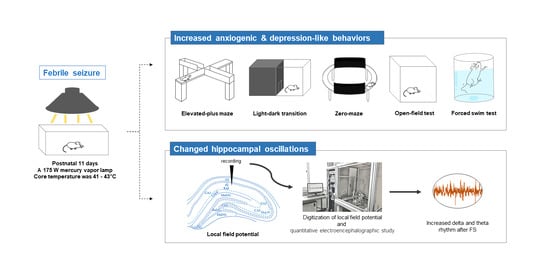
Febrile Seizures Cause Depression and Anxiogenic Behaviors in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. FS Induction
2.3. Behavioral Tests
2.3.1. Open-Field Test
2.3.2. Light–Dark Transition Test
2.3.3. Zero Maze Test
2.3.4. Elevated plus Maze Test
2.3.5. Forced Swim Test
2.4. Local Field Potentials (LFPs)
2.5. Statistical Analysis
3. Results
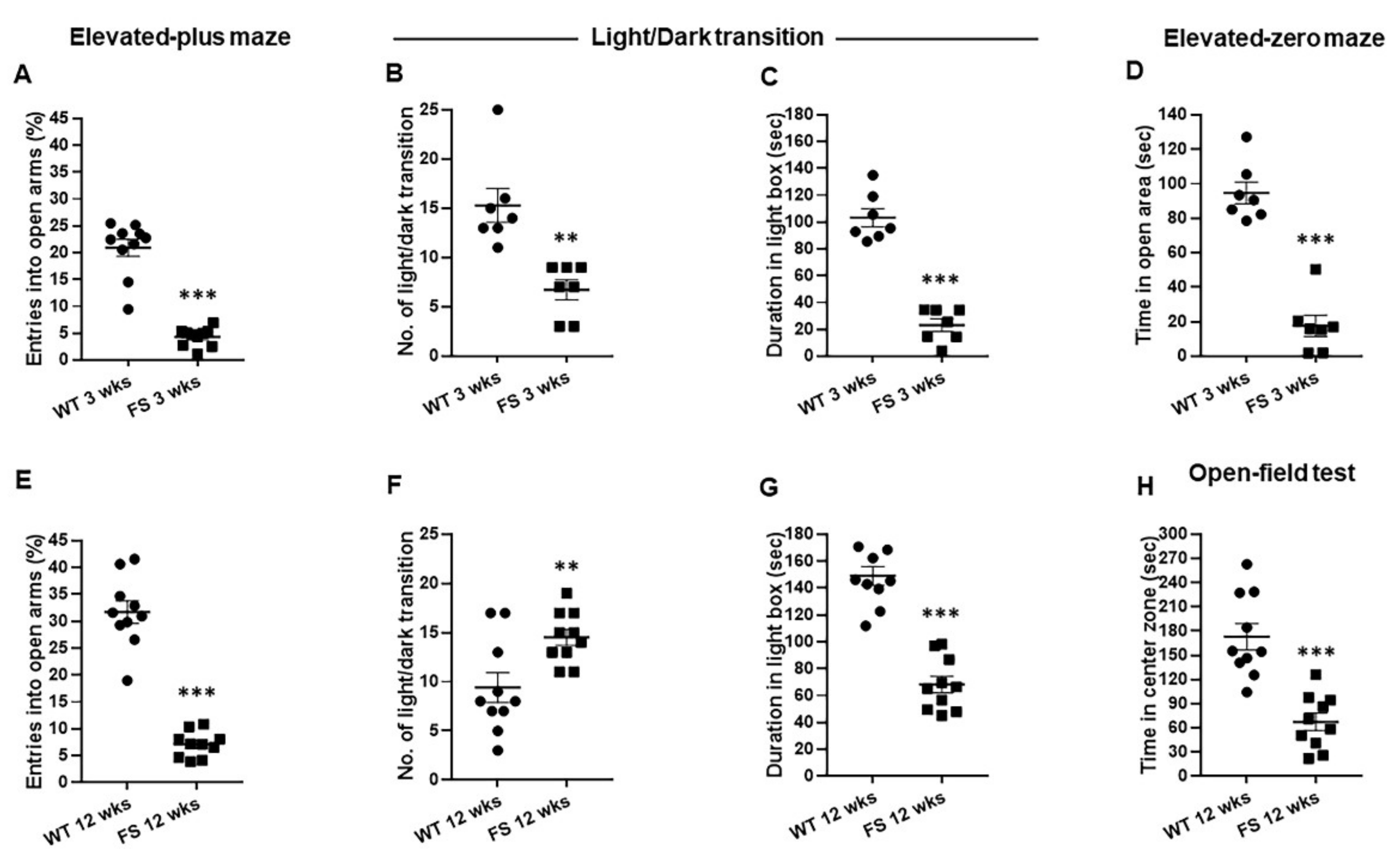
3.1. Increased Levels of Anxiety-Related Behaviors in FS Rat Model
3.2. Increased Levels of Depression in FS Rat Model
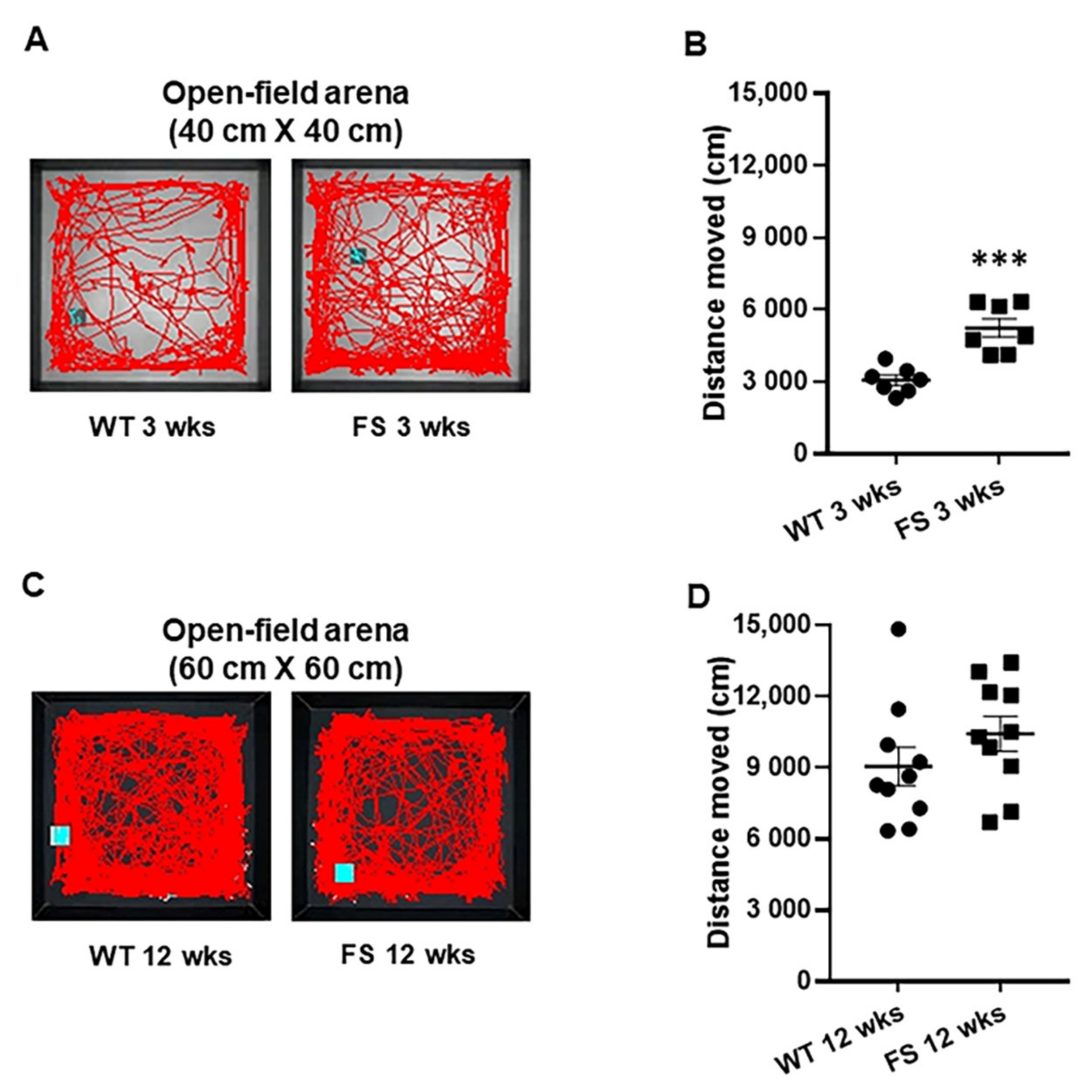
3.3. Increased Levels of Locomotion in FS Juvenile, but Not Adult, Rat Model
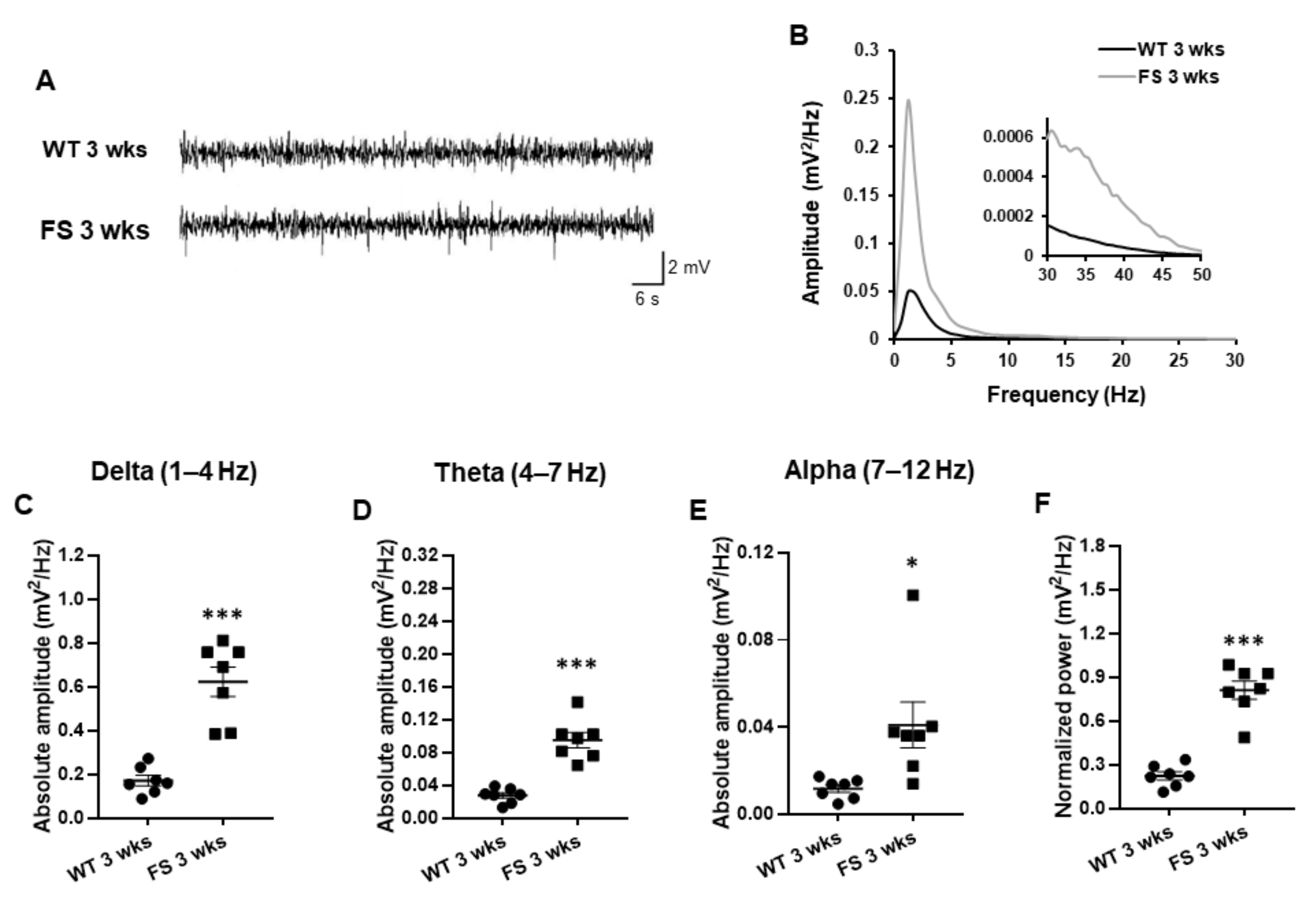
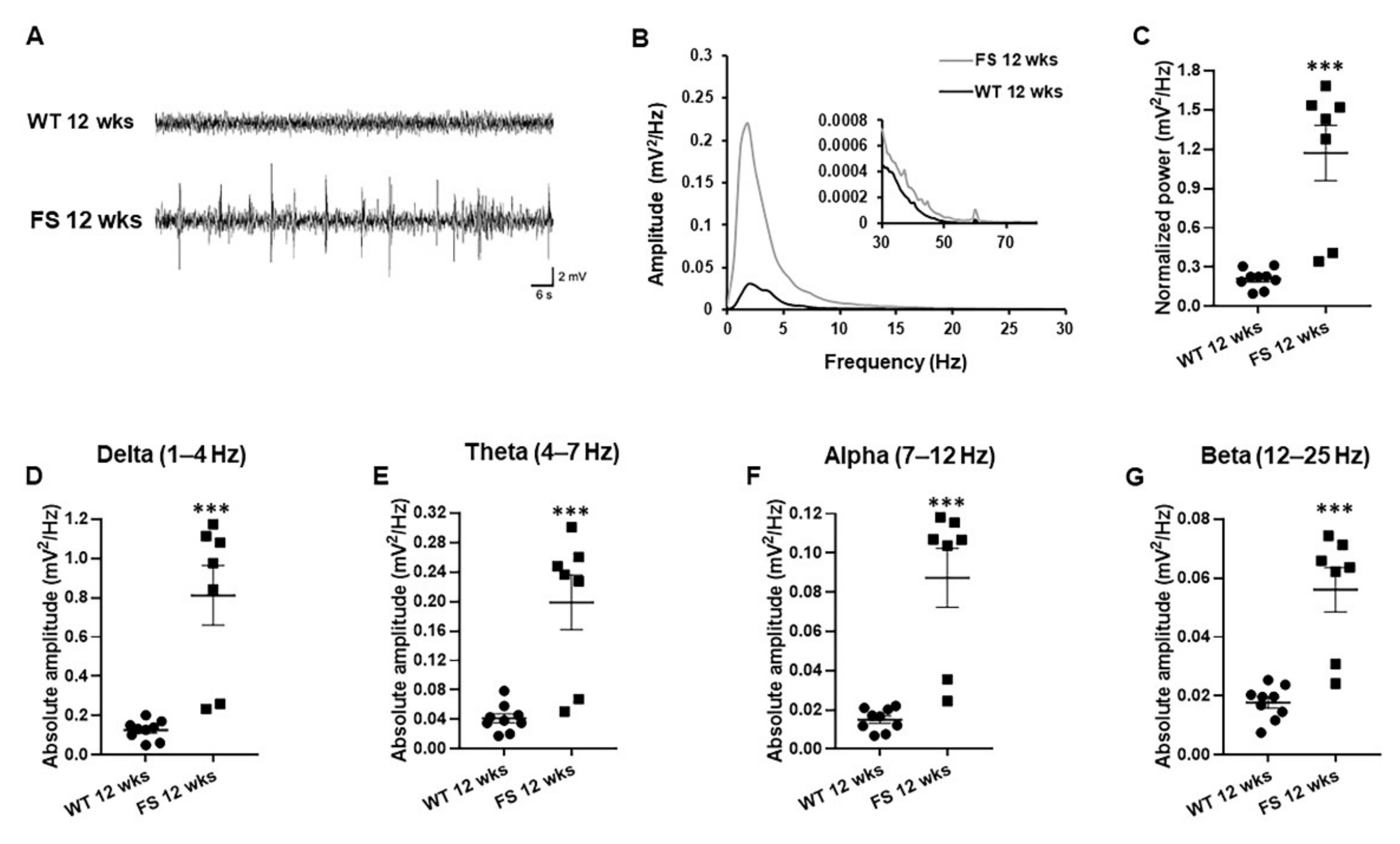
3.4. Representative Profiles of LFP after FS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bender, R.A.; Dubé, C.; Gonzalez-Vega, R.; Mina, E.W.; Baram, T.Z. Mossy Fiber Plasticity and Enhanced Hippocampal Excitability, without Hippocampal Cell Loss or Altered Neurogenesis, in an Animal Model of Prolonged Febrile Seizures. Hippocampus 2003, 13, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Connors, B.W. High Temperatures Alter Physiological Properties of Pyramidal Cells and Inhibitory Interneurons in Hippocampus. Front. Cell. Neurosci. 2012, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.; De Souza, G.E.P. Fever Induction Pathways: Evidence from Responses to Systemic or Local Cytokine Formation. Braz. J. Med. Biol. Res. 2001, 34, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Van Hook, M.J. Temperature Effects on Synaptic Transmission and Neuronal Function in the Visual Thalamus. PLoS ONE 2020, 15, e0232451. [Google Scholar] [CrossRef] [PubMed]
