Probiotics in the Prevention of the Calcium Oxalate Urolithiasis
Abstract
:1. Introduction
2. Oxalobacter formigenes
2.1. Characteristics of Oxalobacter formigenes
2.2. Oxalobacter formigenes and Kidney Stone Course
3. Lactobacillus spp.
3.1. Characteristics of Lactobacillus spp.
3.2. Lactobacillus and Kidney Stone Prevention
4. Bifidobacterium spp.
4.1. Characteristic of Bifidobacterium
4.2. Bifidobacterium and Kidney Stone Prevention
5. Other Bacteria Associated with Oxalate Metabolism
6. B. subtilis and L. plantarum as Novel Recombinant Approaches
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mayans, L. Nephrolithiasis. Prim. Care 2019, 46, 203–212. [Google Scholar] [CrossRef]
- Romero, V.; Akpinar, H.; Assimos, D.G. Kidney stones: A global picture of prevalence, incidence, and associated risk factors. Rev. Urol. 2010, 12, e86–e96. [Google Scholar]
- Ramello, A.; Vitale, C.; Marangella, M. Epidemiology of nephrolithiasis. J. Nephrol. 2000, 13, 45–50. [Google Scholar]
- Pinduli, I.; Spivacow, R.; del Valle, E.; Vidal, S.; Negri, A.L.; Previgliano, H.; Farias Edos, R.; Andrade, J.H.; Negri, G.M.; Boffi-Boggero, H.J. Prevalence of urolithiasis in the autonomous city of Buenos Aires, Argentina. Urol. Res. 2006, 34, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Medina-Escobedo, M.; Zaidi, M.; Real-de Leon, E.; Orozco-Rivadeneyra, S. Urolithiasis prevalence and risk factors in Yucatan, Mexico. Salud Publica Mex. 2002, 44, 541–545. [Google Scholar] [CrossRef] [Green Version]
- Lieske, J.C.; Peña de la Vega, L.S.; Slezak, J.M.; Bergstralh, E.J.; Leibson, C.L.; Ho, K.L.; Gettman, M.T. Renal stone epidemiology in Rochester, Minnesota: An update. Kidney Int. 2006, 69, 760–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scales, C.D., Jr.; Curtis, L.H.; Norris, R.D.; Springhart, W.P.; Sur, R.L.; Schulman, K.A.; Preminger, G.M. Changing gender prevalence of stone disease. J. Urol. 2007, 177, 979–982. [Google Scholar] [CrossRef]
- Uribarri, J.; Oh, M.S.; Carroll, H.J. The first kidney stone. Ann. Intern. Med. 1989, 111, 1006–1009. [Google Scholar] [CrossRef] [PubMed]
- Moe, O.W. Kidney stones: Pathophysiology and medical management. Lancet 2006, 367, 333–344. [Google Scholar] [CrossRef]
- Miano, R.; Germani, S.; Vespasiani, G. Stones and urinary tract infections. Urol. Int. 2007, 79, 32–36. [Google Scholar] [CrossRef]
- Chow, W.H.; Lindblad, P.; Gridley, G.; Nyren, O.; McLaughlin, J.K.; Linet, M.S.; Pennello, G.A.; Adami, H.O.; Fraumeni, J.J. Risk of urinary tract cancers following kidney or ureter stones. J. Natl. Cancer Inst. 1997, 89, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Pearle, M.S.; Calhoun, E.A.; Curhan, G.C. Urologic Diseases of America Project. Urologic diseases in America project: Urolithiasis. J. Urol. 2005, 173, 848–857. [Google Scholar] [CrossRef]
- Antonelli, J.A.; Maalouf, N.M.; Pearle, M.S.; Lotan, Y. Use of the National Health and Nutrition Examination Survey to calculate the impact of obesity and diabetes on cost and prevalence of urolithiasis in 2030. Eur. Urol. 2014, 66, 724–729. [Google Scholar] [CrossRef] [Green Version]
- Chung, M.J. Urolithiasis and nephrolithiasis. JAAPA 2017, 30, 49–50. [Google Scholar] [CrossRef]
