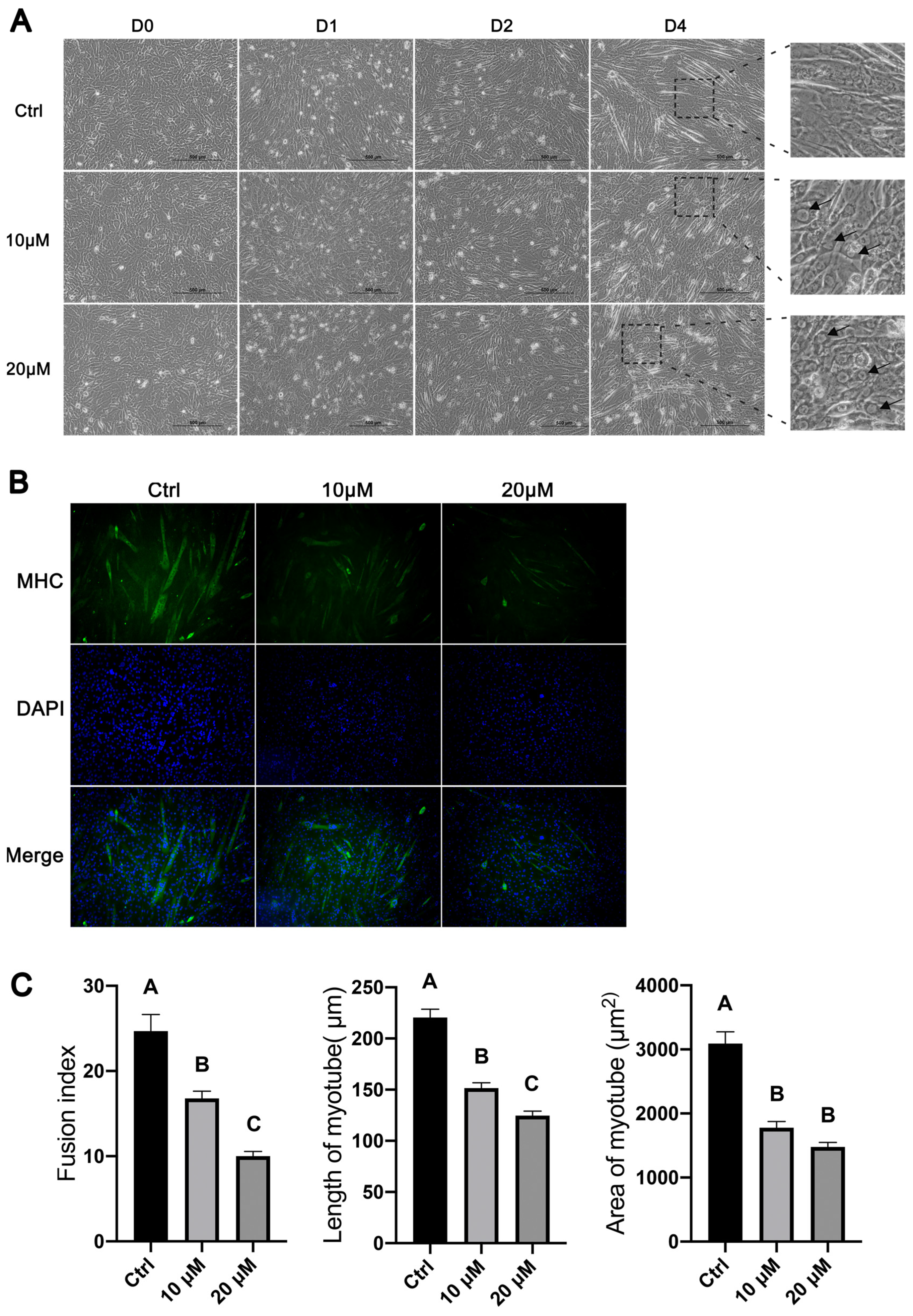
3.4. Inhibition of Autophagy Reduced the Number, Length, and Size of Myotubes Formed during Myoblast Differentiation
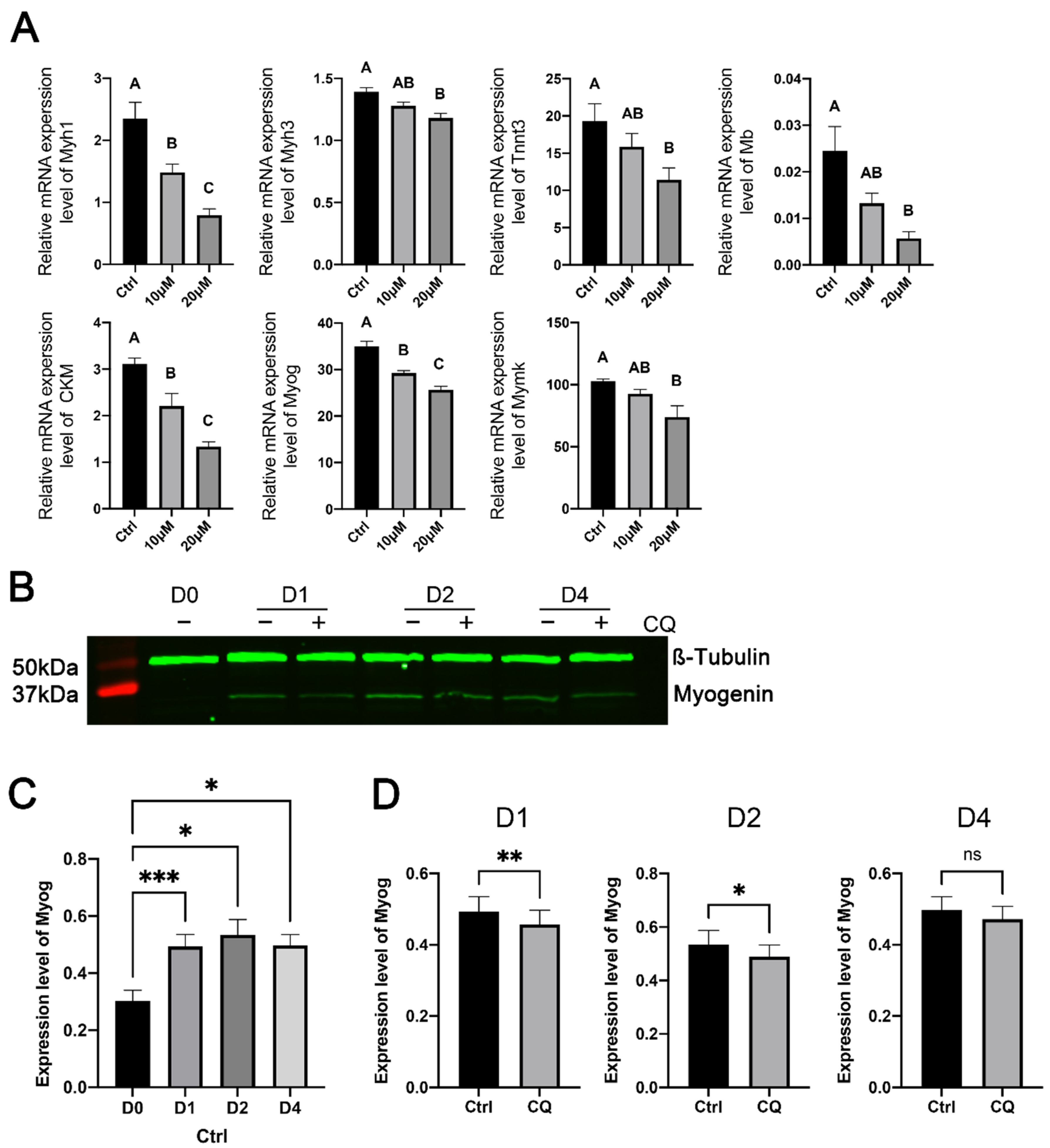
3.5. Inhibition of Autophagy Reduced the Expression of Muscle-Specific Genes during Myoblast Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoppeler, H.; Fluck, M. Plasticity of skeletal muscle mitochondria: Structure and function. Med. Sci. Sports Exerc. 2003, 35, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Ordahl, C.P.; Berdougo, E.; Venters, S.J.; Denetclaw, W.F., Jr. The dermomyotome dorsomedial lip drives growth and morphogenesis of both the primary myotome and dermomyotome epithelium. Development 2001, 128, 1731–1744. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, J.; Rando, T.A. Stem cells in postnatal myogenesis: Molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol. 2005, 15, 666–673. [Google Scholar] [CrossRef]
- Greising, S.M.; Gransee, H.M.; Mantilla, C.B.; Sieck, G.C. Systems biology of skeletal muscle: Fiber type as an organizing principle. Wiley Interdiscip. Rev. Syst. Biol. Med. 2012, 4, 457–473. [Google Scholar] [CrossRef]
- Massenet, J.; Gardner, E.; Chazaud, B.; Dilworth, F.J. Epigenetic regulation of satellite cell fate during skeletal muscle regeneration. Skelet. Muscle 2021, 11, 4. [Google Scholar] [CrossRef]
- Weskamp, K.; Olwin, B.B.; Parker, R. Post-Transcriptional Regulation in Skeletal Muscle Development, Repair, and Disease. Trends Mol. Med. 2021, 27, 469–481. [Google Scholar] [CrossRef]
- Zammit, P.S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 2017, 72, 19–32. [Google Scholar] [CrossRef]
