Treatment of Chronic Hepatitis D with Bulevirtide—A Fight against Two Foes—An Update
Abstract
1. Introduction
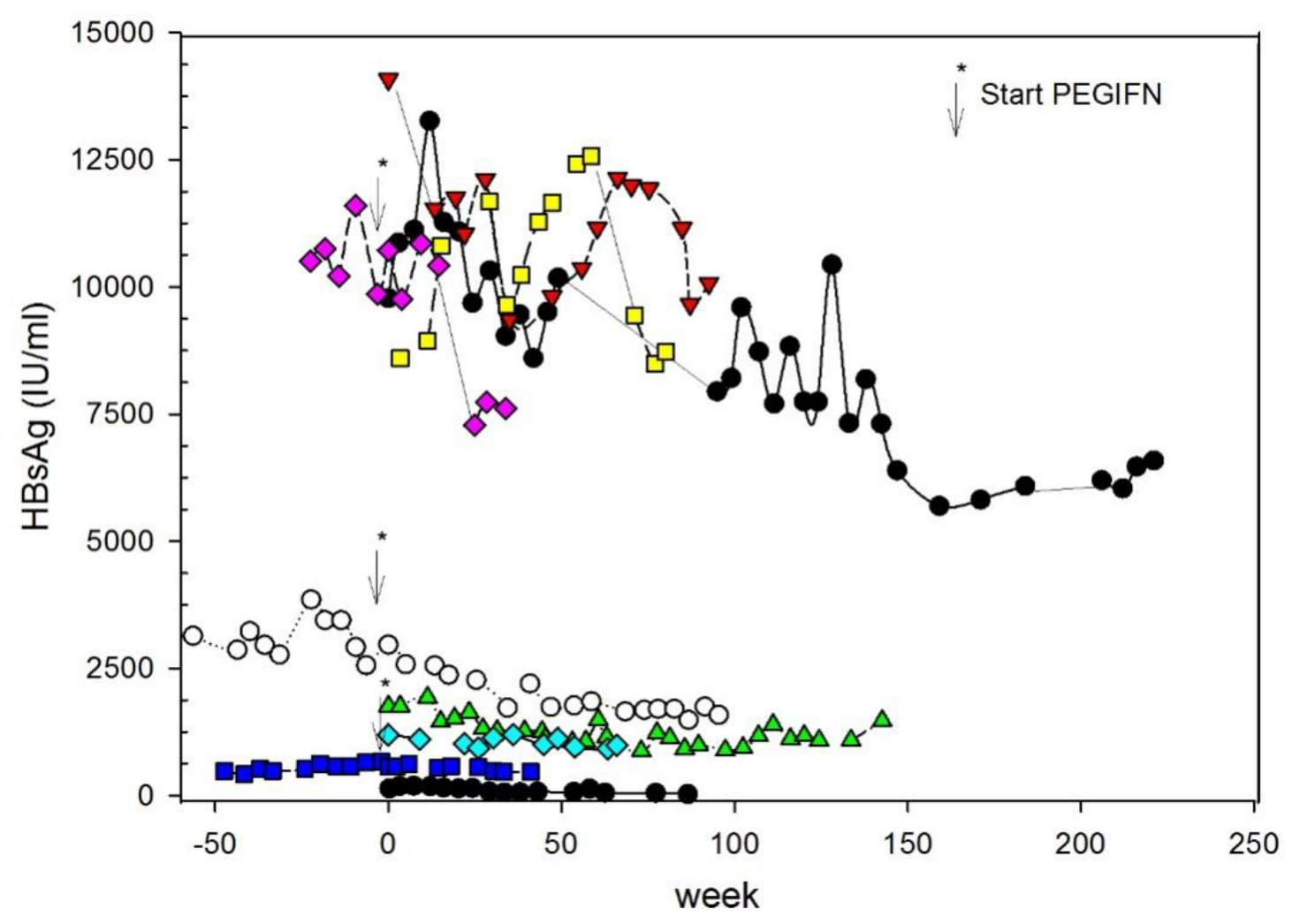
2. Antiviral Efficacy of BLV in Patients with Chronic Hepatitis D
3. Guidance on How to Use BLV in Patients with Chronic Hepatitis D
4. BLV in Patients with Advanced Chronic Liver Disease (Cirrhosis)
5. Role of Chronic Hepatitis B
6. Safety
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Walker, P.J.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Adriaenssens, E.M.; Alfenas-Zerbini, P.; Dempsey, D.M.; BDutilh, B.E.; García, M.L.; Hendrickson, R.C.; et al. Recent Changes to Virus Taxonomy Ratified by the International Committee on Taxonomy of Viruses (2022). Arch. Virol. 2022, 167, 2429–2440. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Zhang, S.; Ou, X.; Li, S.; Ma, Z.; Wang, W.; Peppelenbosch, M.P.; Liu, J.; Pan, Q. Estimating the Global Prevalence, Disease Progression, and Clinical Outcome of Hepatitis Delta Virus Infection. J. Infect. Dis. 2019, 221, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Polaris Observatory, Collaborators. Global Prevalence, Treatment, and Prevention of Hepatitis B Virus Infection in 2016: A Modelling Study. Lancet Gastroenterol. Hepatol. 2018, 3, 383–403. [Google Scholar] [CrossRef]
- Jachs, M.; Binter, T.; Schmidbauer, C.; Hartl, L.; Strasser, M.; Laferl, H.; Hametner-Schreil, S.; Lindorfer, A.; Dax, K.; Stauber, R.E.; et al. Hepatitis D virus (HDV) prevalence in Austria is low but causes considerable morbidity due to fast progression to cirrhosis. United Eur. Gastroenterol. J. 2021, 9, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Farci, P.; Roskams, T.; Chessa, L.; Peddis, G.; Mazzoleni, A.P.; Scioscia, R.; Serra, G.; Lai, M.E.; Loy, M.; Caruso, L.; et al. Long-Term Benefit of Interferon Alpha Therapy of Chronic Hepatitis D: Regression of Advanced Hepatic Fibrosis. Gastroenterology 2004, 126, 1740–1749. [Google Scholar] [CrossRef]
- Hercun, J.; Kim, G.E.; Da, B.L.; Rotman, Y.; Kleiner, D.E.; Chang, R.; Glenn, J.S.; Hoofnagle, J.H.; Koh, C.; Heller, T. Durable Virological Response and Functional Cure of Chronic Hepatitis D after Long-Term Peginterferon Therapy. Aliment. Pharmacol. Ther. 2021, 54, 176–182. [Google Scholar] [CrossRef]
- Ferenci, P.; Formann, E.; Romeo, R. Successful Treatment of Chronic Hepatitis D with a Short Course of Peginterferon alfa-2a. Am. J. Gastroenterol. 2005, 100, 1626–1627. [Google Scholar] [CrossRef]
- Abdrakhman, A.; Ashimkhanova, A.; Almawi, W.Y. Effectiveness of pegylated interferon monotherapy in the treatment of chronic hepatitis D virus infection: A meta-analysis. Antivir. Res. 2020, 185, 104995. [Google Scholar] [CrossRef]
