Clinical Implications of COVID-19 Presence in CSF: Systematic Review of Case Reports
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Search Strategy
2.3. Eligibility Criteria
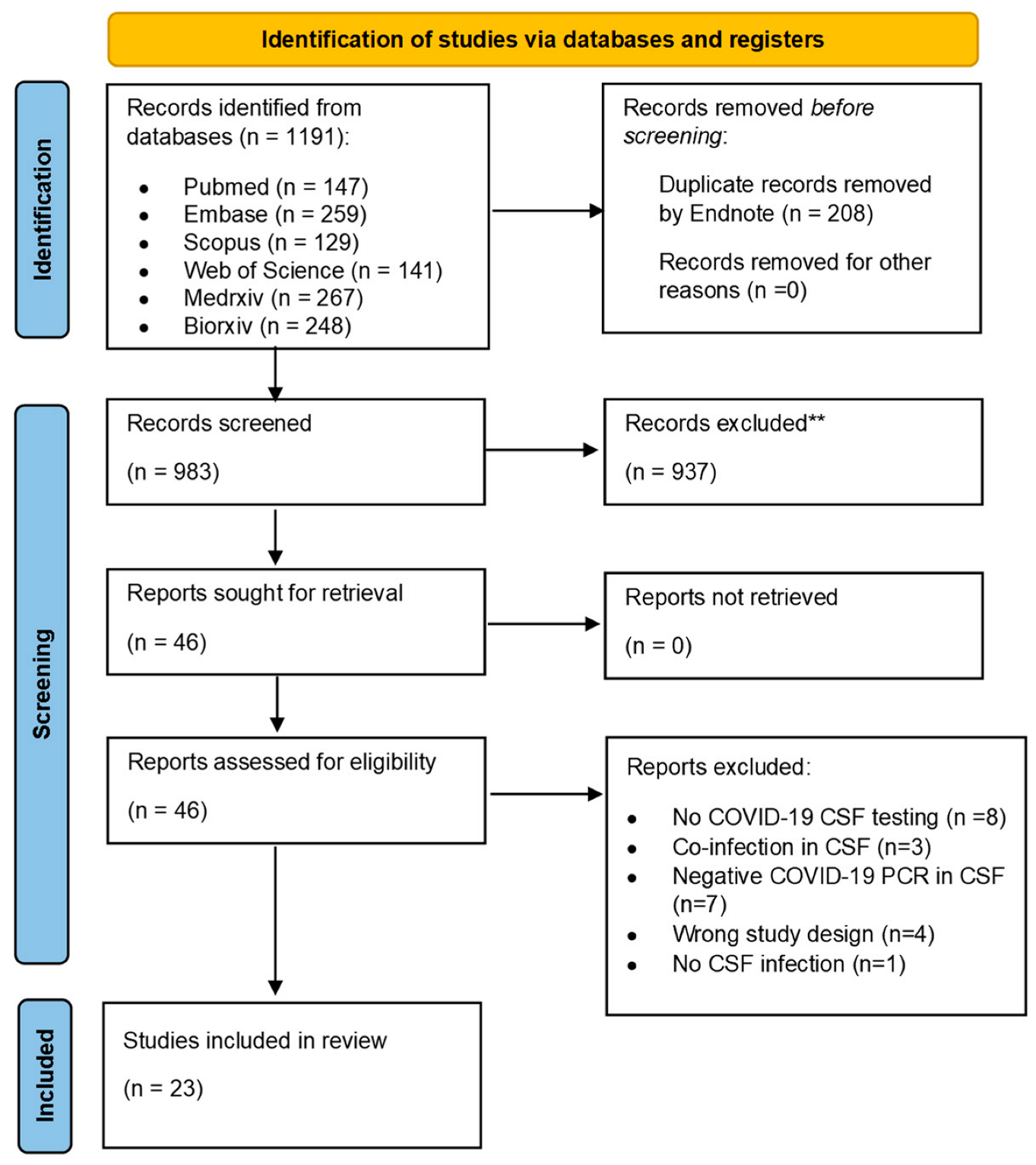
2.4. Study Selection and Screening
2.5. Data Extraction
2.6. Quality of Studies
2.7. Data Analysis
3. Results
3.1. Study Selection
3.2. Study Characteristics and Patient Demographics
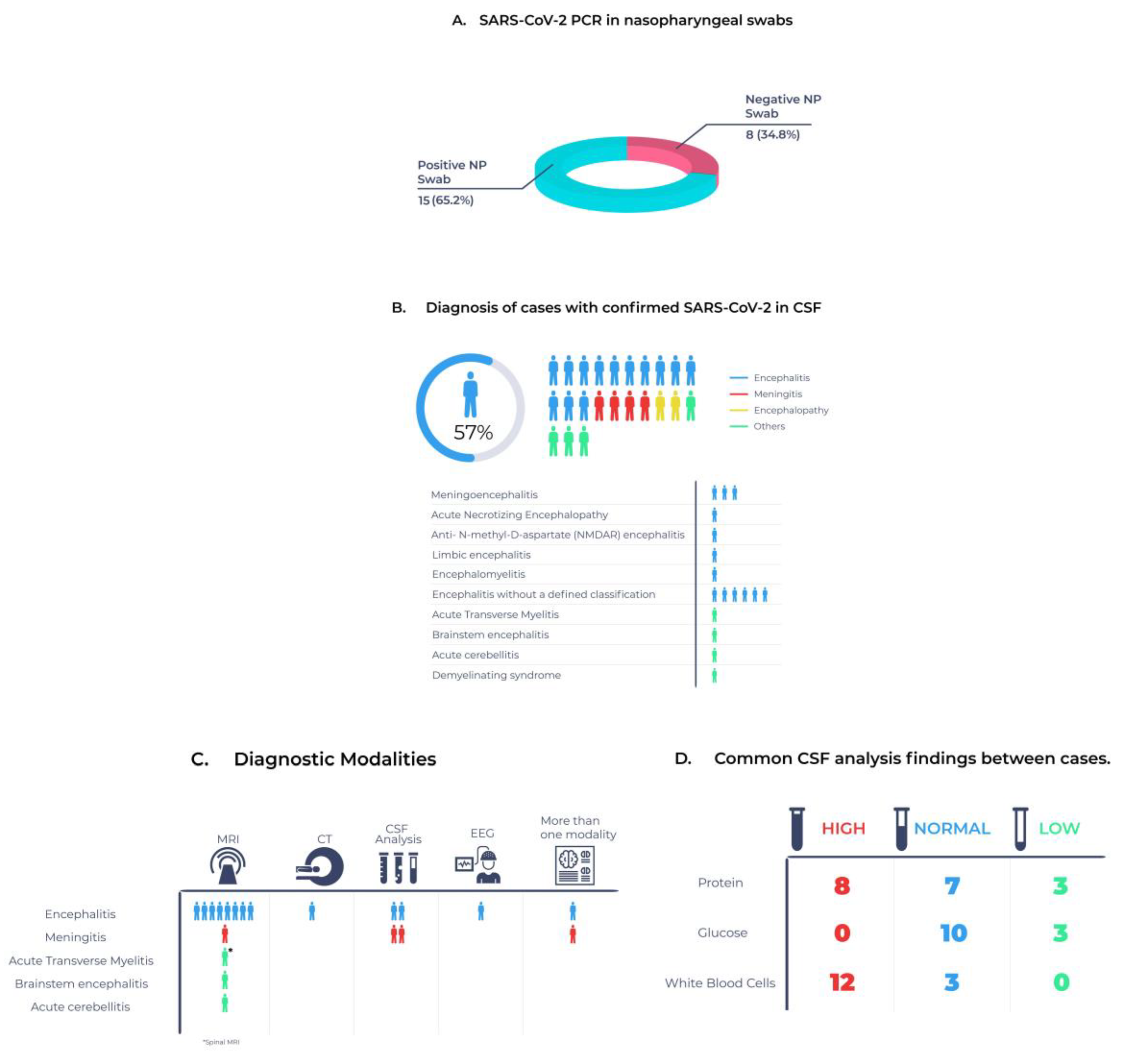
3.3. Clinical Characteristics of Patients with Confirmed COVID-19 in CSF
3.4. Changes in CSF Associated with Confirmed COVID-19 in CSF
3.5. Quality Assessment
4. Discussion
4.1. Principal Findings
4.2. COVID-19 CSF Entry Mechanisms
4.3. Other CSF Changes in SARS-CoV-2
4.4. Issues in Current Practice
4.5. Policy Implications and Future Research
4.6. Strength and Weaknesses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Characterizes COVID-19 as a Pandemic; World Health Organization: Geneva, Switzerland, 2020.