- Verity, C.M.; Greenwood, R.; Golding, J. Long-Term Intellectual and Behavioral Outcomes of Children with Febrile Convulsions. N. Engl. J. Med. 1998, 338, 1723–1728. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.E.; Kim, J.E.; Kim, S.C.; Kwon, O.S.; Choi, S.Y.; Kang, T.C. Hyperthermic Seizure Induces Persistent Alteration in Excitability of the Dentate Gyrus in Immature Rats. Brain Res. 2008, 1216, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.H.; Lee, K.; Sin, D.S.; Park, K.H.; Park, D.K.; Kim, D.S. Altered Functional Efficacy of Hippocampal Interneuron during Epileptogenesis Following Febrile Seizures. Brain Res. Bull. 2017, 131, 25–38. [Google Scholar] [CrossRef]
- Inostroza, M.; Cid, E.; Menendez de la Prida, L.; Sandi, C. Different Emotional Disturbances in Two Experimental Models of Temporal Lobe Epilepsy in Rats. PLoS ONE 2012, 7, e38959. [Google Scholar] [CrossRef]
- Jones, N.C.; Salzberg, M.R.; Kumar, G.; Couper, A.; Morris, M.J.; O’Brien, T.J. Elevated Anxiety and Depressive-like Behavior in a Rat Model of Genetic Generalized Epilepsy Suggesting Common Causation. Exp. Neurol. 2008, 209, 254–260. [Google Scholar] [CrossRef]
- Cornejo, B.J.; Mesches, M.H.; Benke, T.A. A Single Early-Life Seizure Impairs Short-Term Memory but Does Not Alter Spatial Learning, Recognition Memory, or Anxiety. Epilepsy Behav. 2008, 13, 585–592. [Google Scholar] [CrossRef]
- Castelhano, A.S.S.; Cassane, G.D.S.T.; Scorza, F.A.; Cysneiros, R.M. Altered Anxiety-Related and Abnormal Social Behaviors in Rats Exposed to Early Life Seizures. Front. Behav. Neurosci. 2013, 7, 36. [Google Scholar] [CrossRef]
- Cardoso, A.; Lukoyanova, E.A.; Madeira, M.D.; Lukoyanov, N.V. Seizure-Induced Structural and Functional Changes in the Rat Hippocampal Formation: Comparison between Brief Seizures and Status Epilepticus. Behav. Brain Res. 2011, 225, 538. [Google Scholar] [CrossRef]
