Blocking Periostin Prevented Development of Inflammation in Rhabdomyolysis-Induced Acute Kidney Injury Mice Model
Abstract
1. Introduction
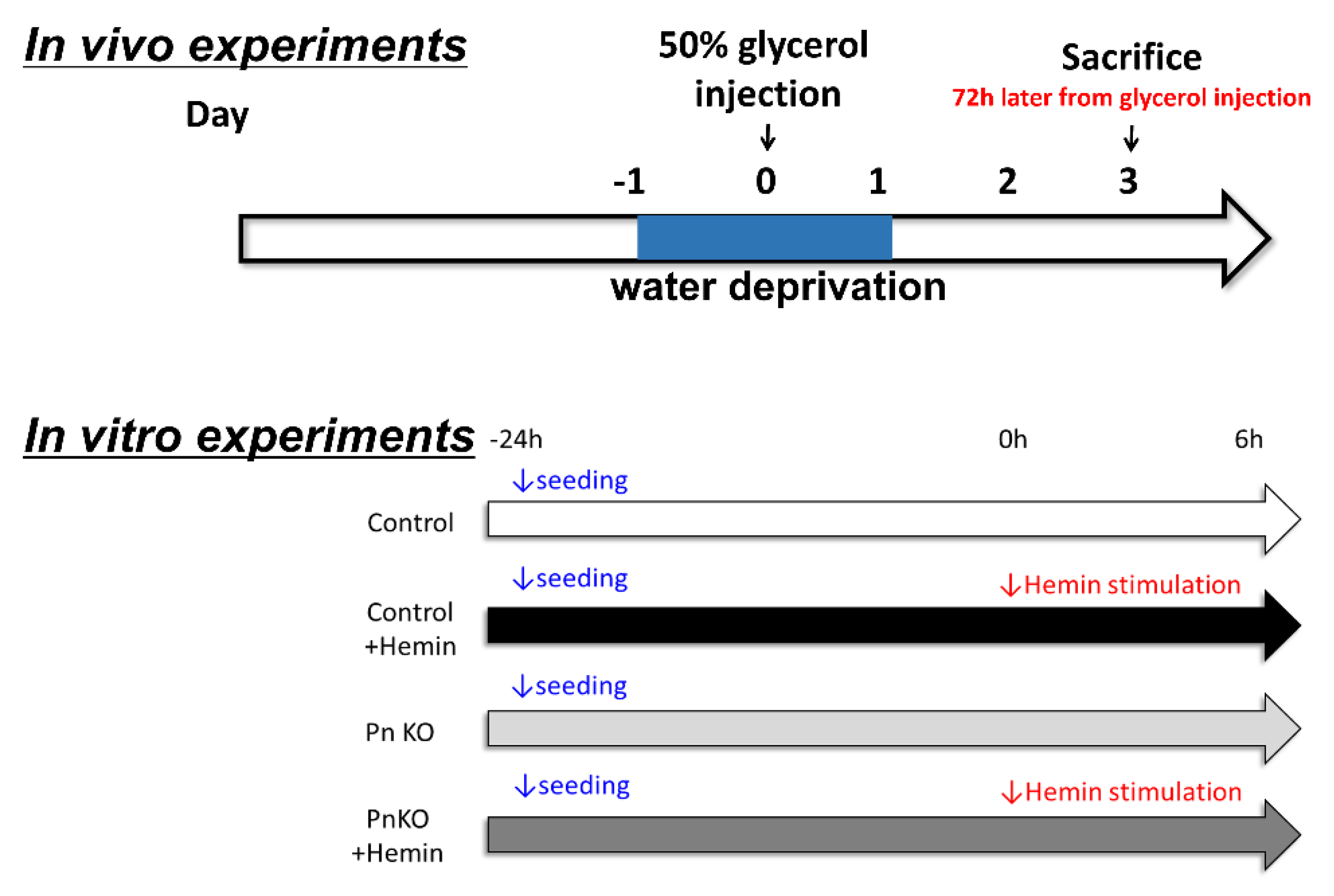
2. Methods
2.1. Ethical Statement
2.2. Cell Cultures
2.3. Evaluation of Renal Histology
2.4. Isolation of Total RNA and RT-PCR
2.5. Statistical Analysis
3. Results
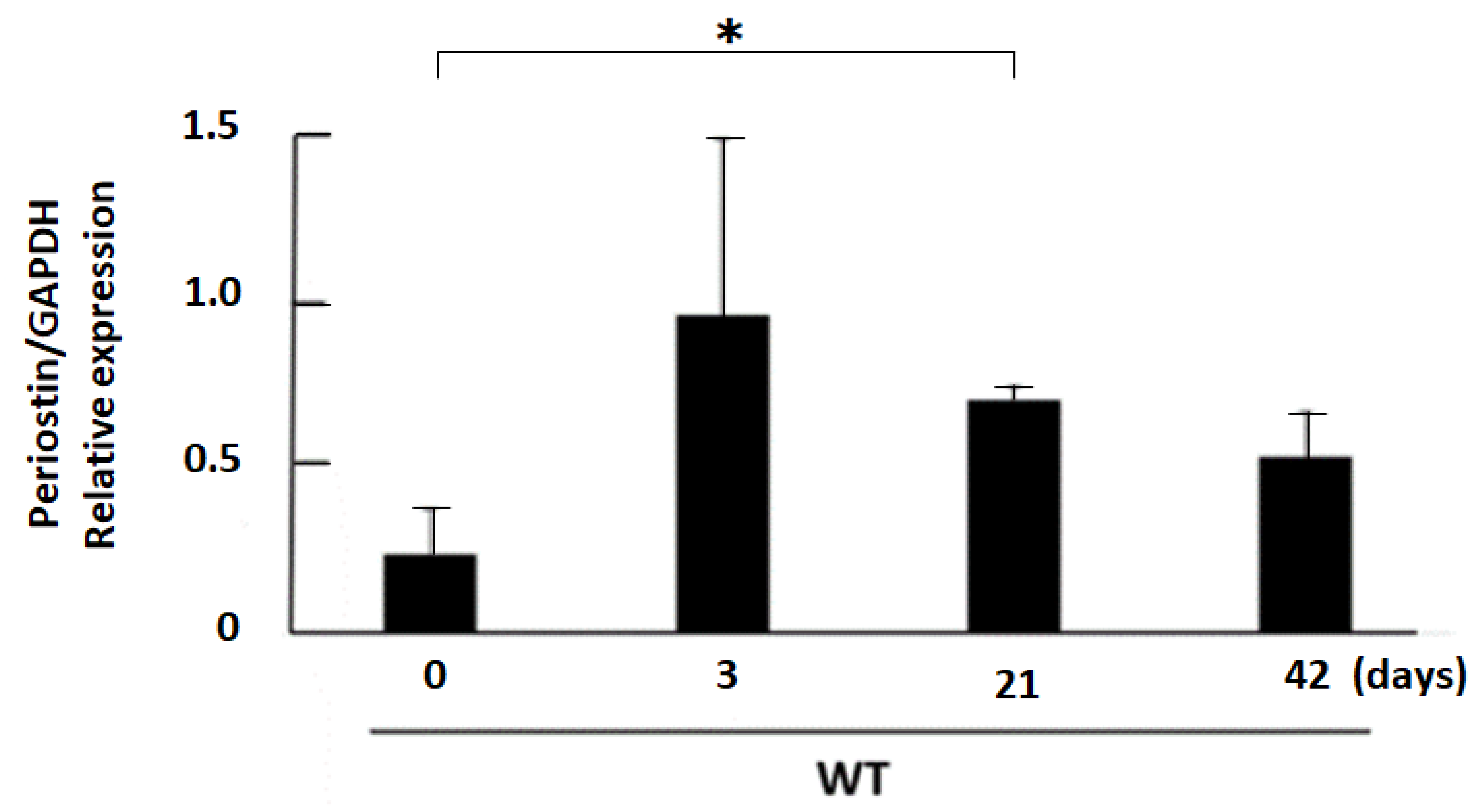
3.1. Expression Profile of Periostin in Kidneys after 50% Glycerol Injection
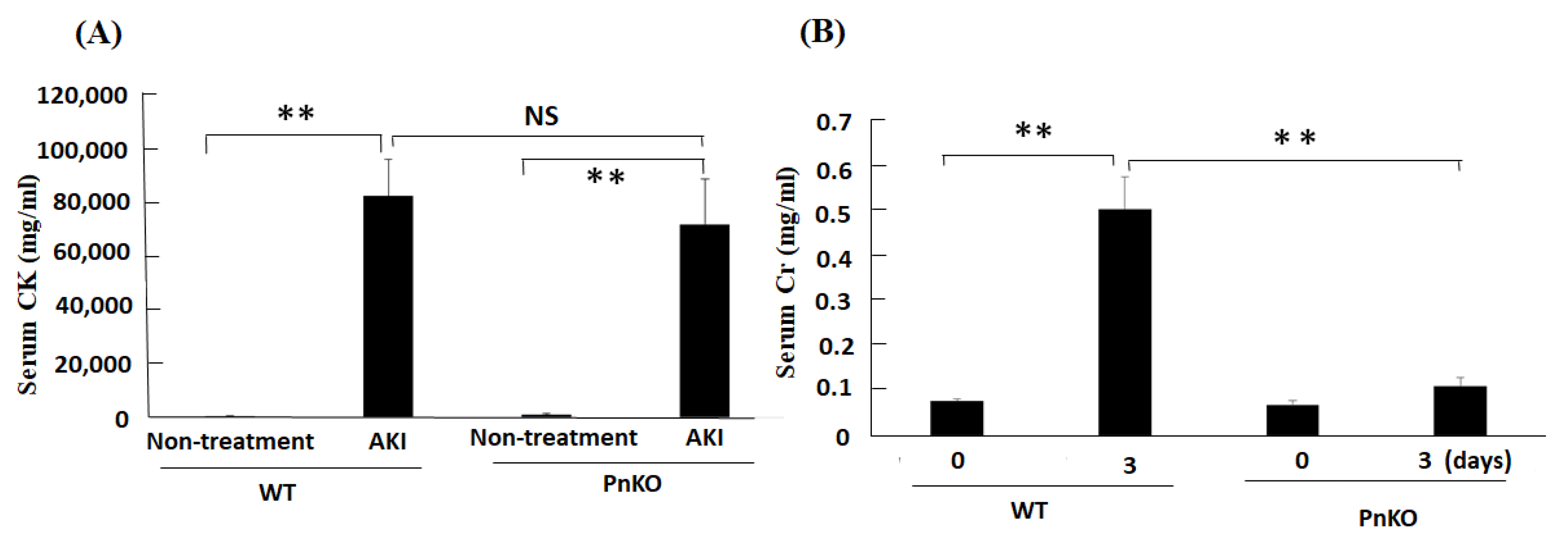
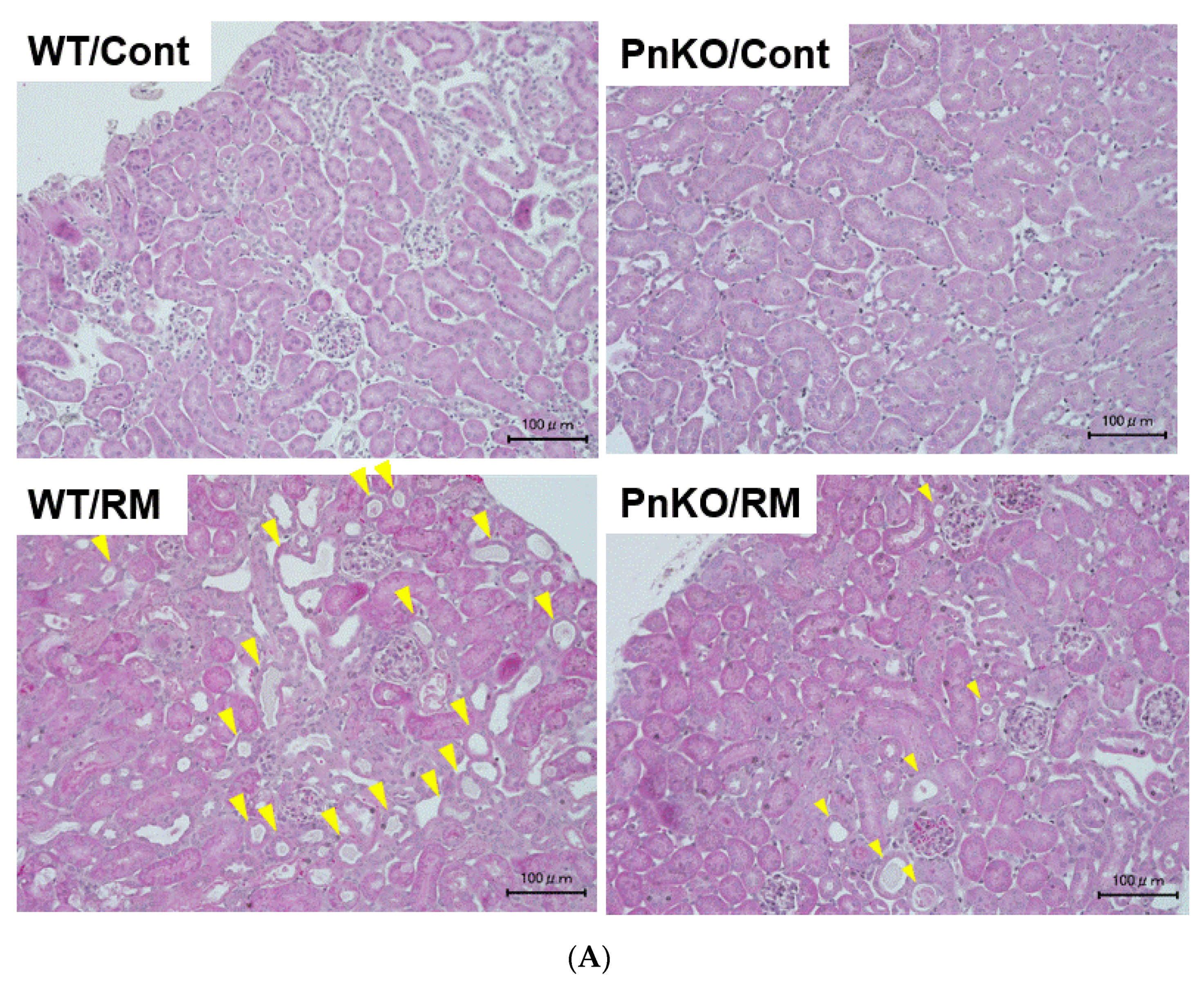
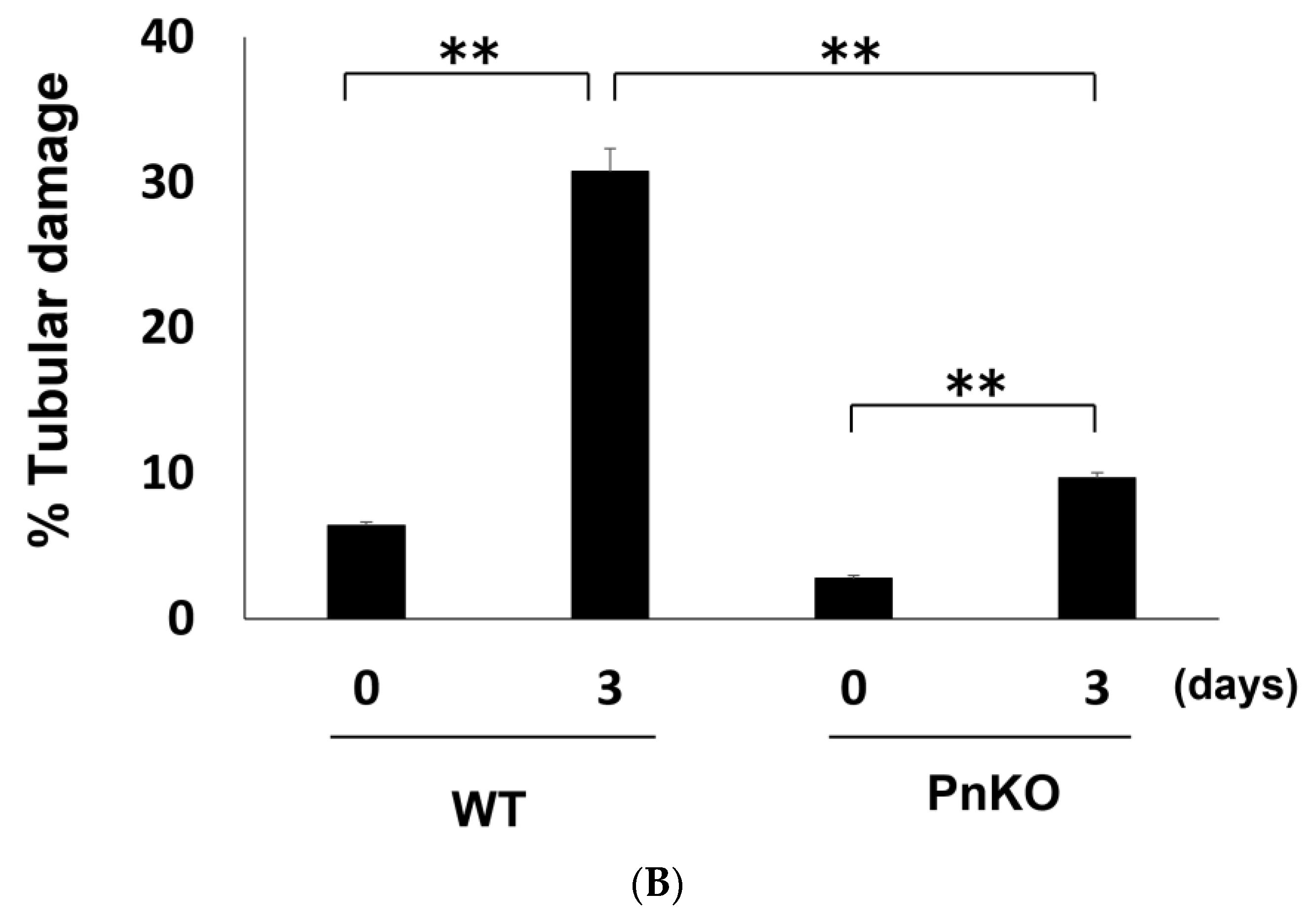
3.2. Genetic Deletion of Periostin Prevents the Development of Inflammation
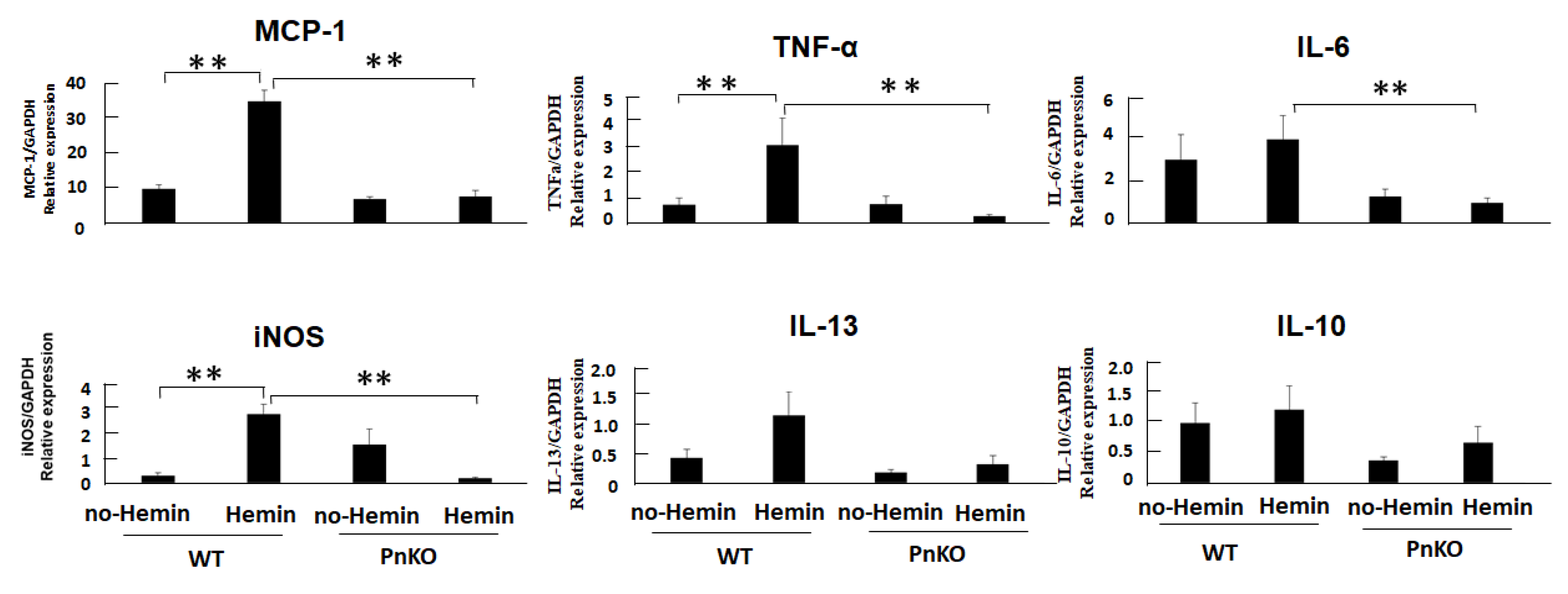
3.3. Expression of Inflammation Markers in NIH3T3 Fibroblasts after Hemin Stimulation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WT | wildtype |
| CKD | chronic kidney disease |
| ESKD | end-stage kidney disease |
| CVD | cardiovascular disease |
| AKI | acute kidney disease |
| NF-kB | nuclear factor kappa beta |
| DMEM | Dulbecco’s modified Eagle’s minimum essential medium |
| FBS | fetal bovine serum |
| H-E | hematoxylin-eosin |
| HMGB1 | high mobility group box proteins-1 |
| TGF-β | transforming growth factor-β |
| TNF-a | tumor necrosis factor alpha |
| CK | creatinine kinase |
| Mets | macrophage extracellular traps |
| iNOS | inducible nitric oxide synthase |
| MCP-1 | Monocyte chemoattractant protein−1 |
| IL-6 | Interleukin-6 |
