Abstract
The fetal origins of adult disease (FOAD) hypothesis holds that events during early development have a profound impact on one’s risk for the development of future adult disease. Studies from humans and animals have demonstrated that many diseases can begin in childhood and are caused by a variety of early life traumas, including maternal malnutrition, maternal disease conditions, lifestyle changes, exposure to toxins/chemicals, improper medication during pregnancy, and so on. Recently, the roles of Peroxisome proliferator-activated receptors (PPARs) in FOAD have been increasingly appreciated due to their wide variety of biological actions. PPARs are members of the nuclear hormone receptor subfamily, consisting of three distinct subtypes: PPARα, β/δ, and γ, highly expressed in the reproductive tissues. By controlling the maturation of the oocyte, ovulation, implantation of the embryo, development of the placenta, and male fertility, the PPARs play a crucial role in the transition from embryo to fetus in developing mammals. Exposure to adverse events in early life exerts a profound influence on the methylation pattern of PPARs in offspring organs, which can affect development and health throughout the life course, and even across generations. In this review, we summarize the latest research on PPARs in the area of FOAD, highlight the important role of PPARs in FOAD, and provide a potential strategy for early prevention of FOAD.
1. Introduction
In 1989, David Barker and his colleagues performed an epidemiological survey. They found that both newborn deaths and the increased risk of death from stroke and coronary heart disease in adults were related to low birth weight [1]. Later studies have confirmed that low birth weight is linked to a variety of chronic disorders, such as hypertension [2], type 2 diabetes (T2DM) [3], autoimmune thyroid disease [4], and chronic bronchitis [5]. This led to the fetal origin of adult diseases (FOAD) hypothesis that the roots of adult metabolic and cardiovascular disorders lay in the effects of malnutrition in fetal life and early infancy [6].
The FOAD hypothesis builds on the “developmental plasticity” that the organisms exhibit plastic or sensitivity in response to environmental influence during critical developmental periods to improve the match between phenotype and environment [7]. For example, a fetus will undergo the process of remodeling and altering the structure or function of various organs, which is critical for survival as well as neurodevelopment when confronted with the adversity of malnutrition [8]. However, it is important to recognize that a person’s response to environmental stimulation or pathological conditions can be limited, and such an evolutionary advantage of “plasticity” is lost over time [9]. This phenomenon called “programming” shows how early-life stimuli may lead to lifelong and irreversible changes [10]. The FOAD hypothesis attracted a lot of attention in the field of developmental plasticity.
With the expansion and deep-going of research, the recognition that “programming” occurs not only during the fetal period but also during the whole process of life development, including the early embryonic period, infancy, and early childhood [11]. FOAD hypothesis has been expanded and recognized as the Developmental Origins of Health and Diseases (DOHaD). The DOHaD theory states that the interplay between genes and environments (nutrition, stress, or environmental chemicals) from fertilization to the neonatal stage affects the disease risks related to lifestyle in later periods of life [12]. Research regarding the potential mechanisms of adverse stimuli in utero or early stage of life increases the risk of diseases later in life has been a focus of the various current animal and clinical studies [13]. One of the most exciting emerging themes in the DOHaD field is epigenetics [14]. Epigenetic mechanisms typically include DNA methylation, histone modifications, and non-coding RNAs (ncRNAs) [11]. These epigenetic modifications may have long-term consequences for gene expression and may be involved in the occurrence and/or progression of various diseases in postnatal life.
Peroxisome proliferator-activated receptors (PPARs) belong to the nuclear receptor superfamily and perform a broad range of physiological functions, including cellular development, differentiation, energy homeostasis, and metabolism [15]. Numerous studies have shed light on the involvement of the PPARs in multiple system impairments or protective effects against impairment, such as the nervous system, cardiovascular system, and metabolism system [16].
To date, three members of PPARs (PPARα, PPARβ/δ, and PPARγ) have been identified [17]. These nuclear receptors play important roles in cell differentiation, development, and reproduction [18]. All of the PPAR isoforms are identified in the rat ovary [19]. PPARα and PPARβ/δ are present primarily in the theca and stroma. PPARγ is localized mainly in the granulosa cells surrounding and supporting the oocyte meiotic maturation [20]. PPARγ expression increases from the primary/secondary follicle stage to the large follicle stage [21]. However, one study found that its expression level remains consistent during follicle development [20]. The absence of PPARα has no discernible impact on the fertility of mice, whereas the deletion of PPARγ and PPARβ/δ does [22]. Chaffin et al. held the opposite opinion; activation of PPARγ appears to play an inhibitory role in follicular growth and differentiation, according to their research [23]. The three PPAR isoforms are expressed in both somatic and germ cells in the testis [24]. Although the action of PPARs in testis development is still unclear [25], several published studies suggest that male fertility may be influenced by PPARs-regulated lipid metabolism, particularly the β-oxidation of fatty acids [25].
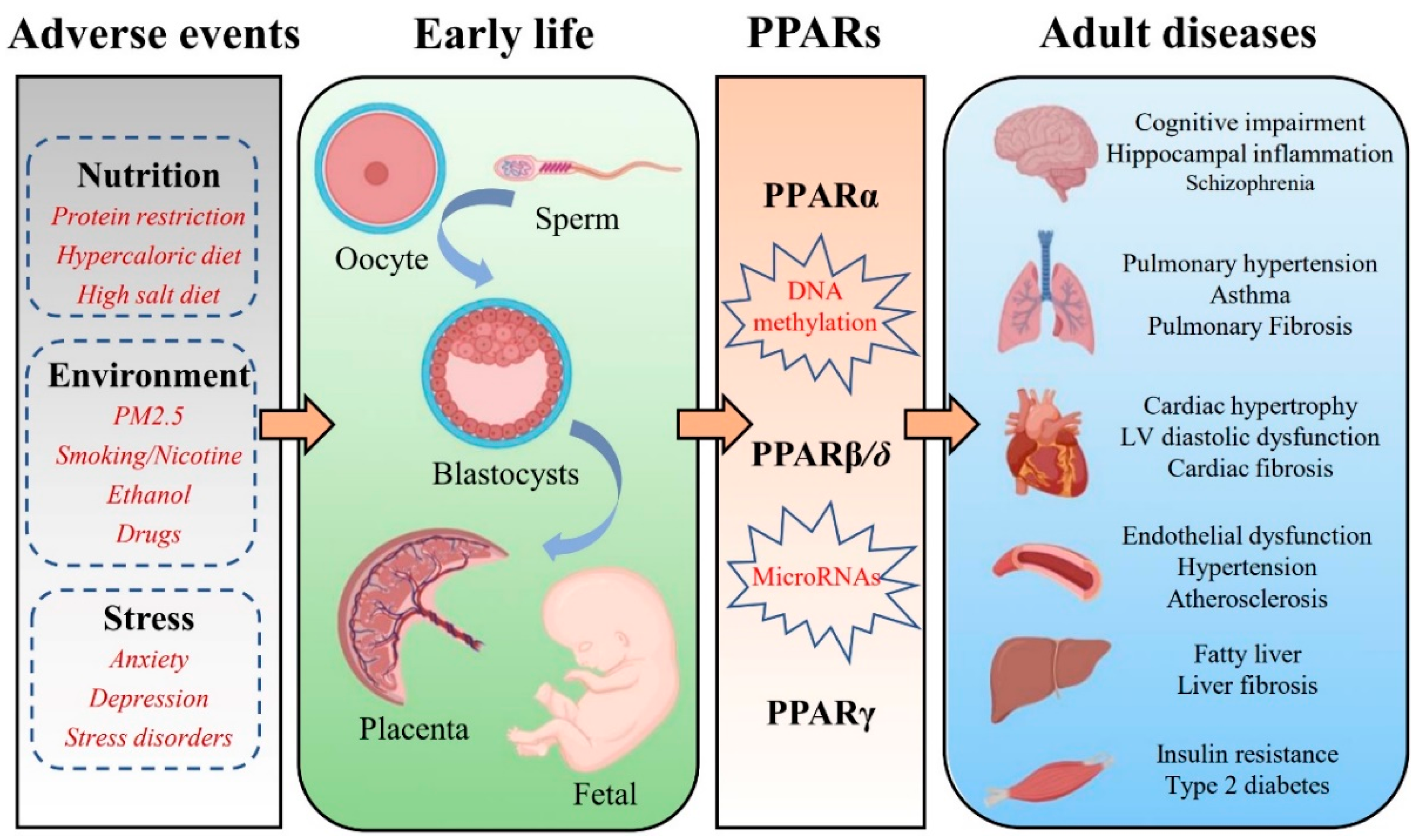
The PPARs isotypes are expressed in the placenta and play an important role in modulating embryo implantation and placental development [26]. Mutation of PPARβ/δ drastically influences placenta development and even embryonic death [27]. PPARγ is necessary for the formation of the labyrinthine layer of the placenta. Mice with deletion of the PPARγ gene exhibit defective placental vascular discourse and embryonic lethality [28]. During fetal development, the interaction of PPARα and its ligands in the liver may be important for the nutrient supply that the fetus may encounter after birth [29]. In addition, there is strong evidence that PPARβ/δ and PPARγ regulate the expression of genes involved in sarcoplasmic and adipose tissue production [30]. Remarkably, PPARs play a crucial role in metabolism during early life, and alterations in PPAR metabolic pathways could be one candidate mechanism contributing to the FOAD [31]. The recent work in developmental epigenetics has significantly expanded our understanding of this interaction. Exposure to adverse events in early life can affect the methylation pattern of PPARs in multiple organs, such as the brain, lungs, heart, blood vessels, liver, and skeletal muscles. Various chronic adult diseases, especially diabetes, cardiovascular disease, and chronic lung disease, show a clear association with PPARs (Figure 1). This review summarizes the contributions of PPARs to the potential mechanisms involved in the FOAD in order to provide a new theoretical direction for the early prevention and even treatment of fetal origin diseases.
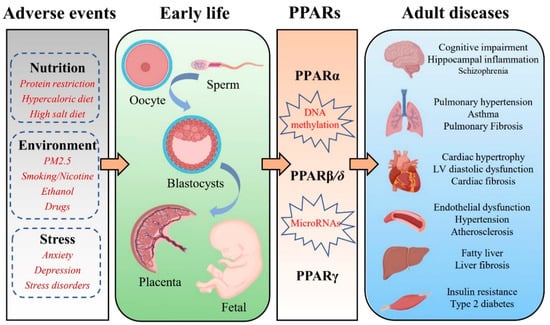
Figure 1.
Schematic illustration of potential mechanisms to support the association between early-life adverse events exposure and epigenetic regulation of PPARs that promote chronic diseases in adulthood.
2. Early Life Adverse Exposure and Future Disease Risk
Epidemiological studies in humans have demonstrated an association between the quality of the early life environment and future disease risk [32]. Animal studies provided insight into the potential mechanisms for these observations by highlighting the environmentally induced changes to epigenetic marks during development [33]. The FOAD theory goes beyond nutrition assumptions and links fetal development to many other exposure factors, such as obesity [34], prenatal maternal stress [35], and environment [36]. Notably, a growing body of research indicates that the paternal environment and dietary habits influence disease onset in offspring [37].
2.1. Nutrition
The investigations have shown a strong correlation between maternal food and nutritional status and fetal development and child health [38]. Maternal diet has often been shown to affect subsequent phenotypic [39]. Many diseases such as type 2 diabetes [40], cardiovascular disease [41], and certain cancers [42] are related to low birth weight. Early epidemiological research used data from several well-documented famines and historical cohorts [43]. The most well-known is the Dutch famine cohort. Studies on the Dutch Hunger Winter have provided convincing evidence. Prenatal exposure to famine, especially in the third trimester, has been reported to be associated with decreased glucose tolerance in adults [44]. Even if the effect on fetal growth is minimal, malnutrition in utero may result in long-term alterations in insulin-glucose metabolism [44]. Additionally, individuals whose mothers were exposed to the Dutch Famine before or during gestation were almost three times more likely to develop hypertension in adulthood than unexposed adults [43]. A study of 290 men born in East Hertfordshire during 1920–1930 showed that the risk of coronary heart disease is increased in children with low birth weight [45]. Apart from the above, the studies of the 1959–1961 mass famine in China and the 1944–1945 Dutch Hunger Winter have found a link between poor nutrition early in life and mental health and cognitive development [46].
Early research focused on evidence linking maternal protein restriction or malnutrition to the long-term health of offspring. Nowadays, a fat-rich diet is prevalent around the world, and 50% of women of childbearing age are overweight or obese in the US [47]. The impact of high-energy-dense, high-glucose, and high-salt foods during pregnancy on the phenotype of the offspring is being studied using animal models. Offspring whose mothers received these various diets showed persistent metabolic changes that were comparable to human cardio-metabolic disorders such as hypertension, insulin resistance, and obesity [48].
Obesity is also highly prevalent among adult men. The prevalence of overweight or obesity (BMI ≥ 25) is 72.1% in men and 61.2% in women, according to recent national statistics on the US population [49]. In 2000, Figueroa et al. published the first research revealing the parental influence on child health in humans [50]. They found that fathers’ total and percentage body fat were predictors of changes in body fat of premenarcheal girls during a 2.7-y period [50]. In 2010, Ng et al. reported that a high-fat diet in male rats resulted in β-cell dysfunction in F1 female offspring [51].
2.2. Environment
In modern society, humans are exposed to a wide range of environmental chemicals, such as endocrine disruptors and other toxins from lifestyle habits [52]. Numerous epidemiological studies have shown that prenatal exposure to multiple environmental pollutants has an impact on fetal development [53]. There have been many investigations that found a connection between four major environmental pollutants (perfluorinated compounds, polyhalogenated aromatic hydrocarbons, heavy metals, and air pollutants) and impaired fetal development and lower birth weight in humans [54]. A study of 1277 children from the European HELIX (Human Early Life Exposure Group) cohort reveals that BP in children may be influenced by early exposure to some substances, as well as the built environment and climatic conditions [55]. Miguel et al. summarize the influence of the early environment on the structural and functional development of children’s brains in their review [56]. For instance, most drugs of abuse (e.g., ecstasy, opiates) can readily cross the placenta and impact fetal brain development [57]. The genes associated with brain growth, myelination, and neuronal migration were down-regulated in the brain of a fetus exposed to tobacco in utero [58]. Several large population-based cohort studies have shown that prenatal exposure to maternal smoking during pregnancy or smoking cessation in early pregnancy was significantly associated with childhood ≈ [59]. Prenatal ethanol exposure (PE) impairs dopaminergic (DA) neuron function in the midbrain [60]. Air pollution can affect the anatomy and physiology of the umbilical cord and placenta [61]. Particles induce antiangiogenesis, resulting in the thinner and less voluminous umbilical cord in mouse models, which affects oxygen transport [62] and replicates in humans [63]. A meta-analysis of epidemiological studies suggests that exposure to air pollution increases the risk of pregnancy-induced hypertensive disorders [64].
A cross-sectional study of 67 men in North Carolina indicated that exposure to environmental chemicals/factors (organophosphates) could alter DNA methylation in human sperm cells, thereby affecting the health of offspring [65]. It has also been shown that human exposure to bisphenol A affects the global methylation of sperm DNA [66]. Environmental toxins also include lifestyle habits such as smoking and alcohol intake. Chronic consumption of smoking and alcohol was associated with epigenetic abnormalities and altered miRNA expression in spermatozoa [67].