- Covolan, L.; Mello, L.E.A.M. Temporal Profile of Neuronal Injury Following Pilocarpine or Kainic Acid-Induced Status Epilepticus. Epilepsy Res. 2000, 39, 133–152. [Google Scholar] [CrossRef]
- Phelps, E.A.; LeDoux, J.E. Contributions of the Amygdala to Emotion Processing: From Animal Models to Human Behavior. Neuron 2005, 48, 175–187. [Google Scholar] [CrossRef]
- Balleine, B.W.; Killcross, S. Parallel Incentive Processing: An Integrated View of Amygdala Function. Trends Neurosci. 2006, 29, 272–279. [Google Scholar] [CrossRef]
- Kornelsen, R.A.; Boon, F.; Leung, L.S.; Cain, D.P. The Effects of a Single Neonatally Induced Convulsion on Spatial Navigation, Locomotor Activity and Convulsion Susceptibility in the Adult Rat. Brain Res. 1996, 706, 155–159. [Google Scholar] [CrossRef]
- Kariuki, S.M.; Newton, C.R.J.C.; Prince, M.J.; Das-Munshi, J. The Association between Childhood Seizures and Later Childhood Emotional and Behavioral Problems: Findings from a Nationally Representative Birth Cohort. Psychosom. Med. 2016, 78, 620–628. [Google Scholar] [CrossRef]
- Mesquita, A.R.; Tavares, H.B.; Silva, R.; Sousa, N. Febrile Convulsions in Developing Rats Induce a Hyperanxious Phenotype Later in Life. Epilepsy Behav. 2006, 9, 401–406. [Google Scholar] [CrossRef]
- Cantalupo, G.; Meletti, S.; Miduri, A.; Mazzotta, S.; Rios-Pohl, L.; Benuzzi, F.; Pisani, F.; Tassinari, C.A.; Cossu, G. Facial Emotion Recognition in Childhood: The Effects of Febrile Seizures in the Developing Brain. Epilepsy Behav. 2013, 29, 211–216. [Google Scholar] [CrossRef]
- Baram, T.Z.; Gerth, A.; Schultz, L. Febrile Seizures: An Appropriate-Aged Model Suitable for Long-Term Studies. Dev. Brain Res. 1997, 98, 265–270. [Google Scholar] [CrossRef]
- Scantlebury, M.H.; Gibbs, S.A.; Foadjo, B.; Lema, P.; Psarropoulou, C.; Carmant, L. Febrile Seizures in the Predisposed Brain: A New Model of Temporal Lobe Epilepsy. Ann. Neurol. 2005, 58, 41–49. [Google Scholar] [CrossRef]