- Kamei, Y.; Hatazawa, Y.; Uchitomi, R.; Yoshimura, R.; Miura, S. Regulation of Skeletal Muscle Function by Amino Acids. Nutrients 2020, 12, 261. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, X.; Yan, J.; Chen, M.; Zhu, M.; Tang, Y.; Liu, S.; Tang, Z. A comprehensive epigenome atlas reveals DNA methylation regulating skeletal muscle development. Nucleic Acids Res. 2021, 49, 1313–1329. [Google Scholar] [CrossRef]
- Carrio, E.; Suelves, M. DNA methylation dynamics in muscle development and disease. Front. Aging Neurosci. 2015, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Thomson, D.M. The Role of AMPK in the Regulation of Skeletal Muscle Size, Hypertrophy, and Regeneration. Int. J. Mol. Sci. 2018, 19, 3125. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Esser, K.A. Cellular mechanisms regulating protein synthesis and skeletal muscle hypertrophy in animals. J. Appl. Physiol. 2009, 106, 1367–1373. [Google Scholar] [CrossRef]
- Horak, M.; Novak, J.; Bienertova-Vasku, J. Muscle-specific microRNAs in skeletal muscle development. Dev. Biol. 2016, 410, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Zheng, Q.; Sui, M.; Zhu, L.; Xu, L.; Zhang, Y.; Liu, Y.; Fang, F.; Chu, M.; Ma, Y.; et al. Comprehensive Analysis of LncRNA Reveals the Temporal-Specific Module of Goat Skeletal Muscle Development. Int. J. Mol. Sci. 2019, 20, 3950. [Google Scholar] [CrossRef]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef]
- Girardi, F.; Le Grand, F. Wnt Signaling in Skeletal Muscle Development and Regeneration. Prog. Mol. Biol. Transl. Sci. 2018, 153, 157–179. [Google Scholar] [CrossRef]
- Wroblewski, O.M.; Vega-Soto, E.E.; Nguyen, M.H.; Cederna, P.S.; Larkin, L.M. Impact of Human Epidermal Growth Factor on Tissue-Engineered Skeletal Muscle Structure and Function. Tissue Eng. Part A 2021, 27, 1151–1159. [Google Scholar] [CrossRef]
- Levine, B.; Mizushima, N.; Virgin, H.W. Autophagy in immunity and inflammation. Nature 2011, 469, 323–335. [Google Scholar] [CrossRef]