- Chaudhary, A.; Singla, S.K.; Tandon, C. In vitro Evaluation of Terminalia arjuna on Calcium Phosphate and Calcium Oxalate Crystallization. Indian J. Pharm. Sci. 2010, 72, 340–345. [Google Scholar]
- Alelign, T.; Petros, B. Kidney Stone Disease: An Update on Current Concepts. Adv. Urol. 2018, 2018, 3068365. [Google Scholar] [CrossRef]
- Scales, C.D., Jr.; Smith, A.C.; Hanley, J.M.; Saigal, C.S. Urologic Diseases in America Project. Prevalence of kidney stones in the United States. Eur. Urol. 2012, 62, 160–165. [Google Scholar] [CrossRef] [Green Version]
- Curhan, G.C.; Willett, W.C.; Rimm, E.B.; Stampfer, M.J. Family history and risk of kidney stones. J. Am. Soc. Nephrol. 1997, 8, 1568–1573. [Google Scholar] [CrossRef]
- Stamatelou, K.K.; Francis, M.E.; Jones, C.A.; Nyberg, L.M.; Curhan, G.C. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int. 2003, 63, 1817–1823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soucie, J.M.; Thun, M.J.; Coates, R.J.; McClellan, W.; Austin, H. Demographic and geographic variability of kidney stones in the United States. Kidney Int. 1994, 46, 893–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferraro, P.M.; Bargagli, M.; Trinchieri, A.; Gambaro, G. Risk of Kidney Stones: Influence of Dietary Factors, Dietary Patterns, and Vegetarian-Vegan Diets. Nutrients 2020, 12, 779. [Google Scholar] [CrossRef] [Green Version]
- Tasian, G.E.; Jemielita, T.; Goldfarb, D.S.; Copelovitch, L.; Gerber, J.S.; Wu, Q.; Denburg, M.R. Oral antibiotic exposure and kidney stone disease. J. Am. Soc. Nephrol. 2018, 29, 1731–1740. [Google Scholar] [CrossRef]
- Tasian, G.; Miller, A.; Lange, D. Antibiotics and Kidney Stones: Perturbation of the Gut-Kidney Axis. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2019, 74, 724–726. [Google Scholar] [CrossRef] [Green Version]
- Ferraro, P.M.; Curhan, G.C.; Gambaro, G.; Taylort, E.N. Antibiotic Use and Risk of Incident Kidney Stones in Female Nurses. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2019, 74, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.M.; Burk, R.D.; Asplin, J.; Krieger, N.S.; Suadicani, S.O.; Wang, Y.; Usyk, M.; Lee, J.A.; Chen, L.; Becker, J.; et al. Kidney stone formation and the gut microbiome are altered by antibiotics in genetic hypercalciuric stone-forming rats. Urolithiasis 2021, 49, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.W.; Orr, T.; Dearing, D.; Monga, M. Loss of function dysbiosis associated with antibiotics and high fat, high sugar diet. ISME J. 2019, 13, 1379–1390. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.M.; Moazami, S.; Qiu, Y.; Kurland, I.; Chen, Z.; Agalliu, I.; Burk, R.; Davies, K.P. Evidence for a distinct gut microbiome in kidney stone formers compared to non-stone formers. Urolithiasis 2016, 44, 399–407. [Google Scholar] [CrossRef]
- Basavaraj, D.R.; Biyani, C.S.; Browning, A.J.; Cartledge, J.J. The role of urinary kidney stone inhibitors and promoters in the pathogenesis of calcium containing renal stones. EAU-EBU Update Ser. 2007, 5, 126–136. [Google Scholar] [CrossRef]
- Taylor, E.N.; Curhan, G.C. Oxalate intake and the risk for nephrolithiasis. J. Am. Soc. Nephrol. 2007, 18, 2198–2204. [Google Scholar] [CrossRef] [Green Version]
- Zabłocka, A.; Janusz, M. The two faces of reactive oxygen species. Postepy Hig. Med. Dosw. 2008, 62, 118–124. [Google Scholar]