- Maytal, J.; Steele, R.; Eviatar, L.; Novak, G. The Value of Early Postictal EEG in Children with Complex Febrile Seizures. Epilepsia 2000, 41, 219–221. [Google Scholar] [CrossRef]
- Graves, R.C.; Oehler, K.; Tingle, L.E. Febrile Seizures: Risks, Evaluation and Prognosis. Am. Fam. Physician 2012, 85, 149–153. [Google Scholar]
- Racine, R.J. Modification of Seizure Activity by Electrical Stimulation: II. Motor Seizure. Electroencephalogr. Clin. Neurophysiol. 1972, 32, 281–294. [Google Scholar] [CrossRef]
- Gangadharan, G.; Shin, J.; Kim, S.W.; Kim, A.; Paydar, A.; Kim, D.S.; Miyazaki, T.; Watanabe, M.; Yanagawa, Y.; Kim, J.; et al. Medial Septal GABAergic Projection Neurons Promote Object Exploration Behavior and Type 2 Theta Rhythm. Proc. Natl. Acad. Sci. USA 2016, 113, 6550–6555. [Google Scholar] [CrossRef]
- Tóthová, Ľ.; Bábíčková, J.; Borbélyová, V.; Filová, B.; Šebeková, K.; Hodosy, J. Chronic Renal Insufficiency Does Not Induce Behavioral and Cognitive Alteration in Rats. Physiol. Behav. 2015, 138, 133–140. [Google Scholar] [CrossRef]
- De La Mora, M.P.; Cárdenas-Cachón, L.; Vázquez-García, M.; Crespo-Ramírez, M.; Jacobsen, K.; Höistad, M.; Agnati, L.; Fuxe, K. Anxiolytic Effects of Intra-Amygdaloid Injection of the D1 Antagonist SCH23390 in the Rat. Neurosci. Lett. 2005, 377, 101–105. [Google Scholar] [CrossRef]
- Braun, A.A.; Skelton, M.R.; Vorhees, C.V.; Williams, M.T. Comparison of the Elevated plus and Elevated Zero Mazes in Treated and Untreated Male Sprague-Dawley Rats: Effects of Anxiolytic and Anxiogenic Agents. Pharmacol. Biochem. Behav. 2011, 97, 406–415. [Google Scholar] [CrossRef]