- World Health Organization. WHO Coronavirus (COVID-19). Available online: https://covid19.who.int/ (accessed on 1 August 2022).
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Niazkar, H.R.; Zibaee, B.; Nasimi, A.; Bahri, N. The neurological manifestations of COVID-19: A review article. Neurol. Sci. 2020, 41, 1667–1671. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, A.; Anson, M.; Harky, A. Neurological Manifestations of COVID-19: A systematic review and current update. Acta Neurol. Scand. 2020, 142, 14–22. [Google Scholar] [CrossRef]
- Jiang, F.; Deng, L.; Zhang, L.; Cai, Y.; Cheung, C.W.; Xia, Z. Review of the Clinical Characteristics of Coronavirus Disease 2019 (COVID-19). J. Gen. Intern. Med. 2020, 35, 1545–1549. [Google Scholar] [CrossRef]
- Miller, E.H.; Namale, V.S.; Kim, C.; Dugue, R.; Waldrop, G.; Ciryam, P.; Chong, A.M.; Zucker, J.; Miller, E.C.; Bain, J.M.; et al. Cerebrospinal Analysis in Patients With COVID-19. Open Forum Infect. Dis. 2020, 7, ofaa501. [Google Scholar] [CrossRef]
- Nuzzo, D.; Vasto, S.; Scalisi, L.; Cottone, S.; Cambula, G.; Rizzo, M.; Giacomazza, D.; Picone, P. Post-Acute COVID-19 Neurological Syndrome: A New Medical Challenge. J. Clin. Med. 2021, 10, 1947. [Google Scholar] [CrossRef]
- Moriguchi, T.; Harii, N.; Goto, J.; Harada, D.; Sugawara, H.; Takamino, J.; Ueno, M.; Sakata, H.; Kondo, K.; Myose, N.; et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020, 94, 55–58. [Google Scholar] [CrossRef]
- Domingues, R.B.; Mendes-Correa, M.C.; de Moura Leite, F.B.V.; Sabino, E.C.; Salarini, D.Z.; Claro, I.; Santos, D.W.; de Jesus, J.G.; Ferreira, N.E.; Romano, C.M.; et al. First case of SARS-CoV-2 sequencing in cerebrospinal fluid of a patient with suspected demyelinating disease. J. Neurol. 2020, 267, 3154–3156. [Google Scholar] [CrossRef]
- Yeh, E.A.; Collins, A.; Cohen, M.E.; Duffner, P.K.; Faden, H. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics 2004, 113, e73–e76. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.M.; Sanders, S.; Carter, M.; Honeyman, D.; Cleo, G.; Auld, Y.; Booth, D.; Condron, P.; Dalais, C.; Bateup, S.; et al. Improving the translation of search strategies using the Polyglot Search Translator: A randomized controlled trial. J. Med. Libr. Assoc. 2020, 108, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Murad, M.H.; Sultan, S.; Haffar, S.; Bazerbachi, F. Methodological quality and synthesis of case series and case reports. BMJ Evid. Based Med. 2018, 23, 60. [Google Scholar] [CrossRef]
- Yousefi, K.; Poorbarat, S.; Abasi, Z.; Rahimi, S.; Khakshour, A. Viral Meningitis Associated with COVID-19 in a 9-year-old Child: A Case Report. Pediatr. Infect. Dis. J. 2021, 40, E87–E88. [Google Scholar] [CrossRef]
- Virhammar, J.; Kumlien, E.; Fällmar, D.; Frithiof, R.; Jackmann, S.; Sköld, M.K.; Kadir, M.; Frick, J.; Lindeberg, J.; Olivero-Reinius, H.; et al. Acute necrotizing encephalopathy with SARS-CoV-2 RNA confirmed in cerebrospinal fluid. Neurology 2020, 95, 445–449. [Google Scholar] [CrossRef]
- Steininger, P.A.; Seifert, F.; Balk, S.; Kuramatsu, J.; Kremer, A.E.; Coras, R.; Engelhorn, T.; Maier, C.; Tenbusch, M.; Korn, K.; et al. Pearls & Oy-sters: SARS-CoV-2 Infection of the CNS in a Patient with Meningeosis Carcinomatosa. Neurology 2021, 96, 496–499. [Google Scholar]
- Shahali, H.; Ghasemi, A.; Farahani, R.H.; Nezami Asl, A.; Hazrati, E. Acute transverse myelitis after SARS-CoV-2 infection: A rare complicated case of rapid onset paraplegia. J. Neurovirol. 2021, 27, 354–358. [Google Scholar] [CrossRef]
- Fadakar, N.; Ghaemmaghami, S.; Masoompour, S.M.; Shirazi Yeganeh, B.; Akbari, A.; Hooshmandi, S.; Ostovan, V.R. A First Case of Acute Cerebellitis Associated with Coronavirus Disease (COVID-19): A Case Report and Literature Review. Cerebellum 2020, 19, 911–914. [Google Scholar] [CrossRef]
- Huang, Y.H.; Jiang, D.; Huang, J.T. SARS-CoV-2 Detected in Cerebrospinal Fluid by PCR in a Case of COVID-19 Encephalitis. Brain Behav. Immun. 2020, 87, 149. [Google Scholar] [CrossRef]
- Khodamoradi, Z.; Hosseini, S.A.; Gholampoor Saadi, M.H.; Mehrabi, Z.; Sasani, M.R.; Yaghoubi, S. COVID-19 meningitis without pulmonary involvement with positive cerebrospinal fluid PCR. Eur. J. Neurol. 2020, 27, 2668–2669. [Google Scholar] [CrossRef] [PubMed]
- Al-olama, M.; Rashid, A.; Garozzo, D. COVID-19-associated meningoencephalitis complicated with intracranial hemorrhage: A case report. Acta Neurochir. 2020, 162, 1495–1499. [Google Scholar] [CrossRef]
- Allahyari, F.; Hosseinzadeh, R.; Nejad, J.H.; Heiat, M.; Ranjbar, R. A case report of simultaneous autoimmune and COVID-19 encephalitis. J. Neurovirol. 2021, 27, 504–506. [Google Scholar] [CrossRef]
- Sattar, S.B.; Haider, M.A.; Zia, Z.S.; Niazi, M.; Iqbal, Q.Z. Clinical, Radiological, and Molecular Findings of Acute Encephalitis in a COVID-19 Patient: A Rare Case Report. Cureus 2020, 12, 10650. [Google Scholar] [CrossRef] [PubMed]
- Braccia, A.; Carta, F.; Fiorillo, D.; Tecilla, G.; Cesnik, E.; Fallica, E.; Govoni, V.; Cultrera, R. A case of limbic encephalitis with CSF detection of SARS-CoV2 virus: Immune-mediated mechanism or direct viral damage? J. Neurol. Sci. 2021, 429, 119783. [Google Scholar] [CrossRef]
- Cheraghali, F.; Tahamtan, A.; Hosseini, S.A.; Gharib, M.H.; Moradi, A.; Nikoo, H.R.; Tabarraei, A. Case Report: Detection of SARS-CoV-2 From Cerebrospinal Fluid in a 34-Month-Old Child with Encephalitis. Front. Pediatr. 2021, 9, 565778. [Google Scholar] [CrossRef]
- de Freitas, G.R.; Figueiredo, M.R.; Vianna, A.; Brandão, C.O.; Torres-Filho, H.M.; Martins, A.F.A.; Tovar-Moll, F.; Barroso, P.F. Clinical and radiological features of severe acute respiratory syndrome coronavirus 2 meningo-encephalitis. Eur. J. Neurol. 2021, 28, 3530–3532. [Google Scholar] [CrossRef]
- Demirci Otluoglu, G.; Yener, U.; Demir, M.K.; Yilmaz, B. Encephalomyelitis associated with COVID-19 infection: Case report. Br. J. Neurosurg. 2020, 7, 1–3. [Google Scholar] [CrossRef]
- Javidarabshahi, Z.; Najafi, S.; Raji, S. Meningitis induced by severe acute respiratory syndrome coronavirus 2: A case report. Iran. Red Crescent Med. J. 2021, 23, 381. [Google Scholar]
- Glavin, D.; Kelly, D.; Gallen, B. COVID-19 encephalitis with SARS-CoV-2 detected in cerebrospinal fluid presenting as a stroke mimic. Eur. Stroke J. 2021, 6, 481. [Google Scholar] [CrossRef] [PubMed]
- Kamal, Y.M.; Abdelmajid, Y.; Al Madani, A.A.R. Cerebrospinal fluid confirmed COVID-19-associated encephalitis treated successfully. BMJ Case Rep. 2020, 13, e237378. [Google Scholar] [CrossRef] [PubMed]
- Matos, A.D.B.; Dahy, F.E.; De Moura, J.V.L.; Marcusso, R.M.N.; Gomes, A.B.F.; Carvalho, F.M.M.; Fernandes, G.B.P.; Felix, A.C.; Smid, J.; Vidal, J.E.; et al. Subacute Cognitive Impairment in Individuals with Mild and Moderate COVID-19: A Case Series. Front. Neurol. 2021, 12, 678924. [Google Scholar] [CrossRef] [PubMed]
- Oosthuizen, K.; Steyn, E.C.; Tucker, L.; Ncube, I.V.; Hardie, D.; Marais, S. SARS-CoV-2 Encephalitis Presenting as a Clinical Cerebellar Syndrome: A Case Report. Neurology 2021, 97, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M. Acute Meningoencephalitis in a Child Secondary to SARS-CoV-2 Virus. Indian Pediatr. 2021, 58, 183–184. [Google Scholar] [CrossRef]
- Tuma, R.; Guedes, B.; Carra, R.; Iepsen, B.; Rodrigues, J.; Camelo-Filho, A.E.; Kubota, G.; Ferrari, M.; Neto, A.S.; Oku, M.H.M.; et al. Clinical, cerebrospinal fluid and neuroimaging findings in COVID-19 encephalopathy: A case series. Neurol. Sci. 2021, 42, 479–489. [Google Scholar] [CrossRef]
- Ellul, M.A.; Benjamin, L.; Singh, B.; Lant, S.; Michael, B.D.; Easton, A.; Kneen, R.; Defres, S.; Sejvar, J.; Solomon, T. Neurological associations of COVID-19. The Lancet. Neurology 2020, 19, 767–783. [Google Scholar] [CrossRef]
- Siow, I.; Lee, K.S.; Zhang, J.J.Y.; Saffari, S.E.; Ng, A. Encephalitis as a neurological complication of COVID-19: A systematic review and meta-analysis of incidence, outcomes, and predictors. Eur. J. Neurol. 2021, 28, 3491–3502. [Google Scholar] [CrossRef]
- Scoppettuolo, P.; Borrelli, S.; Naeije, G. Neurological involvement in SARS-CoV-2 infection: A clinical systematic review. Brain Behav. Immun. Health 2020, 5, 100094. [Google Scholar] [CrossRef]
- Pennisi, M.; Lanza, G.; Falzone, L.; Fisicaro, F.; Ferri, R.; Bella, R. SARS-CoV-2 and the Nervous System: From Clinical Features to Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 5475. [Google Scholar] [CrossRef]
- Venkatesan, A.; Tunkel, A.R.; Bloch, K.C.; Lauring, A.S.; Sejvar, J.; Bitnun, A.; Stahl, J.-P.; Mailles, A.; Drebot, M.; Rupprecht, C.E.; et al. Case Definitions, Diagnostic Algorithms, and Priorities in Encephalitis: Consensus Statement of the International Encephalitis Consortium. Clin. Infect. Dis. 2013, 57, 1114–1128. [Google Scholar] [CrossRef]
- Pezzini, A.; Padovani, A. Lifting the mask on neurological manifestations of COVID-19. Nature reviews. Neurology 2020, 16, 636–644. [Google Scholar] [CrossRef]
- Pilotto, A.; Masciocchi, S.; Volonghi, I.; Crabbio, M.; Magni, E.; De Giuli, V.; Caprioli, F.; Rifino, N.; Sessa, M.; Gennuso, M.; et al. Clinical Presentation and Outcomes of Severe Acute Respiratory Syndrome Coronavirus 2-Related Encephalitis: The ENCOVID Multicenter Study. J. Infect. Dis. 2021, 223, 28–37. [Google Scholar] [CrossRef]
- Caress, J.B.; Castoro, R.J.; Simmons, Z.; Scelsa, S.N.; Lewis, R.A.; Ahlawat, A.; Narayanaswami, P. COVID-19-associated Guillain-Barré syndrome: The early pandemic experience. Muscle Nerve 2020, 62, 485–491. [Google Scholar] [CrossRef]
- Dhiman, K.; Gupta, V.B.; Villemagne, V.L.; Eratne, D.; Graham, P.L.; Fowler, C.; Bourgeat, P.; Li, Q.X.; Collins, S.; Bush, A.I.; et al. Cerebrospinal fluid neurofilament light concentration predicts brain atrophy and cognition in Alzheimer’s disease. Alzheimer’s Dement. 2020, 12, e12005. [Google Scholar] [CrossRef]
- Bodro, M.; Compta, Y.; Llansó, L.; Esteller, D.; Doncel-Moriano, A.; Mesa, A.; Rodríguez, A.; Sarto, J.; Martínez-Hernandez, E.; Vlagea, A.; et al. Increased CSF levels of IL-1β, IL-6, and ACE in SARS-CoV-2-associated encephalitis. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e821. [Google Scholar] [CrossRef]
- Patel, A.B.; Verma, A. COVID-19 and Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers: What Is the Evidence? JAMA 2020, 323, 1769–1770. [Google Scholar] [CrossRef]
- Dias da Costa, M.; Leal Rato, M.; Cruz, D.; Valadas, A.; Antunes, A.P.; Albuquerque, L. Longitudinally extensive transverse myelitis with anti-myelin oligodendrocyte glycoprotein antibodies following SARS-CoV-2 infection. J. Neuroimmunol. 2021, 361, 577739. [Google Scholar] [CrossRef] [PubMed]
- Hepburn, M.; Mullaguri, N.; George, P.; Hantus, S.; Punia, V.; Bhimraj, A.; Newey, C.R. Acute Symptomatic Seizures in Critically Ill Patients with COVID-19: Is There an Association? Neurocrit. Care 2021, 34, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Etemadifar, M.; Salari, M.; Murgai, A.A.; Hajiahmadi, S. Fulminant encephalitis as a sole manifestation of COVID-19. Neurol. Sci. 2020, 41, 3027–3029. [Google Scholar] [CrossRef] [PubMed]
- Hurn, E.; Dickinson, L.; Abraham, J.A. Bacterial meningitis and COVID-19: A complex patient journey. BMJ Case Rep. 2021, 14, e239533. [Google Scholar] [CrossRef]
- Colonna, S.; Sciumé, L.; Giarda, F.; Innocenti, A.; Beretta, G.; Dalla Costa, D. Case Report: Postacute Rehabilitation of Guillain-Barré Syndrome and Cerebral Vasculitis-Like Pattern Accompanied by SARS-CoV-2 Infection. Front. Neurol. 2020, 11, 602554. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, F.A.A.; Palmeira, D.C.C.; Rocha-Filho, P.A.S. Headache and pleocytosis in CSF associated with COVID-19: Case report. Neurol. Sci. 2020, 41, 3021–3022. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, F.A.A.; de Oliveira Filho, J.R.B.; Rocha-Filho, P.A.S. Multiple demyelinating sensory and motor mononeuropathy associated with COVID-19: A case report. J. Neurovirol. 2021, 27, 966–967. [Google Scholar] [CrossRef] [PubMed]
- García-Howard, M.; Herranz-Aguirre, M.; Moreno-Galarraga, L.; Urretavizcaya-Martínez, M.; Alegría-Echauri, J.; Gorría-Redondo, N.; Planas-Serra, L.; Schlüter, A.; Gut, M.; Pujol, A.; et al. Case Report: Benign Infantile Seizures Temporally Associated With COVID-19. Front. Pediatr. 2020, 8, 507. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, E.F.A.; Nasser, A.; Bhargava, A.; Moudgil, S. Post-infectious focal encephalitis due to COVID-19. Germs 2021, 11, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Dharsandiya, M.; Patel, K.; Patel, A. Response to the comments received on a case report SARS-CoV-2 viral sepsis with meningoencephalitis. Indian J. Med. Microbiol. 2021, 39, 565. [Google Scholar] [CrossRef]
- Barreto-Acevedo, E.; Mariños, E.; Espino, P.; Troncoso, J.; Urbina, L.; Valer, N. Acute encephalitis associated with SARS-CoV-2: First case report in Peru. Rev. Neuro-Psiquiatr. 2020, 83, 116–122. [Google Scholar] [CrossRef]
- Al-Janabi, O.; Yousuf, F.; Helgren, L.; Guduru, Z. Two cases of COVID-19 Encephalitis: Case series. Neurology 2021, 96, 1. [Google Scholar]
- Águila-Gordo, D.; Manuel Flores-Barragán, J.; Ferragut-Lloret, F.; Portela-Gutierrez, J.; LaRosa-Salas, B.; Porras-Leal, L.; Carlos Villa Guzmán, J. Acute myelitis and SARS-CoV-2 infection. A new etiology of myelitis? J. Clin. Neurosci. 2020, 80, 280–281. [Google Scholar] [CrossRef]
- Ayatollahi, P.; Tarazi, A.; Wennberg, R. Possible Autoimmune Encephalitis with Claustrum Sign in case of Acute SARS-CoV-2 Infection. Can. J. Neurol. Sci. 2021, 48, 430–432. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, L.; Shekhar, S.; Bansal, A.; Kumar, S. COVID-19 associated arterial ischaemic stroke and multisystem inflammatory syndrome in children: A case report. Lancet Child. Adolesc. Health 2021, 5, 88–90. [Google Scholar] [CrossRef]
- Sohal, S.; Mansur, M. COVID-19 Presenting with Seizures. IDCases 2020, 20, e00782. [Google Scholar] [CrossRef] [PubMed]
- Böger, B.; Fachi, M.M.; Vilhena, R.O.; Cobre, A.F.; Tonin, F.S.; Pontarolo, R. Systematic review with meta-analysis of the accuracy of diagnostic tests for COVID-19. Am. J. Infect. Control. 2021, 49, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.; Jain, R.; Frontera, J.; Placantonakis, D.G.; Galetta, S.; Balcer, L.; Melmed, K.R. COVID-19 associated brain/spinal cord lesions and leptomeningeal enhancement: A meta-analysis of the relationship to CSF SARS-CoV-2. J. Neuroimaging Off. J. Am. Soc. Neuroimaging 2021, 31, 826–848. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, J.M.; Libertin, C.R.; Siegel, J.; Binnicker, M.J.; Harris, D.; Matcha, G.V.; Caulfield, T.; Freeman, W.D. Three-tier stratification for CNS COVID-19 to help decide which patients should undergo lumbar puncture with CSF analysis: A case report and literature review. Rom. J. Intern. Med. 2021, 59, 88–92. [Google Scholar] [CrossRef]
- Hafizi, F.; Kherani, S.; Shams, M. Meningoencephalitis from SARS-CoV-2 infection. IDCases 2020, 21, e00919. [Google Scholar] [CrossRef]
- Nissen, T.; Wynn, R. The clinical case report: A review of its merits and limitations. BMC Res. Notes 2014, 7, 264. [Google Scholar] [CrossRef]
- Rethaningsih, P.B.; Tugasworo, D.; Andhitara, Y.; Ardhini, R.; Kurnianto, A.; Afany, N.; Bunyamin, J.; Utami, F.S.; Sogata, I.A.; Hairuzaman. Meningoencephalitis due to SARS-CoV-2 and tuberculosis co-infection: A case report from Indonesia. Bali Med. J. 2021, 10, 673–676. [Google Scholar] [CrossRef]
- Neumann, B.; Schmidbauer, M.L.; Dimitriadis, K.; Otto, S.; Knier, B.; Niesen, W.-D.; Hosp, J.A.; Günther, A.; Lindemann, S.; Nagy, G.; et al. Cerebrospinal fluid findings in COVID-19 patients with neurological symptoms. J. Neurol. Sci. 2020, 418, 117090. [Google Scholar] [CrossRef]
- Novi, G.; Mikulska, M.; Briano, F.; Toscanini, F.; Tazza, F.; Uccelli, A.; Inglese, M. COVID-19 in a MS patient treated with ocrelizumab: Does immunosuppression have a protective role? Mult. Scler. Relat. Disord. 2020, 42, 102120. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.K.; Paliwal, V.K.; Gupta, A. Encephalopathy in patients with COVID-19: A review. J. Med. Virol. 2020, 93, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Miqdad, M.A.; Enabi, S.; Alshurem, M.; Al-Musawi, T.; Alamri, A. COVID-19–Induced Encephalitis: A Case Report of a Rare Presentation with a Prolonged Electroencephalogram. Cureus 2021, 13, e14476. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Hanif, M.; Haider, M.A.; Ali, M.J.; Ahmed, M.U.; Saleem, S. Meningitis as an Initial Presentation of COVID-19: A Case Report. Front. Public Health 2020, 8, 474. [Google Scholar] [CrossRef] [PubMed]
- Razzack, A.A.; Kassandra-Coronel, M.; Domingo, P.I. Acute disseminated encephalomyelitis and COVID-19: A Systematic review of Case-Reports and Case-Series. Neurology 2021, 96, 1. [Google Scholar]
- Basher, F.; Camargo, J.F.; Diaz-Paez, M.; Lekakis, L.J.; Pereira, D.L. Aseptic Meningitis after Recovery from SARS-CoV-2 in an Allogeneic Stem Cell Transplant Recipient. Clin. Med. Insights Case Rep. 2021, 14, 1–5. [Google Scholar] [CrossRef]
- Affes, Z.; Bouvard, E.-J.; Levy, P.; Dussaule, C.; Grateau, G.; Haymann, J.-P. COVID-19 Presenting With Confusion: An Unusual but Suggestive Electroencephalography Pattern of Encephalitis. J. Clin. Neurophysiol. 2020, 38, e11–e13. [Google Scholar] [CrossRef]
- Vraka, K.; Ram, D.; West, S.; Chia, W.; Kurup, P.; Subramanian, G.; Tan, H.J. Two Paediatric Patients with Encephalopathy and Concurrent COVID-19 Infection: Two Sides of the Same Coin? Case Rep. Neurol. Med. 2021, 2021, 6658000. [Google Scholar] [CrossRef]
- Umanah, T.; Arshad, H.; Noor, E. Acute psychosis in association of COVID19 infection: A case report. Neurology 2021, 96, 4662. [Google Scholar]
- McCuddy, M.; Kelkar, P.; Zhao, Y.; Wicklund, D. Acute Demyelinating Encephalomyelitis (ADEM) in COVID-19 Infection: A Case Series. MedRxiv 2020, 68, 1192–1195. [Google Scholar]
- Zhang, T.; Rodricks, M.B.; Hirsh, E. COVID-19-Associated Acute Disseminated Encephalomyelitis—A Case Report. medRxiv 2020. [Google Scholar] [CrossRef]
- Li, C.X.; Burrell, R.; Dale, R.C.; Kesson, A.; Blyth, C.C.; Clark, J.E.; Crawford, N.; Jones, C.A.; Britton, P.N.; Holmes, E.C.; et al. Diagnosis and analysis of unexplained cases of childhood encephalitis in Australia using metagenomic next-generation sequencing. bioRxiv 2021. [Google Scholar] [CrossRef]
- Ghosh, R.; Dubey, S.; Finsterer, J.; Chatterjee, S.; Ray, B.K. SARS-CoV-2-Associated Acute Hemorrhagic, Necrotizing Encephalitis (AHNE) Presenting with Cognitive Impairment in a 44-Year-Old Woman without Comorbidities: A Case Report. Am. J. Case Rep. 2020, 21, e925641. [Google Scholar] [CrossRef] [PubMed]
- Gunawardhana, C.; Nanayakkara, G.; Gamage, D.; Withanage, I.; Bandara, M.; Siriwimala, C.; Senaratne, N.; Chang, T. Delayed presentation of postinfectious encephalitis associated with SARS-CoV-2 infection: A case report. Neurol. Sci. 2021, 42, 3527–3530. [Google Scholar] [CrossRef]
- Høy Marbjerg, L.; Jacobsen, C.; Fonager, J.; Bøgelund, C.; Rasmussen, M.; Fomsgaard, A.; Banner, J.; Vorobieva Solholm Jensen, V. Possible Involvement of Central Nervous System in COVID-19 and Sequence Variability of SARS-CoV-2 Revealed in Autopsy Tissue Samples: A Case Report. Clin. Pathol. 2021, 14, 1–7. [Google Scholar] [CrossRef]
- Huo, L.; Xu, K.L.; Wang, H. Clinical features of SARS-CoV-2-associated encephalitis and meningitis amid COVID-19 pandemic. World J. Clin. Cases 2021, 9, 1058–1078. [Google Scholar] [CrossRef]
- Maury, A.; Lyoubi, A.; Peiffer-Smadja, N.; de Broucker, T.; Meppiel, E. Neurological manifestations associated with SARS-CoV-2 and other coronaviruses: A narrative review for clinicians. Rev. Neurol. 2021, 177, 51–64. [Google Scholar] [CrossRef]
- Edén, A.; Kanberg, N.; Gostner, J.; Fuchs, D.; Hagberg, L.; Andersson, L.M.; Lindh, M.; Price, R.W.; Zetterberg, H.; Gisslén, M. CSF Biomarkers in Patients with COVID-19 and Neurologic Symptoms A Case Series. Neurology 2021, 96, E294–E300. [Google Scholar] [CrossRef]
| First Author | Article Type | City, Country | Age, Sex Ethnicity | Presenting Symptoms | Physical Exam | COVID-19 Swab | Other Test Result | Diagnosis | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Yousefi et al. (2021) [18] | Case report | Imam Hassan Hospital, Iran | 9-year-old, Iranian Turkish Girl | Fever, headache, low back pain | Head and neck stiffness, +Brudzinski, +Kernig. | Negative PCR in NP swab | CBC: ↑LDH, ↑WBCs, 88% neutrophils. | Meningitis after COVID-19 infection by CSF analysis | Discharged after 10 days of hospitalization with follow-up |
| Virhammar et al. (2020) [19] | Case report | Sweden | 55-year-old, Woman | Day1: fever, myalgia. Day 7: lethargic, unresponsive | Stable, stuporous, multifocal myoclonus. No respiratory problems. | Positive PCR in NP swab | MRI: pathologic signal symmetrically in central thalami, medial temporal lobes, and brain stem. | Acute necrotizing encephalopathy with COVID-19 | Extubated on day 35 and discharged to rehabilitation |
| Steininger et al. (2021) [20] | Case report | Germany | 53-year-old, Man | Fever, headache | Meningism, decreased vigilance. No respiratory problems. | Positive PCR in NP swab | CBC: ↓WBCs, ↓neutrophil, normal CRP. MRI: infratentorial and supratentorial lesions. | Meningeosis carcinomatosis with COVID-19 meningitis | Chemotherapy after viral clearance |
| Shahali et al. (2021) [21] | Case report | Iran | 63-year-old, Caucasian Man | Loss of control in lower limbs, absent sensation below chest, constipation, urinary retention | 91% O2sat, hypotonic lower limbs, hypoesthesia below T8. | Positive PCR in NP swab | Brain CT and MRI: normal. Spinal MRI: increased T2 signal involving central gray matter and dorsal columns. | COVID-19-associated acute transverse myelitis | Neurologic symptoms cleared after 1 week, discharged after 5 weeks |
| Fadakar et al. (2020) [22] | Case report | Iran | 47-year-old, Man | Pain, progressive vertigo, headache, ataxia, fatigue, pain, cough (10 days) | Ataxic gait, dysarthria, impaired tandem gait head titubation, truncal swaying, dysarthria, saccadic pursuit, loss of optokinetic nystagmus, dysmetria. | Positive PCR in NP swab | CBC: normal, ↑ferritin level. Brain MRI: hyperintensities in cerebellar hemispheres and vermis. FLAIR: edema, cortical–meningeal enhancement. | Acute cerebellitis associated with COVID-19 | Improvement of vertigo after 14 days. After 1 month, his ataxia improved |
| Domingues et al. (2020) [12] | Case report | Brazil | 42-year-old, Woman, in São Paulo | Paresthesia in left upper limb, later: left hemithorax, hemiface | Hypoesthesia, coryza, nasal obstruction. | Negative PCR in NP and nasal swabs | CBC: normal. Chest tomography: normal. Brain MRI: normal. | Demyelinating disease, COVID-19-associated | Full recovery after 3 weeks. |
| Huang et al. (2020) [23] | Letter to the Editor | Downtown Los Angeles | 40-year-old, Woman | Fever, syncope | Awake, alert, lethargic but coherent, neck stiffness, photophobia. | Positive PCR in NP swab | Non-contrast head CT: normal. Chest X-ray: clear. EEG: generalized slowing with no epileptic discharges. No MRI. | COVID-19 encephalitis without MRI confirmation | Improved mental status at hospital day 12 |
| Moriguchi et al. (2020) [11] | Case report | Yamanashi University Hospital, Japan | 24-year-old, Man | LOC, lying on the floor in vomit (day 9) | Neck stiffness, transient generalized seizures. | Negative PCR in NP swab | Brain MRI: hyperintensity in wall of right lateral ventricle and hyperintense signal changes in the right mesial temporal lobe and hippocampus. | COVID-19 meningitis based on MRI | Discontinued treatment at day 15 |
| Khodamoradi et al. (2020) [24] | Case report | Iran | 49-year-old, Woman | Chills, fever, nausea, vomiting, malaise | Awake, alert, oriented, febrile (38 °C). | Negative PCR in NP swab | CBC: normal. Chest CT: normal. | COVID-19 meningitis based on CSF analysis | Discharged at day 21 after improvement |
| Al-olama et al. (2020) [25] | Case report | Dubai, United Arab Emirates | 36-year-old, Male | Fever, headache, body pain, cough, diarrhea, vomiting | Pharyngitis. | Positive PCR in NP swab | Brain CT: frontal intracerebral hematoma with subarachnoid hemorrhage. No MRI. | Meningoencephalitis with cerebral and subdural hematoma | Not clear |
| Allahyari et al. (2021) [26] | Case report | Iran | 18-year-old, Female | Generalized tonic–colonic seizures | Bilateral pulmonary crackles, confusion, meningism, neck stiffness. | Positive IgM for COVID-19 | CBC: ↑WBCs, neutrophil dominant, lymphopenia, ↑CRP. CSF: anti-NMDAR antibody. MRI: normal | Anti-NMDAR encephalitis with brain edema due to COVID-19 | Discharged with full recovery after 2 months |
| Sattar et al. (2020) [27] | Case report | New York | 44-year-old, Male | Fever (7 days), cough, SOB | Confusion, minimally responsive. | Positive PCR in NP swab | Chest X-ray: diffuse bilateral opacities. MRI: abnormal cortical signals in cortical frontal lobes. | Acute viral encephalitis secondary to SARS-CoV-2 | Seizures stopped, discharged day 34 |
| Braccia et al. (2021) [28] | Case report | Ferrara, Italy | 70-year-old, Man | Fever, cough, SOB, confessional state | Right focal signs, vigilance fluctuations. | Positive PCR in NP swab | EEG: nonspecific mild background activity. Brain MRI: T2-FLAIR hyper intensity in the mesial temporal lobes. | Limbic encephalitis due to SARS-CoV-2 | Improved cognition and alertness, MRI was similar after 2 months |
| Cheraghali et al. (2021) [29] | Case report | Tamin Ejtemae Hospital in Gonbad, Iran | 34-month-old, Boy | Fever, tonic-clonic seizures, LOC | Upward gaze. | Positive PCR in NP swab | Brain MRI: symmetric, cortical, and juxta-cortical high T1 and T2 signal abnormality, in bilateral parieto-occipital lobes. | Viral SARS-CoV-2 encephalitis, with possible parenchymal hemorrhagic components | Decerebrate posture, ventilator independent then discharged |
| de Freitas et al. (2021) [30] | Case report | Rio de Janeiro, Brazil | 35-year-old male, Man | Fever, diarrhea, vomiting, diplopia, urinary retention, sleepiness, LOC, +Babinski sign | Somnolent, oriented, convergence strabismus, mild ataxia in arms, brisk deep tendon reflexes. | Negative PCR in NP swab | Brain CT: normal. EEG: normal. Ultrasound: DVT. Brain MRI: lesions on white matter hemispheres, the body and splenium of corpus callosum and cerebellar peduncles. | SARS-CoV-2-associated meningitis–encephalitis | Discharged 21 days after admission with diplopia and urinary retention |
| Demirci et al. (2020) [31] | Case report | Turkey | 48-year-old, Male | Headache, cough (10 days), fatigue, myalgia (7 days) | Normal. | Negative PCR in NP swab | MRI: hyperintense lesions in the posterior medial temporal lobe and hyperintense lesions in upper cervical spinal cord. | Viral encephalomyelitis due to SARS-CoV-2 | Stable and under treatment |
| Javidarabshahi et al. (2021) [32] | Case report | Iran | 44-year-old, Male | Febrile, dizzy, convulsion, respiratory symptoms | Not mentioned. | Positive PCR in NP swab | Head contrast-enhanced MRI: revealed a normal image. | Severe acute COVID-19 encephalitis by CSF analysis | Not mentioned |
| Glavin et al. (2021) [33] | Case report | UK | 35-year-old, Male | Dysphasia, confusion, right arm incoordination | Right arm weakness, dysphasia, amnesia, vomiting, pyrexia, GCS 15/15, no meningism. | Negative PCR in NP swab | MRA brain: normal with congenitally hypoplastic left A1 segment of ACA. Other neuroimaging: normal. EEG: excess slow waves. | COVID-19 encephalitis based on CSF and EEG | Full recovery then discharged. |
| Kamal et al. (2020) [34] | Case report | Dubai, UAE | 31-year-old, Male | Mild cough | Afebrile, normal vitals, O2sat 100%, confusion, agitation, fluctuations in loc. | Positive PCR in NP swab | Uncontracted brain CT: Multiple hypodensities in the external capsules. Contrast brain MRI: abnormal signal intensity in the temporal lobe. | COVID-19 encephalitis confirmed by MRI | Patient discharged and given vitamin C tablets and zinc supplements |
| Matos et al. (2021) [35] | Case series | Brazil | 47-year-old, Female | Headache, AMS, sleep disturbance, confusion | MMSE: 30/30, multimedia over Coaxial Alliance 24/30. | Positive PCR in NP swab | CBC and imaging: normal. | COVID-19 encephalopathy | Not mentioned |
| Oosthuizen et al. (2021) [36] | Case report | South Africa | 52-year-old, Male | Gait instability | Pyrexial alerted and oriented, multidirectional nystagmus, dysarthria, truncal appendicular ataxia. | Negative PCR in NP swab | CBC: ↑WBCs, neutrophil predominant, ↑ESR. EEG: normal. MRI brain: brainstem encephalitis. Uncontracted CT brain: central midbrain hypodensity. | SARS-CoV-2 brainstem encephalitis | Discharged, CSF examination remained normal at 6 months |
| Pandey et al. (2021) [37] | Case report | Delhi, India | 11-year-old, Boy | Fever, headache, vomiting, altered sensorium (1 day) | Stable with GCS 9/15. Neck stiffness, +Kernig’s sign, no cranial nerve paresis, increased tone with brisk reflexes and extensor planters in lower limbs. | Positive PCR in NP swab | CBC: severe lymphopenia. Head CECT: scan was normal. | Acute meningoencephalitis confirmed by CSF analysis | Discharged. at day10 of illness |
| Tuma et al. (2020) [38] | Case series | São Paulo, Brazil | 50-year-old, Woman | Fever | Not mentioned. | Positive PCR in NP swab | CBC: ↑WBCs with ↑eosinophils. Brain CT: normal. No MRI. | COVID-19 encephalopathy | Not mentioned |
| Study | Symptoms to Positive NP Swab Collection | CT Threshold for SARS-CoV-2 NP Swab | Symptoms to Positive CSF Collection | CT Threshold for SARS-CoV-2 Positive CSF and Tested Genes |
|---|---|---|---|---|
| Matos et al. (2021) [35] | Not mentioned | Not mentioned | day 16 | Not mentioned |
| Braccia et al. (2021) [28] | Not mentioned | Not mentioned | Not mentioned | Not mentioned |
| Glavin et al. (2021) [33] | Negative swab | Negative swab | 4 days | E gene (CT value: 35.8) S gene (CT value: 35.7) |
| Allahyari et al. (2021) [26] | 3 weeks | Not mentioned | 3 weeks | Not mentioned |
| Cheraghali et al. (2021) [29] | 26 days | PCR 1: E gene PCR 2 N: gene ORF1ab gene (all CT value: 29) | 26 days | PCR 1: E gene PCR 2 N: gene, ORF1ab gene (all CT value: 29) |
| De Freitas et al. (2021) [30] | Negative swab | Negative swab | 3 days | Not mentioned |
| Demirci et al. (2020) [31] | Negative swab | Negative swab | 10 days | Not mentioned |
| Shahali et al. (2021) [21] | 4 days | Not mentioned | 4 days | Not mentioned |
| Javidarabshahi et al. (2021) [32] | 7 days | Not mentioned | 7 days | Not mentioned |
| Virhammar et al. (2020) [19] | 7 days | Not mentioned | 19 days | N gene (CT value: 34.2) |
| Oosthuizen et al. (2021) [36] | Negative swab | Negative swab | 6 days | E gene (CT value: 33) RdRP gene (CT value: 34) N gene (CT value: 35) |
| Yousefi et al. (2021) [18] | Negative swab | Negative swab | 3 days | Not mentioned |
| Al-olama et al. (2020) [25] | 7 days | Not mentioned | 20 days | Not mentioned |
| Pandey et al. (2021) [37] | 1 days | Not mentioned | 1 days | Not mentioned |
| Fadakar et al. (2020) [22] | 13 days | Not mentioned | 13 days | Not mentioned |
| Steininger et al. (2021) [20] | 1 days | Not mentioned | 3 days | E gene (CT value: 19.5) RdRP gene (CT value: 21.6) |
| Tuma et al. (2020) [38] | 1 days | Not mentioned | 12 days | Not mentioned |
| Domingues et al. (2020) [12] | Negative swab | Negative swab | 3 weeks | RdRP-2 gene (CT not mentioned) |
| Sattar et al. (2020) [27] | 7 days | Not mentioned | 32 days | Not mentioned |
| Moriguchi et al. (2020) [11] | Negative swab | Negative swab | 9 days | N gene (CT value: 37) N-2 gene negative |
| Huang et al. (2020) [23] | Not mentioned | Not mentioned | Not mentioned | Not mentioned |
| Kamal et al. (2020) [34] | 5 days | Not mentioned | 5 days | N gene, E gene, RdRP and ORF1ab |
| Khodamoradi et al. (2020) [24] | Negative swab | Negative swab | 3 days | Not mentioned |
| Study | Color | Protein (mg/dL) | Glucose (mg/dL) | WBCs (Cells/µL) | Bacteria | Viruses | COVID-19 | Other |
|---|---|---|---|---|---|---|---|---|
| Yousefi et al. (2021) [18] | Colorless, clear | 81 | 51 | 1870 | Negative | Negative | PCR positive | |
| Virhammar et al. (2020) [19] | Not described | 95 (at day 7), 26 (at day 12) | Not mentioned | 5 | Negative | Negative | PCR positive on day 12 | IL6, NfL, and tau increased. Oligoclonal bands present |
| Steininger et al. (2021) [20] | Not described | 136 | 12 | 57 | Negative | Negative | PCR (in-house method) positive on day 2, positive till day 20 | 3 RBCs/μL |
| Shahali et al. (2021) [21] | Not described | 128 | 68 | 96 | Negative | Negative | PCR positive | |
| Fadakar et al. (2020) [22] | Not described | 58 | 60 | 10 (80% lymphocytes) | Negative | Negative | PCR positive | |
| Domingues et al. (2020) [12] | Not described | 32 | 68 | 1 | Negative | Negative | PCR positive, confirmed by gene sequencing | |
| Huang et al. (2020) [23] | Not described | 100 | 120 | 70 (100% lymphocytes) | Negative | Negative | PCR positive | 65 RBCs |
| Moriguchi et al. (2020) [11] | Clear and colorless | Not mentioned | Not mentioned | 9 | Not mentioned | Negative | PCR positive | |
| Khodamoradi et al. (2020) [24] | Not described | 0.2 (at day 1), 685 (at 1 week) | 45 | 90 | Negative | Negative | PCR positive | 57 RBCs after 1 week |
| Sattar et al. (2020) [27] | Pink | 39 | 75 | Not mentioned | Negative | Negative | PCR positive | 1685 RBCs |
| Allahyari et al. (2021) [26] | Light pink | 241 | 55 | 27 | Negative | Negative | PCR positive | RBCs: 1997, lymphocytes: 93%, PMN: 7% |
| Al-olama et al. (2020) [25] | Not described | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Fluid from the chronic subdural hematoma PCR positive | Not mentioned |
| Braccia et al. (2021) [28] | Not described | Not mentioned | Not mentioned | Not mentioned | Negative | Negative | PCR positive | ND |
| Cheraghali et al. (2021) [29] | Clear and colorless | Normal (levels are not described) | Normal | Not mentioned | Negative | Negative | PCR positive | ND |
| De Freitas et al. (2021) [30] | Not described | 8.6 (at day 4), 6.6 (at day 6), 6.7 (at day 8) | 57 (at day 4), 54 (at day 6), 63 (at day 8) | Not mentioned | Negative | Negative | PCR positive | Lymphocytic pleocytosis, oligoclonal bands, and increased IL6 levels |
| Demirci et al. (2020) [31] | Colorless and clear | 0.04 | 90 | Not mentioned | Negative | Negative | PCR positive | No cell detected microscopically |
| Javidarabshahi et al. (2021) [32] | Not described | 0.0034 | Not mentioned | Not mentioned | Negative | Negative | PCR positive | LDH 40 U/L |
| Glavin et al. (2021) [33] | Clear CSF | 52 | 66:90 CSF to serum glucose ratio | 134 (99% lymphocytes) | Negative | Negative | PCR positive | RBCs 20 × 106/L |
| Kamal et al. (2020) [34] | Clear and colorless | 45 | 60 | <5 | Negative | Negative | PCR positive | CSF chloride: 119 mg/dL, RBCs: 50 cells/cm |
| Matos et al. (2021) [35] | Not described | Not mentioned | Not mentioned | Not mentioned | Negative | Negative | PCR positive | |
| Oosthuizen et al. (2021) [36] | Not described | 37 | 64 | 51 (49 lymphocytes, 2 polymorphonuclear) | Negative | Negative | PCR positive | Increased IGg index, albumin (157 mg/L) |
| Pandey et al. (2021) [37] | Not described | 696 | Normal levels | 75 pleocytosis (lymphocytic predominance 80%) | Negative | Negative | PCR positive | |
| Tuma et al. (2020) [38] | Not described | 54 | Not mentioned | 15 (38% lymphocytes, 8% monocytes, 22% neutrophils, 31% eosinophils, and 1% macrophages) | Negative | Negative | PCR positive | IL6 of 19.57 pg/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmakaty, I.; Ferih, K.; Karen, O.; Ouda, A.; Elsabagh, A.; Amarah, A.; Malki, M.I. Clinical Implications of COVID-19 Presence in CSF: Systematic Review of Case Reports. Cells 2022, 11, 3212. https://doi.org/10.3390/cells11203212
Elmakaty I, Ferih K, Karen O, Ouda A, Elsabagh A, Amarah A, Malki MI. Clinical Implications of COVID-19 Presence in CSF: Systematic Review of Case Reports. Cells. 2022; 11(20):3212. https://doi.org/10.3390/cells11203212
Chicago/Turabian StyleElmakaty, Ibrahim, Khaled Ferih, Omar Karen, Amr Ouda, Ahmed Elsabagh, Ahmed Amarah, and Mohammed Imad Malki. 2022. "Clinical Implications of COVID-19 Presence in CSF: Systematic Review of Case Reports" Cells 11, no. 20: 3212. https://doi.org/10.3390/cells11203212
APA StyleElmakaty, I., Ferih, K., Karen, O., Ouda, A., Elsabagh, A., Amarah, A., & Malki, M. I. (2022). Clinical Implications of COVID-19 Presence in CSF: Systematic Review of Case Reports. Cells, 11(20), 3212. https://doi.org/10.3390/cells11203212






