Immune Transcriptome Study of Human Nucleated Erythroid Cells from Different Tissues by Single-Cell RNA-Sequencing
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Interventions
2.3. Participant Safety
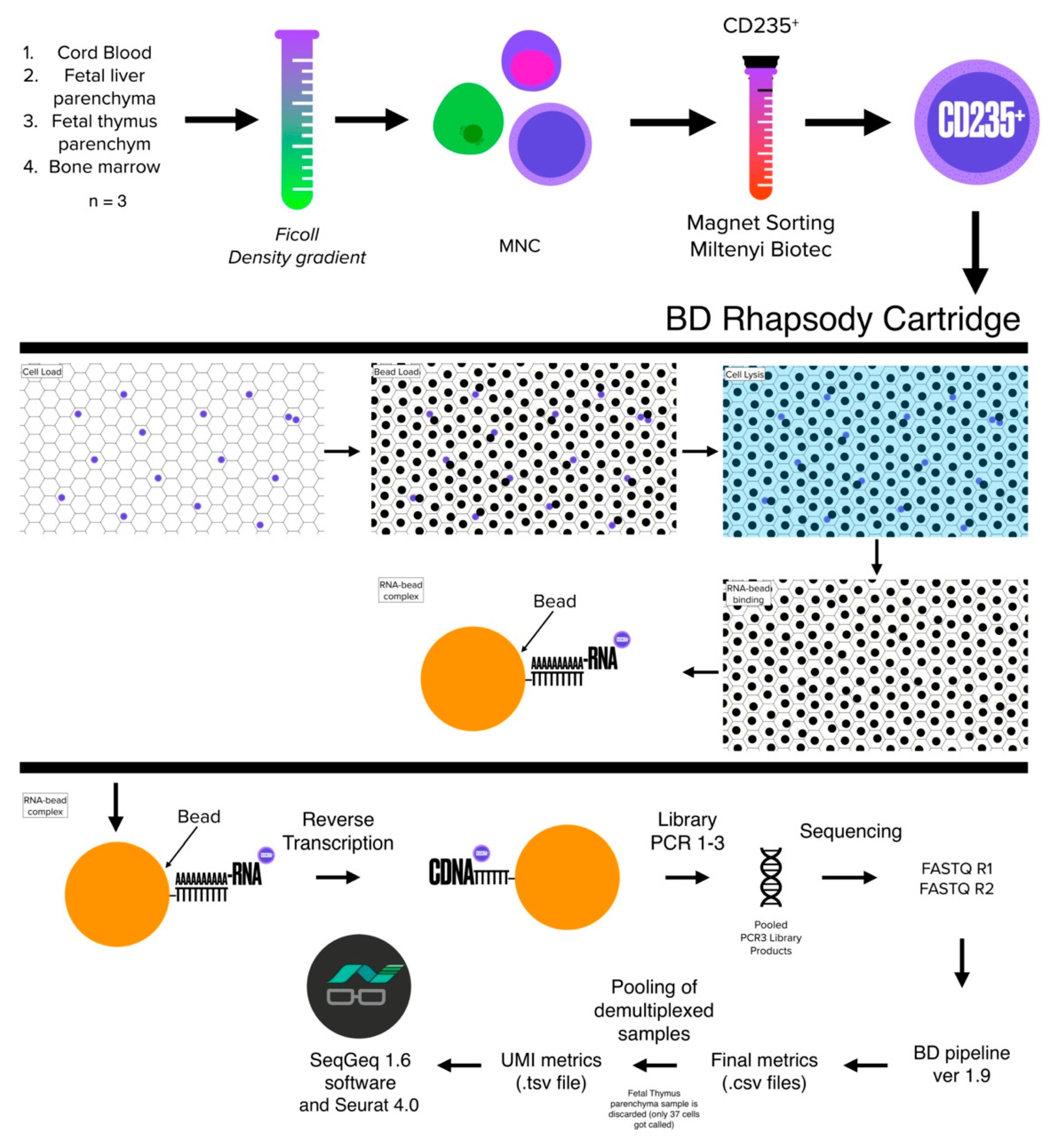
2.4. Cell Isolation
2.5. Cell Sorting
2.6. Viability Staining
2.7. Sample Tag Barcoding
2.8. cDNA Library Preparation and Sequencing
2.9. Data Processing
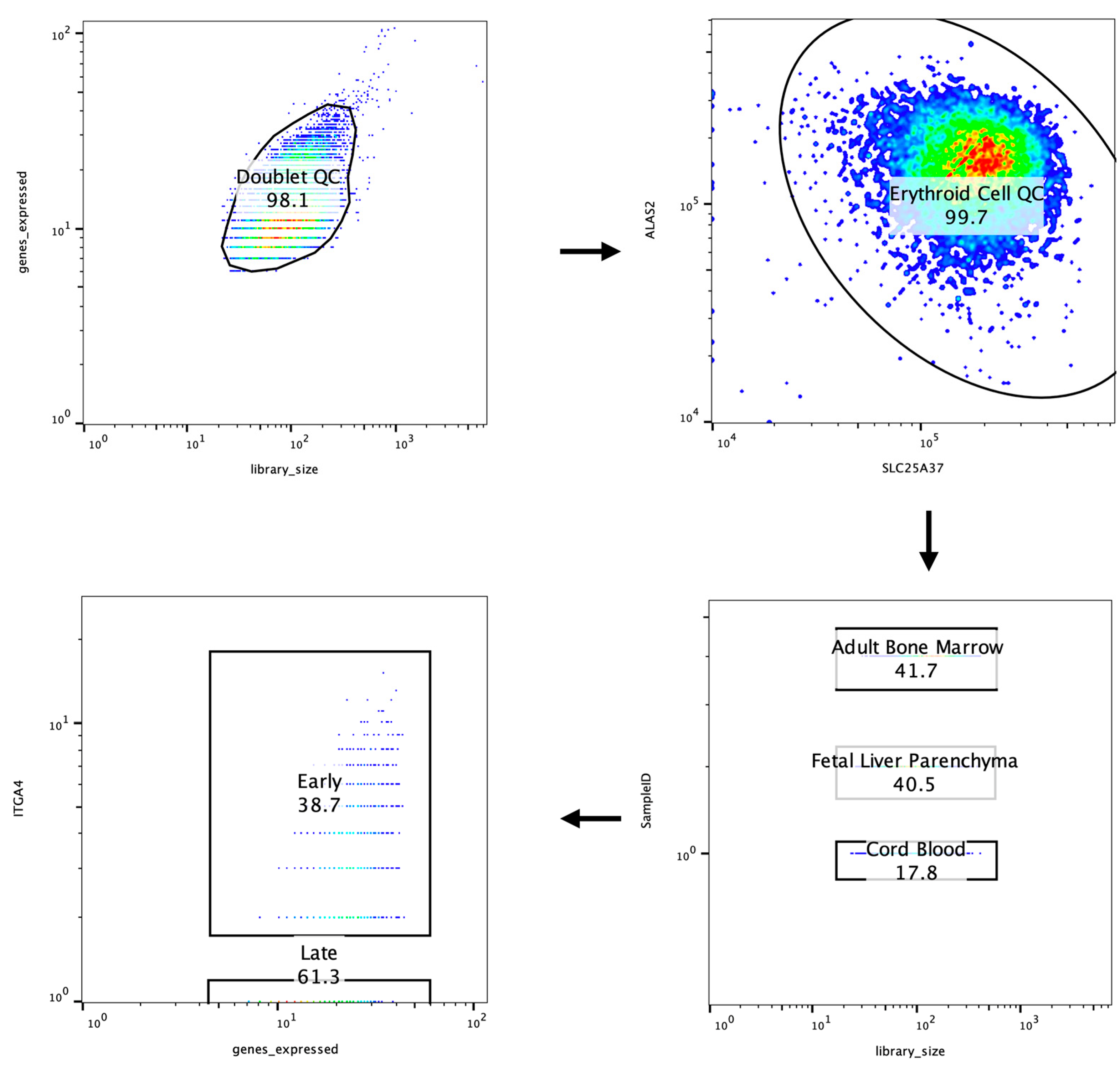
2.10. Data QC and Analysis
3. Results
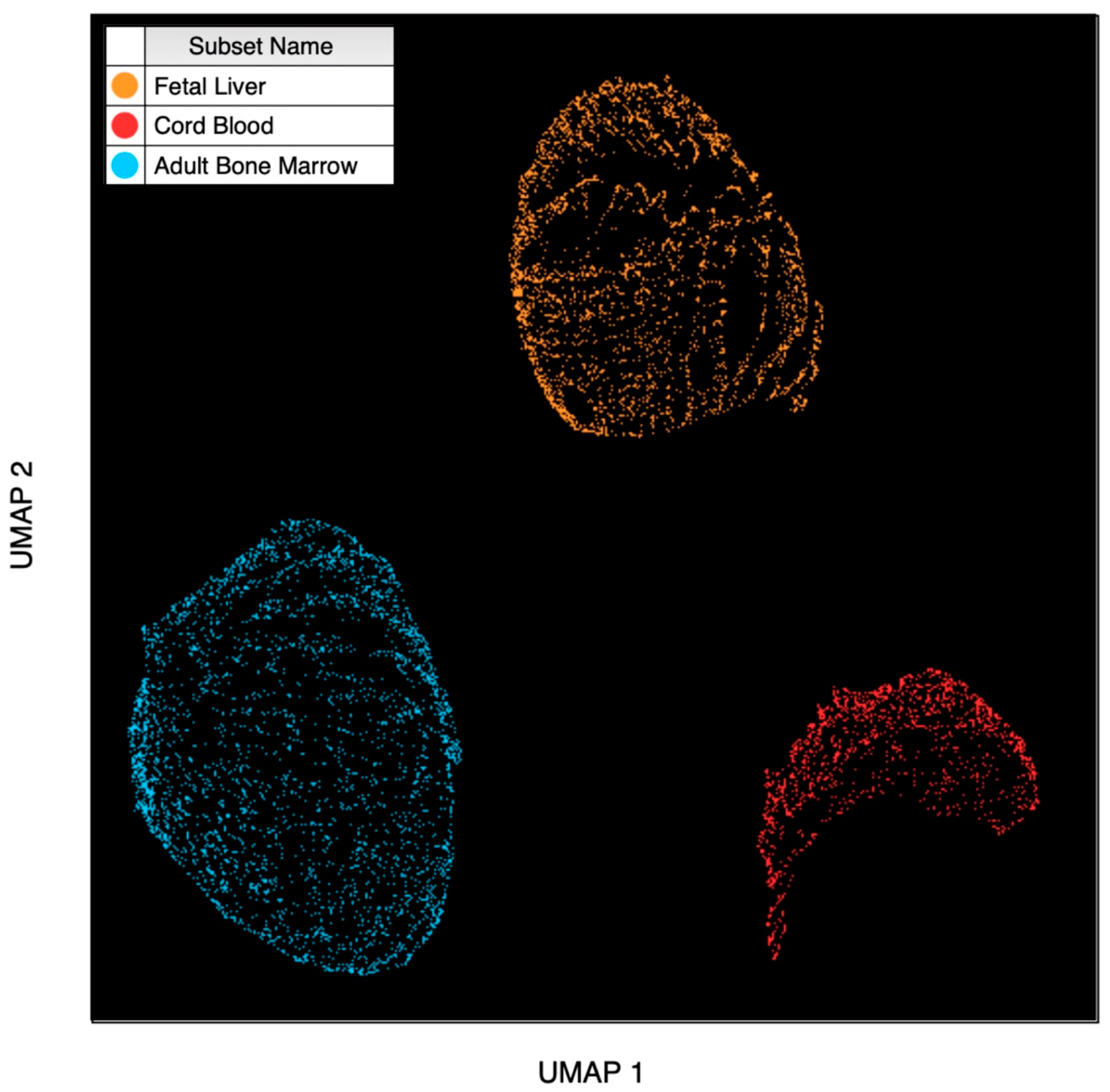
3.1. Dimensionality Reduction
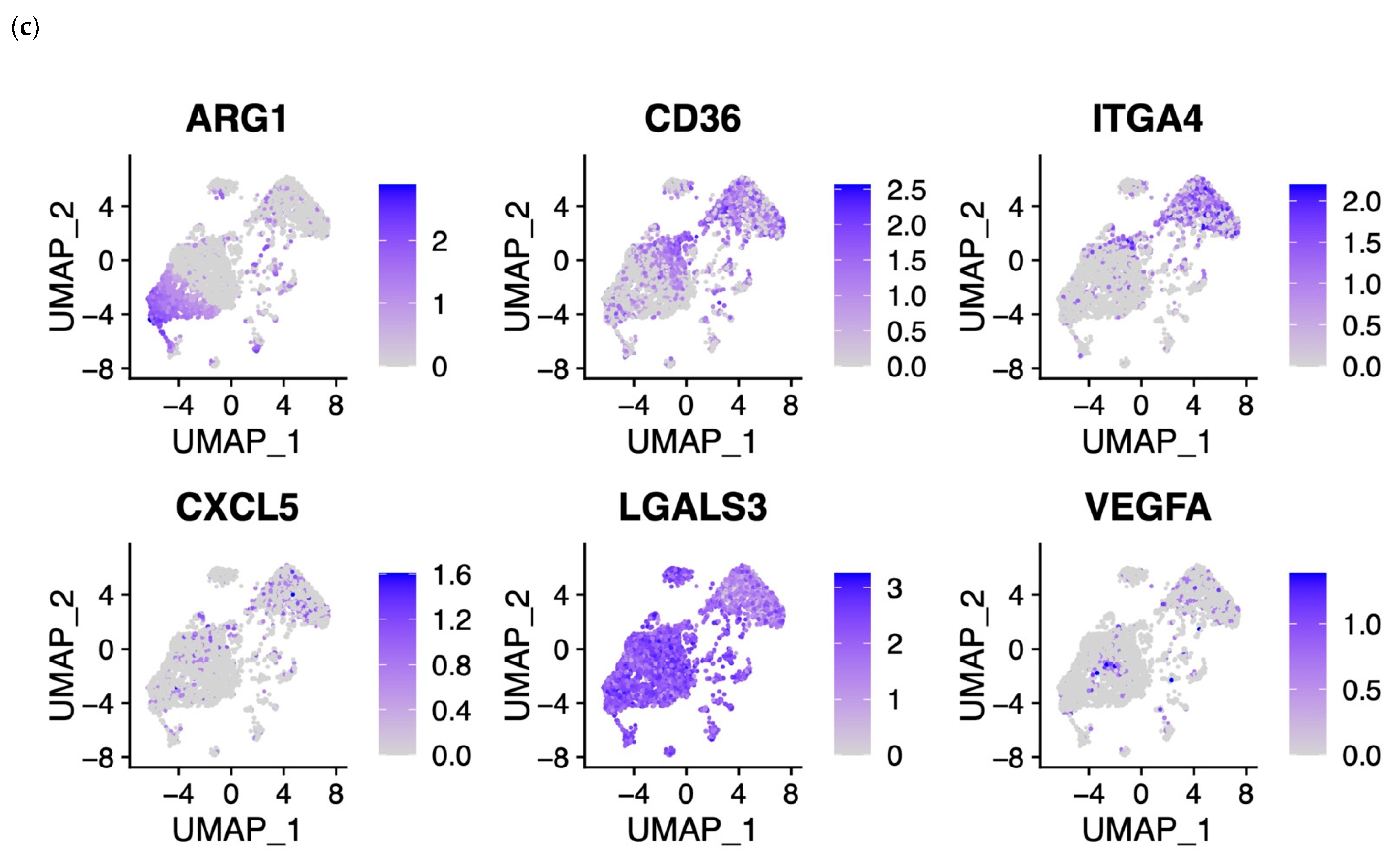
3.2. NECs’ Gene Set Analysis
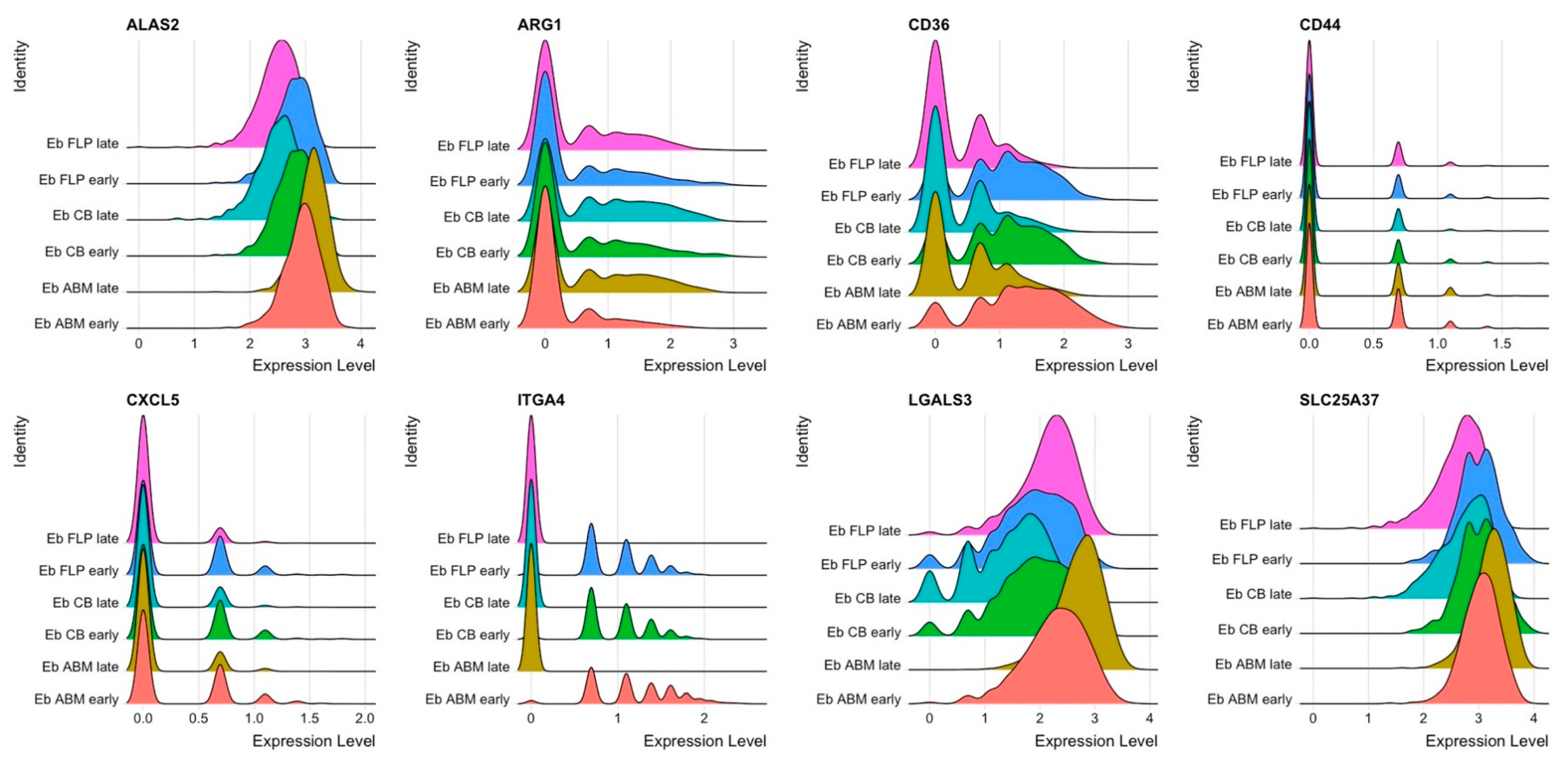
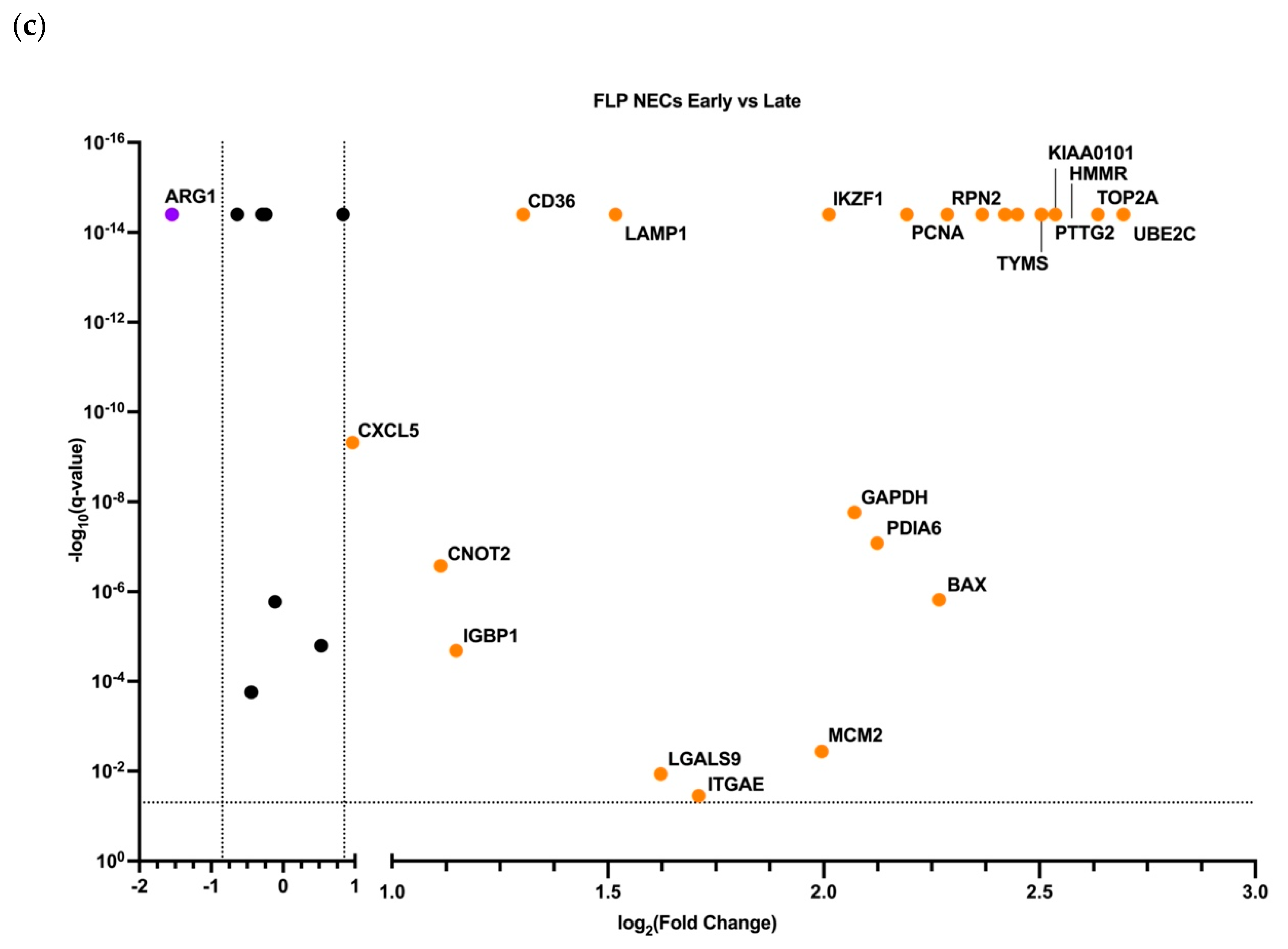
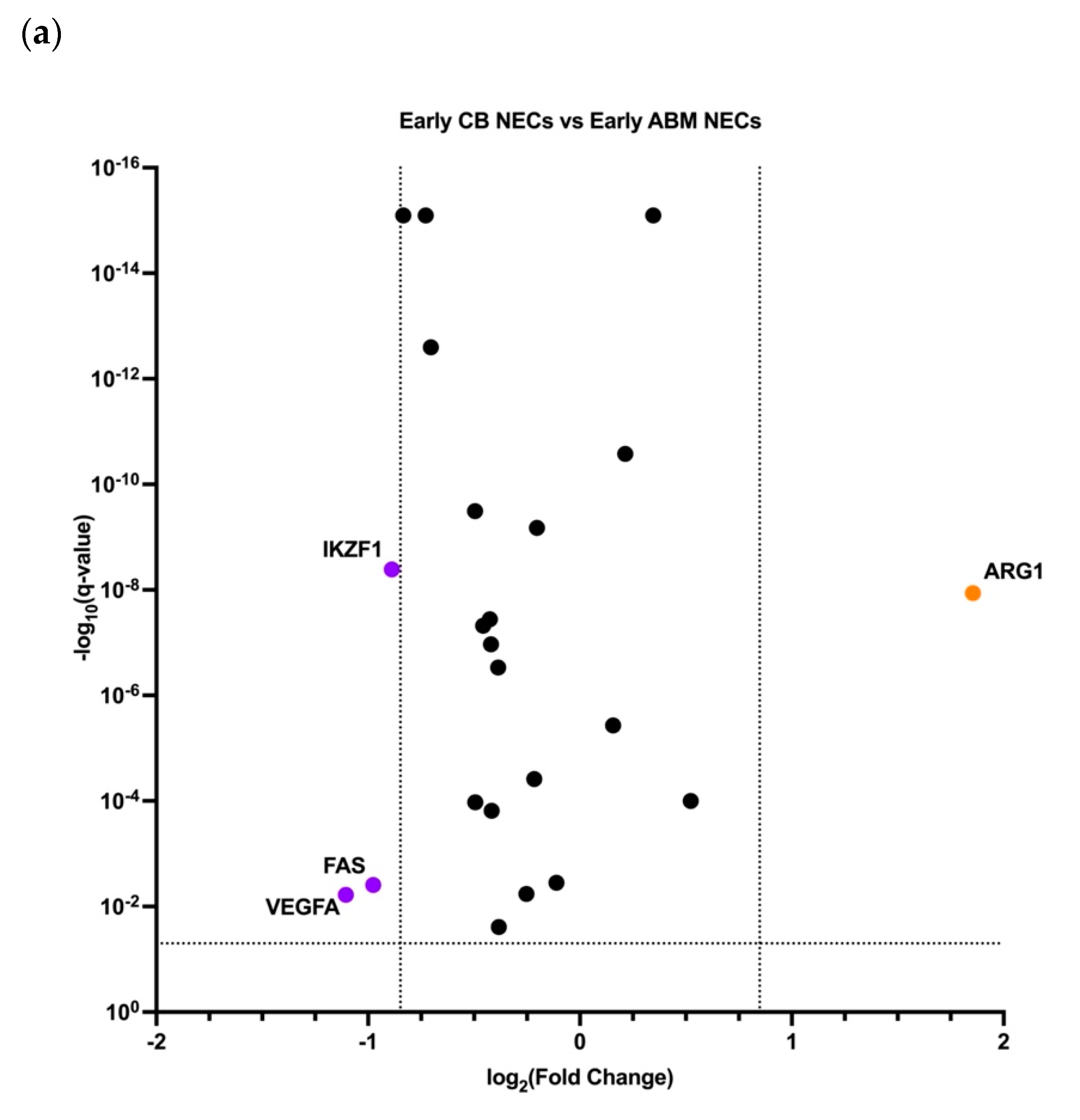
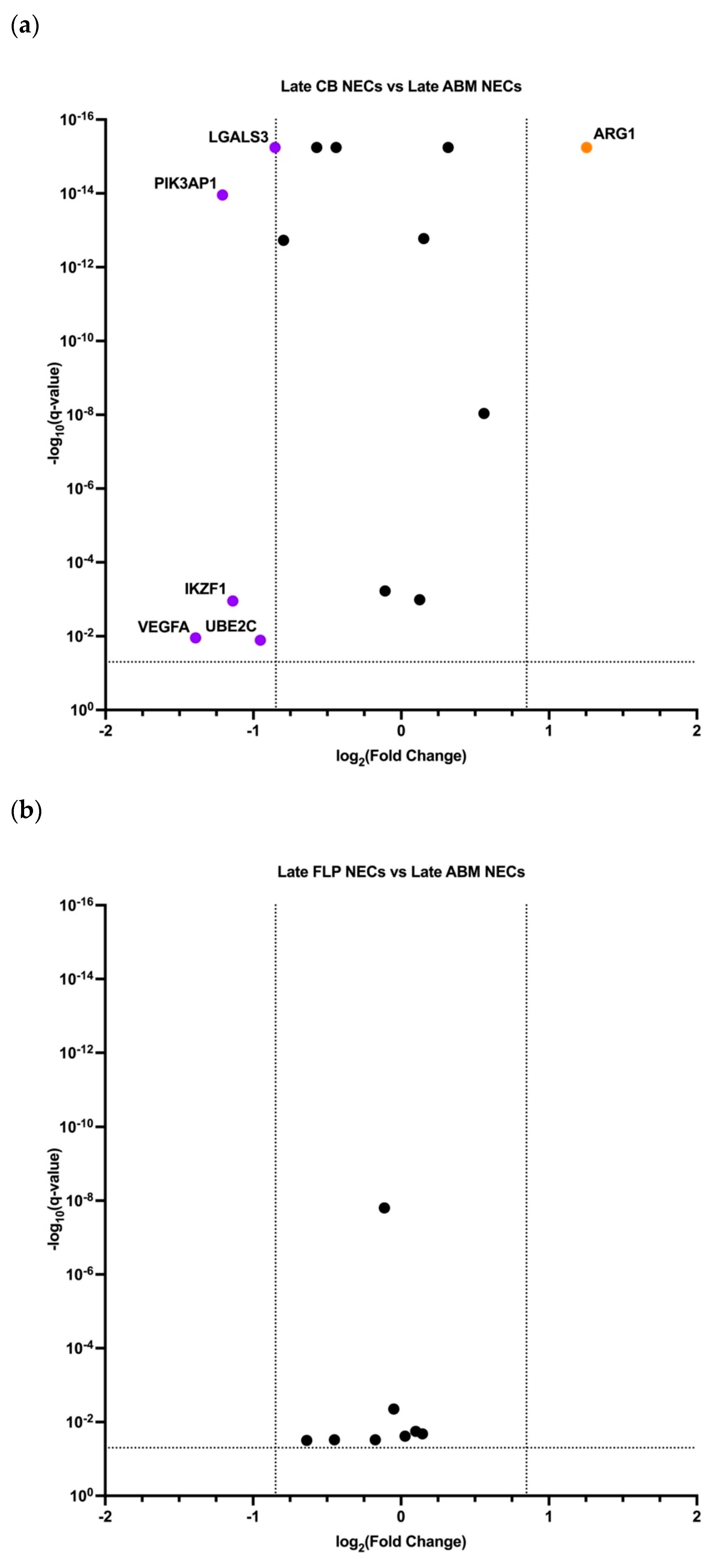
3.3. Early and Late NECs have Differentially Expressed Genes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Ethics Statement
Conflicts of Interest
References
- Fornas, O.; Domingo, J.C.; Marin, P.; Petriz, J. Flow cytometric-based isolation of nucleated erythroid cells during maturation: An approach to cell surface antigen studies. Cytom. J. Int. Soc. Anal. Cytol. 2002, 50, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Delyea, C.; Bozorgmehr, N.; Koleva, P.; Dunsmore, G.; Shahbaz, S.; Huang, V. Elahi CD71+ erythroid suppressor cells promote fetomaternal tolerance through arginase-2 and PDL-1. J. Immunol. 2018, 200, 4044–4058. [Google Scholar] [CrossRef] [PubMed]
- Sennikov, S.V.; Eremina, L.V.; Samarin, D.M.; Avdeev, I.V.; Kozlov, V.A. Cytokine gene expression in erythroid cells. Eur. Cytokine Netw. 1996, 7, 771–774. [Google Scholar] [PubMed]
- Sennikov, S.V.; Injelevskaya, T.V.; Krysov, S.V.; Silkov, A.N.; Kovinev, I.G.; Dyachkova, N.J.; Zenkov, A.N.; Loseva, M.I.; Kozlov, V.A. Production of hemo-and immunoregulatory cytokines by erythroblast antigen+ and glycophorin A+ cells from human bone marrow. BMC Cell Biol. 2004, 5, 39. [Google Scholar] [CrossRef]
- Sennikov, S.V.; Krysov, S.V.; Injelevskaya, T.V.; Silkov, A.N.; Kozlov, V.A. Production of cytokines by immature erythroid cells derived from human embryonic liver. Eur. Cytokine Netw. 2001, 12, 274–279. [Google Scholar]
- Denisova, V.V.; Kulagin, A.D.; Lisukov, I.A.; Kryuchkova, I.V.; Sizikova, S.A.; Sennikov, S.V.; Kozlov, V.A. Cytokine-producing activity of bone marrow erythrokaryocytes and its regulation under normal conditions. Bull. Exp. Biol. Med. 2007, 143, 218–221. [Google Scholar] [CrossRef]
- Seledtsov, V.I.; Seledtsova, G.V.; Samarin, D.M.; Taraban, V.Y.; Sennikov, S.V.; Kozlov, V.A. Characterization of erythroid cell-derived natural suppressor activity. Immunobiology 1998, 198, 361–374. [Google Scholar] [CrossRef]
- Shahbaz, S.; Bozorgmehr, N.; Koleva, P.; Namdar, A.; Jovel, J.; Fava, R.A.; Elahi, S. CD71+ VISTA+ erythroid cells promote the development and function of regulatory T cells through TGF-β. PLoS Biol. 2018, 16, e2006649. [Google Scholar] [CrossRef]
- Satija, R.; Farrell, J.A.; Gennert, D.; Schier, A.F.; Regev, A. Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol. 2015, 33, 495–502. [Google Scholar] [CrossRef]
- Hu, J.; Liu, J.; Xue, F.; Halverson, G.; Reid, M.; Guo, A.; Chen, L.; Raza, A.; Galili, N.; Jaffray, J.; et al. Isolation and functional characterization of human erythroblasts at distinct stages: Implications for understanding of normal and disordered erythropoiesis in vivo. Blood J. Am. Soc. Hematol. 2013, 121, 3246–3253. [Google Scholar] [CrossRef]
- Elahi, S.; Vega-López, M.A.; Herman-Miguel, V.; Ramírez-Estudillo, C.; Mancilla-Ramírez, J.; Motyka, B.; West, L.; Oyegbami, O. CD71+ erythroid cells in human neonates exhibit immunosuppressive properties and compromise immune response against systemic infection in neonatal mice. Front. Immunol. 2020, 11, 597433. [Google Scholar] [CrossRef] [PubMed]
- Grzywa, T.M.; Nowis, D.; Golab, J. The role of CD71+ erythroid cells in the regulation of the immune response. Pharmacol. Ther. 2021, 228, 107927. [Google Scholar] [CrossRef]
- Elahi, S.; Ertelt, J.M.; Kinder, J.M.; Jiang, T.T.; Zhang, X.; Xin, L.; Chaturvedi, V.; Strong, B.S.; Qualls, J.E.; Steinbrecher, K.A.; et al. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature 2013, 504, 158–162. [Google Scholar] [CrossRef]
- Dunsmore, G.; Bozorgmehr, N.; Delyea, C.; Koleva, P.; Namdar, A.; Elahi, S. Erythroid Suppressor Cells Compromise Neonatal Immune Response against Bordetella pertussis. J. Immunol. 2017, 199, 2081–2095. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Jiang, D.; Cai, W.; Chen, X.; Dong, W.; Chen, L.; Hong, G.; Wu, B.; Yao, Y.; Lu, Z. CD71+ erythroid cells expansion in adult sepsis: Potential causes and role in prognosis and nosocomial infection prediction. Front. Immunol. 2022, 13, 830025. [Google Scholar] [CrossRef] [PubMed]
- Mashhouri, S.; Koleva, P.; Huynh, M.; Okoye, I.; Shahbaz, S.; Elahi, S. Sex Matters: Physiological Abundance of Immuno-Regulatory CD71+ Erythroid Cells Impair Immunity in Females. Front. Immunol. 2021, 12, 2893. [Google Scholar] [CrossRef]
- Shahbaz, S.; Xu, L.; Osman, M.; Sligl, W.; Shields, J.; Joyce, M.; Tyrrell, D.L.; Oyegbami, O.; Elahi, S. Erythroid precursors and progenitors suppress adaptive immunity and get invaded by SARS-CoV-2. Stem Cell Rep. 2021, 16, 1165–1181. [Google Scholar] [CrossRef]
- Farhad, M.; Rolig, A.S.; Redmond, W.L. The role of Galectin-3 in modulating tumor growth and immunosuppression within the tumor microenvironment. Oncoimmunology 2018, 7, e1434467. [Google Scholar] [CrossRef]
- Yang, R.; Sun, L.; Li, C.F.; Wang, Y.H.; Yao, J.; Li, H.; Yan, M.; Chang, W.C.; Hsu, J.M.; Cha, J.H.; et al. Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat. Commun. 2021, 12, 832. [Google Scholar] [CrossRef]
- Carmeliet, P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005, 69 (Suppl. S3), 4–10. [Google Scholar] [CrossRef]
- Rajarathnam, K.; Schnoor, M.; Richardson, R.M.; Rajagopal, S. How do chemokines navigate neutrophils to the target site: Dissecting the structural mechanisms and signaling pathways. Cell. Signal. 2019, 54, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, R.I.; Lu, W. α-Defensins in human innate immunity. Immunol. Rev. 2012, 245, 84–112. [Google Scholar] [CrossRef] [PubMed]
- Hattangadi, S.M.; Wong, P.; Zhang, L.; Flygare, J.; Lodish, H.F. From stem cell to red cell: Regulation of erythropoiesis at multiple levels by multiple proteins, RNAs, and chromatin modifications. Blood J. Am. Soc. Hematol. 2011, 118, 6258–6268. [Google Scholar] [CrossRef] [PubMed]
- England, S.J.; McGrath, K.E.; Frame, J.M.; Palis, J. Immature erythroblasts with extensive ex vivo self-renewal capacity emerge from the early mammalian fetus. Blood J. Am. Soc. Hematol. 2011, 117, 2708–2717. [Google Scholar] [CrossRef] [PubMed]
- Mullarky, I.K.; Szaba, F.M.; Kummer, L.W.; Wilhelm, L.B.; Parent, M.A.; Johnson, L.L.; Smiley, S.T. Gamma interferon suppresses erythropoiesis via interleukin-15. Infect. Immun. 2007, 75, 2630–2633. [Google Scholar] [CrossRef]







| Term | Overlap | Adjusted p-Value | Combined Score | Genes |
|---|---|---|---|---|
| Antimicrobial humoral immune response mediated by antimicrobial peptide | 6/64 | 1.96838 × 10−5 | 782.554935 | CXCL8, DEFA3, S100A12, S100A9, GAPDH, CXCL5 |
| Response to lipopolysaccharide | 7/159 | 0.000113823 | 305.7517678 | CXCL8, IL23R, DEFA3, LGALS9, S100A9, CXCL5, SNCA |
| Positive regulation of cellular biosynthetic process | 7/180 | 0.000176087 | 253.7105023 | SLC25A37, CXCL8, PCNA, MYC, CD36, PASK, SNCA |
| Positive regulation of cysteine-type endopeptidase activity involved in apoptotic process | 6/119 | 0.000203856 | 317.1125977 | MYC, FAS, BAX, LGALS9, S100A9, SNCA |
| Neutrophil chemotaxis | 5/70 | 0.000230242 | 435.9051686 | LGALS3, CXCL8, S100A12, S100A9, CXCL5 |
| Granulocyte chemotaxis | 5/73 | 0.000230242 | 410.2177586 | LGALS3, CXCL8, S100A12, S100A9, CXCL5 |
| Response to molecule of bacterial origin | 5/73 | 0.000230242 | 410.2177586 | CXCL8, IL23R, LGALS9, S100A9, SNCA |
| Neutrophil migration | 5/77 | 0.000262892 | 379.6970674 | LGALS3, CXCL8, S100A12, S100A9, CXCL5 |
| Regulation of cysteine-type endopeptidase activity involved in apoptotic process | 5/89 | 0.000479286 | 307.5642872 | IGBP1, MYC, CD44, VEGFA, SNCA |
| Cellular response to cytokine stimulus | 9/482 | 0.000502164 | 103.8739458 | CXCL8, DUSP1, MYC, IL23R, RORA, CD36, LGALS9, GAPDH, VEGFA |
| Regulation of stress-activated MAPK cascade | 4/49 | 0.000884791 | 421.4278016 | IGBP1, MYC, FAS, LGALS9 |
| Response to lipid | 5/114 | 0.001105979 | 213.7226208 | IL23R, CD36, LGALS9, S100A9, SNCA |
| Positive regulation of protein-containing complex assembly | 5/118 | 0.001143908 | 203.0533426 | LGALS3, BAX, CD36, VEGFA, SNCA |
| Positive regulation of macromolecule biosynthetic process | 5/129 | 0.001650333 | 177.7596539 | SLC25A37, PCNA, MYC, NAMPT, PASK |
| Cytokine-mediated signaling pathway | 9/621 | 0.002169213 | 66.66591591 | IL15RA, CXCL8, MYC, IL23R, RORA, CD36, CXCL5, CD44, VEGFA |
| Defense response to fungus | 3/24 | 0.002169213 | 585.4575138 | S100A12, S100A9, GAPDH |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perik-Zavodskii, R.; Perik-Zavodskaia, O.; Shevchenko, J.; Denisova, V.; Alrhmoun, S.; Volynets, M.; Tereshchenko, V.; Zaitsev, K.; Sennikov, S. Immune Transcriptome Study of Human Nucleated Erythroid Cells from Different Tissues by Single-Cell RNA-Sequencing. Cells 2022, 11, 3537. https://doi.org/10.3390/cells11223537
Perik-Zavodskii R, Perik-Zavodskaia O, Shevchenko J, Denisova V, Alrhmoun S, Volynets M, Tereshchenko V, Zaitsev K, Sennikov S. Immune Transcriptome Study of Human Nucleated Erythroid Cells from Different Tissues by Single-Cell RNA-Sequencing. Cells. 2022; 11(22):3537. https://doi.org/10.3390/cells11223537
Chicago/Turabian StylePerik-Zavodskii, Roman, Olga Perik-Zavodskaia, Julia Shevchenko, Vera Denisova, Saleh Alrhmoun, Marina Volynets, Valeriy Tereshchenko, Konstantin Zaitsev, and Sergey Sennikov. 2022. "Immune Transcriptome Study of Human Nucleated Erythroid Cells from Different Tissues by Single-Cell RNA-Sequencing" Cells 11, no. 22: 3537. https://doi.org/10.3390/cells11223537
APA StylePerik-Zavodskii, R., Perik-Zavodskaia, O., Shevchenko, J., Denisova, V., Alrhmoun, S., Volynets, M., Tereshchenko, V., Zaitsev, K., & Sennikov, S. (2022). Immune Transcriptome Study of Human Nucleated Erythroid Cells from Different Tissues by Single-Cell RNA-Sequencing. Cells, 11(22), 3537. https://doi.org/10.3390/cells11223537