2.3. Stress
In addition to physical status, the effects of altered maternal mental health and psychological stress during pregnancy on the offspring have been extensively documented in the literature. Maternal anxiety, depression, and stress disorders are common in pregnant women. A wide range of acute and chronic maternal stress exposures, such as daily hassles [68], life event stress [69], and unusual and extremely stressful events [70], have a negative impact on child development [71]. Low birth weight in infants is linked to chronic maternal stress, racism exposure, and depressive symptoms during pregnancy [72]. Accumulating research indicates that prenatal stress and depression during pregnancy are associated with cognitive and academic performance difficulties [73]. Maternal anxiety during pregnancy is associated with subsequent infant development, increased risk of behavioral/emotional disorders, and depression later in children [74]. According to electroencephalography and MRI research results, infants whose mothers had prenatal anxiety may have less volume and/or thickness in their frontal, temporal, and limbic regions and more frontal activity [75]. Natural changes in maternal care during the first day of life are associated with long-term changes in stress reactivity and hippocampal morphology and function in rodent studies [76]. These effects are mediated by epigenetic changes in the promoter of the progeny hippocampal glucocorticoid receptor gene [77]. In humans, childhood abuse was similarly associated with increased DNA methylation and decreased hippocampal glucocorticoid receptor expression [78]. Prenatal stress exposure has been linked to neurodevelopment and the risk of neuropsychiatric disorders in offspring [79]. Retrospective epidemiological studies have provided compelling evidence linking lifetime stress exposure in men with disease risk in offspring [80]. The rodent studies have demonstrated the susceptibility of germ cells to stressful environments throughout the paternal lifetime [81].
3. Peroxisome Proliferator-Activated Receptors
Peroxisome proliferator-activated receptors (PPARs) belong to nuclear hormone receptors (NRs) and ligand-activated transcription factors that regulate genes crucial for cell differentiation and a variety of metabolic processes such as glucose and lipid homeostasis. The PPAR family consists of three different isoforms: PPARα, PPARβ/δ, and PPARγ. These three isotypes have different tissue distribution, biological activity, and affinity for ligands [82]. The essential roles of PPARs in regulating mitochondrial function and energy metabolism have been clearly established. Notably, all three PPAR subtypes have overlapping and also distinct functions in regulating metabolic processes. Six functional domains (from A to F) make up the PPARs [83]. A C structural domain is present at the N-terminus of PPAR, also known as the DNA binding domain (DBD). The DNA sequence in the promoter region of genes, called the peroxisome proliferator response element (PPRE), is recognized by DBD. On the other hand, a ligand-binding domain (LBD) in the C-terminus is responsible for the specificity of the ligand and dimerization of the receptor with the retinoid X receptors (RXR) [84]. PPARs translocate to the nucleus after interacting with specific ligands (synthetic or non-synthetic) [85]. PPARs interact with RXR, peroxisome proliferator-activated receptor gamma-coactivators (PGC), steroid receptor coactivators, and CREB binding protein (CBP/p300) after translocating to the nucleus, then bind to the sequences of PPRE, which subsequently initiate the transcription of target genes involved in different physiological processes [86]. The target genes are involved primarily in the metabolism of fat, as well as in cellular proliferation and differentiation, protein and glucose, inflammation, and tumorigenesis [86]. Their aberrant expression is related to a variety of disorders, such as neurodegenerative disorders, cardiovascular disease, obesity, type 2 diabetes, pancreatic cancer, and so on [16].
Given their central roles in regulating metabolic flexibility, it is essential to understand the manner in which PPARs regulate gene expression. The function of PPARs is principally modulated by ligand binding, which induces structural changes, further recruiting co-activator or co-repressor complexes, which stimulate or inhibit their functions [87]. In addition to ligand binding, post-translational modifications of PPARs are emerging as one such way PPARs are regulated, including phosphorylation, ubiquitination, SUMOylation, acetylation, and O-GlcNAcylation, which contribute to fine-tuning of the transcriptional activities [88]. Recent studies have suggested that post-translational modifications are observed in all three PPAR isoforms [87,88]. A detailed view of the functional regulation of PPARs through post-translational modifications can be found in a recent review by Xu et al. [87].
3.1. PPARα
PPARα is known to be important for regulating the transcriptional expression of key enzymes that are involved in mitochondrial dynamics and metabolic functions, including glucose metabolism, fatty acids β-oxidation, and fatty acid transport [89]. Moreover, PPARα receptors are found largely in metabolically active tissues, such as brown adipose, skeletal muscle, heart, liver, and intestinal mucosa tissues. Natural ligands for the PPAR receptor include saturated, monounsaturated, and polyunsaturated fatty acids and their metabolites, such as leukotrienes B4, oxidized phospholipids, lipolytic lipoprotein products, etc. Nature ligands bind to PPARα and activate PPAR-responsive genes, increasing hepatic intracellular fatty acid absorption [90]. PPARα also plays an important role in extracellular lipid homeostasis by modulating the transcriptional regulation of major very-low-density and high-density apolipoproteins [91]. Furthermore, PPARα seems to modulate the bioactivity of leptin in the liver and adipose tissue [92].
The transcriptional activity of PPARα is enhanced by binding to the ligands, after which transcriptional coactivators contribute to the activation of target genes [93]. In addition, PPARα trans-activity is regulated by post-translational modifications such as phosphorylation, SUMOylation, and ubiquitination. As a phosphoprotein, PPARα is phosphorylated exclusively on serine residues in vivo [94]. It was reported that treatment with insulin or ciprofibrate (a PPARα agonist) increased the phosphorylation of PPARα [95,96]. SUMOylation is a reversible post-translational modification that has been established as one of the key regulatory protein modifications in eukaryotic cells. Two lysine residues of PPARα, K185, and K358, have been reported to be modified by SUMOylation [97,98]. Moreover, several studies have shown that the ubiquitin–proteasome system is involved in the regulation of PPARα activity. These studies suggest the ubiquitination of PPARα in a ligand-dependent manner, and that effect of ubiquitination on PPARα activity depends on the systems studied [87,88].
3.2. PPARβ/δ
PPARβ/δ is generally expressed in nearly all tissues, such as the brain, skin, liver, skeletal muscle, heart, and various types of cancer [99]. Polyunsaturated fatty acids (arachidonic and linoleic acids) and their metabolites, such as prostacyclin PGI2, are suggested as natural ligands. Similar to the other PPAR family members, it mainly participates in the oxidation of fatty acids and affects lipid metabolism, both reducing fat and hence preventing the development of obesity and controlling blood sugar and cholesterol levels in the heart and skeletal muscle [100]. Overall, PPARβ/δ has significant functional overlap with PPARα in most tissues. For example, PPARβ/δ also stimulates fatty acid oxidation in muscle and heart [101]. However, PPARβ/δ and PPARα carry out different roles in regulating hepatic energy metabolism. Unlike PPARα, PPARβ/δ regulates gene expression associated with lipogenesis and glucose utilization rather than inducing fatty acid oxidation [102]. Additionally, several investigations have shown a large expression of PPARβ/δ in the central nervous system (CNS) [103]. PPARβ/δ may affect the differentiation of neural and glial cells and alter cholesterol metabolism in the brain. It is well known that PPARs regulate inflammatory processes associated with lipid signaling pathways [104]. Inhibiting inflammatory processes in the CNS may reduce brain damage and enhance motor and cognitive outcomes [105]. Comparatively, PPARβ/δ is the least reported PPAR family member in terms of a post-translational modification. To date, we are aware of only one previous study showing that SUMOization of PPARβ/δ at K104 is removed by SENP2 and promotes FAO gene expression in muscle [106].
3.3. PPARγ
PPARγ is widely expressed in brown and white adipose tissue, spleen, and large intestine. PPARγ has two isoforms in mice and four different isoforms in humans [107]. Unsaturated fatty acids and their metabolites are the primary natural modulators of PPARγ. Activated PPARγ by these natural ligands regulates adipogenesis and fat distribution, the levels of adipokines such as adiponectin, which involve insulin sensitivity and lipid and glucose metabolism [108]. PPARγ are ligand-inducible transcription factors involved in regulating the expression of genes, including glucose sensitivity (IRS-1, IRS-2, GLUT-4, and PI3K), fatty acid uptake and mobilization (FAT/CD36, FABPs, and LPL) and triglyceride synthesis (ACSL, GK, and PEPCK) [102]. In addition, PPARγ also induces the expression of mitochondrial proteins, such as CPT-1 and UCPs, which play an important role in the regulation of mitochondrial metabolism. PPARγ is associated with the pathology of many diseases, such as obesity, atherosclerosis, diabetes, and cancer. PPARγ agonists, including troglitazone, rosiglitazone, ciglitazone, and pioglitazone, have been used in the treatment of hyperlipidemia and hyperglycemia [109]. The role of PPARγ in cancer initiation/progression is contradictory. Numerous studies show that PPARγ has a tumor-promoting effect. Conversely, some literature has reported that PPARγ plays a key role in tumorigenesis as a tumor suppressor. PPARγ activation by many agonists has been demonstrated to have antiproliferative and proapoptotic actions in prostate, thyroid, and lung cancers [110].
Thiazolidinediones, such as rosiglitazone, pioglitazone, and lobeglitazone, are PPARγ agonists that modulate the transcriptional activity of PPARγ. Like PPARα, PPARγ activity is also regulated by post-translational modifications. PPARγ is now known to be phosphorylated upon stimulation of the MAPK activation pathway [87]. A variety of stimuli (growth factors, platelet-derived growth factors, transforming growth factor beta, insulin, and prostaglandin F2 alpha, etc.) can activate PPARγ phosphorylation via specific activation of MAPKs [87]. Moreover, PPARγ is regulated by SUMO1 and SUMO2 sumoylation. The targeted lysine residue was identified as K107, K33, K64, K68, and K77, respectively [111,112]. In addition to this, other post-translational modifications of PPARγ, such as acetylation, ubiquitination, and O-GlcNAcylation, have also been reviewed by Xu et al. [87].
4. Effects of PPARs in the Placenta and the Fetus
During pregnancy, the placental metabolism can adapt to the environment throughout pregnancy to adapt to the maternal nutritional status and meet the demands of the fetus [31]. All three PPAR isoforms are expressed in the placenta [26,113]. The PPARs promote placental developmental plasticity by regulating lipid, hormone, and glucose metabolic pathways, including lipidogenesis, steroidogenesis, glucose transporters, and placental signaling pathways [114]. Although the role of each PPAR in placental function has not been fully determined, unique and common functions between these isoforms have been observed. Among the PPAR-isoforms, PPARγ appears to be a major regulator of the mammalian placenta [115]. PPARγ was the first to be demonstrated in the placenta [116]. In rodent placenta, PPARγ is largely expressed in the trophoblastic layer of the labyrinth zone [117,118]. In human placenta, PPARγ is present in villous trophoblast and extravillous trophoblast [119,120]. There is some evidence suggesting that PPARγ modulates villous trophoblast differentiation, oxidative pathways, inflammatory response, and barrier formation [121,122]. Furthermore, dysregulation of both PPARα and PPARγ in the placenta has been implicated in common complications of pregnancy, such as gestational diabetes mellitus, intrauterine growth restriction, and preeclampsia [123]. Their expression pattern is regulated at least partially by DNA methylation in the placenta, and the involvement of other PPAR-regulated processes, such as placenta-specific miRNAs, has just been discovered [124]. Placental epigenetic regulation appears to provide a plausible connection between environmental exposures and fetal development. Studies have shown that changes in placental DNA methylation patterns have been associated with fetal growth after exposure to maternal risk conditions such as GDM, obesity, and preeclampsia [125,126].
During the development of the human embryo and fetus, three isoforms are expressed in cells of the endoderm and mesoderm at early time points in gestation [127]. PPARβ/δ was the first allotype to be expressed during embryonic development in rodents [128]. PPARα and PPARγ are expressed first in the placenta and then in the fetus [128,129]. The role of PPAR in development has been revealed by studies in PPAR knockout mice [130]. The important role of PPARα in lipid catabolism in the fetal liver and heart is consistent with the function of PPARα in adult tissues [131,132,133]. Knockout of PPARα in mice causes a high miscarriage rate, hepatic lipid accumulation, obesity, and prolonged inflammation [134,135]. In the early stages of organogenesis in the rat embryo, only the PPARβ/δ isotype is expressed [128] and plays an important role in the closure of the neural tube [136]. The signaling pathway involved in PPARβ/δ activation associated with nervous system development is profoundly altered by maternal diabetes [136]. PPARγ null mutations are lethal. The developmental defects in the placenta occur in parallel to developmental defects in the embryo [137]. In fetuses from diabetic rats, the concentration of PPARγ endogenous is reduced [138]. The capacity of PPARγ endogenous to prevent the overproduction of both NO and MMPs in fetuses from diabetic rats demonstrates its anti-inflammatory effects [138]. PPARγ activation increases lipid concentrations in midgestation fetuses from diabetic rats [139]. Collectively, these data indicate that PPARs-mediated mechanisms are involved in the fetal origins of health and disease.
5. PPARs and FOAD
It is now well recognized that adverse events exposure in early life contribute to the development of the chronic diseases of adulthood, including hypertension, type 2 diabetes, stroke, cognitive impairment, and pulmonary hypertension. Additionally, the role of PPARs in numerous chronic diseases such as diabetes, cardiovascular diseases, autoimmune diseases, chronic fatigue, depression, and neurodegenerative diseases is well established. PPARs are ubiquitously expressed in almost all mammalian tissues and organs. Altering PPARs methylation patterns during early development may be maintained throughout the life course and even across generations [31]. In the following sections, we review the expression pattern of PPARs in various organs, including the brain, lung, heart, vessel, liver, and skeletal muscle, and discuss the potential roles of PPARs in FOAD (Table 1).

Table 1.
Summary of studies on PPARs in the fetal origins of adult disease.
5.1. Brain
PPARα is expressed in several regions of the central nervous system (CNS), and its specific biological function remains unclear [171]. Various inflammatory parameters were significantly enhanced in PPARα KO mice [172]. Neuroinflammation is considered a cause and/or contributing factor to neuronal degeneration [173]. It suggests that PPARα attenuates the inflammatory response after ischemia/brain injury [174]. Moreover, the activation of PPARα has anti-inflammatory properties and a beneficial impact on certain neurologic diseases, including Alzheimer’s disease (AD) [175], Multiple sclerosis (MS) [176], Huntington’s disease (HD) [177], and Parkinson’s disease (PD) [178]. Malnutrition during pregnancy affects sleep homeostasis and increases sleep pressure in offspring, which may be related to the increased PPARα mRNA expression in the hypothalamus [140]. In a study by Felice et al., it was found that prenatal administration of fenofibrate (PPARα agonist) reduced the risk of schizophrenia-like behavior in male offspring of maternal immune activation (MIA) and emphasizes PPARα as a possible target for schizophrenia therapies [141].
Although PPARβ/δ is the most abundant PPAR subtype in the CNS, its role is rarely studied [171]. It has been suggested that the most important roles of PPARβ/δ in brain cells are antioxidant and anti-inflammatory effects [179]. There also a study identified that the differentiation of neural and glial cells might be impacted by PPARβ/δ, which may also affect the metabolism of cholesterol in the brain [103]. One study found that prenatal exposure to a high-fat diet increased the density of cells immunoreactive for PPARβ/δ in the hypothalamic paraventricular nucleus, perifornical lateral hypothalamus, and central nucleus of the amygdala [180]. However, the clinical significance of this change and the potential role of PPARβ/δ in fetal origins of CNS diseases remains unclear.
PPARγ is the most studied subgroup of the PPAR family and has an important role in the CNS, including relieving endoplasmic reticulum stress and the inflammatory response [181], the balance of cerebral metabolite [182] and the maintenance of glucose homeostasis [183]. Animal studies have demonstrated that maternal vitamin D deficiency leads to decreased PPARγ levels in the offspring’s brain and affects angiogenesis in the brain [142]. Fetal hippocampal inflammation is significantly increased in immune-activated mothers, followed by cognitive deficits, which are highly correlated with hippocampal neurogenesis disorders in pre-pubertal male offspring. The PPARγ agonist pioglitazone improves neurogenesis, cognitive impairment, and anxious behavior in MIA offspring [143]. Maternal high-fructose-induced hippocampal neuroinflammation in the adult female offspring. Adult female offspring exposed to high maternal fructose have decreased levels of PPARγ and endogenous antioxidant expression in the hippocampus, which leads to hippocampal neuroinflammation. An oral dose of pioglitazone (PPARγ agonist) effectively increases the expression of antioxidants and blocks neuroinflammation [144]. Based on the findings described above, synthetic PPARγ agonists have been suggested as therapeutic medicines for the treatment of CNS diseases such as PD [184], AD [185], HD, and Autism spectrum disorder [186].