- Ryter, S.W.; Lee, S.J.; Smith, A.; Choi, A.M. Autophagy in vascular disease. Proc. Am. Thorac. Soc. 2010, 7, 40–47. [Google Scholar] [CrossRef]
- Zhang, J. Teaching the basics of autophagy and mitophagy to redox biologists--mechanisms and experimental approaches. Redox Biol. 2015, 4, 242–259. [Google Scholar] [CrossRef] [PubMed]
- Ilha, J.; do Espirito-Santo, C.C.; de Freitas, G.R. mTOR Signaling Pathway and Protein Synthesis: From Training to Aging and Muscle Autophagy. Adv. Exp. Med. Biol. 2018, 1088, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Milan, G.; Romanello, V.; Pescatore, F.; Armani, A.; Paik, J.H.; Frasson, L.; Seydel, A.; Zhao, J.; Abraham, R.; Goldberg, A.L.; et al. Regulation of autophagy and the ubiquitin-proteasome system by the FoxO transcriptional network during muscle atrophy. Nat. Commun. 2015, 6, 6670. [Google Scholar] [CrossRef] [PubMed]
- McMillan, E.M.; Quadrilatero, J. Autophagy is required and protects against apoptosis during myoblast differentiation. Biochem. J. 2014, 462, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Balnis, J.; Drake, L.A.; Singer, D.V.; Vincent, C.E.; Korponay, T.C.; D’Armiento, J.; Lee, C.G.; Elias, J.A.; Singer, H.A.; Jaitovich, A. Deaccelerated Myogenesis and Autophagy in Genetically Induced Pulmonary Emphysema. Am. J. Respir. Cell Mol. Biol. 2022, 66, 623–637. [Google Scholar] [CrossRef]
- Han, S.; Cui, C.; He, H.; Shen, X.; Chen, Y.; Wang, Y.; Li, D.; Zhu, Q.; Yin, H. FHL1 regulates myoblast differentiation and autophagy through its interaction with LC3. J. Cell. Physiol. 2020, 235, 4667–4678. [Google Scholar] [CrossRef]
- Fortini, P.; Ferretti, C.; Iorio, E.; Cagnin, M.; Garribba, L.; Pietraforte, D.; Falchi, M.; Pascucci, B.; Baccarini, S.; Morani, F.; et al. The fine tuning of metabolism, autophagy and differentiation during in vitro myogenesis. Cell Death Dis. 2016, 7, e2168. [Google Scholar] [CrossRef]