- Blanchet, M.; Sureau, C. Infectivity Determinants of the Hepatitis B Virus Pre-S Domain Are Confined to the N-Terminal 75 Amino Acid Residues. J. Virol. 2007, 81, 5841–5849. [Google Scholar] [CrossRef]
- Ni, Y.; Lempp, F.A.; Mehrle, S.; Nkongolo, S.; Kaufman, C.; Fälth, M.; Stindt, J.; Königer, C.; Nassal, M.; Kubitz, R.; et al. Hepatitis B and D Viruses Exploit Sodium Taurocholate Co-transporting Polypeptide for Species-Specific Entry into Hepatocytes. Gastroenterology 2014, 146, 1070–1083.e6. [Google Scholar] [CrossRef]
- Urban, S.; Neumann-Haefelin, C.; Lampertico, P. Hepatitis D virus in 2021: Virology, immunology and new treatment approaches for a difficult-to-treat disease. Gut 2021, 70, 1782–1794. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Urban, S. New insights into HDV persistence: The role of interferon response and implications for upcoming novel therapies. J. Hepatol. 2020, 74, 686–699. [Google Scholar] [CrossRef] [PubMed]
- Wedemeyer, H.; Schöneweis, K.; Bogomolov, P.O.; Voronkova, N.; Chulanov, V.P.; Stepanova, T.; Bremer, B.; Allweiss, L.; Dandri, M.; Burhenne, J.; et al. GS-13-Final results of a multicenter, open-label phase 2 clinical trial (MYR203) to assess safety and efficacy of myrcludex B in cwith PEG-interferon Alpha 2a in patients with chronic HBV/HDV co-infection. Hepatology 2019, 70, e81. [Google Scholar] [CrossRef]
- Wedemeyer, H.; Schöneweis, K.; Bogomolov, P.; Blank, A.; Voronkova, N.; Stepanova, T.; Sagalova, O.; Chulanov, V.; Osipenko, M.; Morozov, V.; et al. Safety and Efficacy of Bulevirtide in Combination with Tenofovir Disoproxil Fumarate in Patients with Hepatitis B Virus and Hepatitis D Virus Coinfection (Myr202): A Multicentre, Randomised, Parallel-Group, Open-Label, Phase 2 Trial. Lancet Infect. Dis. 2022, 13, S1473. [Google Scholar] [CrossRef]
- Asselah, T.; Arama, S.S.; Bogomolov, P.; Bourliere, M.; Fontaine, H.; Gherlan, S.G.; Gorodin, V.; Hilleret, M.; Lazar, S.; Mamonova, N.; et al. Safety and Efficacy of Bulevirtide Monotherapy and in Combination with Peginterferon Alfa-2a in Patients with Chronic Hepatitis Delta: 24 Weeks Interim Data of Myr204 Phase 2b Study. J. Hepatol. 2021, 75, S291. [Google Scholar]
- Wedemeyer, H.; Aleman, S.; Andreone, P.; Blank, A.; Brunetto, M.; Bogomolov, P.; Chulanov, V.; Geyvandova, N.; Hilgard, G.; Mamonova, N.; et al. Bulevirtide Monotherapy at Low and High Dose in Patients with Chronic Hepatitis Delta: 24 Weeks Interim Data of the Phase 3 Myr301 Study. J. Hepatol 2021, 75, S294–S803. [Google Scholar] [CrossRef]
- Lampertico, P.; Roulot, D.; Wedemeyer, H. Bulevirtide with or without pegIFNα for patients with compensated chronic hepatitis delta: From clinical trials to real-world studies. J. Hepatol. 2022, 77, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Lok, A.S.; Negro, F.; Asselah, T.; Farci, P.; Rizzetto, M. Endpoints and New Options for Treatment of Chronic Hepatitis D. Hepatology 2021, 74, 3479–3485. [Google Scholar] [CrossRef] [PubMed]
- Da, B.L. Clinical trials in hepatitis D virus: Measuring success. Hepatology 2022. [Google Scholar] [CrossRef]
- Kim, W.R.; Flamm, S.L.; Di Bisceglie, A.M.; Bodenheimer, H.C. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology 2008, 47, 1363–1370. [Google Scholar] [CrossRef]
- De Lédinghen, V.; Guyader, D.; Metivier, S.; Hilleret, M.-N.; Fontaine, H.; Roche, B.; Ganne-Carrié, N.; d’Alteroche, L.; Loustaud-Ratti, V.; Gervais, A.; et al. Safety and Efficacy of 2mg Bulevirtide in Patients with Chronic Hbv/Hdv Co-Infection. First Real-World Results (French Early Access Program). Hepatology 2021, 74, 16–17. [Google Scholar]
- Wedemeyer, H.; Schöneweis, K.; Bogomolov, P.; Chulanov, V.; Stepanova, T.; Viacheslav, M.; Allweiss, L.; Dandri, M.; Ciesek, S.; Dittmer, U.; et al. 48 Weeks of High Dose (10 Mg) Bulevirtide as Monotherapy or with Peginterferon Alfa-2a in Patients with Chronic Hbv/Hdv Co-Infection. J. Hepatol. 2020, 73, S52–S53. [Google Scholar] [CrossRef]
- Jachs, M.; Schwarz, C.; Panzer, M.; Binter, T.; Aberle, S.W.; Hartl, L.; Dax, K.; Aigner, E.; Stättermayer, A.F.; Munda, P.; et al. Response-Guided Long-Term Treatment of Chronic Hepatitis D Patients with Bulevirtide-Results of a “Real World” Study. Aliment. Pharm. 2022, 56, 144–154. [Google Scholar] [CrossRef] [PubMed]
- European Association For The Study Of The Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. Hepatology 2017, 67, 370–398. [Google Scholar] [CrossRef]
- Chan, H.L.Y.; Ahn, S.H.; Chang, T.T.; Peng, C.Y.; Wong, D.; Coffin, C.S.; Lim, S.G.; Chen, P.J.; Janssen, H.L.A.; Marcellin, P.; et al. Peginterferon Lambda for the Treatment of Hbeag-Positive Chronic Hepatitis B: A Randomized Phase 2b Study (Lira-B). J. Hepatol. 2016, 64, 1011–1019. [Google Scholar] [CrossRef]
- Koh, C.; Hercun, J.; Rahman, F.; Huang, A.; Da, B.L.; Surana, P.; Kapuria, D.; Rotman, Y.; Vittal, A.; Gilman, C.A.; et al. A Phase 2 Study of Peginterferon Lambda, Lonafarnib and Ritonavir for 24 Weeks: End-of-Treatment Results from the Lift Hdv Study. J. Hepatol 2020, 73, S130. [Google Scholar] [CrossRef]
- Bazinet, M.; Pântea, V.; Cebotarescu, V.; Cojuhari, L.; Jimbei, P.; Anderson, M.; Gersch, J.; Holzmayer, V.; Elsner, C.; Krawczyk, A.; et al. Persistent Control of Hepatitis B Virus and Hepatitis Delta Virus Infection Following REP 2139-Ca and Pegylated Interferon Therapy in Chronic Hepatitis B Virus/Hepatitis Delta Virus Coinfection. Hepatol. Commun. 2020, 5, 189–202. [Google Scholar] [CrossRef]
- Lampertico, P.; Aleman, S.; Blank, A.; Bogomolov, P.; Chulanov, V.; Mamanova, N.; Morozov, V.; Sagalova, O.; Stepanova, T.; Suri, V.; et al. Integrated Efficacy Analysis of 24-Week Data from Two Phase 2 and One Phase 3 Clinical Trials of Bulevirtide Monotherapy Given at 2 Mg or 10 Mg Dose Level for Treatment of Chronic Hepatitis Delta. J. Hepatol. 2022, 77, S828. [Google Scholar] [CrossRef]
- Degasperi, E.; Anolli, M.P.; Renteria, S.C.U.; Sambarion, D.; Borghi, M.; Perbellini, R.; Scholtes, C.; Facchetti, F.; Loglio, A.; Monico, S.; et al. Bulevirtide Monotherapy for 48 Weeks in Patients with Hdv-Related Compensated Cirrhosis and Clinically Significant Portal Hypertension. J. Hepatol. 2022. [Google Scholar] [CrossRef]
- Mandorfer, M.; Kozbial, K.; Schwabl, P.; Freissmuth, C.; Schwarzer, R.; Stern, R.; Chromy, D.; Stättermayer, A.F.; Reiberger, T.; Beinhardt, S.; et al. Sustained Virologic Response to Interferon-Free Therapies Ameliorates Hcv-Induced Portal Hypertension. J. Hepatol. 2016, 65, 692–699. [Google Scholar] [CrossRef]
- Mandorfer, M.; Kozbial, K.; Schwabl, P.; Chromy, D.; Semmler, G.; Stättermayer, A.F.; Pinter, M.; Hernández-Gea, V.; Fritzer-Szekeres, M.; Steindl-Munda, P.; et al. Changes in Hepatic Venous Pressure Gradient Predict Hepatic Decompensation in Patients Who Achieved Sustained Virologic Response to Interferon-Free Therapy. Hepatology 2020, 71, 1023–1036. [Google Scholar] [CrossRef] [PubMed]
- Lens, S.; Baiges, A.; Alvarado-Tapias, E.; Lop, L.; Martinez, E.J.; Fortea, J.I.; Ibanez-Samaniego, L.; Marino, Z.; Rodriguez-Tajes, S.; Gallego, A.; et al. Clinical Outcome and Hemodynamic Changes Following Hcv Eradication with Oral Antiviral Therapy in Patients with Clinically Significant Portal Hypertension. J. Hepatol. 2020, 73, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Semmler, G.; Lens, S.; Meyer, E.L.; Baiges, A.; Alvarado-Tapias, E.; Llop, E.; Telle, L.; Schwabl, P.; Mauro, E.; Escudé, L.; et al. Non-Invasive Tests for Clinically Significant Portal Hypertension after HCV Cure. J. Hepatol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Giersch, K.; Bhadra, O.D.; Volz, T.; Allweiss, L.; Riecken, K.; Fehse, B.; Lohse, A.W.; Petersen, J.; Sureau, C.; Urban, S.; et al. Hepatitis Delta Virus Persists During Liver Regeneration and Is Amplified through Cell Division Both in Vitro and in Vivo. Gut 2019, 68, 150–157. [Google Scholar] [CrossRef]
- Zhang, Z.; Ni, Y.; Lempp, F.A.; Walter, L.; Mutz, P.; Bartenschlager, R.; Urban, S. Hepatitis D virus-induced interferon response and administered interferons control cell division-mediated virus spread. J. Hepatol. 2022, 77, 957–966. [Google Scholar] [CrossRef]
- Fung, S.; Choi, H.S.J.; Gehring, A.; Janssen, H.L.A. Getting to HBV cure: The promising paths forward. Hepatology 2022, 76, 233–250. [Google Scholar] [CrossRef]
- Moucari, R.; Marcellin, P. Quantification of hepatitis B surface antigen: A new concept for the management of chronic hepatitis B. Liver Int. 2011, 31, 122–128. [Google Scholar] [CrossRef]
- Rijckborst, V.; Hansen, B.E.; Cakaloglu, Y.; Ferenci, P.; Tabak, F.; Akdogan, M.; Simon, K.; Akarca, U.S.; Flisiak, R.; Verhey, E.A.; et al. Early on-Treatment Prediction of Response to Peginterferon Alfa-2a for Hbeag-Negative Chronic Hepatitis B Using Hbsag and Hbv DNA Levels. Hepatology 2010, 52, 454–461. [Google Scholar] [CrossRef]
- Schwarz, C.; Chromy, D.; Bangert, C.; Schwarz, M.; Jachs, M.; Reiberger, T.; Gschwantler, M. Immediate-Type Hypersensitivity Reaction to Bulevirtide and M. Successful Desensitization in a Patient with HBV/HDV-Associated Compensated Cirrhosis. J. Hepatol. 2022, 77, 254–255. [Google Scholar] [CrossRef]
- Behrendt, P.; Traidl, S.; Böker, K.H.W.; Wedemeyer, H.; Deterding, K. T-Cell Driven Allergic Cutaneous Reaction Complicating Treatment of Hepatitis Delta Virus Infection with Bulevirtide. Liver Int. 2022, 42, 1770–1771. [Google Scholar] [CrossRef]
| Study | Phase | Treatment | Duration | End of Tx Virologic Response */** | Follow-Up |
|---|---|---|---|---|---|
| Myr202 [14] | 2 | BLV 2 mg + TDF vs. BLV 5 mg + TDF vs. BLV10 mg + TDF vs. TDF | 24 | 54% 55% 77% 0 | Not reported |
| Myr203 [13] | 2 | BLV 5 mg BID + TDF vs. BLV10 mg + PEGIFN | 48 | 40% 87% | Not reported |
| Myr204 [15] ** | 2b | PEGIFN (48 weeks) BLV 2 mg (96 weeks) + PEGIFN (48 weeks) BLV 10 mg (96 weeks) + PEGIFN (48 weeks) BLV 10 mg (96 weeks) | 48 96 96 96 | ongoing | ongoing |
| Myr301 [16] ** | 3 | BLV 10 mg (48 weeks) BLV 2 mg (144 weeks) BLV 10 mg (144 weeks) | 48 144 144 | ongoing | ongoing |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferenci, P.; Reiberger, T.; Jachs, M. Treatment of Chronic Hepatitis D with Bulevirtide—A Fight against Two Foes—An Update. Cells 2022, 11, 3531. https://doi.org/10.3390/cells11223531
Ferenci P, Reiberger T, Jachs M. Treatment of Chronic Hepatitis D with Bulevirtide—A Fight against Two Foes—An Update. Cells. 2022; 11(22):3531. https://doi.org/10.3390/cells11223531
Chicago/Turabian StyleFerenci, Peter, Thomas Reiberger, and Mathias Jachs. 2022. "Treatment of Chronic Hepatitis D with Bulevirtide—A Fight against Two Foes—An Update" Cells 11, no. 22: 3531. https://doi.org/10.3390/cells11223531
APA StyleFerenci, P., Reiberger, T., & Jachs, M. (2022). Treatment of Chronic Hepatitis D with Bulevirtide—A Fight against Two Foes—An Update. Cells, 11(22), 3531. https://doi.org/10.3390/cells11223531