| IL-13 | Interleukin-13 |
References
- Keith, D.S.; Nichols, G.A.; Gullion, C.M.; Brown, J.B.; Smith, D.H. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch. Intern. Med. 2004, 164, 659–663. [Google Scholar] [CrossRef]
- Tonelli, M.; Muntner, P.; Lloyd, A.; Manns, B.J.; Klarenbach, S.; Pannu, N.; James, M.T.; Hemmelgarn, B.R.; Alberta Kidney Disease Network. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: A population-level cohort study. Lancet 2012, 380, 807–814. [Google Scholar] [CrossRef]
- Chronic Kidney Disease Prognosis Consortium; Matsushita, K.; van der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; de Jong, P.E.; Coresh, J.; Gansevoort, R.T. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010, 375, 2073–2081. [Google Scholar] [PubMed]
- Sato, Y.; Yanagita, M. Immune cells and inflammation in AKI to CKD progression. Am. J. Physiol. Renal. Physiol. 2018, 315, F1501–F1512. [Google Scholar] [CrossRef] [PubMed]
- Muratsu, J.; Kamide, K.; Fujimoto, T.; Takeya, Y.; Sugimoto, K.; Taniyama, Y.; Morishima, A.; Sakaguchi, K.; Matsuzawa, Y.; Rakugi, H. The Combination of High Levels of Adiponectin and Insulin Resistance Are Affected by Aging in Non-Obese Old Peoples. Front. Endocrinol. (Lausanne) 2022, 12, 805244. [Google Scholar] [CrossRef]
- GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Carnethon, M.R. Physical Activity and Cardiovascular Disease: How Much is Enough? Am. J. Lifestyle Med. 2009, 3, 44S–49S. [Google Scholar] [CrossRef]
- Muratsu, J.; Kamide, K.; Fujimoto, T.; Takeya, Y.; Sugimoto, K.; Taniyama, Y.; Morishima, A.; Sakaguchi, K.; Rakugi, H. Lower body mass index potentiates the association between skipping breakfast and prevalence of proteinuria. Front. Endocrinol. (Lausanne) 2022, 13, 916374. [Google Scholar] [CrossRef]
- Garofalo, C.; Borrelli, S.; Minutolo, R.; Chiodini, P.; De Nicola, L.; Conte, G. A systematic review and meta-analysis suggests obesity predicts onset of chronic kidney disease in the general population. Kidney Int. 2017, 91, 1224–1235. [Google Scholar] [CrossRef]
- Kushiyama, A.; Yoshida, Y.; Kikuchi, T.; Suzawa, N.; Yamamoto, M.; Tanaka, K.; Okayasu, M.; Tahara, T.; Takao, T.; Onishi, Y.; et al. Twenty-year trend of increasing obesity in young patients with poorly controlled type 2 diabetes at first diagnosis in urban Japan. J. Diabetes Investig. 2013, 4, 540–545. [Google Scholar] [CrossRef][Green Version]
- Bellou, V.; Belbasis, L.; Tzoulaki, I.; Evangelou, E. Risk factors for type 2 diabetes mellitus: An exposure-wide umbrella review of meta-analyses. PLoS ONE 2018, 13, e0194127. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Monforte, M.; Sanchez, E.; Barrio, F.; Costa, B.; Flores-Mateo, G. Metabolic syndrome and dietary patterns: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2017, 56, 925–947. [Google Scholar] [CrossRef] [PubMed]
- Carey, R.M.; Muntner, P.; Bosworth, H.B.; Whelton, P.K. Prevention and Control of Hypertension: JACC Health Promotion Series. J. Am. Coll. Cardiol. 2018, 72, 1278–1293. [Google Scholar] [CrossRef]
- Bagley, W.H.; Yang, H.; Shah, K.H. Rhabdomyolysis. Intern. Emerg. Med. 2007, 2, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Holt, S.G.; Moore, K.P. Pathogenesis and treatment of renal dysfunction in rhabdomyolysis. Intensive Care Med. 2001, 27, 803–811. [Google Scholar] [CrossRef]
- Sakamoto, K.; Kimura, J. Mechanism of statin-induced rhabdomyolysis. J. Pharmacol. Sci. 2013, 123, 289–294. [Google Scholar] [CrossRef]
- Bosch, X.; Poch, E.; Grau, J.M. Rhabdomyolysis and acute kidney injury. N. Engl. J. Med. 2009, 361, 62–72. [Google Scholar] [CrossRef]
- Matsumoto, H. Role of serum periostin in the management of asthma and its comorbidities. Respir. Investig. 2020, 58, 144–154. [Google Scholar] [CrossRef]
- Izuhara, K.; Nunomura, S.; Nanri, Y.; Ogawa, M.; Ono, J.; Mitamura, Y.; Yoshihara, T. Periostin in inflammation and allergy. Cell Mol. Life Sci. 2017, 74, 4293–4303. [Google Scholar] [CrossRef]
- Kormann, R.; Kavvadas, P.; Placier, S.; Vandermeersch, S.; Dorison, A.; Dussaule, J.-C.; Chadjichristos, C.E.; Prakoura, N.; Chatziantoniou, C. Periostin Promotes Cell Proliferation and Macrophage Polarization to Drive Repair after AKI. J. Am. Soc. Nephrol. 2020, 31, 85–100. [Google Scholar] [CrossRef]
- Prakoura, N.; Kavvadas, P.; Kormann, R.; Dussaule, J.C.; Chadjichristos, C.E.; Chatziantoniou, C. NFkappaB-Induced Periostin Activates Integrin-beta3 Signaling to Promote Renal Injury in GN. J. Am. Soc. Nephrol. 2017, 28, 1475–1490. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.C.; Stefansson, B.V.; Jongs, N.; Chertow, G.M.; Greene, T.; Hou, F.F.; McMurray, J.J.V.; Correa-Rotter, R.; Rossing, P.; Toto, R.D.; et al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: A prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021, 9, 22–31. [Google Scholar] [CrossRef]
- Pitt, B.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Schloemer, P.; et al. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 2252–2263. [Google Scholar] [CrossRef]
- Uchida, A.; Kidokoro, K.; Sogawa, Y.; Itano, S.; Nagasu, H.; Satoh, M.; Sasaki, T.; Kashihara, N. 5-Aminolevulinic acid exerts renoprotective effect via Nrf2 activation in murine rhabdomyolysis-induced acute kidney injury. Nephrology (Carlton) 2019, 24, 28–38. [Google Scholar] [CrossRef]
- Thiel, G.; Wilson, D.R.; Arce, M.L.; Oken, D.E. Glycerol induced hemoglobinuric acute renal failure in the rat. II. The experimental model, predisposing factors, and pathophysiologic features. Nephron 1967, 4, 276–297. [Google Scholar] [CrossRef]
- Cannon, J.B.; Yunker, M.H.; Luoma, N. The effect of aggregation inhibitors and antioxidants on the stability of hemin solutions. PDA J. Pharm. Sci. Technol. 1995, 49, 77–82. [Google Scholar]
- Chiabrando, D.; Vinchi, F.; Fiorito, V.; Mercurio, S.; Tolosano, E. Heme in pathophysiology: A matter of scavenging, metabolism and trafficking across cell membranes. Front. Pharmacol. 2014, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Yuqiang, C.; Lisha, Z.; Jiejun, W.; Qin, X.; Niansong, W. Pifithrin-α ameliorates glycerol induced rhabdomyolysis and acute kidney injury by reducing p53 activation. Ren. Fail. 2022, 44, 473–481. [Google Scholar] [CrossRef]
- An, J.N.; Yang, S.H.; Kim, Y.C.; Hwang, J.H.; Park, J.Y.; Kim, D.K.; Kim, J.H.; Kim, D.W.; Hur, D.G.; Oh, Y.K.; et al. Periostin induces kidney fibrosis after acute kidney injury via the p38 MAPK pathway. Am. J. Physiol. Renal. Physiol. 2019, 316, F426–F437. [Google Scholar] [CrossRef]
- Moreno, J.A.; Martin-Cleary, C.; Gutierrez, E.; Toldos, O.; Blanco-Colio, L.M.; Praga, M.; Ortiz, A.; Egido, J. AKI associated with macroscopic glomerular hematuria: Clinical and pathophysiologic consequences. Clin. J. Am. Soc. Nephrol. 2012, 7, 175–184. [Google Scholar] [CrossRef]
- Vuletich, J.L.; Osawa, Y.; Aviram, M. Enhanced lipid oxidation by oxidatively modified myoglobin: Role of protein-bound heme. Biochem. Biophys. Res. Commun. 2000, 269, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Hill, W.D.; Su, Y.; Huang, S.; Dong, Z. Heme oxygenase-1 induction contributes to renoprotection by G-CSF during rhabdomyolysis-associated acute kidney injury. Am. J. Physiol. Renal. Physiol. 2011, 301, F162–F170. [Google Scholar] [CrossRef]
- Oishi, S.; Tsukiji, N.; Otake, S.; Oishi, N.; Sasaki, T.; Shirai, T.; Yoshikawa, Y.; Takano, K.; Shinmori, H.; Inukai, T.; et al. Heme activates platelets and exacerbates rhabdomyolysis-induced acute kidney injury via CLEC-2 and GPVI/FcRgamma. Blood Adv. 2021, 5, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, L.; Xie, J.; Li, R.; Li, G.-N.; Chen, Q.-H.; Zhang, X.-L.; Kang, L.-N.; Xu, B. Periostin expression induced by oxidative stress contributes to myocardial fibrosis in a rat model of high salt-induced hypertension. Mol. Med. Rep. 2016, 14, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Abbad, L.; Prakoura, N.; Michon, A.; Chalghoumi, R.; Reichelt-Wurm, S.; Banas, M.C.; Chatziantoniou, C. Role of Periostin and Nuclear Factor-κB Interplay in the Development of Diabetic Nephropathy. Cells 2022, 11, 2212. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Sun, M.; Wu, J.; Fang, H.; Cai, S.; An, S.; Huang, Q.; Chen, Z.; Wu, C.; Zhou, Z.; et al. SIRT1 attenuates sepsis-induced acute kidney injury via Beclin1 deacetylation-mediated autophagy activation. Cell Death Dis. 2021, 12, 217. [Google Scholar] [CrossRef]
- Kirita, Y.; Wu, H.; Uchimura, K.; Wilson, P.C.; Humphreys, B.D. Cell profiling of mouse acute kidney injury reveals conserved cellular responses to injury. Proc. Natl. Acad. Sci. USA 2020, 117, 15874–15883. [Google Scholar] [CrossRef]
- McMahon, G.M.; Zeng, X.; Waikar, S.S. A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA Intern. Med. 2013, 173, 1821–1827. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muratsu, J.; Sanada, F.; Koibuchi, N.; Shibata, K.; Katsuragi, N.; Ikebe, S.; Tsunetoshi, Y.; Rakugi, H.; Morishita, R.; Taniyama, Y. Blocking Periostin Prevented Development of Inflammation in Rhabdomyolysis-Induced Acute Kidney Injury Mice Model. Cells 2022, 11, 3388. https://doi.org/10.3390/cells11213388
Muratsu J, Sanada F, Koibuchi N, Shibata K, Katsuragi N, Ikebe S, Tsunetoshi Y, Rakugi H, Morishita R, Taniyama Y. Blocking Periostin Prevented Development of Inflammation in Rhabdomyolysis-Induced Acute Kidney Injury Mice Model. Cells. 2022; 11(21):3388. https://doi.org/10.3390/cells11213388
Chicago/Turabian StyleMuratsu, Jun, Fumihiro Sanada, Nobutaka Koibuchi, Kana Shibata, Naruto Katsuragi, Shoji Ikebe, Yasuo Tsunetoshi, Hiromi Rakugi, Ryuichi Morishita, and Yoshiaki Taniyama. 2022. "Blocking Periostin Prevented Development of Inflammation in Rhabdomyolysis-Induced Acute Kidney Injury Mice Model" Cells 11, no. 21: 3388. https://doi.org/10.3390/cells11213388
APA StyleMuratsu, J., Sanada, F., Koibuchi, N., Shibata, K., Katsuragi, N., Ikebe, S., Tsunetoshi, Y., Rakugi, H., Morishita, R., & Taniyama, Y. (2022). Blocking Periostin Prevented Development of Inflammation in Rhabdomyolysis-Induced Acute Kidney Injury Mice Model. Cells, 11(21), 3388. https://doi.org/10.3390/cells11213388