- Park, S.; Choi, Y.; Jung, N.; Kim, J.; Oh, S.; Yu, Y.; Ahn, J.H.; Jo, I.; Choi, B.O.; Jung, S.C. Autophagy induction in the skeletal myogenic differentiation of human tonsil-derived mesenchymal stem cells. Int. J. Mol. Med. 2017, 39, 831–840. [Google Scholar] [CrossRef]
- Li, J.; Shi, M.; Liu, L.; Wang, J.; Zhu, M.; Chen, H. Tetrandrine Inhibits Skeletal Muscle Differentiation by Blocking Autophagic Flux. Int. J. Mol. Sci. 2022, 23, 8148. [Google Scholar] [CrossRef]
- Ge, X.; Zhang, Y.; Park, S.; Cong, X.; Gerrard, D.E.; Jiang, H. Stac3 inhibits myoblast differentiation into myotubes. PLoS ONE 2014, 9, e95926. [Google Scholar] [CrossRef]
- Duleh, S.; Wang, X.; Komirenko, A.; Margeta, M. Activation of the Keap1/Nrf2 stress response pathway in autophagic vacuolar myopathies. Acta Neuropathol. Commun. 2016, 4, 115. [Google Scholar] [CrossRef] [PubMed]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef] [PubMed]
- Nnah, I.C.; Wang, B.; Saqcena, C.; Weber, G.F.; Bonder, E.M.; Bagley, D.; De Cegli, R.; Napolitano, G.; Medina, D.L.; Ballabio, A.; et al. TFEB-driven endocytosis coordinates MTORC1 signaling and autophagy. Autophagy 2019, 15, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Putri, G.H.; Anders, S.; Pyl, P.T.; Pimanda, J.E.; Zanini, F. Analysing high-throughput sequencing data in Python with HTSeq 2.0. Bioinformatics 2022, 38, 2943–2945. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Cong, X.; Doering, J.; Grange, R.W.; Jiang, H. Defective excitation-contraction coupling is partially responsible for impaired contractility in hindlimb muscles of Stac3 knockout mice. Sci. Rep. 2016, 6, 26194. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Rasband, W.S. Image J. U.S. National Institutes of Health, Bethesda, Maryland, USA. Available online: https://imagej.nih.gov/ij/ (accessed on 1 April 2022).