- Holmes, R.P.; Goodman, H.O.; Assimos, D.G. Contribution of dietary oxalate to urinary oxalate excretion. Kidney Int. 2001, 59, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ticinesi, A.; Milani, C.; Guerra, A.; Allegri, F.; Lauretani, F.; Nouvenne, A.; Mancabelli, L.; Lugli, G.A.; Turroni, F.; Duranti, S.; et al. Understanding the gut-kidney axis in nephrolithiasis: An analysis of the gut microbiota composition and functionality of stone formers. Gut 2018, 67, 2097–2106. [Google Scholar] [CrossRef]
- Lieske, J.C. Probiotics for prevention of urinary stones. Ann. Transl. Med. 2017, 5, 29. [Google Scholar] [CrossRef]
- Aggarwal, K.P.; Narula, S.; Kakkar, M.; Tandon, C. Nephrolithiasis: Molecular mechanism of renal stone formation and the critical role played by modulators. BioMed Res. Int. 2013, 2013, 21. [Google Scholar] [CrossRef] [Green Version]
- Ratkalkar, V.N.; Kleinman, J.G. Mechanisms of stone formation. Clin. Rev. Bone Mineral. Metab. 2011, 9, 187–197. [Google Scholar] [CrossRef] [Green Version]
- Courbebaisse, M.; Prot-Bertoye, C.; Bertocchio, J.; Baron, S.; Maruani, G.; Briand, S.; Daudon, M.; Houillier, P. Nephrolithiasis of adult: From mechanisms to preventive medical treatment. Rev. Med Int. 2017, 38, 4452. [Google Scholar]
- Moryama, M.T.; Domiki, C.; Miyazawa, K.; Tanaka, T.; Suzuki, K. Effects of oxalate exposure on Madin-Darby canine kidney cells in culture: Renal prothrombin fragment-1 mRNA expression. Urol. Res. 2005, 33, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R.; Glenton, P.A.; Backov, R.; Talham, D.R. Presence of lipids in urine, crystals and stones: Implications for the formation of kidney stones. Kidney Int. 2002, 62, 2062–2072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhadja, P.; Lunagariya, J.; Ouyang, J.M. Seaweed sulphated polysaccharide as an inhibitor of calcium oxalate renal stone formation. J. Funct. Foods 2016, 27, 685–694. [Google Scholar] [CrossRef]
- Allison, M.J.; Dawson, K.A.; Mayberry, W.R.; Foss, J.G. Oxalobacter formigenes gen. nov., sp. Nov.: Oxalate-degrading anaerobes that inhabit the gastrointestinal tract. Arch. Microbiol. 1985, 141, 1–7. [Google Scholar] [CrossRef]
- Mehta, M.; Goldfarb, D.S.; Nazzal, L. The role of the microbiome in kidney stone formation. Int. J. Surg. 2016, 36, 607–612. [Google Scholar] [CrossRef]
- Dawson, K.A.; Allison, M.J.; Hartman, P.A. Isolation and some characteristics of anaerobic oxalate-degrading bacteria from the rumen. Appl. Environ. Microbiol. 1980, 40, 833–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allison, M.J.; Cook, H.M.; Milne, D.B.; Gallagher, S.; Clayman, R.V. Oxalate degradation by gastrointestinal bacteria from humans. J. Nutr. 1986, 116, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, H.; Hoppe, B.; Hesse, A.; Tenbrock, K.; Brömme, S.; Rietschel, E.; Peck, A.B. Absence of Oxalobacter formigenes in cystic fibrosis patients: A risk factor for hyperoxaluria. Lancet 1998, 352, 1026–1029. [Google Scholar] [CrossRef]
- Sidhu, H.; Enatska, L.; Ogden, S.; Williams, W.N.; Allison, M.J.; Peck, A.B. Evaluating Children in the Ukraine for Colonization with the Intestinal Bacterium Oxalobacter formigenes, Using a Polymerase Chain Reaction-based Detection System. Mol. Diagn. 1997, 2, 89–97. [Google Scholar] [CrossRef]