5.2. Lung
PPARα has been implicated in the control of airway inflammation, but as yet, little is known about its role in lung disease. There is a mouse model of pulmonary fibrosis suggesting that PPARα regulates fibrosis [187]. A study by Genovese et al. revealed that endogenous and exogenous PPARα ligands reduced bleomycin-induced lung injury in mice [188]. Liu et al. found that the activity of PPARα was inhibited in lipopolysaccharide (LPS) induced acute lung injury (ALI) [189]. By reducing oxidative stress and inflammation, which are both directly related to the activation of PPARα, eupatilin has a protective function in ALI [190]. Taken together, they proposed that PPARα could be a potential therapeutic target for lung injury.
PPARβ/δ agonist inhibited the proliferation of lung fibroblasts and enhanced the antifibrotic properties of PPARγ agonist [187]. The role of PPARβ/δ in pulmonary hypertension and lung cancer has received attention in recent years. According to epidemiological and experimental animal studies, prenatal hypoxia, intrauterine growth restriction (IUGR), and obesity raise the risk of pulmonary hypertension in offspring [191]. Prostacyclin and prostacyclin mimetics are the cornerstone of treatment for patients with pulmonary arterial hypertension (PAH) [192]. One study suggests that PPARβ/δ may be a potent target for prostacyclin mimics in the treatment of pulmonary hypertension. PPARβ/δ agonist (GW0742) mediates vascular relaxation and prevents the right heart from hypertrophy associated with pulmonary arterial hypertension [193]. The role of PPARβ/δ in the negative growth regulation of lung cancer cells was first reported in an in vitro study [194]. Using a variety of lung cancer models, one research group demonstrated that increased synthesis of the PPARβ/δ agonist (prostacyclin) inhibited lung tumorigenesis [195]. These findings imply that PPARβ/δ may play a protective function in PAH and lung cancer.
PPARγ is expressed in many lung cells, including bronchial epithelial cells, airway smooth muscle (HASM) cells, fibroblasts, alveolar type II pneumocytes, and mononuclear phagocytes [187,196]. The activation of PPARγ signaling is involved in the paracrine effect of interstitial fibroblasts and alveolar type II (ATII) cells, which is necessary to maintain alveolar homeostasis [197]. The PPARγ gene depends on developmentally specific transcription of mRNA variants and epigenetics for normal tissue. Therefore, it is susceptible to epigenetic changes [198]. There is evidence that perinatal damage, including exposure to nicotine or maternal tobacco smoke (MTS), IUGR, and preterm delivery, altered both epigenetic determinants and gene expression in the lung [198]. It has been demonstrated that IUGR caused epigenetic modifications to the PPARγ gene in rat lungs [199]. The levels of PPARγ mRNA variants, PPARγ protein, and downstream targets were decreased in the lung of neonatal rats [149]. Numerous studies have shown an increase in asthma in offspring whose mothers smoked during pregnancy [200]. Perinatal smoke/nicotine exposure is a recognized factor that affects lung growth and differentiation by down-regulating the expression of PPARγ [201]. Downregulation of PPARγ causes lipid-rich alveolar mesenchymal fibroblasts to transdifferentiate into myofibroblasts, which is the cellular hallmark of chronic lung diseases such as asthma [202,203]. PPARγ agonist (rosiglitazone) can effectively block asthma induced by perinatal smoke exposure [148].
5.3. Heart
PPARs are the physiological master switches of the heart, which guide the energy metabolism of cardiomyocytes, thereby influencing pathological heart failure and diabetic cardiomyopathy [204]. However, the roles of PPARs in heart function and the results of their respective agonists differ greatly in preclinical animal models and clinical studies [205]. PPARα is highly expressed in the heart and can affect the expression of numerous genes implicated in the uptake and oxidation of cellular fatty acid (FA) [206]. Therefore, it plays a major role in cardiac fatty acid homeostasis. Down-regulation of PPARα expression altered the expression of fatty acid-metabolizing proteins that lead to myocardial damage and fibrosis [207]. The expression of fetal cardiac lipid metabolism genes (PPARα, fatty acid translocase, lipoprotein lipase, etc.) was reduced in offspring from mothers with high blood glucose levels, not accompanied by cardiac triglyceride deposition or cardiac hypertrophy [132]. However, it has subsequently been suggested that the heart of adult offspring from diabetic rats showed increased lipid concentrations. The increased expression of PPARα in offspring from diabetic rats can prevent toxic lipid accumulation in the heart [208]. There is also solid evidence that PPARα exerts a protective effect on cellular oxidative damage [209]. Thus, chronic deactivation of the PPARα signaling pathway may disrupt the balance between oxidant production and antioxidant defenses and ultimately contribute to heart damage [210]. In the 2-day-old and pre-pubertal stage progeny from diabetic rats, there was an increase in the expression of prooxidative/proinflammatory markers and PPARα protein expression in the hearts. Maternal treatment with mitochondrial antioxidants led to reductions in PPARα protein expression and pro-oxidant/ro-inflammatory markers and prevented the adverse programming of heart alterations in prepubertal offspring from diabetic rats [151]. Both neonatal and adult hearts from the offspring of maternal protein restriction (PR) during pregnancy showed a reduction in the level of PPARα promoter methylation and an increase in PPARα mRNA expression [150]. The possible implication of these findings is that the enhanced capacity of fatty acid β-oxidation leads to an increased risk of oxidative damage to offspring hearts.
PPARγ is expressed at very low levels in the adult heart [211]. PPARγ activation in cardiomyocytes is associated with impaired cardiac function due to its lipogenic effect [211]. Maternal obesity leads to cardiac hypertrophy, and left ventricular diastolic dysfunction in offspring might be related to persistent upregulation of PPARγ expression [152]. In rat offspring programmed by the reduced protein diet during gestation, the PPARγ agonist (rosiglitazone) was shown to have beneficial effects by reducing cardiac fibrosis and enhancing myocardial vascularization [153]. PPARγ activator therapy has a beneficial impact on risk factors for cardiovascular disease, but it also appears to have adverse effects on the cardiovascular system. It has been reported that treatment with rosiglitazone is associated with an increase in myocardial infarction (MI) or heart failure in humans [212].
5.4. Vessel
Studies have shown that PPARs are present in all essential vascular cells, including monocyte-macrophages, endothelial cells, and vascular smooth muscle cells [213]. PPARs influence lipid metabolism and vascular diseases such as atherosclerosis and hypertension [214]. PPARα has been implicated in blood pressure regulation and vascular inflammation [215]. PPARα was expressed in both vascular endothelial cells and vascular smooth muscle cells [216]. Activation of PPARα blocks multiple pathways such as NF-κB and MAPK, which in turn inhibit the expression of many genes involved in vascular inflammation, oxidative stress, and cell growth and migration [217]. In experimental hypertension models, PPARα ligands can reportedly lower blood pressure [218]. PPARα was also associated with atherosclerotic processes [219]. The administration of the fibrate class of PPARα agonists to patients with type 2 diabetes or dyslipidemia significantly slowed the development of atherosclerosis and reduced their risk of cardiovascular events [220], but surprisingly, high-fat diet PPARα-null mice are more responsive to insulin, have lower blood pressures, and develop less atherosclerosis [219].
Activation of PPARβ/δ has a significant effect on anti-hypertension [221]. However, it is argued that PPARβ/δ agonist acts via interference with the ET-1 signaling and lower blood pressure through a PPARβ/δ-independent mechanism [222]. Moreover, the reduction of vascular oxidative stress markers and improvement of endothelial dysfunction were observed after a high dose of the PPARβ/δ antagonist GSK0660 [223]. It has been shown that the offspring of rats with maternal diabetes have abnormal fetal programming of vascular endothelial function, which is linked to increased ER stress and may be attributed to the down-regulation of the AMPK/PPAR signaling cascade [224].
Whether PPARγ is hypotensive or hypertensive is still under debate so far [225]. Genetic studies showed impaired vascular smooth muscle contraction in response to alpha-adrenergic drugs and hypotension in a generalized PPARγ knockout mouse model [226], which is very well in agreement with the findings by Tontonoz [227]. These findings suggest that PPARγ has a hypertensive function in controlling blood pressure. However, activation of PPARγ has beneficial effects on hypertension in a number of animal and human studies [228]. PPARγ activation may regulate blood pressure via modulating endothelial vasoactive factors such as prostacyclin, nitric oxide, and endothelin-1. Additionally, PPARγ may also be involved in vessel tone regulation by down-regulating ANG II receptor 1 (AT1-R) in vascular smooth muscle cells [229]. Angiotensin II-induced endothelial dysfunction in adult offspring of pregnancy complicated with hypertension is associated with impaired endothelial PPARγ [155]. Rosiglitazone (a PPARγ agonist) reduced blood pressure and attenuated vascular remodeling in perinatal low-protein offspring rats [156]. Chronic treatment with rosiglitazone has also been shown to prevent impaired nitric oxide synthase-dependent responses induced by prenatal alcohol exposure [230]. Collectively, it is widely believed that activation of PPARγ can moderately lower blood pressure and plays a protective role in endothelial dysfunction, vascular inflammation, and other pathological processes that lead to atherosclerosis [231].
5.5. Liver
The liver is a major organ that regulates whole-body nutrient and energy homeostasis. PPARs are involved in the regulation of adipogenesis, lipid metabolism, inflammation, and metabolic homeostasis [232]. PPARα is a major regulator of lipid metabolism in the liver, especially at fasting. In addition to fatty acid oxidation and ketogenesis, PPARα controls the expression of almost all genes involved in lipid metabolism in the liver [233]. Free fatty acids and other lipids are known to activate PPARα to increase lipid clearance in the liver [234]. In the liver of the PPARα-null mice (lacking the PPARα gene), constitutive mitochondrial β-oxidative activity was significantly reduced [235]. Polyunsaturated fatty Acids (PUFAs) are endogenous PPARα activators. Mice on a high-fat diet supplemented with PUFAs showed enhanced hepatic FA β-oxidation and ameliorated fatty liver [236]. Maternal exposure to perfluorooctanoic acid (PFOA) significantly decreased the expression of the PPARα gene in female offspring mice, leading to reduced fatty acid oxidation and histone acetylation and increased liver oxidative stress [157]. Other authors have found a lower expression of PPARα in the liver of rat offspring exposed to vitamin B12 deficient diets before and during pregnancy due to increased global methylation levels. [237]. The offspring born to an obese mother has a greater likelihood of progression to the fatty liver, which may be associated with PPARα dysfunction [238]. Similar works showed that a high-fat diet during pregnancy impairs the demethylation of PPARα, therefore inducing lipid metabolism disorders and obesity in offspring [161]. Maternal high-fat diet decreased the expression of PPARα and genes for fatty acid oxidation, which contributes to nonalcoholic fatty liver disease (NAFLD) in offspring [158]. Prenatal 1,2-Cyclohexane dicarboxylic acid diisononyl ester (DINCH) plasticizer exposure downregulates PPARα expression, which, in turn, affects the liver function of offspring [239]. Maternal nicotine exposure leads to lipid metabolism disorders and insulin resistance by activating PI3K/Akt signaling, inhibiting PPARα protein expression, and promoting the progression of MAFLD in adult offspring [159].
PPARγ is expressed at much lower levels in the liver and muscle than in adipose tissue and macrophages [240]. Many studies have demonstrated a link between elevated PPARγ expression and hepatic steatosis [241]. Specific disruption of liver PPARγ in mice can effectively improve fatty liver [242]. Overexpression of PPARγ in mouse liver can lead to the development of adipogenic hepatic steatosis [243]. Activation of PPARγ is steatogenic. Paradoxically, treatment of PPARγ-null mice with PPARγ ligands protects other tissues from TAG accumulation and insulin resistance [244]. In A-ZIP/F-1 mice, disrupting hepatocyte PPARγ reduced hepatic steatosis but worsened hyperlipidemia and muscle insulin resistance [244]. PPARγ has anti-inflammatory effects; PPARγ activation decreases inflammatory response by negatively interfering with NF-κB and signal transducers and transcriptional activators [245]. PPARγ agonists may have potential in the prevention of liver fibrosis/cirrhosis [246]. The NAFLD induced by gestational BPA exposure in male offspring may be related to the dysregulation of the HNF1b/PPARγ pathway [165]. Co-agonists of PPARα and PPARγ attenuated liver and white adipose tissue inflammation in male offspring of obese mothers [247]. The reduction of PPARγ level plays a crucial role in arsenic-induced hepatic autophagy in progeny [248]. Metabolic and reproductive disturbances in the female offspring of polycystic ovary syndrome may be associated with the upregulation of PPARγ in the liver [249]. Prenatal exposure to a low-protein diet exhibited a lower expression of PPARγ and hepatic steatosis [250].
5.6. Skeletal Muscle
Skeletal muscle is a metabolic organ that accounts for 40% of the total body weight in a healthy person. It produces adenosine triphosphate (ATP) through insulin-mediated glucose uptake, stores excess glucose as glycogen, and is involved in fatty acid oxidation. All three PPAR isotypes have significant effects on muscle homeostasis, either directly or indirectly. PPARα participates in glucose metabolism and fatty acid catabolism, which is crucial in regulating inflammation and energy expenditure [251]. PPARβ/δ is the major PPAR isotype in skeletal muscle. It is involved in lipid and glucose metabolism, energy expenditure, inflammation, tissue repair and regeneration, and muscle fiber type switching associated with physical exercise [252]. One of the main functions of PPARγ in skeletal muscle is fat deposition [253]. Several observations suggest that PPAR isotypes are at least partially related and overlapping in muscle.
Maternal protein restriction impaired the expression of genes that increased the ability to oxidize fat in response to fasting and exhibited an enhanced expression of PPARα in adult offspring [250]. The study by Zhou et al. showed that miR-29a was upregulated in the skeletal muscles of IUGR offspring. The direct interaction between miR-29a and PPARβ/δ inhibited the expression of PPARβ/δ, which was associated with the progression of insulin resistance (IR) [167]. The reduced mitochondrial content in the muscle of IR offspring may be in part due to decreased PPARβ/δ activation [168]. Maternal cafeteria diet during gestation and lactation Maternal cafeteria diets during pregnancy and lactation were associated with the increased PPARγ mRNA level in pups [170]. The adult mice suffered from maternal caloric restriction during late pregnancy, and a post-weaning high-fat diet, the expressions of PPARγ in their skeletal muscle tissue were significantly increased [169]. PPARγ agonist can improve skeletal muscle insulin sensitivity in the pregestational intrauterine growth-restricted rat offspring [254]. In conclusion, skeletal muscle insulin resistance and impaired fat or glucose metabolism may be closely related to PPARs changes in offspring exposed to adverse factors during pregnancy.
6. Conclusions and Outlook
The concept of FOAD (or DOHaD) has provided new insights into the origin of lifestyle diseases, and the field of FOAD has grown rapidly to high prominence in biomedical science and public health. This review is concerned with understanding how stressful environmental conditions during sensitive periods of early development influence the risk of chronic disease later in life, particularly the role of PPARs in this process. Notably, agonists of PPARs have been intensively evaluated as a potential strategy for the early prevention of FOAD. A growing body of exciting evidence demonstrates that PPAR activators reverse some of the adverse effects of adverse exposure during pregnancy on offspring. These data provide important proof that the epigenetic state of a particular gene can be modified. It provides a novel therapeutic strategy to prevent or delay the fetal origin of adult diseases through epigenetic regulation of metabolic genes. Here, we briefly summarize the relevant studies in Table 2. Nevertheless, studies on PPARs in the area of FOAD are currently in the nascent stage, especially the application of PPARs agonists in the primary prevention and treatment of FOAD remains controversial. Therefore, further research is necessary to enhance our understanding of the PPAR-mediated mechanisms involved in the fetal origins of health and disease. Connecting early-life adverse events exposures and PPARs epigenomic measures with later-life health outcomes is a proven strategy for investigating such underlying mechanisms. Recent research has begun to identify features of the PPARs-related regulation of non-coding RNAs, histone modification, and DNA methylation in FOAD. These advances drive the development of the complex transcriptional and epigenetic regulation of PPARs in FOAD. We believe that the studies of such new perspectives will open up new avenues in FOAD research, as well as potential strategies for early prevention of FOAD.

Table 2.
Summary of studies on PPAR agonists in the fetal origins of adult disease.
Author Contributions
Conceptualization: Y.L., J.G. and J.W.; Writing—original draft preparation: J.G., J.W., Q.H. and M.Z.; writing—review and editing: J.G., H.L. and Y.L.; supervision: H.L. and Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Special Scientific Research Cultivation of Gusu School of Nanjing Medical University (GSKY20220523, Szslyyrc2022003), the Suzhou introduce expert team of clinical medicine (SZYJTD201708).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barker, D.J.; Osmond, C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1986, 1, 1077–1081. [Google Scholar] [CrossRef]
- Barker, D.J.; Osmond, C. Low birth weight and hypertension. BMJ 1988, 297, 134–135. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.N.; Barker, D.J. Type 2 (non-insulin-dependent) diabetes mellitus: The thrifty phenotype hypothesis. Diabetologia 1992, 35, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.I.; Cooper, C.; Fall, C.; Prentice, L.; Osmond, C.; Barker, D.J.; Rees Smith, B. Fetal growth and autoimmune thyroid disease. Q. J. Med. 1993, 86, 247–253. [Google Scholar]
- Barker, D.J.; Osmond, C.; Law, C.M. The intrauterine and early postnatal origins of cardiovascular disease and chronic bronchitis. J. Epidemiol. Community Health 1989, 43, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Bateson, P.; Barker, D.; Clutton-Brock, T.; Deb, D.; D’Udine, B.; Foley, R.A.; Gluckman, P.; Godfrey, K.; Kirkwood, T.; Lahr, M.M.; et al. Developmental plasticity and human health. Nature 2004, 430, 419–421. [Google Scholar] [CrossRef]
- Calkins, K.; Devaskar, S.U. Fetal origins of adult disease. Curr. Probl. Pediatric Adolesc. Health Care 2011, 41, 158–176. [Google Scholar] [CrossRef]
- Hochberg, Z.; Feil, R.; Constancia, M.; Fraga, M.; Junien, C.; Carel, J.C.; Boileau, P.; Le Bouc, Y.; Deal, C.L.; Lillycrop, K.; et al. Child health, developmental plasticity, and epigenetic programming. Endocr. Rev. 2011, 32, 159–224. [Google Scholar] [CrossRef]
- Koletzko, B. Developmental origins of adult disease: Barker’s or Dorner’s hypothesis? Am. J. Hum. Biol. 2005, 17, 381–382. [Google Scholar] [CrossRef]
- Hoffman, D.J.; Reynolds, R.M.; Hardy, D.B. Developmental origins of health and disease: Current knowledge and potential mechanisms. Nutr. Rev. 2017, 75, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Arima, Y.; Fukuoka, H. Developmental origins of health and disease theory in cardiology. J. Cardiol. 2020, 76, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Krontira, A.C.; Cruceanu, C.; Binder, E.B. Glucocorticoids as Mediators of Adverse Outcomes of Prenatal Stress. Trends Neurosci. 2020, 43, 394–405. [Google Scholar] [CrossRef]
- Bird, A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002, 16, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.S.; Tan, W.R.; Low, Z.S.; Marvalim, C.; Lee, J.Y.H.; Tan, N.S. Exploration and Development of PPAR Modulators in Health and Disease: An Update of Clinical Evidence. Int. J. Mol. Sci. 2019, 20, 5055. [Google Scholar] [CrossRef]
- Wójtowicz, S.; Strosznajder, A.K.; Jeżyna, M.; Strosznajder, J.B. The Novel Role of PPARα in the Brain: Promising Target in Therapy of Alzheimer’s Disease and Other Neurodegenerative Disorders. Neurochem. Res. 2020, 45, 972–988. [Google Scholar] [CrossRef]
- Han, S.W.; Roman, J. Anticancer actions of PPARγ ligands: Current state and future perspectives in human lung cancer. World J. Biol. Chem. 2010, 1, 31–40. [Google Scholar] [CrossRef]
- Jia, Z.; Xinhua, X.; Qian, Z.; Miao, Y.; Jianping, X.; Zhixin, W.; Yijing, L.; Mingmin, L. PPARγ links maternal malnutrition and abnormal glucose and lipid metabolism in the offspring of mice. Yi Chuan 2015, 37, 70–76. [Google Scholar] [CrossRef]
- Braissant, O.; Foufelle, F.; Scotto, C.; Dauça, M.; Wahli, W. Differential expression of peroxisome proliferator-activated receptors (PPARs): Tissue distribution of PPAR-α, -β, and -γ in the adult rat. Endocrinology 1996, 137, 354–366. [Google Scholar] [CrossRef]
- Komar, C.M.; Braissant, O.; Wahli, W.; Curry, T.E., Jr. Expression and localization of PPARs in the rat ovary during follicular development and the periovulatory period. Endocrinology 2001, 142, 4831–4838. [Google Scholar] [CrossRef]
- Froment, P.; Fabre, S.; Dupont, J.; Pisselet, C.; Chesneau, D.; Staels, B.; Monget, P. Expression and functional role of peroxisome proliferator-activated receptor-gamma in ovarian folliculogenesis in the sheep. Biol. Reprod. 2003, 69, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Miyoshi, K.; Claudio, E.; Siebenlist, U.K.; Gonzalez, F.J.; Flaws, J.; Wagner, K.U.; Hennighausen, L. Loss of the peroxisome proliferation-activated receptor gamma (PPARγ) does not affect mammary development and propensity for tumor formation but leads to reduced fertility. J. Biol. Chem. 2002, 277, 17830–17835. [Google Scholar] [CrossRef] [PubMed]
- Lovekamp-Swan, T.; Chaffin, C.L. The peroxisome proliferator-activated receptor gamma ligand troglitazone induces apoptosis and p53 in rat granulosa cells. Mol. Cell. Endocrinol. 2005, 233, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, N.; Dufour, J.M.; Vo, M.N.; Okita, J.; Okita, R.; Kim, K.H. Differential effects of phthalates on the testis and the liver. Biol. Reprod. 2005, 72, 745–754. [Google Scholar] [CrossRef]
- Froment, P.; Gizard, F.; Defever, D.; Staels, B.; Dupont, J.; Monget, P. Peroxisome proliferator-activated receptors in reproductive tissues: From gametogenesis to parturition. J. Endocrinol. 2006, 189, 199–209. [Google Scholar] [CrossRef]
- Fournier, T.; Tsatsaris, V.; Handschuh, K.; Evain-Brion, D. PPARs and the placenta. Placenta 2007, 28, 65–76. [Google Scholar] [CrossRef]
- Nadra, K.; Anghel, S.I.; Joye, E.; Tan, N.S.; Basu-Modak, S.; Trono, D.; Wahli, W.; Desvergne, B. Differentiation of trophoblast giant cells and their metabolic functions are dependent on peroxisome proliferator-activated receptor beta/delta. Mol. Cell. Biol. 2006, 26, 3266–3281. [Google Scholar] [CrossRef]
- Asami-Miyagishi, R.; Iseki, S.; Usui, M.; Uchida, K.; Kubo, H.; Morita, I. Expression and function of PPARγ in rat placental development. Biochem. Biophys. Res. Commun. 2004, 315, 497–501. [Google Scholar] [CrossRef]
- Rees, W.D.; McNeil, C.J.; Maloney, C.A. The Roles of PPARs in the Fetal Origins of Metabolic Health and Disease. PPAR Res. 2008, 2008, 459030. [Google Scholar] [CrossRef]
- Rosen, E.D.; MacDougald, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896. [Google Scholar] [CrossRef]
- Lendvai, Á.; Deutsch, M.J.; Plösch, T.; Ensenauer, R. The peroxisome proliferator-activated receptors under epigenetic control in placental metabolism and fetal development. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E797–E810. [Google Scholar] [CrossRef] [PubMed]
- Tobi, E.W.; Slieker, R.C.; Luijk, R.; Dekkers, K.F.; Stein, A.D.; Xu, K.M.; Slagboom, P.E.; van Zwet, E.W.; Lumey, L.H.; Heijmans, B.T. DNA methylation as a mediator of the association between prenatal adversity and risk factors for metabolic disease in adulthood. Sci. Adv. 2018, 4, eaao4364. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, P.; Wang, E. Fetal programming and metabolic syndrome. Annu. Rev. Physiol. 2012, 74, 107–130. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.E.; Barry, C.; Sabhlok, A.; Russell, K.; Majors, A.; Kollins, S.H.; Fuemmeler, B.F. Maternal pre-pregnancy obesity and child neurodevelopmental outcomes: A meta-analysis. Obes. Rev. 2018, 19, 464–484. [Google Scholar] [CrossRef] [PubMed]
- Lipner, E.; Murphy, S.K.; Ellman, L.M. Prenatal Maternal Stress and the Cascade of Risk to Schizophrenia Spectrum Disorders in Offspring. Curr. Psychiatry Rep. 2019, 21, 99. [Google Scholar] [CrossRef] [PubMed]
- Araya, F.; Stingone, J.A.; Claudio, L. Inequalities in Exposure to Ambient Air Neurotoxicants and Disparities in Markers of Neurodevelopment in Children by Maternal Nativity Status. Int. J. Environ. Res. Public Health 2021, 18, 7512. [Google Scholar] [CrossRef] [PubMed]
- Houfflyn, S.; Matthys, C.; Soubry, A. Male Obesity: Epigenetic Origin and Effects in Sperm and Offspring. Curr. Mol. Biol. Rep. 2017, 3, 288–296. [Google Scholar] [CrossRef]
- Berti, C.; Agostoni, C.; Davanzo, R.; Hyppönen, E.; Isolauri, E.; Meltzer, H.M.; Steegers-Theunissen, R.P.; Cetin, I. Early-life nutritional exposures and lifelong health: Immediate and long-lasting impacts of probiotics, vitamin D, and breastfeeding. Nutr. Rev. 2017, 75, 83–97. [Google Scholar] [CrossRef]
- Lillycrop, K.A.; Burdge, G.C. Maternal diet as a modifier of offspring epigenetics. J. Dev. Orig. Health Dis. 2015, 6, 88–95. [Google Scholar] [CrossRef]
- Zhang, Z.; Kris-Etherton, P.M.; Hartman, T.J. Birth weight and risk factors for cardiovascular disease and type 2 diabetes in US children and adolescents: 10 year results from NHANES. Matern. Child Health J. 2014, 18, 1423–1432. [Google Scholar] [CrossRef]
- Liang, J.; Xu, C.; Liu, Q.; Fan, X.; Xu, J.; Zhang, L.; Hang, D.; Shang, H.; Gu, A. Association between birth weight and risk of cardiovascular disease: Evidence from UK Biobank. Nutr. Metab. Cardiovasc. Dis. NMCD 2021, 31, 2637–2643. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.O.; Reeves, G.K.; Green, J.; Beral, V.; Cairns, B.J. Birth weight and adult cancer incidence: Large prospective study and meta-analysis. Ann. Oncol. 2014, 25, 1836–1843. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.D.; Zybert, P.A.; van der Pal-de Bruin, K.; Lumey, L.H. Exposure to famine during gestation, size at birth, and blood pressure at age 59 y: Evidence from the Dutch Famine. Eur. J. Epidemiol. 2006, 21, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, A.C.; van der Meulen, J.H.; Michels, R.P.; Osmond, C.; Barker, D.J.; Hales, C.N.; Bleker, O.P. Glucose tolerance in adults after prenatal exposure to famine. Lancet 1998, 351, 173–177. [Google Scholar] [CrossRef]
- Fall, C.H.; Vijayakumar, M.; Barker, D.J.; Osmond, C.; Duggleby, S. Weight in infancy and prevalence of coronary heart disease in adult life. BMJ 1995, 310, 17–19. [Google Scholar] [CrossRef]
- St Clair, D.; Xu, M.; Wang, P.; Yu, Y.; Fang, Y.; Zhang, F.; Zheng, X.; Gu, N.; Feng, G.; Sham, P.; et al. Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959–1961. JAMA 2005, 294, 557–562. [Google Scholar] [CrossRef]
- Mirpuri, J. Evidence for maternal diet-mediated effects on the offspring microbiome and immunity: Implications for public health initiatives. Pediatric Res. 2021, 89, 301–306. [Google Scholar] [CrossRef]
- Ozkan, H.; Topsakal, S.; Ozmen, O. Investigation of the diabetic effects of maternal high-glucose diet on rats. Biomed. Pharmacother. 2019, 110, 609–617. [Google Scholar] [CrossRef]
- Soubry, A. POHaD: Why we should study future fathers. Environ. Epigenetics 2018, 4, dvy007. [Google Scholar] [CrossRef]
- Figueroa-Colon, R.; Arani, R.B.; Goran, M.I.; Weinsier, R.L. Paternal body fat is a longitudinal predictor of changes in body fat in premenarcheal girls. Am. J. Clin. Nutr. 2000, 71, 829–834. [Google Scholar] [CrossRef]
- Ng, S.F.; Lin, R.C.; Laybutt, D.R.; Barres, R.; Owens, J.A.; Morris, M.J. Chronic high-fat diet in fathers programs β-cell dysfunction in female rat offspring. Nature 2010, 467, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, K.; Butt, C.M.; Webster, T.F.; Preston, E.V.; Hammel, S.C.; Makey, C.; Lorenzo, A.M.; Cooper, E.M.; Carignan, C.; Meeker, J.D.; et al. Temporal Trends in Exposure to Organophosphate Flame Retardants in the United States. Environ. Sci. Technol. Lett. 2017, 4, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Zhang, J.; Sommer, K.; Bassig, B.A.; Zhang, X.; Braun, J.; Xu, S.; Boyle, P.; Zhang, B.; Shi, K.; et al. Effects of Environmental Exposures on Fetal and Childhood Growth Trajectories. Ann. Glob. Health 2016, 82, 41–99. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Li, R.; Zhao, Y.; Zhu, Q.; Xia, B.; Chen, S.; Zhang, Y. Evaluating effects of prenatal exposure to phthalates on neonatal birth weight: Structural equation model approaches. Chemosphere 2018, 205, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Warembourg, C.; Maitre, L.; Tamayo-Uria, I.; Fossati, S.; Roumeliotaki, T.; Aasvang, G.M.; Andrusaityte, S.; Casas, M.; Cequier, E.; Chatzi, L.; et al. Early-Life Environmental Exposures and Blood Pressure in Children. J. Am. Coll. Cardiol. 2019, 74, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Miguel, P.M.; Pereira, L.O.; Silveira, P.P.; Meaney, M.J. Early environmental influences on the development of children’s brain structure and function. Dev. Med. Child Neurol. 2019, 61, 1127–1133. [Google Scholar] [CrossRef]
- Ross, E.J.; Graham, D.L.; Money, K.M.; Stanwood, G.D. Developmental consequences of fetal exposure to drugs: What we know and what we still must learn. Neuropsychopharmacology 2015, 40, 61–87. [Google Scholar] [CrossRef]
- Salihu, H.M.; Paothong, A.; Das, R.; King, L.M.; Pradhan, A.; Riggs, B.; Naik, E.; Siegel, E.M.; Whiteman, V.E. Evidence of altered brain regulatory gene expression in tobacco-exposed fetuses. J. Perinat. Med. 2017, 45, 1045–1053. [Google Scholar] [CrossRef]
- Dong, T.; Hu, W.; Zhou, X.; Lin, H.; Lan, L.; Hang, B.; Lv, W.; Geng, Q.; Xia, Y. Prenatal exposure to maternal smoking during pregnancy and attention-deficit/hyperactivity disorder in offspring: A meta-analysis. Reprod. Toxicol. 2018, 76, 63–70. [Google Scholar] [CrossRef]
- Aghaie, C.I.; Hausknecht, K.A.; Wang, R.; Dezfuli, P.H.; Haj-Dahmane, S.; Kane, C.J.M.; Sigurdson, W.J.; Shen, R.Y. Prenatal Ethanol Exposure and Postnatal Environmental Intervention Alter Dopaminergic Neuron and Microglia Morphology in the Ventral Tegmental Area During Adulthood. Alcohol. Clin. Exp. Res. 2020, 44, 435–444. [Google Scholar] [CrossRef]
- Ghosh, R.; Causey, K.; Burkart, K.; Wozniak, S.; Cohen, A.; Brauer, M. Ambient and household PM2.5 pollution and adverse perinatal outcomes: A meta-regression and analysis of attributable global burden for 204 countries and territories. PLoS Med. 2021, 18, e1003718. [Google Scholar] [CrossRef] [PubMed]
- Veras, M.M.; Guimarães-Silva, R.M.; Caldini, E.G.; Saldiva, P.H.; Dolhnikoff, M.; Mayhew, T.M. The effects of particulate ambient air pollution on the murine umbilical cord and its vessels: A quantitative morphological and immunohistochemical study. Reprod. Toxicol. 2012, 34, 598–606. [Google Scholar] [CrossRef] [PubMed]
- van den Hooven, E.H.; Pierik, F.H.; de Kluizenaar, Y.; Hofman, A.; van Ratingen, S.W.; Zandveld, P.Y.; Russcher, H.; Lindemans, J.; Miedema, H.M.; Steegers, E.A.; et al. Air pollution exposure and markers of placental growth and function: The generation R study. Environ. Health Perspect. 2012, 120, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.; Stayner, L.; Slama, R.; Sørensen, M.; Figueras, F.; Nieuwenhuijsen, M.J.; Raaschou-Nielsen, O.; Dadvand, P. Ambient air pollution and pregnancy-induced hypertensive disorders: A systematic review and meta-analysis. Hypertension 2014, 64, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Soubry, A.; Hoyo, C.; Butt, C.M.; Fieuws, S.; Price, T.M.; Murphy, S.K.; Stapleton, H.M. Human exposure to flame-retardants is associated with aberrant DNA methylation at imprinted genes in sperm. Environ. Epigenetics 2017, 3, dvx003. [Google Scholar] [CrossRef]
- Miao, M.; Zhou, X.; Li, Y.; Zhang, O.; Zhou, Z.; Li, T.; Yuan, W.; Li, R.; Li, D.K. LINE-1 hypomethylation in spermatozoa is associated with Bisphenol A exposure. Andrology 2014, 2, 138–144. [Google Scholar] [CrossRef]
- Marczylo, E.L.; Amoako, A.A.; Konje, J.C.; Gant, T.W.; Marczylo, T.H. Smoking induces differential miRNA expression in human spermatozoa: A potential transgenerational epigenetic concern? Epigenetics 2012, 7, 432–439. [Google Scholar] [CrossRef]
- Huizink, A.C.; Robles de Medina, P.G.; Mulder, E.J.; Visser, G.H.; Buitelaar, J.K. Stress during pregnancy is associated with developmental outcome in infancy. J. Child Psychol. Psychiatry 2003, 44, 810–818. [Google Scholar] [CrossRef]
- Paarlberg, K.M.; Vingerhoets, A.J.; Passchier, J.; Dekker, G.A.; Heinen, A.G.; van Geijn, H.P. Psychosocial predictors of low birthweight: A prospective study. Br. J. Obstet. Gynaecol. 1999, 106, 834–841. [Google Scholar] [CrossRef]
- King, S.; Mancini-Marïe, A.; Brunet, A.; Walker, E.; Meaney, M.J.; Laplante, D.P. Prenatal maternal stress from a natural disaster predicts dermatoglyphic asymmetry in humans. Dev. Psychopathol. 2009, 21, 343–353. [Google Scholar] [CrossRef]
- Korja, R.; Nolvi, S.; Grant, K.A.; McMahon, C. The Relations Between Maternal Prenatal Anxiety or Stress and Child’s Early Negative Reactivity or Self-Regulation: A Systematic Review. Child Psychiatry Hum. Dev. 2017, 48, 851–869. [Google Scholar] [CrossRef] [PubMed]
- Dunkel Schetter, C.; Tanner, L. Anxiety, depression and stress in pregnancy: Implications for mothers, children, research, and practice. Curr. Opin. Psychiatry 2012, 25, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.J.; Yung, A.R.; Pantelis, C. Cognitive precursors of severe mental disorders. Cogn. Neuropsychiatry 2013, 18, 1–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Davis, E.P.; Sandman, C.A. Prenatal psychobiological predictors of anxiety risk in preadolescent children. Psychoneuroendocrinology 2012, 37, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Adamson, B.; Letourneau, N.; Lebel, C. Prenatal maternal anxiety and children’s brain structure and function: A systematic review of neuroimaging studies. J. Affect. Disord. 2018, 241, 117–126. [Google Scholar] [CrossRef]
- Meaney, M.J. Epigenetics and the biological definition of gene x environment interactions. Child Dev. 2010, 81, 41–79. [Google Scholar] [CrossRef]
- Weaver, I.C.; Cervoni, N.; Champagne, F.A.; D’Alessio, A.C.; Sharma, S.; Seckl, J.R.; Dymov, S.; Szyf, M.; Meaney, M.J. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004, 7, 847–854. [Google Scholar] [CrossRef]
- McGowan, P.O.; Sasaki, A.; D’Alessio, A.C.; Dymov, S.; Labonté, B.; Szyf, M.; Turecki, G.; Meaney, M.J. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci. 2009, 12, 342–348. [Google Scholar] [CrossRef]
- Chan, J.C.; Nugent, B.M.; Bale, T.L. Parental Advisory: Maternal and Paternal Stress Can Impact Offspring Neurodevelopment. Biol. Psychiatry 2018, 83, 886–894. [Google Scholar] [CrossRef]
- González, C.R.; González, B. Exploring the Stress Impact in the Paternal Germ Cells Epigenome: Can Catecholamines Induce Epigenetic Reprogramming? Front. Endocrinol. 2020, 11, 630948. [Google Scholar] [CrossRef]
- Ly, L.; Chan, D.; Trasler, J.M. Developmental windows of susceptibility for epigenetic inheritance through the male germline. Semin. Cell Dev. Biol. 2015, 43, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Grygiel-Górniak, B. Peroxisome proliferator-activated receptors and their ligands: Nutritional and clinical implications—A review. Nutr. J. 2014, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Lazennec, G.; Canaple, L.; Saugy, D.; Wahli, W. Activation of peroxisome proliferator-activated receptors (PPARs) by their ligands and protein kinase A activators. Mol. Endocrinol. 2000, 14, 1962–1975. [Google Scholar] [CrossRef] [PubMed]
- Basilotta, R.; Lanza, M.; Casili, G.; Chisari, G.; Munao, S.; Colarossi, L.; Cucinotta, L.; Campolo, M.; Esposito, E.; Paterniti, I. Potential Therapeutic Effects of PPAR Ligands in Glioblastoma. Cells 2022, 11, 621. [Google Scholar] [CrossRef]
- Chan, L.S.; Wells, R.A. Cross-Talk between PPARs and the Partners of RXR: A Molecular Perspective. PPAR Res. 2009, 2009, 925309. [Google Scholar] [CrossRef]
- Yousefnia, S.; Momenzadeh, S.; Seyed Forootan, F.; Ghaedi, K.; Nasr Esfahani, M.H. The influence of peroxisome proliferator-activated receptor γ (PPARγ) ligands on cancer cell tumorigenicity. Gene 2018, 649, 14–22. [Google Scholar] [CrossRef]
- Brunmeir, R.; Xu, F. Functional Regulation of PPARs through Post-Translational Modifications. Int. J. Mol. Sci. 2018, 19, 1738. [Google Scholar] [CrossRef]
- Wadosky, K.M.; Willis, M.S. The story so far: Post-translational regulation of peroxisome proliferator-activated receptors by ubiquitination and SUMOylation. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H515–H526. [Google Scholar] [CrossRef]
- Takeyama, K.; Kodera, Y.; Suzawa, M.; Kato, S. Peroxisome proliferator-activated receptor(PPAR)—Structure, function, tissue distribution, gene expression. Nihon Rinsho Jpn. J. Clin. Med. 2000, 58, 357–363. [Google Scholar]
- Tan, N.S.; Michalik, L.; Desvergne, B.; Wahli, W. Multiple expression control mechanisms of peroxisome proliferator-activated receptors and their target genes. J. Steroid Biochem. Mol. Biol. 2005, 93, 99–105. [Google Scholar] [CrossRef]
- Lee, Y.; Yu, X.; Gonzales, F.; Mangelsdorf, D.J.; Wang, M.Y.; Richardson, C.; Witters, L.A.; Unger, R.H. PPAR alpha is necessary for the lipopenic action of hyperleptinemia on white adipose and liver tissue. Proc. Natl. Acad. Sci. USA 2002, 99, 11848–11853. [Google Scholar] [CrossRef] [PubMed]
- Storlien, L.H.; Kraegen, E.W.; Chisholm, D.J.; Ford, G.L.; Bruce, D.G.; Pascoe, W.S. Fish oil prevents insulin resistance induced by high-fat feeding in rats. Science 1987, 237, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Feige, J.N.; Gelman, L.; Michalik, L.; Desvergne, B.; Wahli, W. From molecular action to physiological outputs: Peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Prog. Lipid Res. 2006, 45, 120–159. [Google Scholar] [CrossRef] [PubMed]
- Burns, K.A.; Vanden Heuvel, J.P. Modulation of PPAR activity via phosphorylation. Biochim. Et Biophys. Acta 2007, 1771, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Shalev, A.; Siegrist-Kaiser, C.A.; Yen, P.M.; Wahli, W.; Burger, A.G.; Chin, W.W.; Meier, C.A. The peroxisome proliferator-activated receptor alpha is a phosphoprotein: Regulation by insulin. Endocrinology 1996, 137, 4499–4502. [Google Scholar] [CrossRef]
- Passilly, P.; Schohn, H.; Jannin, B.; Cherkaoui Malki, M.; Boscoboinik, D.; Dauça, M.; Latruffe, N. Phosphorylation of peroxisome proliferator-activated receptor alpha in rat Fao cells and stimulation by ciprofibrate. Biochem. Pharmacol. 1999, 58, 1001–1008. [Google Scholar] [CrossRef]
- Pourcet, B.; Pineda-Torra, I.; Derudas, B.; Staels, B.; Glineur, C. SUMOylation of human peroxisome proliferator-activated receptor alpha inhibits its trans-activity through the recruitment of the nuclear corepressor NCoR. J. Biol. Chem. 2010, 285, 5983–5992. [Google Scholar] [CrossRef]
- Leuenberger, N.; Pradervand, S.; Wahli, W. Sumoylated PPARalpha mediates sex-specific gene repression and protects the liver from estrogen-induced toxicity in mice. J. Clin. Investig. 2009, 119, 3138–3148. [Google Scholar] [CrossRef]
- Hong, F.; Pan, S.; Guo, Y.; Xu, P.; Zhai, Y. PPARs as Nuclear Receptors for Nutrient and Energy Metabolism. Molecules 2019, 24, 2545. [Google Scholar] [CrossRef]
- Rogue, A.; Lambert, C.; Jossé, R.; Antherieu, S.; Spire, C.; Claude, N.; Guillouzo, A. Comparative gene expression profiles induced by PPARγ and PPARα/γ agonists in human hepatocytes. PLoS ONE 2011, 6, e18816. [Google Scholar] [CrossRef]
- Cheng, L.; Ding, G.; Qin, Q.; Huang, Y.; Lewis, W.; He, N.; Evans, R.M.; Schneider, M.D.; Brako, F.A.; Xiao, Y.; et al. Cardiomyocyte-restricted peroxisome proliferator-activated receptor-delta deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nat. Med. 2004, 10, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Mello, T.; Materozzi, M.; Galli, A. PPARs and Mitochondrial Metabolism: From NAFLD to HCC. PPAR Res. 2016, 2016, 7403230. [Google Scholar] [CrossRef] [PubMed]
- Strosznajder, A.K.; Wójtowicz, S.; Jeżyna, M.J.; Sun, G.Y.; Strosznajder, J.B. Recent Insights on the Role of PPAR-β/δ in Neuroinflammation and Neurodegeneration, and Its Potential Target for Therapy. Neuromolecular Med. 2021, 23, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Dietschy, J.M.; Turley, S.D. Thematic review series: Brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J. Lipid Res. 2004, 45, 1375–1397. [Google Scholar] [CrossRef]
- Villapol, S. Roles of Peroxisome Proliferator-Activated Receptor Gamma on Brain and Peripheral Inflammation. Cell. Mol. Neurobiol. 2018, 38, 121–132. [Google Scholar] [CrossRef]
- Koo, Y.D.; Choi, J.W.; Kim, M.; Chae, S.; Ahn, B.Y.; Kim, M.; Oh, B.C.; Hwang, D.; Seol, J.H.; Kim, Y.B.; et al. SUMO-Specific Protease 2 (SENP2) Is an Important Regulator of Fatty Acid Metabolism in Skeletal Muscle. Diabetes 2015, 64, 2420–2431. [Google Scholar] [CrossRef]
- Taheri, M.; Salamian, A.; Ghaedi, K.; Peymani, M.; Izadi, T.; Nejati, A.S.; Atefi, A.; Nematollahi, M.; Ahmadi Ghahrizjani, F.; Esmaeili, M.; et al. A ground state of PPARγ activity and expression is required for appropriate neural differentiation of hESCs. Pharmacol. Rep. PR 2015, 67, 1103–1114. [Google Scholar] [CrossRef]
- Marion-Letellier, R.; Savoye, G.; Ghosh, S. Fatty acids, eicosanoids and PPAR gamma. Eur. J. Pharmacol. 2016, 785, 44–49. [Google Scholar] [CrossRef]
- Li, Y.; Qi, Y.; Huang, T.H.; Yamahara, J.; Roufogalis, B.D. Pomegranate flower: A unique traditional antidiabetic medicine with dual PPAR-alpha/-gamma activator properties. Diabetes Obes. Metab. 2008, 10, 10–17. [Google Scholar] [CrossRef]
- Ravi Kiran Ammu, V.V.V.; Garikapati, K.K.; Krishnamurthy, P.T.; Chintamaneni, P.K.; Pindiprolu, S. Possible role of PPAR-γ and COX-2 receptor modulators in the treatment of Non-Small Cell lung carcinoma. Med. Hypotheses 2019, 124, 98–100. [Google Scholar] [CrossRef]
- Ohshima, T.; Koga, H.; Shimotohno, K. Transcriptional activity of peroxisome proliferator-activated receptor gamma is modulated by SUMO-1 modification. J. Biol. Chem. 2004, 279, 29551–29557. [Google Scholar] [CrossRef] [PubMed]
- Diezko, R.; Suske, G. Ligand binding reduces SUMOylation of the peroxisome proliferator-activated receptor γ (PPARγ) activation function 1 (AF1) domain. PLoS ONE 2013, 8, e66947. [Google Scholar] [CrossRef] [PubMed]
- Nadra, K.; Quignodon, L.; Sardella, C.; Joye, E.; Mucciolo, A.; Chrast, R.; Desvergne, B. PPARgamma in placental angiogenesis. Endocrinology 2010, 151, 4969–4981. [Google Scholar] [CrossRef] [PubMed]
- Murthi, P.; Kalionis, B.; Cocquebert, M.; Rajaraman, G.; Chui, A.; Keogh, R.J.; Evain-Brion, D.; Fournier, T. Homeobox genes and down-stream transcription factor PPARγ in normal and pathological human placental development. Placenta 2013, 34, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Q.; Cook, T.J.; Knipp, G.T. Effect of placental fatty acid metabolism and regulation by peroxisome proliferator activated receptor on pregnancy and fetal outcomes. J. Pharm. Sci. 2007, 96, 2582–2606. [Google Scholar] [CrossRef]
- Matsuo, H.; Strauss, J.F., 3rd. Peroxisome proliferators and retinoids affect JEG-3 choriocarcinoma cell function. Endocrinology 1994, 135, 1135–1145. [Google Scholar] [CrossRef]
- Kubota, N.; Terauchi, Y.; Miki, H.; Tamemoto, H.; Yamauchi, T.; Komeda, K.; Satoh, S.; Nakano, R.; Ishii, C.; Sugiyama, T.; et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol. Cell 1999, 4, 597–609. [Google Scholar] [CrossRef]
- Barak, Y.; Nelson, M.C.; Ong, E.S.; Jones, Y.Z.; Ruiz-Lozano, P.; Chien, K.R.; Koder, A.; Evans, R.M. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol. Cell 1999, 4, 585–595. [Google Scholar] [CrossRef]
- Waite, L.L.; Person, E.C.; Zhou, Y.; Lim, K.H.; Scanlan, T.S.; Taylor, R.N. Placental peroxisome proliferator-activated receptor-gamma is up-regulated by pregnancy serum. J. Clin. Endocrinol. Metab. 2000, 85, 3808–3814. [Google Scholar] [CrossRef][Green Version]
- Tarrade, A.; Schoonjans, K.; Pavan, L.; Auwerx, J.; Rochette-Egly, C.; Evain-Brion, D.; Fournier, T. PPARgamma/RXRalpha heterodimers control human trophoblast invasion. J. Clin. Endocrinol. Metab. 2001, 86, 5017–5024. [Google Scholar] [CrossRef][Green Version]
- Qiao, L.; Wattez, J.S.; Lee, S.; Guo, Z.; Schaack, J.; Hay, W.W., Jr.; Zita, M.M.; Parast, M.; Shao, J. Knockout maternal adiponectin increases fetal growth in mice: Potential role for trophoblast IGFBP-1. Diabetologia 2016, 59, 2417–2425. [Google Scholar] [CrossRef] [PubMed]
- Tarrade, A.; Schoonjans, K.; Guibourdenche, J.; Bidart, J.M.; Vidaud, M.; Auwerx, J.; Rochette-Egly, C.; Evain-Brion, D. PPAR gamma/RXR alpha heterodimers are involved in human CG beta synthesis and human trophoblast differentiation. Endocrinology 2001, 142, 4504–4514. [Google Scholar] [CrossRef] [PubMed]
- Holdsworth-Carson, S.J.; Lim, R.; Mitton, A.; Whitehead, C.; Rice, G.E.; Permezel, M.; Lappas, M. Peroxisome proliferator-activated receptors are altered in pathologies of the human placenta: Gestational diabetes mellitus, intrauterine growth restriction and preeclampsia. Placenta 2010, 31, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, T.; Shi, Z.; Ding, H.; Ling, X. MicroRNA-518d regulates PPARα protein expression in the placentas of females with gestational diabetes mellitus. Mol. Med. Rep. 2014, 9, 2085–2090. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Lambertini, L.; Rialdi, A.; Lee, M.; Mystal, E.Y.; Grabie, M.; Manaster, I.; Huynh, N.; Finik, J.; Davey, M.; et al. Global methylation in the placenta and umbilical cord blood from pregnancies with maternal gestational diabetes, preeclampsia, and obesity. Reprod. Sci. 2014, 21, 131–137. [Google Scholar] [CrossRef]
- Ruchat, S.M.; Houde, A.A.; Voisin, G.; St-Pierre, J.; Perron, P.; Baillargeon, J.P.; Gaudet, D.; Hivert, M.F.; Brisson, D.; Bouchard, L. Gestational diabetes mellitus epigenetically affects genes predominantly involved in metabolic diseases. Epigenetics 2013, 8, 935–943. [Google Scholar] [CrossRef]
- Huin, C.; Corriveau, L.; Bianchi, A.; Keller, J.M.; Collet, P.; Krémarik-Bouillaud, P.; Domenjoud, L.; Bécuwe, P.; Schohn, H.; Ménard, D.; et al. Differential expression of peroxisome proliferator-activated receptors (PPARs) in the developing human fetal digestive tract. J. Histochem. Cytochem. 2000, 48, 603–611. [Google Scholar] [CrossRef]
- Braissant, O.; Wahli, W. Differential expression of peroxisome proliferator-activated receptor-alpha, -beta, and -gamma during rat embryonic development. Endocrinology 1998, 139, 2748–2754. [Google Scholar] [CrossRef]
- Wieser, F.; Waite, L.; Depoix, C.; Taylor, R.N. PPAR Action in Human Placental Development and Pregnancy and Its Complications. PPAR Res. 2008, 2008, 527048. [Google Scholar] [CrossRef]
- Jawerbaum, A.; Capobianco, E. Review: Effects of PPAR activation in the placenta and the fetus: Implications in maternal diabetes. Placenta 2011, 32 (Suppl. 2), S212–S217. [Google Scholar] [CrossRef]
- Ringseis, R.; Gutgesell, A.; Dathe, C.; Brandsch, C.; Eder, K. Feeding oxidized fat during pregnancy up-regulates expression of PPARalpha-responsive genes in the liver of rat fetuses. Lipids Health Dis. 2007, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Lindegaard, M.L.; Nielsen, L.B. Maternal diabetes causes coordinated down-regulation of genes involved with lipid metabolism in the murine fetal heart. Metab. Clin. Exp. 2008, 57, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Martínez, N.; White, V.; Kurtz, M.; Higa, R.; Capobianco, E.; Jawerbaum, A. Activation of the nuclear receptor PPARα regulates lipid metabolism in foetal liver from diabetic rats: Implications in diabetes-induced foetal overgrowth. Diabetes Metab. Res. Rev. 2011, 27, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Devchand, P.R.; Keller, H.; Peters, J.M.; Vazquez, M.; Gonzalez, F.J.; Wahli, W. The PPARα-leukotriene B4 pathway to inflammation control. Nature 1996, 384, 39–43. [Google Scholar] [CrossRef]
- Kersten, S.; Seydoux, J.; Peters, J.M.; Gonzalez, F.J.; Desvergne, B.; Wahli, W. Peroxisome proliferator-activated receptor α mediates the adaptive response to fasting. J. Clin. Investig. 1999, 103, 1489–1498. [Google Scholar] [CrossRef]
- Higa, R.; González, E.; Pustovrh, M.C.; White, V.; Capobianco, E.; Martínez, N.; Jawerbaum, A. PPARdelta and its activator PGI2 are reduced in diabetic embryopathy: Involvement of PPARdelta activation in lipid metabolic and signalling pathways in rat embryo early organogenesis. Mol. Hum. Reprod. 2007, 13, 103–110. [Google Scholar] [CrossRef]
- Barak, Y.; Liao, D.; He, W.; Ong, E.S.; Nelson, M.C.; Olefsky, J.M.; Boland, R.; Evans, R.M. Effects of peroxisome proliferator-activated receptor delta on placentation, adiposity, and colorectal cancer. Proc. Natl. Acad. Sci. USA 2002, 99, 303–308. [Google Scholar] [CrossRef]
- Pustovrh, M.C.; Capobianco, E.; Martínez, N.; Higa, R.; White, V.; Jawerbaum, A. MMP/ TIMP balance is modulated in vitro by 15dPGJ(2) in fetuses and placentas from diabetic rats. Eur. J. Clin. Investig. 2009, 39, 1082–1090. [Google Scholar] [CrossRef]
- Capobianco, E.; Martínez, N.; Higa, R.; White, V.; Jawerbaum, A. The effects of maternal dietary treatments with natural PPAR ligands on lipid metabolism in fetuses from control and diabetic rats. Prostaglandins Leukot. Essent. Fat. Acids 2008, 79, 191–199. [Google Scholar] [CrossRef]
- Shimizu, N.; Chikahisa, S.; Nishi, Y.; Harada, S.; Iwaki, Y.; Fujihara, H.; Kitaoka, K.; Shiuchi, T.; Séi, H. Maternal dietary restriction alters offspring’s sleep homeostasis. PLoS ONE 2013, 8, e64263. [Google Scholar] [CrossRef]
- De Felice, M.; Melis, M.; Aroni, S.; Muntoni, A.L.; Fanni, S.; Frau, R.; Devoto, P.; Pistis, M. The PPARα agonist fenofibrate attenuates disruption of dopamine function in a maternal immune activation rat model of schizophrenia. CNS Neurosci. Ther. 2019, 25, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Nema, J.; Randhir, K.; Wadhwani, N.; Sundrani, D.; Joshi, S. Maternal vitamin D deficiency reduces docosahexaenoic acid, placental growth factor and peroxisome proliferator activated receptor gamma levels in the pup brain in a rat model of preeclampsia. Prostaglandins Leukot. Essent. Fat. Acids 2021, 175, 102364. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, Q.; Wang, J.; Tang, M.; Huang, S.; Peng, K.; Han, Y.; Zhang, J.; Liu, G.; Fang, Q.; et al. Maternal immune activation-induced PPARγ-dependent dysfunction of microglia associated with neurogenic impairment and aberrant postnatal behaviors in offspring. Neurobiol. Dis. 2019, 125, 1–13. [Google Scholar] [CrossRef]
- Liu, W.C.; Wu, C.W.; Fu, M.H.; Tain, Y.L.; Liang, C.K.; Hung, C.Y.; Chen, I.C.; Lee, Y.C.; Wu, C.Y.; Wu, K.L.H. Maternal high fructose-induced hippocampal neuroinflammation in the adult female offspring via PPARγ-NF-κB signaling. J. Nutr. Biochem. 2020, 81, 108378. [Google Scholar] [CrossRef]
- Ke, X.; Xing, B.; Yu, B.; Yu, X.; Majnik, A.; Cohen, S.; Lane, R.; Joss-Moore, L. IUGR disrupts the PPARγ-Setd8-H4K20me(1) and Wnt signaling pathways in the juvenile rat hippocampus. Int. J. Dev. Neurosci. 2014, 38, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Rehan, V.K.; Liu, J.; Sakurai, R.; Torday, J.S. Perinatal nicotine-induced transgenerational asthma. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, L501–L507. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ji, B.; Zhao, G.; Su, H.; Ge, Y.; Dai, J.; Lu, Y.; Sakurai, R.; Rehan, V.K. Protective effect of electro-acupuncture at maternal different points on perinatal nicotine exposure-induced pulmonary dysplasia in offspring based on HPA axis and signal transduction pathway. Biochem. Biophys. Res. Commun. 2018, 505, 586–592. [Google Scholar] [CrossRef]
- Cannon, D.T.; Liu, J.; Sakurai, R.; Rossiter, H.B.; Rehan, V.K. Impaired Lung Mitochondrial Respiration Following Perinatal Nicotine Exposure in Rats. Lung 2016, 194, 325–328. [Google Scholar] [CrossRef]
- Joss-Moore, L.A.; Wang, Y.; Baack, M.L.; Yao, J.; Norris, A.W.; Yu, X.; Callaway, C.W.; McKnight, R.A.; Albertine, K.H.; Lane, R.H. IUGR decreases PPARγ and SETD8 Expression in neonatal rat lung and these effects are ameliorated by maternal DHA supplementation. Early Hum. Dev. 2010, 86, 785–791. [Google Scholar] [CrossRef]
- Slater-Jefferies, J.L.; Lillycrop, K.A.; Townsend, P.A.; Torrens, C.; Hoile, S.P.; Hanson, M.A.; Burdge, G.C. Feeding a protein-restricted diet during pregnancy induces altered epigenetic regulation of peroxisomal proliferator-activated receptor-α in the heart of the offspring. J. Dev. Orig. Health Dis. 2011, 2, 250–255. [Google Scholar] [CrossRef]
- Higa, R.; Roberti, S.L.; Capobianco, E.; Fornes, D.; White, V.; Jawerbaum, A. Pro-oxidant/pro-inflammatory alterations in the offspring’s heart of mild diabetic rats are regulated by maternal treatments with a mitochondrial antioxidant. Reprod. Toxicol. 2017, 73, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, O.R.; Rosario, F.J.; Chan, J.; Cox, L.A.; Ferchaud-Roucher, V.; Zemski-Berry, K.A.; Reusch, J.E.B.; Keller, A.C.; Powell, T.L.; Jansson, T. Maternal obesity causes fetal cardiac hypertrophy and alters adult offspring myocardial metabolism in mice. J. Physiol. 2022, 600, 3169–3191. [Google Scholar] [CrossRef] [PubMed]
- Torres Tda, S.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Rosiglitazone reverses cardiac adverse remodeling (fibrosis and vascularization) in perinatal low protein rat offspring. Pathol. Res. Pract. 2010, 206, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.L.; Sébert, S.P.; Hyatt, M.A.; Stephenson, T.; Budge, H.; Symonds, M.E.; Gardner, D.S. Effect of maternal nutrient restriction from early to midgestation on cardiac function and metabolism after adolescent-onset obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1455–R1463. [Google Scholar] [CrossRef]
- Nair, A.R.; Silva, S.D., Jr.; Agbor, L.N.; Wu, J.; Nakagawa, P.; Mukohda, M.; Lu, K.T.; Sandgren, J.A.; Pierce, G.L.; Santillan, M.K.; et al. Endothelial PPARγ (Peroxisome Proliferator-Activated Receptor-γ) Protects from Angiotensin II-Induced Endothelial Dysfunction in Adult Offspring Born from Pregnancies Complicated by Hypertension. Hypertension 2019, 74, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Torres, T.S.; D’Oliveira Silva, G.; Aguila, M.B.; de Carvalho, J.J.; Mandarim-De-Lacerda, C.A. Effects of rosiglitazone (a peroxysome proliferator-activated receptor gamma agonist) on the blood pressure and aortic structure in metabolically programmed (perinatal low protein) rats. Hypertens. Res. 2008, 31, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, L.; Zhang, Y.; Guan, S.; Gong, X.; Wang, X. Maternal exposure to perfluorooctanoic acid (PFOA) causes liver toxicity through PPAR-α pathway and lowered histone acetylation in female offspring mice. Environ. Sci. Pollut. Res. Int. 2019, 26, 18866–18875. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Xu, H.; Wu, J.; Li, J.; Zhou, Y.; Ding, Z.; Siwko, S.K.; Yuan, X.; Schalinske, K.L.; Alpini, G.; et al. Maternal high-fat diet disrupted one-carbon metabolism in offspring, contributing to nonalcoholic fatty liver disease. Liver Int. 2021, 41, 1305–1319. [Google Scholar] [CrossRef]
- Huang, S.J.; Chen, S.Q.; Lin, Y.; Yang, H.Y.; Ran, J.; Yan, F.F.; Huang, M.; Liu, X.L.; Hong, L.C.; Zhang, X.D.; et al. Maternal nicotine exposure aggravates metabolic associated fatty liver disease via PI3K/Akt signaling in adult offspring mice. Liver Int. 2021, 41, 1867–1878. [Google Scholar] [CrossRef]
- Castaño-Moreno, E.; Castillo, V.; Peñailillo, R.; Llanos, M.N.; Valenzuela, R.; Ronco, A.M. Fatty acid and lipid metabolism in liver of pregnant mice and their offspring is influenced by unbalanced folates/vitamin B12 diets. Prostaglandins Leukot. Essent. Fat. Acids 2020, 154, 102057. [Google Scholar] [CrossRef]
- Pang, H.; Ling, D.; Cheng, Y.; Akbar, R.; Jin, L.; Ren, J.; Wu, H.; Chen, B.; Zhou, Y.; Zhu, H.; et al. Gestational high-fat diet impaired demethylation of Pparα and induced obesity of offspring. J. Cell. Mol. Med. 2021, 25, 5404–5416. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Liu, Z.; Gong, J.; Zhang, L.; Wang, L.; Magdalou, J.; Chen, L.; Wang, H. Prenatal ethanol exposure programs an increased susceptibility of non-alcoholic fatty liver disease in female adult offspring rats. Toxicol. Appl. Pharmacol. 2014, 274, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shi, X.; Hou, Y.; Cao, X.; Gong, L.; Wang, H.; Li, J.; Li, J.; Wu, C.; Xiao, D.; et al. Paternal hyperglycemia induces transgenerational inheritance of susceptibility to hepatic steatosis in rats involving altered methylation on Pparα promoter. Biochim. Et Biophys. Acta. Mol. Basis Dis. 2019, 1865, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Chen, H.; Zaky, A.; Pollock, C.; Saad, S. SIRT1 overexpression attenuates offspring metabolic and liver disorders as a result of maternal high-fat feeding. J. Physiol. 2019, 597, 467–480. [Google Scholar] [CrossRef]
- Long, Z.; Fan, J.; Wu, G.; Liu, X.; Wu, H.; Liu, J.; Chen, Y.; Su, S.; Cheng, X.; Xu, Z.; et al. Gestational bisphenol A exposure induces fatty liver development in male offspring mice through the inhibition of HNF1b and upregulation of PPARγ. Cell Biol. Toxicol. 2021, 37, 65–84. [Google Scholar] [CrossRef]
- da Silva Aragão, R.; Guzmán-Quevedo, O.; Pérez-García, G.; Manhães-de-Castro, R.; Bolaños-Jiménez, F. Maternal protein restriction impairs the transcriptional metabolic flexibility of skeletal muscle in adult rat offspring. Br. J. Nutr. 2014, 112, 328–337. [Google Scholar] [CrossRef]
- Zhou, Y.; Gu, P.; Shi, W.; Li, J.; Hao, Q.; Cao, X.; Lu, Q.; Zeng, Y. MicroRNA-29a induces insulin resistance by targeting PPARδ in skeletal muscle cells. Int. J. Mol. Med. 2016, 37, 931–938. [Google Scholar] [CrossRef]
- Morino, K.; Petersen, K.F.; Sono, S.; Choi, C.S.; Samuel, V.T.; Lin, A.; Gallo, A.; Zhao, H.; Kashiwagi, A.; Goldberg, I.J.; et al. Regulation of mitochondrial biogenesis by lipoprotein lipase in muscle of insulin-resistant offspring of parents with type 2 diabetes. Diabetes 2012, 61, 877–887. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, H.; Yang, L.; Wang, X.; Yang, L.; Xing, Y.; Lv, X.; Ma, H.; Song, G. The role of CD36-Fabp4-PPARγ in skeletal muscle involves insulin resistance in intrauterine growth retardation mice with catch-up growth. BMC Endocr. Disord. 2022, 22, 10. [Google Scholar] [CrossRef]
- Bayol, S.A.; Simbi, B.H.; Stickland, N.C. A maternal cafeteria diet during gestation and lactation promotes adiposity and impairs skeletal muscle development and metabolism in rat offspring at weaning. J. Physiol. 2005, 567, 951–961. [Google Scholar] [CrossRef]
- Zolezzi, J.M.; Santos, M.J.; Bastías-Candia, S.; Pinto, C.; Godoy, J.A.; Inestrosa, N.C. PPARs in the central nervous system: Roles in neurodegeneration and neuroinflammation. Biol. Rev. Camb. Philos. Soc. 2017, 92, 2046–2069. [Google Scholar] [CrossRef] [PubMed]
- Cuzzocrea, S.; Mazzon, E.; Di Paola, R.; Peli, A.; Bonato, A.; Britti, D.; Genovese, T.; Muià, C.; Crisafulli, C.; Caputi, A.P. The role of the peroxisome proliferator-activated receptor-alpha (PPAR-alpha) in the regulation of acute inflammation. J. Leukoc. Biol. 2006, 79, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, M.A.; Yadav, S.; Gupta, R.K.; Waggoner, G.R.; Deloach, A.; Calingasan, N.Y.; Beal, M.F.; Kiaei, M. Preferential PPAR-α activation reduces neuroinflammation, and blocks neurodegeneration in vivo. Hum. Mol. Genet. 2016, 25, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Besson, V.C.; Chen, X.R.; Plotkine, M.; Marchand-Verrecchia, C. Fenofibrate, a peroxisome proliferator-activated receptor alpha agonist, exerts neuroprotective effects in traumatic brain injury. Neurosci. Lett. 2005, 388, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Jana, M.; Kundu, M.; Corbett, G.T.; Rangaswamy, S.B.; Mishra, R.K.; Luan, C.H.; Gonzalez, F.J.; Pahan, K. HMG-CoA Reductase Inhibitors Bind to PPARα to Upregulate Neurotrophin Expression in the Brain and Improve Memory in Mice. Cell Metab. 2015, 22, 253–265. [Google Scholar] [CrossRef]
- Xu, J.; Racke, M.K.; Drew, P.D. Peroxisome proliferator-activated receptor-alpha agonist fenofibrate regulates IL-12 family cytokine expression in the CNS: Relevance to multiple sclerosis. J. Neurochem. 2007, 103, 1801–1810. [Google Scholar] [CrossRef]
- Johri, A.; Calingasan, N.Y.; Hennessey, T.M.; Sharma, A.; Yang, L.; Wille, E.; Chandra, A.; Beal, M.F. Pharmacologic activation of mitochondrial biogenesis exerts widespread beneficial effects in a transgenic mouse model of Huntington’s disease. Hum. Mol. Genet. 2012, 21, 1124–1137. [Google Scholar] [CrossRef]
- Kreisler, A.; Gelé, P.; Wiart, J.F.; Lhermitte, M.; Destée, A.; Bordet, R. Lipid-lowering drugs in the MPTP mouse model of Parkinson’s disease: Fenofibrate has a neuroprotective effect, whereas bezafibrate and HMG-CoA reductase inhibitors do not. Brain Res. 2007, 1135, 77–84. [Google Scholar] [CrossRef]
- Schnegg, C.I.; Robbins, M.E. Neuroprotective Mechanisms of PPARδ: Modulation of Oxidative Stress and Inflammatory Processes. PPAR Res. 2011, 2011, 373560. [Google Scholar] [CrossRef]
- Chang, G.Q.; Karatayev, O.; Lukatskaya, O.; Leibowitz, S.F. Prenatal fat exposure and hypothalamic PPAR β/δ: Possible relationship to increased neurogenesis of orexigenic peptide neurons. Peptides 2016, 79, 16–26. [Google Scholar] [CrossRef]
- Gold, P.W.; Licinio, J.; Pavlatou, M.G. Pathological parainflammation and endoplasmic reticulum stress in depression: Potential translational targets through the CNS insulin, klotho and PPAR-γ systems. Mol. Psychiatry 2013, 18, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Cramer, P.E.; Cirrito, J.R.; Wesson, D.W.; Lee, C.Y.; Karlo, J.C.; Zinn, A.E.; Casali, B.T.; Restivo, J.L.; Goebel, W.D.; James, M.J.; et al. ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models. Science 2012, 335, 1503–1506. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhong, C. Decoding Alzheimer’s disease from perturbed cerebral glucose metabolism: Implications for diagnostic and therapeutic strategies. Prog. Neurobiol. 2013, 108, 21–43. [Google Scholar] [CrossRef] [PubMed]
- P, P.; Justin, A.; Ananda Kumar, T.D.; Chinaswamy, M.; Kumar, B.R.P. Glitazones Activate PGC-1α Signaling via PPAR-γ: A Promising Strategy for Antiparkinsonism Therapeutics. ACS Chem. Neurosci. 2021, 12, 2261–2272. [Google Scholar] [CrossRef] [PubMed]
- Landreth, G. PPARgamma agonists as new therapeutic agents for the treatment of Alzheimer’s disease. Exp. Neurol. 2006, 199, 245–248. [Google Scholar] [CrossRef] [PubMed]
- d’Angelo, M.; Castelli, V.; Catanesi, M.; Antonosante, A.; Dominguez-Benot, R.; Ippoliti, R.; Benedetti, E.; Cimini, A. PPARγ and Cognitive Performance. Int. J. Mol. Sci. 2019, 20, 5068. [Google Scholar] [CrossRef]
- Lakatos, H.F.; Thatcher, T.H.; Kottmann, R.M.; Garcia, T.M.; Phipps, R.P.; Sime, P.J. The Role of PPARs in Lung Fibrosis. PPAR Res. 2007, 2007, 71323. [Google Scholar] [CrossRef]
- Failla, M.; Genovese, T.; Mazzon, E.; Fruciano, M.; Fagone, E.; Gili, E.; Barera, A.; La Rosa, C.; Conte, E.; Crimi, N.; et al. 16,16-Dimethyl prostaglandin E2 efficacy on prevention and protection from bleomycin-induced lung injury and fibrosis. Am. J. Respir. Cell Mol. Biol. 2009, 41, 50–58. [Google Scholar] [CrossRef]
- Liu, P.; Feng, Y.; Li, H.; Chen, X.; Wang, G.; Xu, S.; Li, Y.; Zhao, L. Ferrostatin-1 alleviates lipopolysaccharide-induced acute lung injury via inhibiting ferroptosis. Cell. Mol. Biol. Lett. 2020, 25, 10. [Google Scholar] [CrossRef]
- Liu, H.; Hao, J.; Wu, C.; Liu, G.; Wang, X.; Yu, J.; Liu, Y.; Zhao, H. Eupatilin Alleviates Lipopolysaccharide-Induced Acute Lung Injury by Inhibiting Inflammation and Oxidative Stress. Med. Sci. Monit. 2019, 25, 8289–8296. [Google Scholar] [CrossRef]
- Selle, J.; Dinger, K.; Jentgen, V.; Zanetti, D.; Will, J.; Georgomanolis, T.; Vohlen, C.; Wilke, R.; Kojonazarov, B.; Klymenko, O.; et al. Maternal and perinatal obesity induce bronchial obstruction and pulmonary hypertension via IL-6-FoxO1-axis in later life. Nat. Commun. 2022, 13, 4352. [Google Scholar] [CrossRef] [PubMed]
- Mandras, S.; Kovacs, G.; Olschewski, H.; Broderick, M.; Nelsen, A.; Shen, E.; Champion, H. Combination Therapy in Pulmonary Arterial Hypertension-Targeting the Nitric Oxide and Prostacyclin Pathways. J. Cardiovasc. Pharmacol. Ther. 2021, 26, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Harrington, L.S.; Moreno, L.; Reed, A.; Wort, S.J.; Desvergne, B.; Garland, C.; Zhao, L.; Mitchell, J.A. The PPARbeta/delta agonist GW0742 relaxes pulmonary vessels and limits right heart hypertrophy in rats with hypoxia-induced pulmonary hypertension. PLoS ONE 2010, 5, e9526. [Google Scholar] [CrossRef]
- Fukumoto, K.; Yano, Y.; Virgona, N.; Hagiwara, H.; Sato, H.; Senba, H.; Suzuki, K.; Asano, R.; Yamada, K.; Yano, T. Peroxisome proliferator-activated receptor delta as a molecular target to regulate lung cancer cell growth. FEBS Lett. 2005, 579, 3829–3836. [Google Scholar] [CrossRef] [PubMed]
- Keith, R.L.; Miller, Y.E.; Hudish, T.M.; Girod, C.E.; Sotto-Santiago, S.; Franklin, W.A.; Nemenoff, R.A.; March, T.H.; Nana-Sinkam, S.P.; Geraci, M.W. Pulmonary prostacyclin synthase overexpression chemoprevents tobacco smoke lung carcinogenesis in mice. Cancer Res. 2004, 64, 5897–5904. [Google Scholar] [CrossRef]
- Huang, T.H.; Razmovski-Naumovski, V.; Kota, B.P.; Lin, D.S.; Roufogalis, B.D. The pathophysiological function of peroxisome proliferator-activated receptor-gamma in lung-related diseases. Respir. Res. 2005, 6, 102. [Google Scholar] [CrossRef] [PubMed]
- Cerny, L.; Torday, J.S.; Rehan, V.K. Prevention and treatment of bronchopulmonary dysplasia: Contemporary status and future outlook. Lung 2008, 186, 75–89. [Google Scholar] [CrossRef]
- Joss-Moore, L.A.; Albertine, K.H.; Lane, R.H. Epigenetics and the developmental origins of lung disease. Mol. Genet. Metab. 2011, 104, 61–66. [Google Scholar] [CrossRef]
- Joss-Moore, L.A.; Metcalfe, D.B.; Albertine, K.H.; McKnight, R.A.; Lane, R.H. Epigenetics and fetal adaptation to perinatal events: Diversity through fidelity. J. Anim. Sci. 2010, 88, E216–E222. [Google Scholar] [CrossRef][Green Version]
- Pattenden, S.; Antova, T.; Neuberger, M.; Nikiforov, B.; De Sario, M.; Grize, L.; Heinrich, J.; Hruba, F.; Janssen, N.; Luttmann-Gibson, H.; et al. Parental smoking and children’s respiratory health: Independent effects of prenatal and postnatal exposure. Tob. Control 2006, 15, 294–301. [Google Scholar] [CrossRef]
- Gong, M.; Liu, J.; Sakurai, R.; Corre, A.; Anthony, S.; Rehan, V.K. Perinatal nicotine exposure suppresses PPARγ epigenetically in lung alveolar interstitial fibroblasts. Mol. Genet. Metab. 2015, 114, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Krebs, M.; Sakurai, R.; Torday, J.S.; Rehan, V.K. Evidence for in vivo nicotine-induced alveolar interstitial fibroblast-to-myofibroblast transdifferentiation. Exp. Lung Res. 2010, 36, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Rehan, V.K.; Wang, Y.; Sugano, S.; Santos, J.; Patel, S.; Sakurai, R.; Boros, L.G.; Lee, W.P.; Torday, J.S. In utero nicotine exposure alters fetal rat lung alveolar type II cell proliferation, differentiation, and metabolism. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, L323–L333. [Google Scholar] [CrossRef] [PubMed]
- Montaigne, D.; Butruille, L.; Staels, B. PPAR control of metabolism and cardiovascular functions. Nat. Rev. Cardiol. 2021, 18, 809–823. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Kim, J. Peroxisome Proliferator-Activated Receptors and the Heart: Lessons from the Past and Future Directions. PPAR Res. 2015, 2015, 271983. [Google Scholar] [CrossRef]
- Kersten, S.; Desvergne, B.; Wahli, W. Roles of PPARs in health and disease. Nature 2000, 405, 421–424. [Google Scholar] [CrossRef]
- Watanabe, K.; Fujii, H.; Takahashi, T.; Kodama, M.; Aizawa, Y.; Ohta, Y.; Ono, T.; Hasegawa, G.; Naito, M.; Nakajima, T.; et al. Constitutive regulation of cardiac fatty acid metabolism through peroxisome proliferator-activated receptor alpha associated with age-dependent cardiac toxicity. J. Biol. Chem. 2000, 275, 22293–22299. [Google Scholar] [CrossRef]
- Capobianco, E.; Pelesson, M.; Careaga, V.; Fornes, D.; Canosa, I.; Higa, R.; Maier, M.; Jawerbaum, A. Intrauterine programming of lipid metabolic alterations in the heart of the offspring of diabetic rats is prevented by maternal diets enriched in olive oil. Mol. Nutr. Food Res. 2015, 59, 1997–2007. [Google Scholar] [CrossRef]
- Toyama, T.; Nakamura, H.; Harano, Y.; Yamauchi, N.; Morita, A.; Kirishima, T.; Minami, M.; Itoh, Y.; Okanoue, T. PPARalpha ligands activate antioxidant enzymes and suppress hepatic fibrosis in rats. Biochem. Biophys. Res. Commun. 2004, 324, 697–704. [Google Scholar] [CrossRef]
- Lecarpentier, Y.; Claes, V.; Hébert, J.L. PPARs, Cardiovascular Metabolism, and Function: Near- or Far-from-Equilibrium Pathways. PPAR Res. 2010, 2010, 783273. [Google Scholar] [CrossRef]
- Son, N.H.; Park, T.S.; Yamashita, H.; Yokoyama, M.; Huggins, L.A.; Okajima, K.; Homma, S.; Szabolcs, M.J.; Huang, L.S.; Goldberg, I.J. Cardiomyocyte expression of PPARgamma leads to cardiac dysfunction in mice. J. Clin. Investig. 2007, 117, 2791–2801. [Google Scholar] [CrossRef]
- Mahaffey, K.W.; Hafley, G.; Dickerson, S.; Burns, S.; Tourt-Uhlig, S.; White, J.; Newby, L.K.; Komajda, M.; McMurray, J.; Bigelow, R.; et al. Results of a reevaluation of cardiovascular outcomes in the RECORD trial. Am. Heart J. 2013, 166, 240–249.e1. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.E.; Fu, M.; Zhang, J.; Zhu, X.; Lin, Y.; Akinbami, M.A.; Song, Q. Peroxisome proliferator-activated receptors and the cardiovascular system. Vitam. Horm. 2003, 66, 157–188. [Google Scholar] [CrossRef] [PubMed]
- Plutzky, J. The PPAR-RXR transcriptional complex in the vasculature: Energy in the balance. Circ. Res. 2011, 108, 1002–1016. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Zheng, F.; Guan, Y. PPARs and the kidney in metabolic syndrome. Am. J. Physiol. Ren. Physiol. 2008, 294, F1032–F1047. [Google Scholar] [CrossRef] [PubMed]
- Marx, N.; Sukhova, G.K.; Collins, T.; Libby, P.; Plutzky, J. PPARalpha activators inhibit cytokine-induced vascular cell adhesion molecule-1 expression in human endothelial cells. Circulation 1999, 99, 3125–3131. [Google Scholar] [CrossRef]
- Kintscher, U.; Lyon, C.; Wakino, S.; Bruemmer, D.; Feng, X.; Goetze, S.; Graf, K.; Moustakas, A.; Staels, B.; Fleck, E.; et al. PPARalpha inhibits TGF-beta-induced beta5 integrin transcription in vascular smooth muscle cells by interacting with Smad4. Circ. Res. 2002, 91, e35–e44. [Google Scholar] [CrossRef]
- Usuda, D.; Kanda, T. Peroxisome proliferator-activated receptors for hypertension. World J. Cardiol. 2014, 6, 744–754. [Google Scholar] [CrossRef]
- Tordjman, K.; Bernal-Mizrachi, C.; Zemany, L.; Weng, S.; Feng, C.; Zhang, F.; Leone, T.C.; Coleman, T.; Kelly, D.P.; Semenkovich, C.F. PPARalpha deficiency reduces insulin resistance and atherosclerosis in apoE-null mice. J. Clin. Investig. 2001, 107, 1025–1034. [Google Scholar] [CrossRef]
- Elkeles, R.S.; Diamond, J.R.; Poulter, C.; Dhanjil, S.; Nicolaides, A.N.; Mahmood, S.; Richmond, W.; Mather, H.; Sharp, P.; Feher, M.D. Cardiovascular outcomes in type 2 diabetes. A double-blind placebo-controlled study of bezafibrate: The St. Mary’s, Ealing, Northwick Park Diabetes Cardiovascular Disease Prevention (SENDCAP) Study. Diabetes Care 1998, 21, 641–648. [Google Scholar] [CrossRef]
- Zarzuelo, M.J.; Jiménez, R.; Galindo, P.; Sánchez, M.; Nieto, A.; Romero, M.; Quintela, A.M.; López-Sepúlveda, R.; Gómez-Guzmán, M.; Bailón, E.; et al. Antihypertensive effects of peroxisome proliferator-activated receptor-β activation in spontaneously hypertensive rats. Hypertension 2011, 58, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.H.; Kim, I.J.; Ma, S.K.; Kim, S.W. Rosiglitazone prevents the progression of renal injury in DOCA-salt hypertensive rats. Hypertens. Res. 2010, 33, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Zarzuelo, M.J.; Gómez-Guzmán, M.; Jiménez, R.; Quintela, A.M.; Romero, M.; Sánchez, M.; Zarzuelo, A.; Tamargo, J.; Pérez-Vizcaíno, F.; Duarte, J. Effects of peroxisome proliferator-activated receptor-β activation in endothelin-dependent hypertension. Cardiovasc. Res. 2013, 99, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Lan, C.; Fan, C.; Gong, X.; Chen, C.; Yu, C.; Wang, J.; Luo, X.; Hu, C.; Jose, P.A.; et al. Down-regulation of AMPK/PPARδ signalling promotes endoplasmic reticulum stress-induced endothelial dysfunction in adult rat offspring exposed to maternal diabetes. Cardiovasc. Res. 2022, 118, 2304–2316. [Google Scholar] [CrossRef] [PubMed]
- Weatherford, E.T.; Itani, H.; Keen, H.L.; Sigmund, C.D. Is peroxisome proliferator-activated receptor-gamma a new “pal” of renin? Hypertension 2007, 50, 844–846. [Google Scholar] [CrossRef]
- Duan, S.Z.; Ivashchenko, C.Y.; Whitesall, S.E.; D’Alecy, L.G.; Duquaine, D.C.; Brosius, F.C., 3rd; Gonzalez, F.J.; Vinson, C.; Pierre, M.A.; Milstone, D.S.; et al. Hypotension, lipodystrophy, and insulin resistance in generalized PPARgamma-deficient mice rescued from embryonic lethality. J. Clin. Investig. 2007, 117, 812–822. [Google Scholar] [CrossRef]
- Tontonoz, P.; Nagy, L.; Alvarez, J.G.; Thomazy, V.A.; Evans, R.M. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell 1998, 93, 241–252. [Google Scholar] [CrossRef]
- Bakris, G.; Viberti, G.; Weston, W.M.; Heise, M.; Porter, L.E.; Freed, M.I. Rosiglitazone reduces urinary albumin excretion in type II diabetes. J. Hum. Hypertens. 2003, 17, 7–12. [Google Scholar] [CrossRef]
- Dormandy, J.A.; Charbonnel, B.; Eckland, D.J.; Erdmann, E.; Massi-Benedetti, M.; Moules, I.K.; Skene, A.M.; Tan, M.H.; Lefèbvre, P.J.; Murray, G.D.; et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): A randomised controlled trial. Lancet 2005, 366, 1279–1289. [Google Scholar] [CrossRef]
- Saha, P.S.; Kim Sawtelle, K.R.; Bamberg, B.N.; Arrick, D.M.; Watt, M.J.; Scholl, J.L.; Zheng, H.; Mayhan, W.G. Rosiglitazone restores nitric oxide synthase-dependent reactivity of cerebral arterioles in rats exposed to prenatal alcohol. Alcohol. Clin. Exp. Res. 2021, 45, 1359–1369. [Google Scholar] [CrossRef]
- Jones, J.R.; Barrick, C.; Kim, K.A.; Lindner, J.; Blondeau, B.; Fujimoto, Y.; Shiota, M.; Kesterson, R.A.; Kahn, B.B.; Magnuson, M.A. Deletion of PPARgamma in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 2005, 102, 6207–6212. [Google Scholar] [CrossRef] [PubMed]
- Dubois, V.; Eeckhoute, J.; Lefebvre, P.; Staels, B. Distinct but complementary contributions of PPAR isotypes to energy homeostasis. J. Clin. Investig. 2017, 127, 1202–1214. [Google Scholar] [CrossRef] [PubMed]
- Kersten, S. Integrated physiology and systems biology of PPARα. Mol. Metab. 2014, 3, 354–371. [Google Scholar] [CrossRef] [PubMed]
- Patsouris, D.; Reddy, J.K.; Müller, M.; Kersten, S. Peroxisome proliferator-activated receptor alpha mediates the effects of high-fat diet on hepatic gene expression. Endocrinology 2006, 147, 1508–1516. [Google Scholar] [CrossRef]
- Aoyama, T.; Peters, J.M.; Iritani, N.; Nakajima, T.; Furihata, K.; Hashimoto, T.; Gonzalez, F.J. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPARalpha). J. Biol. Chem. 1998, 273, 5678–5684. [Google Scholar] [CrossRef]
- Nakajima, T.; Yang, Y.; Lu, Y.; Kamijo, Y.; Yamada, Y.; Nakamura, K.; Koyama, M.; Yamaguchi, S.; Sugiyama, E.; Tanaka, N.; et al. Decreased Fatty Acid β-Oxidation Is the Main Cause of Fatty Liver Induced by Polyunsaturated Fatty Acid Deficiency in Mice. Tohoku J. Exp. Med. 2017, 242, 229–239. [Google Scholar] [CrossRef]
- Meher, A.; Joshi, A.; Joshi, S. Differential regulation of hepatic transcription factors in the Wistar rat offspring born to dams fed folic acid, vitamin B12 deficient diets and supplemented with omega-3 fatty acids. PLoS ONE 2014, 9, e90209. [Google Scholar] [CrossRef]
- Heinecke, F.; Mazzucco, M.B.; Fornes, D.; Roberti, S.; Jawerbaum, A.; White, V. The offspring from rats fed a fatty diet display impairments in the activation of liver peroxisome proliferator activated receptor alpha and features of fatty liver disease. Mol. Cell. Endocrinol. 2020, 511, 110818. [Google Scholar] [CrossRef]
- Campioli, E.; Lau, M.; Papadopoulos, V. Effect of subacute and prenatal DINCH plasticizer exposure on rat dams and male offspring hepatic function: The role of PPAR-α. Environ. Res. 2019, 179, 108773. [Google Scholar] [CrossRef]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; Evans, R.M. PPARγ signaling and metabolism: The good, the bad and the future. Nat. Med. 2013, 19, 557–566. [Google Scholar] [CrossRef]
- Lee, Y.J.; Ko, E.H.; Kim, J.E.; Kim, E.; Lee, H.; Choi, H.; Yu, J.H.; Kim, H.J.; Seong, J.K.; Kim, K.S.; et al. Nuclear receptor PPARγ-regulated monoacylglycerol O-acyltransferase 1 (MGAT1) expression is responsible for the lipid accumulation in diet-induced hepatic steatosis. Proc. Natl. Acad. Sci. USA 2012, 109, 13656–13661. [Google Scholar] [CrossRef] [PubMed]
- Matsusue, K.; Haluzik, M.; Lambert, G.; Yim, S.H.; Gavrilova, O.; Ward, J.M.; Brewer, B., Jr.; Reitman, M.L.; Gonzalez, F.J. Liver-specific disruption of PPARgamma in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J. Clin. Investig. 2003, 111, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Matsusue, K.; Kashireddy, P.; Cao, W.Q.; Yeldandi, V.; Yeldandi, A.V.; Rao, M.S.; Gonzalez, F.J.; Reddy, J.K. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor γ1 (PPARγ1) overexpression. J. Biol. Chem. 2003, 278, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Gavrilova, O.; Haluzik, M.; Matsusue, K.; Cutson, J.J.; Johnson, L.; Dietz, K.R.; Nicol, C.J.; Vinson, C.; Gonzalez, F.J.; Reitman, M.L. Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J. Biol. Chem. 2003, 278, 34268–34276. [Google Scholar] [CrossRef] [PubMed]
- Konstantinopoulos, P.A.; Vandoros, G.P.; Sotiropoulou-Bonikou, G.; Kominea, A.; Papavassiliou, A.G. NF-κB/PPARγ and/or AP-1/PPARγ ‘on/off’ switches and induction of CBP in colon adenocarcinomas: Correlation with COX-2 expression. Int. J. Colorectal Dis. 2007, 22, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.A.; Rhee, S.D.; Jung, W.H.; Ahn, J.H.; Song, B.J.; Cheon, H.G. Selective inhibition of activated stellate cells and protection from carbon tetrachloride-induced liver injury in rats by a new PPARgamma agonist KR62776. Arch. Pharmacal Res. 2010, 33, 433–442. [Google Scholar] [CrossRef]
- Magliano, D.C.; Bargut, T.C.; de Carvalho, S.N.; Aguila, M.B.; Mandarim-de-Lacerda, C.A.; Souza-Mello, V. Peroxisome proliferator-activated receptors-alpha and gamma are targets to treat offspring from maternal diet-induced obesity in mice. PLoS ONE 2013, 8, e64258. [Google Scholar] [CrossRef]
- Bai, J.; Yao, X.; Jiang, L.; Zhang, Q.; Guan, H.; Liu, S.; Wu, W.; Qiu, T.; Gao, N.; Yang, L.; et al. Taurine protects against As2O3-induced autophagy in livers of rat offsprings through PPARγ pathway. Sci. Rep. 2016, 6, 27733. [Google Scholar] [CrossRef]
- Huang, Y.; Gao, J.M.; Zhang, C.M.; Zhao, H.C.; Zhao, Y.; Li, R.; Yu, Y.; Qiao, J. Assessment of growth and metabolism characteristics in offspring of dehydroepiandrosterone-induced polycystic ovary syndrome adults. Reproduction 2016, 152, 705–714. [Google Scholar] [CrossRef]
- Erhuma, A.; Salter, A.M.; Sculley, D.V.; Langley-Evans, S.C.; Bennett, A.J. Prenatal exposure to a low-protein diet programs disordered regulation of lipid metabolism in the aging rat. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1702–E1714. [Google Scholar] [CrossRef]
- Huang, J.; Jia, Y.; Fu, T.; Viswakarma, N.; Bai, L.; Rao, M.S.; Zhu, Y.; Borensztajn, J.; Reddy, J.K. Sustained activation of PPARα by endogenous ligands increases hepatic fatty acid oxidation and prevents obesity in ob/ob mice. FASEB J. 2012, 26, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.S.; Vázquez-Carrera, M.; Montagner, A.; Sng, M.K.; Guillou, H.; Wahli, W. Transcriptional control of physiological and pathological processes by the nuclear receptor PPARβ/δ. Prog. Lipid Res. 2016, 64, 98–122. [Google Scholar] [CrossRef] [PubMed]
- Lazar, M.A. PPAR gamma, 10 years later. Biochimie 2005, 87, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Oak, S.; Tran, C.; Castillo, M.O.; Thamotharan, S.; Thamotharan, M.; Devaskar, S.U. Peroxisome proliferator-activated receptor-gamma agonist improves skeletal muscle insulin signaling in the pregestational intrauterine growth-restricted rat offspring. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E514–E524. [Google Scholar] [CrossRef][Green Version]
- Yuan, X.; Tsujimoto, K.; Hashimoto, K.; Kawahori, K.; Hanzawa, N.; Hamaguchi, M.; Seki, T.; Nawa, M.; Ehara, T.; Kitamura, Y.; et al. Epigenetic modulation of Fgf21 in the perinatal mouse liver ameliorates diet-induced obesity in adulthood. Nat. Commun. 2018, 9, 636. [Google Scholar] [CrossRef]
- Liu, J.; Sakurai, R.; Rehan, V.K. PPAR-γ agonist rosiglitazone reverses perinatal nicotine exposure-induced asthma in rat offspring. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L788–L796. [Google Scholar] [CrossRef][Green Version]
- Mirza, R.; Sharma, B. A selective peroxisome proliferator-activated receptor-γ agonist benefited propionic acid induced autism-like behavioral phenotypes in rats by attenuation of neuroinflammation and oxidative stress. Chem.-Biol. Interact. 2019, 311, 108758. [Google Scholar] [CrossRef]
- Sağır, D. Dose-dependent effects of prenatal exposure of pioglitazone, the PPARγ agonist, on the hippocampus development and learning and memory performance of rat offspring. Toxicol. Appl. Pharmacol. 2021, 421, 115544. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).