- Millay, D.P.; O’Rourke, J.R.; Sutherland, L.B.; Bezprozvannaya, S.; Shelton, J.M.; Bassel-Duby, R.; Olson, E.N. Myomaker is a membrane activator of myoblast fusion and muscle formation. Nature 2013, 499, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.H.A.; Liang, C.; Guohua, W.; Pant, S.D.; Mohammedsaleh, Z.M.; Shater, A.F.; Alotaibi, M.A.; Khan, R.; Schreurs, N.; Cheng, G.; et al. Screening and Identification of Muscle-Specific Candidate Genes via Mouse Microarray Data Analysis. Front. Vet. Sci. 2021, 8, 794628. [Google Scholar] [CrossRef]
- Shen, X.; Collier, J.M.; Hlaing, M.; Zhang, L.; Delshad, E.H.; Bristow, J.; Bernstein, H.S. Genome-wide examination of myoblast cell cycle withdrawal during differentiation. Dev. Dyn. 2003, 226, 128–138. [Google Scholar] [CrossRef]
- Zebedee, Z.; Hara, E. Id proteins in cell cycle control and cellular senescence. Oncogene 2001, 20, 8317–8325. [Google Scholar] [CrossRef]
- Al-Shanti, N.; Stewart, C.E. Ca2+/calmodulin-dependent transcriptional pathways: Potential mediators of skeletal muscle growth and development. Biol. Rev. Camb. Philos. Soc. 2009, 84, 637–652. [Google Scholar] [CrossRef]
- Mukund, K.; Subramaniam, S. Skeletal muscle: A review of molecular structure and function, in health and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, e1462. [Google Scholar] [CrossRef]
- Mizushima, N.; Yoshimori, T. How to interpret LC3 immunoblotting. Autophagy 2007, 3, 542–545. [Google Scholar] [CrossRef]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 and Autophagy. Methods Mol. Biol. 2008, 445, 77–88. [Google Scholar] [CrossRef]
- Pan, X.; Liu, B.; Chen, S.; Ding, H.; Yao, X.; Cheng, Y.; Xu, D.; Yin, Y.; Dai, X.; Sun, J.; et al. Nr4a1 as a myogenic factor is upregulated in satellite cells/myoblast under proliferation and differentiation state. Biochem. Biophys. Res. Commun. 2019, 513, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, Y.; Chen, C.; Hu, Q.; Fu, Y.; Xu, L.; Wang, C.; Liu, Y. Identification of Robust and Key Differentially Expressed Genes during C2C12 Cell Myogenesis Based on Multiomics Data. Int. J. Mol. Sci. 2022, 23, 6002. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, K.K.; Marinescu, V.D.; Ramoni, M.F.; Sanoudou, D.; Montanaro, F.; Han, M.; Kunkel, L.M.; Kohane, I.S.; Beggs, A.H. Expression profiling and identification of novel genes involved in myogenic differentiation. FASEB J. 2004, 18, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jin, P.; Duan, R.; Chen, E.H. Mechanisms of myoblast fusion during muscle development. Curr. Opin. Genet. Dev. 2015, 32, 162–170. [Google Scholar] [CrossRef]
- Zhao, S.; Fung-Leung, W.P.; Bittner, A.; Ngo, K.; Liu, X. Comparison of RNA-Seq and microarray in transcriptome profiling of activated T cells. PLoS ONE 2014, 9, e78644. [Google Scholar] [CrossRef]
- Rao, M.S.; Van Vleet, T.R.; Ciurlionis, R.; Buck, W.R.; Mittelstadt, S.W.; Blomme, E.A.G.; Liguori, M.J. Comparison of RNA-Seq and Microarray Gene Expression Platforms for the Toxicogenomic Evaluation of Liver from Short-Term Rat Toxicity Studies. Front. Genet. 2018, 9, 636. [Google Scholar] [CrossRef]
- Wang, C.; Gong, B.; Bushel, P.R.; Thierry-Mieg, J.; Thierry-Mieg, D.; Xu, J.; Fang, H.; Hong, H.; Shen, J.; Su, Z.; et al. The concordance between RNA-seq and microarray data depends on chemical treatment and transcript abundance. Nat. Biotechnol. 2014, 32, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Liu, K.; Li, R.; Cao, Y.; Shen, M.; Tao, J.; Liu, H. Trillin inhibits myoblast differentiation via increasing autophagy. Phytomedicine 2022, 99, 153962. [Google Scholar] [CrossRef]
- You, J.S.; Anderson, G.B.; Dooley, M.S.; Hornberger, T.A. The role of mTOR signaling in the regulation of protein synthesis and muscle mass during immobilization in mice. Dis. Model. Mech. 2015, 8, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Reggiani, C.; Akimoto, T.; Blaauw, B. Molecular Mechanisms of Skeletal Muscle Hypertrophy. J. Neuromuscul. Dis. 2021, 8, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N. Autophagy: Process and function. Genes Dev. 2007, 21, 2861–2873. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.A.; Al-Khalaf, M.; Megeney, L.A. The beneficial role of proteolysis in skeletal muscle growth and stress adaptation. Skelet. Muscle 2016, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Baechler, B.L.; Bloemberg, D.; Quadrilatero, J. Mitophagy regulates mitochondrial network signaling, oxidative stress, and apoptosis during myoblast differentiation. Autophagy 2019, 15, 1606–1619. [Google Scholar] [CrossRef]
- Sin, J.; Andres, A.M.; Taylor, D.J.; Weston, T.; Hiraumi, Y.; Stotland, A.; Kim, B.J.; Huang, C.; Doran, K.S.; Gottlieb, R.A. Mitophagy is required for mitochondrial biogenesis and myogenic differentiation of C2C12 myoblasts. Autophagy 2016, 12, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Baker, N.E. E Proteins and ID Proteins: Helix-Loop-Helix Partners in Development and Disease. Dev. Cell 2015, 35, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Leng, X.; Ji, X.; Hou, Y.; Settlage, R.; Jiang, H. Roles of the proteasome and inhibitor of DNA binding 1 protein in myoblast differentiation. FASEB J. 2019, 33, 7403–7416. [Google Scholar] [CrossRef] [PubMed]
- Brennan, T.J.; Edmondson, D.G.; Li, L.; Olson, E.N. Transforming growth factor beta represses the actions of myogenin through a mechanism independent of DNA binding. Proc. Natl. Acad. Sci. USA 1991, 88, 3822–3826. [Google Scholar] [CrossRef] [PubMed]
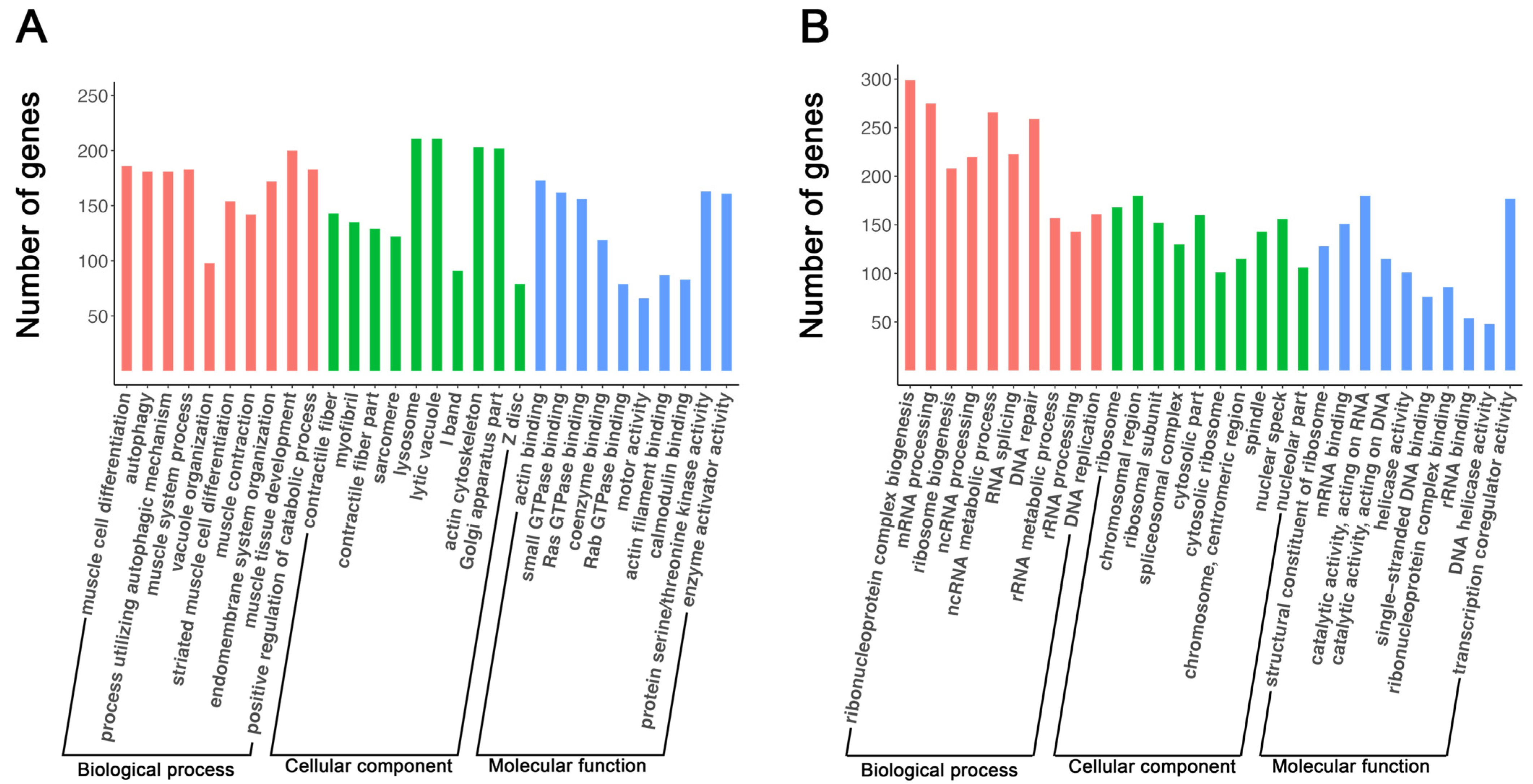

| ID | Description | Gene Ratio | p-Value | GeneID |
|---|---|---|---|---|
| GO:0042692 | muscle cell differentiation | 186/5118 | 1.28 × 10−28 | Pgm5, Myl2, Cacna1s, Lmod2, Tnnt3, Mybpc2, Myoz2, Mybpc1, Actc1, Casq1, Trim72, Actn2, Neb, Casq2, Myom3, Myh6, Cav3, Ankrd2, Csrp3, Mypn, Tcap, Rbm24, Klhl41, Tnnt1, Acta1, Myom1, Myom2, Fbxo40, Lmod3, Igf2, Igf1, Dmd, Myog, Alpk3, Ins2, Klhl40, Ryr1, Ankrd23, Tmod1, Myo18b, Ttn, Sypl2, Mef2c, Neu2, Smyd1, Synpo2l, Hopx, Nrap, Dysf, Fgf9, et al. |
| GO:0006914 | autophagy | 181/5118 | 1.32 × 10−27 | Dcn, Fez1, Mapt, Trem2, Synpo2, Epm2a, Bmf, Htr2b, Casp1, Prkaa2, Trp53inp2, Ifng, Pink1, Lzts1, Map1lc3a, Pik3c2b, Srpx, Rnf152, Rasip1, Mtm1, Tlr2, Irgm2, Lix1, Fbxw7, Usp13, Snapin, Adrb2, Fyco1, Trp53inp1, Stat3, Atp6v0a1, Nupr1, Tfeb, Dram2, Trib3, Nod1, A tg4a, Ctsd, Qsox1, Atg10, Zfyve1, Trim13, Atg13, Vps13d, Flcn, Wipi1, Nod2, Fbxl2, Sirt2, Hspb8, et al. |
| ID | Description | Gene Ratio | p-Value | GeneID |
|---|---|---|---|---|
| GO:0022613 | ribonucleoprotein complex biogenesis | 299/4657 | 1.47 × 10−107 | Celf4, Suv39h1, Lyar, Exosc8, Nop56, Lsm2, Npm1, Lsm3, Dkc1, Nhp2, Fbl, Gar1, Gemin6, Snrpd1, Dis3, Ppan, Ruvbl2, Hsp90aa1, Nop2, Ncl, Ran, Noc2l, Pa2g4, Exosc2, Xpo1, Nop58, Rcl1, Snrpg, Npm3, Rrp15, Snrpe, Rbmxl1, Mybbp1a, Ddx20, Ddx18, Rrp9, Ruvbl1, Ddx21, Rrs1, Snrpd3, Mrto4, Utp20, Rpsa, Eri1, Lsm6, Gemin5, Lsm4, Srsf1, Wdr43, Rps2, et al. |
| GO:0006397 | mRNA processing | 275/4657 | 5.32 × 10−83 | Ccnb1, Celf4, Hmx2, Ptbp1, Lsm2, Slbp, Npm1, Lsm3, Hnrnpa1, Lsm5, Snrpa1, Gemin6, Snrpd1, Ppil1, Rbmx2, Alyref, Pabpc1, Mbnl3, Khdrbs3, Adarb1, Srsf7, Papolb, Ddx39, Snrpg, Zfp473, Srsf9, Dazap1, Lsm8, Tbrg4, Snrnp40, Magohb, Snrpe, Rbmxl1, Ddx20, Srrt, U2af2, Lsm7, Snrpd3, Hnrnph1, Hnrnpm, Tra2a, Ttf2, Hnrnpa3, Snrpa, Rbm19, Cstf2, U2af1, Lsm6, Gemin5, Lsm4, et al. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, P.; Jiang, H. RNA-Sequencing Reveals Upregulation and a Beneficial Role of Autophagy in Myoblast Differentiation and Fusion. Cells 2022, 11, 3549. https://doi.org/10.3390/cells11223549
Lyu P, Jiang H. RNA-Sequencing Reveals Upregulation and a Beneficial Role of Autophagy in Myoblast Differentiation and Fusion. Cells. 2022; 11(22):3549. https://doi.org/10.3390/cells11223549
Chicago/Turabian StyleLyu, Pengcheng, and Honglin Jiang. 2022. "RNA-Sequencing Reveals Upregulation and a Beneficial Role of Autophagy in Myoblast Differentiation and Fusion" Cells 11, no. 22: 3549. https://doi.org/10.3390/cells11223549
APA StyleLyu, P., & Jiang, H. (2022). RNA-Sequencing Reveals Upregulation and a Beneficial Role of Autophagy in Myoblast Differentiation and Fusion. Cells, 11(22), 3549. https://doi.org/10.3390/cells11223549