- Anantharam, V.; Allison, M.J.; Maloney, P.C. Oxalate: Formate exchange. The basis for energy coupling in Oxalobacter. J. Biol. Chem. 1989, 264, 7244. [Google Scholar] [CrossRef]
- Troxel, S.A.; Sidhu, H.; Kaul, P.; Low, R.K. Intestinal Oxalobacter formigenes colonization in calcium oxalate stone formers and its relation to urinary oxalate. J. Endourol. 2003, 17, 173–176. [Google Scholar] [CrossRef]
- Hatch, M.; Cornelius, J.; Allison, M.; Sidhu, H.; Peck, A.; Freel, R.W. Oxalobacter sp. Reduces urinary oxalate excretion by promoting enteric oxalate secretion. Kidney Int. 2006, 69, 691–698. [Google Scholar] [CrossRef] [Green Version]
- Ellis, M.E.; Mobley, J.A.; Holmes, R.P.; Knight, J. Proteome Dynamics of the Specialist Oxalate Degrader Oxalobacter formigenes. J. Proteom. Bioinform. 2016, 9, 19–24. [Google Scholar]
- Ellis, M.L.; Dowell, A.E.; Li, X.; Knight, J. Probiotic properties of Oxalobacter formigenes: An in vitro examination. Arch. Microbiol. 2016, 198, 1019–1026. [Google Scholar] [CrossRef] [Green Version]
- Duncan, S.H.; Richardson, A.J.; Kaul, P.; Holmes, R.P.; Allison, M.J.; Stewart, C.S. Oxalobacter formigenes and its potential role in human health. Appl. Environ. Microbiol. 2002, 68, 3841–3847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoppe, B.; Beck, B.; Gatter, N.; von Unruh, G.; Tischer, A.; Hesse, A.; Laube, N.; Kaul, P.; Sidhu, H. Oxalobacter formigenes: A potential tool for the treatment of primary hyperoxaluria type 1. Kidney Int. 2006, 70, 1305–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidhu, H.; Schmidt, M.E.; Cornelius, J.G.; Thamilselvan, S.; Khan, S.R.; Hesse, A.; Peck, A.B. Direct correlation between hyperoxaluria/oxalate stone disease and the absence of the gastrointestinal tract-dwelling bacterium Oxalobacter formigenes: Possible prevention by gut recolonization or enzyme replacement therapy. J. Am. Soc. Nephrol. 1999, 10, 334–340. [Google Scholar]
- Sidhu, H.; Allison, M.J.; Chow, J.M.; Clark, A.; Peck, A.B. Rapid reversal of hyperoxaluria in a rat model after probiotic administration of Oxalobacter formigenes. J. Urol. 2001, 166, 1487–1491. [Google Scholar] [CrossRef]
- Siener, R.; Bangen, U.; Sidhu, H.; Hönow, R.; von Unruh, G.; Hesse, A. The role of Oxalobacter formigenes colonization in calcium oxalate stone disease. Kidney Int. 2013, 83, 1144–1149. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, D.W. Oxalobacter formigenes may reduce the risk of calcium oxalate kidney stones. J. Am. Soc. Nephrol. 2008, 19, 1197–1203. [Google Scholar] [CrossRef] [Green Version]
- Kwak, C.; Jeong, B.C.; Lee, J.H.; Kim, H.K.; Kim, E.C.; Kim, H.H. Molecular identification of Oxalobacter formigenes with the polymerase chain reaction in fresh or frozen fecal samples. BJU Int. 2001, 88, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Kodama, T.; Mikami, K.; Akakura, K.; Takei, K.; Naya, Y.; Ueda, T.; Ito, H. Detection of Oxalobacter formigenes in human feces and study of related genes in a new oxalate-degrading bacterium. Hinyokika Kiyo 2003, 49, 371–376. [Google Scholar]
- Kumar, R.; Mukherjee, M.; Bhandari, M.; Kumar, A.; Sidhu, H.; Mittal, R.D. Role of Oxalobacter formigenes in calcium oxalate stone disease: A study from North India. Eur. Urol. 2002, 41, 318–322. [Google Scholar] [CrossRef]
- Kharlamb, V.; Schelker, J.; Francois, F.; Jiang, J.; Holmes, R.P.; Goldfarb, D.S. Oral antibiotic treatment of Helicobacter pylori leads to persistently reduced intestinal colonization rates with Oxalobacter formigenes. J. Endourol. 2011, 25, 1781–1785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- PeBenito, A.; Nazzal, L.; Wang, C.; Li, H.; Jay, M.; Noya-Alarcon, O.; Contreras, M.; Lander, O.; Leach, J.; Dominguez-Bello, M.G.; et al. Comparative prevalence of Oxalobacter formigenes in three human populations. Sci. Rep. 2019, 9, 574. [Google Scholar] [CrossRef] [Green Version]
- Nazzal, L.; Francois, F.; Henderson, N.; Liu, M.; Li, H.; Koh, H.; Wang, C.; Gao, Z.; Perez, G.P.; Asplin, J.R.; et al. Effect of antibiotic treatment on Oxalobacter formigenes colonization of the gut microbiome and urinary oxalate excretion. Sci. Rep. 2021, 11, 16428. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Knight, J.; Easter, L.H.; Neiberg, R.; Holmes, R.P.; Assimos, D.G. Impact of dietary calcium and oxalate, and Oxalobacter formigenes colonization on urinary oxalate excretion. J. Urol. 2011, 186, 135–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ticinesi, A.; Nouvenne, A.; Meschi, T. Gut microbiome and kidney stone disease: Not just an Oxalobacter story. Kidney Int. 2019, 96, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Chen, D.Q.; Chen, L.; Liu, J.R.; Vaziri, N.D.; Guo, Y.; Zhao, Y.Y. Microbiome-metabolome reveals the contribution of gut-kidney axis on kidney disease. J. Transl. Med. 2019, 17, 5. [Google Scholar] [CrossRef] [Green Version]
- Pedro, R.N.; Aslam, A.U.; Bello, J.O.; Bhatti, K.H.; Philipraj, J.; Sissoko, I.; Vasconcellos, G.S.; Trinchieri, A.; Buchholz, N. Nutrients, vitamins, probiotics and herbal products: An update of their role in urolithogenesis. Urolithiasis 2020, 48, 285–301. [Google Scholar] [CrossRef]
- Daniel, S.L.; Moradi, L.; Paiste, H.; Wood, K.D.; Assimos, D.G.; Holmes, R.P.; Nazzal, L.; Hatch, M.; Knight, J. Forty Years of Oxalobacter formigenes, a Gutsy Oxalate-Degrading Specialist. Appl. Environ. Microbiol. 2021, 87, 0054421. [Google Scholar] [CrossRef] [PubMed]
- Milliner, D.; Hoppe, B.; Groothoff, J. A randomised Phase II/III study to evaluate the efficacy and safety of orally administered Oxalobacter formigenes to treat primary hyperoxaluria. Urolithiasis 2018, 46, 313–323. [Google Scholar] [CrossRef]
- Hoppe, B.; Niaudet, P.; Salomon, R.; Harambat, J.; Hulton, S.A.; Van’t Hoff, W.; Moochhala, S.H.; Deschênes, G.; Lindner, E.; Sjögren, A.; et al. A randomised Phase I/II trial to evaluate the efficacy and safety of orally administered Oxalobacter formigenes to treat primary hyperoxaluria. Pediatr. Nephrol. 2017, 32, 781–790. [Google Scholar] [CrossRef]
- Gänzle, M.G. Lactic metabolism revisited: Metabolism of lactic acid bacteria in food fermentations and food spoilage. Curr. Opin. Food Sci. Food Microbiol. Funct. Foods Nutr. 2015, 2, 106–117. [Google Scholar] [CrossRef]
- Lewanika, T.R.; Reid, S.J.; Abratt, V.R.; Macfarlane, G.T.; Macfarlane, S. Lactobacillus gasseri Gasser AM63T degrades oxalate in a multistage continuous culture simulator of the human colonic microbiota. FEMS Microbiol. Ecol. 2007, 61, 110–120. [Google Scholar] [CrossRef] [Green Version]
- Martín, R.; Langella, P. Emerging Health Concepts in the Probiotics Field: Streamlining the Definitions. Front. Microbiol. 2019, 10, 1047. [Google Scholar] [CrossRef] [Green Version]
- Le, B.; Yang, S.H. Efficacy of Lactobacillus plantarum in Prevention of Inflammatory Bowel disease. Toxicol. Rep. 2018, 5, 314–317. [Google Scholar] [CrossRef]
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus reuteri in Human Health and Diseases. Front. Microbiol. 2018, 9, 757. [Google Scholar] [CrossRef] [PubMed]
- Bai, A.P.; Ouyang, Q. Probiotics and Inflammatory Bowel Disease. Postgrad. Med. J. 2006, 82, 376–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heeney, D.D.; Gareau, M.G.; Marco, M.L. Intestinal Lactobacillus in health and disease, a driver or just along for the ride? Curr. Opin. Biotechnol. 2018, 49, 140–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinross, J.M.; von Roon, A.C.; Holmes, E.; Darzi, A.; Nicholson, J.K. The human gut microbiome: Implications for future health care. Curr. Gastroenterol. Rep. 2008, 10, 396–403. [Google Scholar] [CrossRef]
- Dang, F.; Jiang, Y.; Pan, R.; Zhou, Y.; Wu, S.; Wang, R.; Zhuang, K.; Zhang, W.; Li, T.; Man, C. Administration of Lactobacillus paracasei ameliorates type 2 diabetes in mice. Food Funct. 2018, 9, 3630–3639. [Google Scholar] [CrossRef] [PubMed]
- Oriach, C.S.; Robertson, R.C.; Stanton, C.; Cryan, J.F.; Dinanad, T.G. Food for Thought: The Role of Nutrition in the Microbiota-Gut-Brain Axis. Clin. Nutr. Exp. 2016, 6, 25–38. [Google Scholar] [CrossRef] [Green Version]
- Campieri, C.; Campieri, M.; Bertuzzi, V.; Swennen, E.; Matteuzzi, D.; Stefoni, S.; Pirovano, F.; Centi, C.; Ulisse, S.; Famularo, G.; et al. Reduction of oxaluria after an oral course of lactic acid bacteria at high concentration. Kidney Int. 2001, 60, 1097–1105. [Google Scholar] [CrossRef] [Green Version]
- Turroni, S.; Vitali, B.; Bendazzoli, C.; Candela, M.; Gotti, R.; Federici, F.; Pirovano, F.; Brigidi, P. Oxalate consumption by lactobacilli: Evaluation of oxalyl-CoA decarboxylase and formyl-CoA transferase activity in Lactobacillus acidophilus. J. Appl. Microbiol. 2007, 103, 1600–1609. [Google Scholar] [CrossRef]
- Turroni, S.; Bendazzoli, C.; Dipalo, S.C.; Candela, M.; Vitali, B.; Gotti, R.; Brigidi, P. Oxalate-degrading activity in Bifidobacterium animalis subsp. lactis: Impact of acidic conditions on the transcriptional levels of the oxalyl coenzyme A (CoA) decarboxylase and formyl-CoA transferase genes. Appl. Environ. Microbiol. 2010, 76, 5609–5620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mogna, L.; Pane, M.; Nicola, S.; Raiteri, E. Screening of different probiotic strains for their in vitro ability to metabolise oxalates: Any prospective use in humans? J. Clin. Gastroenterol. 2014, 48, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Azcarate-Peril, M.A.; Bruno-Barcena, J.M.; Hassan, H.M.; Klaenhammer, T.R. Transcriptional and functional analysis of oxalyl-coenzyme A (CoA) decarboxylase and formyl-CoA transferase genes from Lactobacillus acidophilus. Appl. Environ. Microbiol. 2006, 72, 1891–1899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, C.; Murphy, S.; O’Brien, F.; O’Donoghue, M.; Boileau, T.; Sunvold, G.; Reinhart, G.; Kiely, B.; Shanahan, F.; O’Mahony, L. Metabolic activity of probiotics-oxalate degradation. Vet. Microbiol. 2009, 136, 100–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomathi, S.; Sasikumar, P.; Anbazhagan, K.; Sasikumar, S.; Kavitha, M.; Selvi, M.S.; Selvam, G.S. Screening of indigenous oxalate degrading lactic acid bacteria from human faeces and South Indian fermented foods: Assessment of probiotic potential. Sci. World J. 2014, 2014, 648059. [Google Scholar] [CrossRef] [Green Version]
- Giardina, S.; Scilironi, C.; Michelotti, A.; Samuele, A.; Borella, F.; Daglia, M.; Marzatico, F. In vitro anti-inflammatory activity of selected oxalate-degrading probiotic bacteria: Potential applications in the prevention and treatment of hyperoxaluria. J. Food Sci. 2014, 79, M384–M390. [Google Scholar] [CrossRef]
- Okombo, J.; Liebman, M. Probiotic-induced reduction of gastrointestinal oxalate absorption in healthy subjects. Urol. Res. 2010, 38, 169–178. [Google Scholar] [CrossRef]
- Kwak, C.; Jeong, B.C.; Ku, J.H.; Kim, H.H.; Lee, J.J.; Huh, C.S.; Baek, Y.J.; Lee, S.E. Prevention of nephrolithiasis by Lactobacillus in stone-forming rats: A preliminary study. Urol. Res. 2006, 34, 265–270. [Google Scholar] [CrossRef]
- Wei, Z.; Cui, Y.; Tian, L.; Liu, Y.; Yu, Y.; Jin, X.; Li, H.; Wang, K.; Sun, Q. Probiotic Lactiplantibacillus plantarum N-1 could prevent ethylene glycol-induced kidney stones by regulating gut microbiota and enhancing intestinal barrier function. FASEB J. 2021, 35, e21937. [Google Scholar] [CrossRef]
- Chapman, C.M.; Gibson, G.R.; Rowland, I. Health benefits of probiotics: Are mixtures more effective than single strains? Eur. J. Nutr. 2011, 50, 1–17. [Google Scholar] [CrossRef]
- Lieske, J.C.; Goldfarb, D.S.; De Simone, C.; Regnier, C. Use of a probiotic to decrease enteric hyperoxaluria. Kidney Int. 2005, 68, 1244–1249. [Google Scholar] [CrossRef] [Green Version]
- Lieske, J.C.; Tremaine, W.J.; De Simone, C.; O’Connor, H.M.; Li, X.; Bergstralh, E.J.; Goldfarb, D.S. Diet, but not oral probiotics, effectively reduces urinary oxalate excretion and calcium oxalate supersaturation. Kidney Int. 2010, 78, 1178–1185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldfarb, D.S.; Modersitzki, F.; Asplin, J.R. A randomized, controlled trial of lactic acid bacteria for idiopathic hyperoxaluria. Clin. J. Am. Soc. Nephrol. 2007, 2, 745–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siener, R.; Bade, D.J.; Hesse, A.; Hoppe, B. Dietary hyperoxaluria is not reduced by treatment with lactic acid bacteria. J. Transl. Med. 2013, 11, 306. [Google Scholar] [CrossRef] [Green Version]
- Ferraz, R.R.; Marques, N.C.; Froeder, L.; Menon, V.B.; Siliano, P.R.; Baxmann, A.C.; Heilberg, I.P. Effects of Lactobacillus casei and Bifidobacterium breve on urinary oxalate excretion in nephrolithiasis patients. Urol. Res. 2009, 37, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Al-Wahsh, I.; Wu, Y.; Liebman, M. Acute probiotic ingestion reduces gastrointestinal oxalate absorption in healthy subjects. Urol. Res. 2012, 40, 191–196. [Google Scholar] [CrossRef]
- Tisser, M.H. La reaction chromophile d’Escherichia et le Bacterium coli. C. R. Soc. Biol. 1899, 51, 943. [Google Scholar]
- Picard, C.; Fioramonti, J.; Francois, A.; Robinson, T.; Neant, F.; Matuchansky, C. Review article: Bifidobacteria as probiotic agents—physiological effects and clinical benefits. