Mitochondria-Targeted Antioxidant SkQ1 Prevents the Development of Experimental Colitis in Mice and Impairment of the Barrier Function of the Intestinal Epithelium
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Induction of Colitis
2.3. Histological Analysis
2.4. Reverse Transcription-PCR
2.5. Cell Culture
2.6. Fluorescence Microscopy
2.7. Statistical Analysis
3. Results
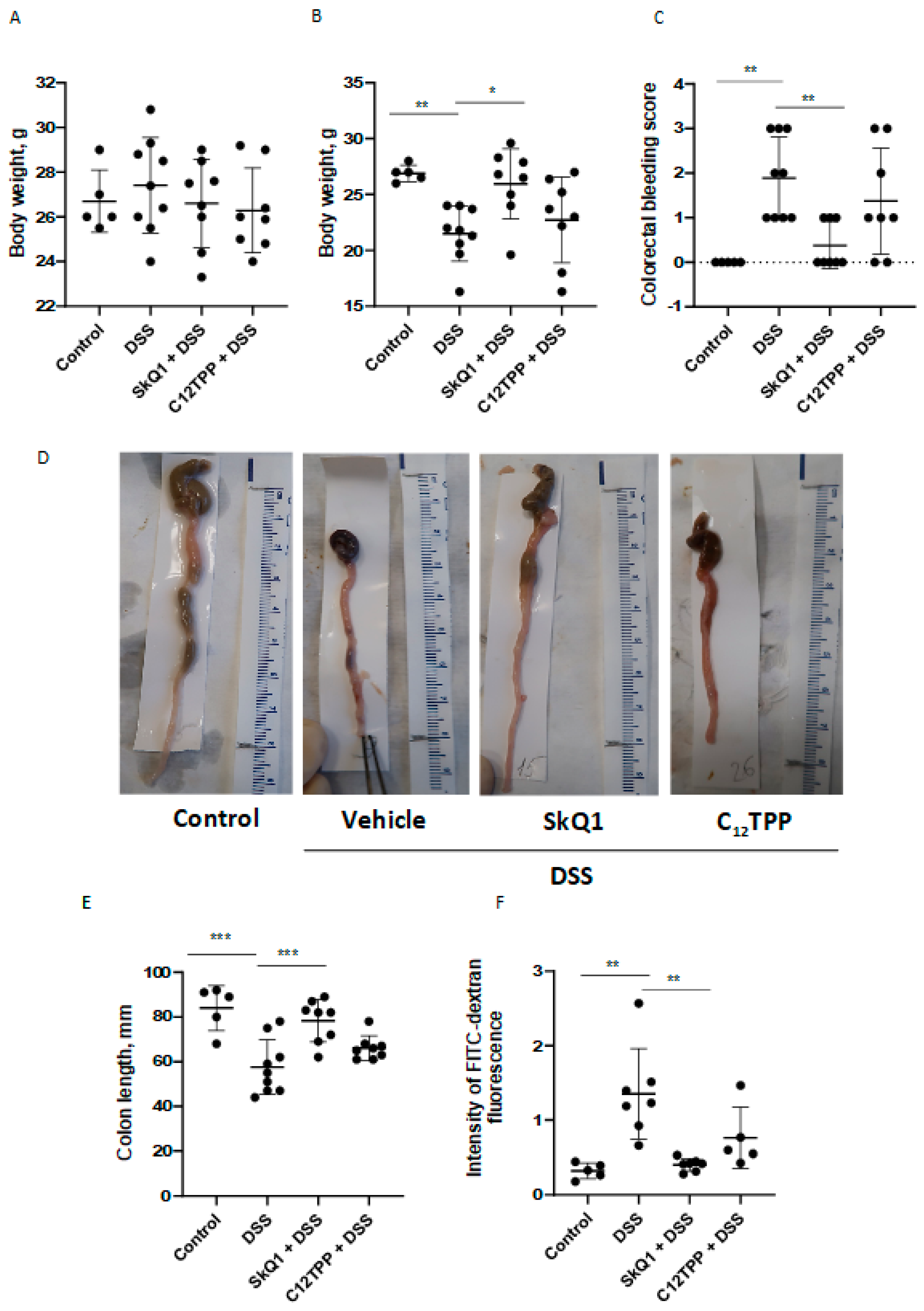
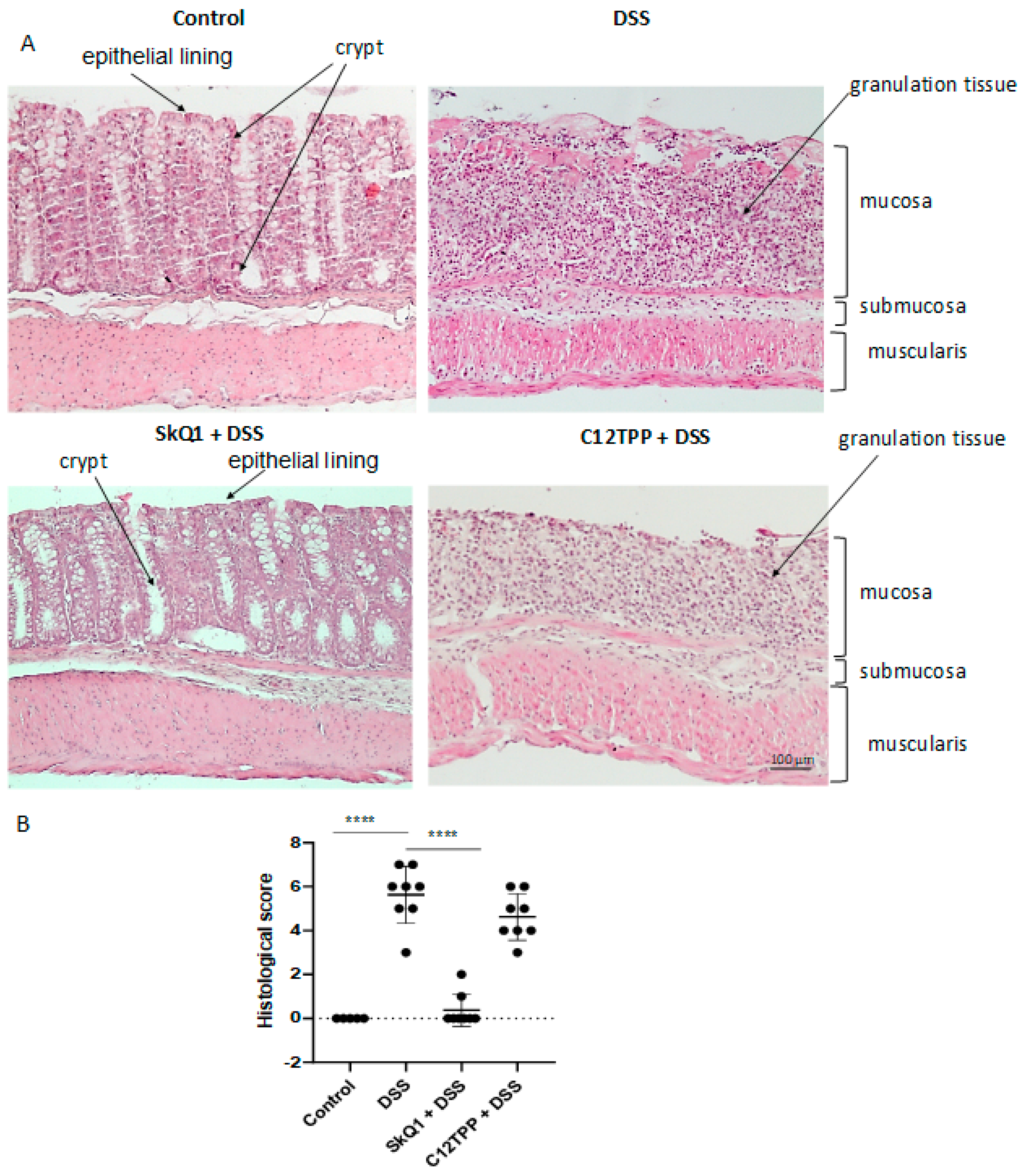
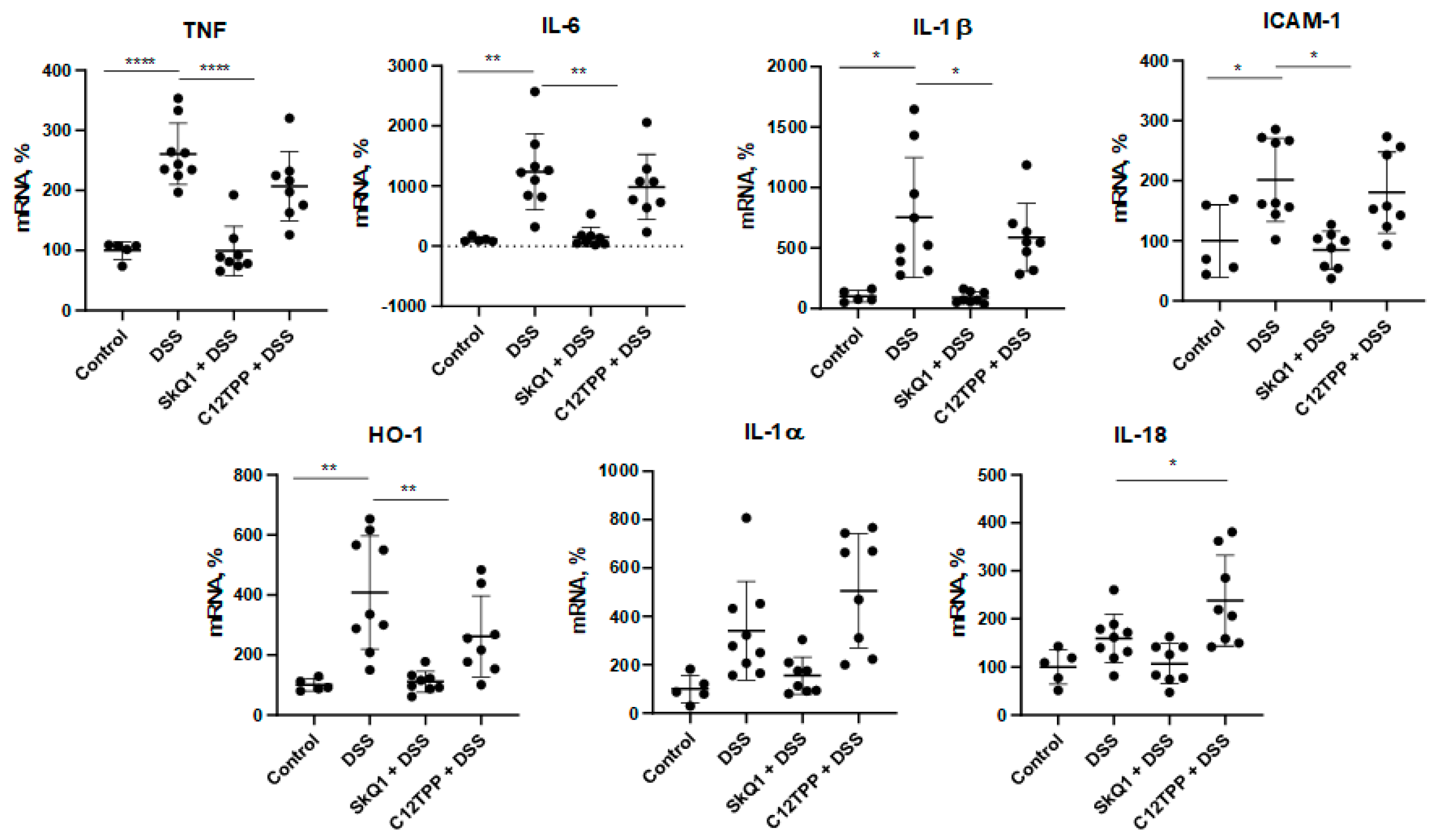
3.1. SkQ1 Prevents the Development of Dextran Sulfate Sodium-Induced Colitis in Mice
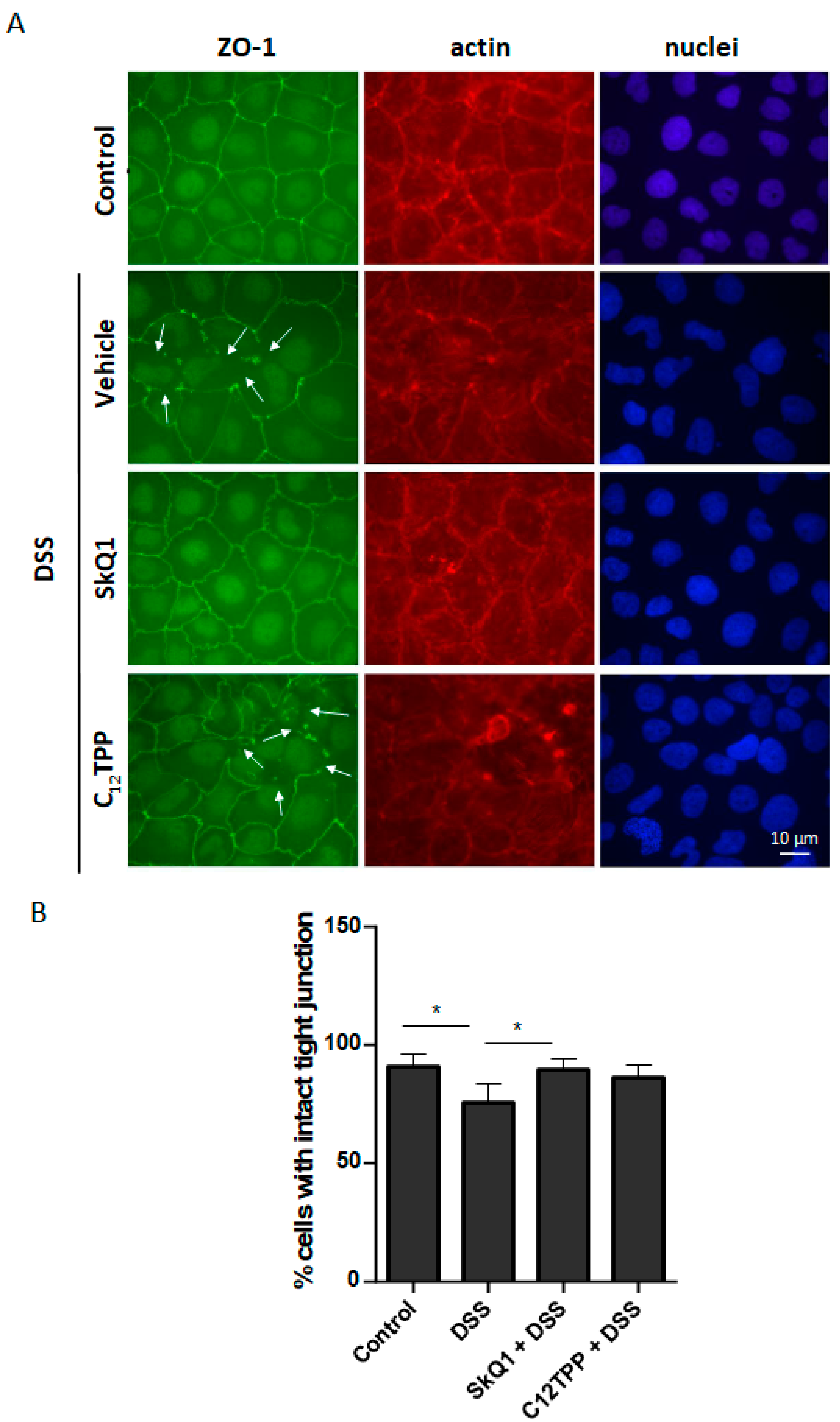
3.2. SkQ1 Prevents the DSS-Induced Disassembly of Intercellular Contacts in the Caco-2 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collins, P.; Rhodes, J. Ulcerative Colitis: Diagnosis and Management. BMJ 2006, 333, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Ray, K. IBD: The Changing Epidemiology of IBD. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 690. [Google Scholar] [PubMed]
- Kaur, A.; Goggolidou, P. Ulcerative Colitis: Understanding Its Cellular Pathology Could Provide Insights into Novel Therapies. J. Inflamm. 2020, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Regulation of Intestinal Epithelial Permeability by Tight Junctions. Cell. Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight Junctions: From Simple Barriers to Multifunctional Molecular Gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef]
- John, L.J.; Fromm, M.; Schulzke, J.-D. Epithelial Barriers in Intestinal Inflammation. Antioxid. Redox Signal. 2011, 15, 1255–1270. [Google Scholar] [CrossRef]
- Keshavarzian, A.; Banan, A.; Farhadi, A.; Komanduri, S.; Mutlu, E.; Zhang, Y.; Fields, J.Z. Increases in Free Radicals and Cytoskeletal Protein Oxidation and Nitration in the Colon of Patients with Inflammatory Bowel Disease. Gut 2003, 52, 720–728. [Google Scholar] [CrossRef]
- Yasukawa, K.; Miyakawa, R.; Yao, T.; Tsuneyoshi, M.; Utsumi, H. Non-Invasive Monitoring of Redox Status in Mice with Dextran Sodium Sulphate-Induced Colitis. Free Radic. Res. 2009, 43, 505–513. [Google Scholar] [CrossRef]
- Yasukawa, K.; Hirago, A.; Yamada, K.; Tun, X.; Ohkuma, K.; Utsumi, H. In Vivo Redox Imaging of Dextran Sodium Sulfate-Induced Colitis in Mice Using Overhauser-Enhanced Magnetic Resonance Imaging. Free Radic. Biol. Med. 2019, 136, 1–11. [Google Scholar] [CrossRef]
- Dashdorj, A.; Jyothi, K.R.; Lim, S.; Jo, A.; Nguyen, M.N.; Ha, J.; Yoon, K.-S.; Kim, H.J.; Park, J.-H.; Murphy, M.P.; et al. Mitochondria-Targeted Antioxidant MitoQ Ameliorates Experimental Mouse Colitis by Suppressing NLRP3 Inflammasome-Mediated Inflammatory Cytokines. BMC Med. 2013, 11, 178. [Google Scholar] [CrossRef]
- Rath, E.; Berger, E.; Messlik, A.; Nunes, T.; Liu, B.; Kim, S.C.; Hoogenraad, N.; Sans, M.; Sartor, R.B.; Haller, D. Induction of dsRNA-Activated Protein Kinase Links Mitochondrial Unfolded Protein Response to the Pathogenesis of Intestinal Inflammation. Gut 2012, 61, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Haberman, Y.; Karns, R.; Dexheimer, P.J.; Schirmer, M.; Somekh, J.; Jurickova, I.; Braun, T.; Novak, E.; Bauman, L.; Collins, M.H.; et al. Ulcerative Colitis Mucosal Transcriptomes Reveal Mitochondriopathy and Personalized Mechanisms Underlying Disease Severity and Treatment Response. Nat. Commun. 2019, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Yang, L.; Jiang, S.; Qian, D.; Duan, J. Excessive Apoptosis in Ulcerative Colitis: Crosstalk Between Apoptosis, ROS, ER Stress, and Intestinal Homeostasis. Inflamm. Bowel Dis. 2022, 28, 639–648. [Google Scholar] [CrossRef]
- Zinovkin, R.A.; Romaschenko, V.P.; Galkin, I.I.; Zakharova, V.V.; Pletjushkina, O.Y.; Chernyak, B.V.; Popova, E.N. Role of Mitochondrial Reactive Oxygen Species in Age-Related Inflammatory Activation of Endothelium. Aging 2014, 6, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, V.V.; Pletjushkina, O.Y.; Galkin, I.I.; Zinovkin, R.A.; Chernyak, B.V.; Krysko, D.V.; Bachert, C.; Krysko, O.; Skulachev, V.P.; Popova, E.N. Low Concentration of Uncouplers of Oxidative Phosphorylation Decreases the TNF-Induced Endothelial Permeability and Lethality in Mice. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Rogler, G.; Brand, K.; Vogl, D.; Page, S.; Hofmeister, R.; Andus, T.; Knuechel, R.; Baeuerle, P.A.; Schölmerich, J.; Gross, V. Nuclear Factor kappaB Is Activated in Macrophages and Epithelial Cells of Inflamed Intestinal Mucosa. Gastroenterology 1998, 115, 357–369. [Google Scholar] [CrossRef]
- Kaminsky, L.W.; Al-Sadi, R.; Ma, T.Y. IL-1β and the Intestinal Epithelial Tight Junction Barrier. Front. Immunol. 2021, 12, 767456. [Google Scholar] [CrossRef]
- Zielonka, J.; Joseph, J.; Sikora, A.; Hardy, M.; Ouari, O.; Vasquez-Vivar, J.; Cheng, G.; Lopez, M.; Kalyanaraman, B. Mitochondria-Targeted Triphenylphosphonium-Based Compounds: Syntheses, Mechanisms of Action, and Therapeutic and Diagnostic Applications. Chem. Rev. 2017, 117, 10043–10120. [Google Scholar] [CrossRef]
- Zinovkin, R.A.; Zamyatnin, A.A. Mitochondria-Targeted Drugs. Curr. Mol. Pharmacol. 2019, 12, 202–214. [Google Scholar] [CrossRef]
- Formentini, L.; Santacatterina, F.; de Arenas, C.N.; Stamatakis, K.; López-Martínez, D.; Logan, A.; Fresno, M.; Smits, R.; Murphy, M.P.; Cuezva, J.M. Mitochondrial ROS Production Protects the Intestine from Inflammation through Functional M2 Macrophage Polarization. Cell Rep. 2017, 19, 1202–1213. [Google Scholar] [CrossRef]
- Gwyer Findlay, E.; Sutton, G.; Ho, G.-T. The MARVEL Trial: A Phase 2b Randomised Placebo-Controlled Trial of Oral MitoQ in Moderate Ulcerative Colitis. Immunoth. Adv. 2020, 1, ltaa002. [Google Scholar] [CrossRef] [PubMed]
- Antonenko, Y.N.; Roginsky, V.A.; Pashkovskaya, A.A.; Rokitskaya, T.I.; Kotova, E.A.; Zaspa, A.A.; Chernyak, B.V.; Skulachev, V.P. Protective Effects of Mitochondria-Targeted Antioxidant SkQ in Aqueous and Lipid Membrane Environments. J. Membr. Biol. 2008, 222, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Shabalina, I.G.; Vyssokikh, M.Y.; Gibanova, N.; Csikasz, R.I.; Edgar, D.; Hallden-Waldemarson, A.; Rozhdestvenskaya, Z.; Bakeeva, L.E.; Vays, V.B.; Pustovidko, A.V.; et al. Improved Health-Span and Lifespan in mtDNA Mutator Mice Treated with the Mitochondrially Targeted Antioxidant SkQ1. Aging 2017, 9, 315–339. [Google Scholar] [CrossRef]
- Demyanenko, I.A.; Zakharova, V.V.; Ilyinskaya, O.P.; Vasilieva, T.V.; Fedorov, A.V.; Manskikh, V.N.; Zinovkin, R.A.; Pletjushkina, O.Y.; Chernyak, B.V.; Skulachev, V.P.; et al. Mitochondria-Targeted Antioxidant SkQ1 Improves Dermal Wound Healing in Genetically Diabetic Mice. Oxid. Med. Cell. Longev. 2017, 2017, 6408278. [Google Scholar] [CrossRef] [PubMed]
- Bakeeva, L.E.; Barskov, I.V.; Egorov, M.V.; Isaev, N.K.; Kapelko, V.I.; Kazachenko, A.V.; Kirpatovsky, V.I.; Kozlovsky, S.V.; Lakomkin, V.L.; Levina, S.B.; et al. Mitochondria-Targeted Plastoquinone Derivatives as Tools to Interrupt Execution of the Aging Program. 2. Treatment of Some ROS- and Age-Related Diseases (heart Arrhythmia, Heart Infarctions, Kidney Ischemia, and Stroke). Biochemistry 2008, 73, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, E.Y.; Pevzner, I.B.; Zorova, L.D.; Chernikov, V.P.; Prusov, A.N.; Kireev, I.I.; Silachev, D.N.; Skulachev, V.P.; Zorov, D.B. Mitochondrial Damage and Mitochondria-Targeted Antioxidant Protection in LPS-Induced Acute Kidney Injury. Antioxidants 2019, 8, 176. [Google Scholar] [CrossRef]
- Plotnikov, E.Y.; Morosanova, M.A.; Pevzner, I.B.; Zorova, L.D.; Manskikh, V.N.; Pulkova, N.V.; Galkina, S.I.; Skulachev, V.P.; Zorov, D.B. Protective Effect of Mitochondria-Targeted Antioxidants in an Acute Bacterial Infection. Proc. Natl. Acad. Sci. USA 2013, 110, E3100–E3108. [Google Scholar] [CrossRef]
- Demyanenko, I.A.; Popova, E.N.; Zakharova, V.V.; Ilyinskaya, O.P.; Vasilieva, T.V.; Romashchenko, V.P.; Fedorov, A.V.; Manskikh, V.N.; Skulachev, M.V.; Zinovkin, R.A.; et al. Mitochondria-Targeted Antioxidant SkQ1 Improves Impaired Dermal Wound Healing in Old Mice. Aging 2015, 7, 475–485. [Google Scholar] [CrossRef]
- Vorobjeva, N.; Prikhodko, A.; Galkin, I.; Pletjushkina, O.; Zinovkin, R.; Sud’ina, G.; Chernyak, B.; Pinegin, B. Mitochondrial Reactive Oxygen Species Are Involved in Chemoattractant-Induced Oxidative Burst and Degranulation of Human Neutrophils in Vitro. Eur. J. Cell Biol. 2017, 96, 254–265. [Google Scholar] [CrossRef]
- Vorobjeva, N.; Galkin, I.; Pletjushkina, O.; Golyshev, S.; Zinovkin, R.; Prikhodko, A.; Pinegin, V.; Kondratenko, I.; Pinegin, B.; Chernyak, B. Mitochondrial Permeability Transition Pore Is Involved in Oxidative Burst and NETosis of Human Neutrophils. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165664. [Google Scholar] [CrossRef]
- Galkin, I.I.; Pletjushkina, O.Y.; Zinovkin, R.A.; Zakharova, V.V.; Birjukov, I.S.; Chernyak, B.V.; Popova, E.N. Mitochondria-Targeted Antioxidants Prevent TNFα-Induced Endothelial Cell Damage. Biochemistry 2014, 79, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, S.; Takuma, S.; Morimoto, M. Histological Analysis of Murine Colitis Induced by Dextran Sulfate Sodium of Different Molecular Weights. Exp. Anim. 2000, 49, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Eissa, N.; Hussein, H.; Wang, H.; Rabbi, M.F.; Bernstein, C.N.; Ghia, J.-E. Stability of Reference Genes for Messenger RNA Quantification by Real-Time PCR in Mouse Dextran Sodium Sulfate Experimental Colitis. PLoS ONE 2016, 11, e0156289. [Google Scholar] [CrossRef]
- Hu, Q.; Ren, J.; Li, G.; Wu, J.; Wu, X.; Wang, G.; Gu, G.; Ren, H.; Hong, Z.; Li, J. The Mitochondrially Targeted Antioxidant MitoQ Protects the Intestinal Barrier by Ameliorating Mitochondrial DNA Damage via the Nrf2/ARE Signaling Pathway. Cell Death Dis. 2018, 9, 403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhou, Q.; Li, Y.; Zhang, Y.; Wu, Y. MitoQ Modulates Lipopolysaccharide-Induced Intestinal Barrier Dysfunction via Regulating Nrf2 Signaling. Mediators Inflamm. 2020, 2020, 3276148. [Google Scholar] [CrossRef]
- Piotrowska, M.; Swierczynski, M.; Fichna, J.; Piechota-Polanczyk, A. The Nrf2 in the Pathophysiology of the Intestine: Molecular Mechanisms and Therapeutic Implications for Inflammatory Bowel Diseases. Pharmacol. Res. 2021, 163, 105243. [Google Scholar] [CrossRef]
- Wang, Y.; Hekimi, S. Understanding Ubiquinone. Trends Cell Biol. 2016, 26, 367–378. [Google Scholar] [CrossRef]
- Furfaro, A.L.; Traverso, N.; Domenicotti, C.; Piras, S.; Moretta, L.; Marinari, U.M.; Pronzato, M.A.; Nitti, M. The Nrf2/HO-1 Axis in Cancer Cell Growth and Chemoresistance. Oxid. Med. Cell Longev. 2016, 2016, 1958174. [Google Scholar] [CrossRef]
- Antonenko, Y.N.; Avetisyan, A.V.; Bakeeva, L.E.; Chernyak, B.V.; Chertkov, V.A.; Domnina, L.V.; Ivanova, O.Y.; Izyumov, D.S.; Khailova, L.S.; Klishin, S.S.; et al. Mitochondria-Targeted Plastoquinone Derivatives as Tools to Interrupt Execution of the Aging Program. 1. Cationic Plastoquinone Derivatives: Synthesis and in Vitro Studies. Biochemistry 2008, 73, 1273–1287. [Google Scholar] [CrossRef]
- Wang, A.; Keita, Å.V.; Phan, V.; McKay, C.M.; Schoultz, I.; Lee, J.; Murphy, M.P.; Fernando, M.; Ronaghan, N.; Balce, D.; et al. Targeting Mitochondria-Derived Reactive Oxygen Species to Reduce Epithelial Barrier Dysfunction and Colitis. Am. J. Pathol. 2014, 184, 2516–2527. [Google Scholar] [CrossRef]
- Jackson, D.N.; Panopoulos, M.; Neumann, W.L.; Turner, K.; Cantarel, B.L.; Thompson-Snipes, L.; Dassopoulos, T.; Feagins, L.A.; Souza, R.F.; Mills, J.C.; et al. Mitochondrial Dysfunction during Loss of Prohibitin 1 Triggers Paneth Cell Defects and Ileitis. Gut 2020, 69, 1928–1938. [Google Scholar] [CrossRef] [PubMed]
- Theiss, A.L.; Idell, R.D.; Srinivasan, S.; Klapproth, J.-M.; Jones, D.P.; Merlin, D.; Sitaraman, S.V. Prohibitin Protects against Oxidative Stress in Intestinal Epithelial Cells. FASEB J. 2007, 21, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Poritz, L.S.; Garver, K.I.; Green, C.; Fitzpatrick, L.; Ruggiero, F.; Koltun, W.A. Loss of the Tight Junction Protein ZO-1 in Dextran Sulfate Sodium Induced Colitis. J. Surg. Res. 2007, 140, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Zehendner, C.M.; Librizzi, L.; Hedrich, J.; Bauer, N.M.; Angamo, E.A.; de Curtis, M.; Luhmann, H.J. Moderate Hypoxia Followed by Reoxygenation Results in Blood-Brain Barrier Breakdown via Oxidative Stress-Dependent Tight-Junction Protein Disruption. PLoS ONE 2013, 8, e82823. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.K.; Basuroy, S.; Rao, V.U.; Karnaky, K.J., Jr.; Gupta, A. Tyrosine Phosphorylation and Dissociation of Occludin-ZO-1 and E-Cadherin-Beta-Catenin Complexes from the Cytoskeleton by Oxidative Stress. Biochem. J. 2002, 368, 471–481. [Google Scholar] [CrossRef]
- Fukui, A.; Naito, Y.; Handa, O.; Kugai, M.; Tsuji, T.; Yoriki, H.; Qin, Y.; Adachi, S.; Higashimura, Y.; Mizushima, K.; et al. Acetyl Salicylic Acid Induces Damage to Intestinal Epithelial Cells by Oxidation-Related Modifications of ZO-1. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G927–G936. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Fink, M.P.; Delude, R.L. Proinflammatory Cytokines Cause NO*-Dependent and -Independent Changes in Expression and Localization of Tight Junction Proteins in Intestinal Epithelial Cells. Shock 2003, 19, 229–237. [Google Scholar] [CrossRef]
- Gangwar, R.; Meena, A.S.; Shukla, P.K.; Nagaraja, A.S.; Dorniak, P.L.; Pallikuth, S.; Waters, C.M.; Sood, A.; Rao, R. Calcium-Mediated Oxidative Stress: A Common Mechanism in Tight Junction Disruption by Different Types of Cellular Stress. Biochem. J. 2017, 474, 731–749. [Google Scholar] [CrossRef]
- Ivanov, A.I.; Parkos, C.A.; Nusrat, A. Cytoskeletal Regulation of Epithelial Barrier Function during Inflammation. Am. J. Pathol. 2010, 177, 512–524. [Google Scholar] [CrossRef]
- Tojkander, S.; Gateva, G.; Lappalainen, P. Actin Stress Fibers—Assembly, Dynamics and Biological Roles. J. Cell Sci. 2012, 125, 1855–1864. [Google Scholar] [CrossRef]
- Yu, D.; Marchiando, A.M.; Weber, C.R.; Raleigh, D.R.; Wang, Y.; Shen, L.; Turner, J.R. MLCK-Dependent Exchange and Actin Binding Region-Dependent Anchoring of ZO-1 Regulate Tight Junction Barrier Function. Proc. Natl. Acad. Sci. USA 2010, 107, 8237–8241. [Google Scholar] [CrossRef] [PubMed]
- Graham, W.V.; He, W.; Marchiando, A.M.; Zha, J.; Singh, G.; Li, H.-S.; Biswas, A.; Ong, M.L.D.M.; Jiang, Z.-H.; Choi, W.; et al. Intracellular MLCK1 Diversion Reverses Barrier Loss to Restore Mucosal Homeostasis. Nat. Med. 2019, 25, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Seidner, D.L.; Lashner, B.A.; Brzezinski, A.; Banks, P.L.C.; Goldblum, J.; Fiocchi, C.; Katz, J.; Lichtenstein, G.R.; Anton, P.A.; Kam, L.Y.; et al. An Oral Supplement Enriched with Fish Oil, Soluble Fiber, and Antioxidants for Corticosteroid Sparing in Ulcerative Colitis: A Randomized, Controlled Trial. Clin. Gastroenterol. Hepatol. 2005, 3, 358–369. [Google Scholar] [CrossRef]
- Aghdassi, E.; Wendland, B.E.; Steinhart, A.H.; Wolman, S.L.; Jeejeebhoy, K.; Allard, J.P. Antioxidant Vitamin Supplementation in Crohn’s Disease Decreases Oxidative Stress. a Randomized Controlled Trial. Am. J. Gastroenterol. 2003, 98, 348–353. [Google Scholar]
- Neroev, V.V.; Archipova, M.M.; Bakeeva, L.E.; Fursova, A.Z.; Grigorian, E.N.; Grishanova, A.Y.; Iomdina, E.N.; Ivashchenko, Z.N.; Katargina, L.A.; Khoroshilova-Maslova, I.P.; et al. Mitochondria-Targeted Plastoquinone Derivatives as Tools to Interrupt Execution of the Aging Program. 4. Age-Related Eye Disease. SkQ1 Returns Vision to Blind Animals. Biochemistry 2008, 73, 1317–1328. [Google Scholar] [CrossRef]
- Brzheskiy, V.V.; Efimova, E.L.; Vorontsova, T.N.; Alekseev, V.N.; Gusarevich, O.G.; Shaidurova, K.N.; Ryabtseva, A.A.; Andryukhina, O.M.; Kamenskikh, T.G.; Sumarokova, E.S.; et al. Results of a Multicenter, Randomized, Double-Masked, Placebo-Controlled Clinical Study of the Efficacy and Safety of Visomitin Eye Drops in Patients with Dry Eye Syndrome. Adv. Ther. 2015, 32, 1263–1279. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedorov, A.V.; Chelombitko, M.A.; Chernyavskij, D.A.; Galkin, I.I.; Pletjushkina, O.Y.; Vasilieva, T.V.; Zinovkin, R.A.; Chernyak, B.V. Mitochondria-Targeted Antioxidant SkQ1 Prevents the Development of Experimental Colitis in Mice and Impairment of the Barrier Function of the Intestinal Epithelium. Cells 2022, 11, 3441. https://doi.org/10.3390/cells11213441
Fedorov AV, Chelombitko MA, Chernyavskij DA, Galkin II, Pletjushkina OY, Vasilieva TV, Zinovkin RA, Chernyak BV. Mitochondria-Targeted Antioxidant SkQ1 Prevents the Development of Experimental Colitis in Mice and Impairment of the Barrier Function of the Intestinal Epithelium. Cells. 2022; 11(21):3441. https://doi.org/10.3390/cells11213441
Chicago/Turabian StyleFedorov, Artem V., Maria A. Chelombitko, Daniil A. Chernyavskij, Ivan I. Galkin, Olga Yu. Pletjushkina, Tamara V. Vasilieva, Roman A. Zinovkin, and Boris V. Chernyak. 2022. "Mitochondria-Targeted Antioxidant SkQ1 Prevents the Development of Experimental Colitis in Mice and Impairment of the Barrier Function of the Intestinal Epithelium" Cells 11, no. 21: 3441. https://doi.org/10.3390/cells11213441
APA StyleFedorov, A. V., Chelombitko, M. A., Chernyavskij, D. A., Galkin, I. I., Pletjushkina, O. Y., Vasilieva, T. V., Zinovkin, R. A., & Chernyak, B. V. (2022). Mitochondria-Targeted Antioxidant SkQ1 Prevents the Development of Experimental Colitis in Mice and Impairment of the Barrier Function of the Intestinal Epithelium. Cells, 11(21), 3441. https://doi.org/10.3390/cells11213441