- Buddenberg, T.E.; Komorowski, M.; Ruocco, L.A.; Silva, M.A.D.S.; Topic, B. Attenuating Effects of Testosterone on Depressive-like Behavior in the Forced Swim Test in Healthy Male Rats. Brain Res. Bull. 2009, 79, 182–186. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Brossard, G.; Hautbois, C.; Roux, S. Rodent Models of Depression: Forced Swimming and Tail Suspension Behavioral Despair Tests in Rats and Mice. Curr. Protoc. Neurosci. 2001, 14, 8–10. [Google Scholar] [CrossRef]
- Scheffer-Teixeira, R.; Belchior, H.; Leão, R.N.; Ribeiro, S.; Tort, A.B.L. On High-Frequency Field Oscillations (>100 Hz) and the Spectral Leakage of Spiking Activity. J. Neurosci. 2013, 33, 1535–1539. [Google Scholar] [CrossRef] [PubMed]
- Fuggetta, G.; Bennett, M.A.; Duke, P.A.; Young, A.M.J. Quantitative Electroencephalography as a Biomarker for Proneness toward Developing Psychosis. Schizophr. Res. 2014, 153, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.H.; Kim, S.W.; Park, D.K.; Song, H.Y.; Kim, D.S.; Gil, H.W. Altered Emotional Phenotypes in Chronic Kidney Disease Following 5/6 Nephrectomy. Brain Sci. 2021, 11, 882. [Google Scholar] [CrossRef] [PubMed]
- Di Gennaro, G.; Quarato, P.P.; Onorati, P.; Colazza, G.B.; Mari, F.; Grammaldo, L.G.; Ciccarelli, O.; Meldolesi, N.G.; Sebastiano, F.; Manfredi, M.; et al. Localizing Significance of Temporal Intermittent Rhythmic Delta Activity (TIRDA) in Drug-Resistant Focal Epilepsy. Clin. Neurophysiol. 2003, 114, 70–78. [Google Scholar] [CrossRef]
- Gelisse, P.; Serafini, A.; Velizarova, R.; Genton, P.; Crespel, A. Temporal Intermittent Delta Activity: A Marker of Juvenile Absence Epilepsy? Seizure 2011, 20, 38–41. [Google Scholar] [CrossRef]
- Danzer, S.C. Depression, Stress, Epilepsy and Adult Neurogenesis. Exp. Neurol. 2012, 233, 22–32. [Google Scholar] [CrossRef]
- Leung, A.K.C.; Hon, K.L.; Leung, T.N.H. Febrile Seizures: An Overview. Drugs Context 2018, 7, 212536. [Google Scholar] [CrossRef]
- Ettinger, A.B.; Weisbrot, D.M.; Nolan, E.E.; Gadow, K.D.; Vitale, S.A.; Andriola, M.R.; Lenn, N.J.; Novak, G.P.; Hermann, B.P. Symptoms of Depression and Anxiety in Pediatric Epilepsy Patients. Epilepsia 1998, 39, 595–599. [Google Scholar] [CrossRef]
- Castelhano, A.S.S.; Ramos, F.O.; Scorza, F.A.; Cysneiros, R.M. Early Life Seizures in Female Rats Lead to Anxiety-Related Behavior and Abnormal Social Behavior Characterized by Reduced Motivation to Novelty and Deficit in Social Discrimination. J. Neural Transm. 2015, 122, 349. [Google Scholar] [CrossRef]
- Dharmadhikari, A.S.; Tandle, A.L.; Jaiswal, S.V.; Sawant, V.A.; Vahia, V.N.; Jog, N. Frontal Theta Asymmetry as a Biomarker of Depression. East Asian Arch. Psychiatry 2018, 28, 17–22. [Google Scholar] [CrossRef]
- Shin, J.; Gireesh, G.; Kim, S.W.; Kim, D.S.; Lee, S.; Kim, Y.S.; Watanabe, M.; Shin, H.S. Phospholipase C Β4 in the Medial Septum Controls Cholinergic Theta Oscillations and Anxiety Behaviors. J. Neurosci. 2009, 29, 15375–15378. [Google Scholar] [CrossRef]
- Hoeller, A.A.; Duzzioni, M.; Duarte, F.S.; Leme, L.R.; Costa, A.P.R.; Santos, E.C.D.S.; De Pieri, C.H.; Dos Santos, A.A.; Naime, A.A.; Farina, M.; et al. GABA-A Receptor Modulators Alter Emotionality and Hippocampal Theta Rhythm in an Animal Model of Long-Lasting Anxiety. Brain Res. 2013, 1532, 21–31. [Google Scholar] [CrossRef]
- Adhikari, A.; Topiwala, M.A.; Gordon, J.A. Synchronized Activity between the Ventral Hippocampus and the Medial Prefrontal Cortex during Anxiety. Neuron 2010, 65, 257–269. [Google Scholar] [CrossRef]
- Adhikari, A.; Topiwala, M.A.; Gordon, J.A. Single Units in the Medial Prefrontal Cortex with Anxiety-Related Firing Patterns Are Preferentially Influenced by Ventral Hippocampal Activity. Neuron 2011, 71, 898–910. [Google Scholar] [CrossRef]
- Visser, A.M.; Jaddoe, V.W.; Ghassabian, A.; Schenk, J.J.; Verhulst, F.C.; Hofman, A.; Tiemeier, H.; Moll, H.A.; Arts, W.F.M. Febrile Seizures and Behavioural and Cognitive Outcomes in Preschool Children: The Generation R Study. Dev. Med. Child Neurol. 2012, 54, 1006–1011. [Google Scholar] [CrossRef]
- Epps, S.A.; Weinshenker, D. Rhythm and Blues: Animal Models of Epilepsy and Depression Comorbidity. Biochem. Pharmacol. 2013, 85, 135–146. [Google Scholar] [CrossRef]
- Russo, E.; Citraro, R.; Scicchitano, F.; Urzino, A.; Marra, R.; Rispoli, V.; De Sarro, G. Vigabatrin Has Antiepileptogenic and Antidepressant Effects in an Animal Model of Epilepsy and Depression Comorbidity. Behav. Brain Res. 2011, 225, 373–376. [Google Scholar] [CrossRef]
- Sarkisova, K.Y.; Kuznetsova, G.D.; Kulikov, M.A.; Van Luijtelaar, G. Spike-Wave Discharges Are Necessary for the Expression of Behavioral Depression-like Symptoms. Epilepsia 2010, 51, 146–160. [Google Scholar] [CrossRef]
- Bird, C.M. The Role of the Hippocampus in Recognition Memory. Cortex 2017, 93, 155–165. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, H.; Tong, L.; Li, Z.L.; Wang, L.; Zhang, C.; Yang, Q.; Yan, B. Emotion Regulation of Hippocampus Using Real-Time FMRI Neurofeedback in Healthy Human. Front. Hum. Neurosci. 2019, 13, 242. [Google Scholar] [CrossRef]
- Yamasaki, N.; Maekawa, M.; Kobayashi, K.; Kajii, Y.; Maeda, J.; Soma, M.; Takao, K.; Tanda, K.; Ohira, K.; Toyama, K.; et al. Alpha-CaMKII Deficiency Causes Immature Dentate Gyrus, a Novel Candidate Endophenotype of Psychiatric Disorders. Mol. Brain 2008, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Hagihara, H.; Takao, K.; Walton, N.M.; Matsumoto, M.; Miyakawa, T. Immature Dentate Gyrus: An Endophenotype of Neuropsychiatric Disorders. Neural Plast. 2013, 2013, 318596. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Ikeda, Y.; Sakai, A.; Yamasaki, N.; Haneda, E.; Miyakawa, T.; Suzuki, H. Reversal of Hippocampal Neuronal Maturation by Serotonergic Antidepressants. Proc. Natl. Acad. Sci. USA 2010, 107, 8434–8439. [Google Scholar] [CrossRef] [PubMed]
- Franois, J.; Ferrandon, A.; Koning, E.; Angst, M.J.; Sandner, G.; Nehlig, A. Selective Reorganization of GABAergic Transmission in Neonatal Ventral Hippocampal-Lesioned Rats. Int. J. Neuropsychopharmacol. 2009, 12, 1097–1110. [Google Scholar] [CrossRef]
- Holmes, G.L. Drug Treatment of Epilepsy Neuropsychiatric Comorbidities in Children. Pediatr. Drugs 2021, 23, 55–73. [Google Scholar] [CrossRef]
- Müller, C.J.; Gröticke, I.; Bankstahl, M.; Löscher, W. Behavioral and Cognitive Alterations, Spontaneous Seizures, and Neuropathology Developing after a Pilocarpine-Induced Status Epilepticus in C57BL/6 mice. Exp. Neurol. 2009, 219, 284–297. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.H.; Kim, S.-W.; Im, H.; Song, Y.; Kim, S.J.; Lee, Y.R.; Kim, G.W.; Hwang, C.; Park, D.-K.; Kim, D.-S. Febrile Seizures Cause Depression and Anxiogenic Behaviors in Rats. Cells 2022, 11, 3228. https://doi.org/10.3390/cells11203228
Yu YH, Kim S-W, Im H, Song Y, Kim SJ, Lee YR, Kim GW, Hwang C, Park D-K, Kim D-S. Febrile Seizures Cause Depression and Anxiogenic Behaviors in Rats. Cells. 2022; 11(20):3228. https://doi.org/10.3390/cells11203228
Chicago/Turabian StyleYu, Yeon Hee, Seong-Wook Kim, Hyuna Im, Yejin Song, Seo Jeong Kim, Yu Ran Lee, Gun Woo Kim, Changmin Hwang, Dae-Kyoon Park, and Duk-Soo Kim. 2022. "Febrile Seizures Cause Depression and Anxiogenic Behaviors in Rats" Cells 11, no. 20: 3228. https://doi.org/10.3390/cells11203228
APA StyleYu, Y. H., Kim, S.-W., Im, H., Song, Y., Kim, S. J., Lee, Y. R., Kim, G. W., Hwang, C., Park, D.-K., & Kim, D.-S. (2022). Febrile Seizures Cause Depression and Anxiogenic Behaviors in Rats. Cells, 11(20), 3228. https://doi.org/10.3390/cells11203228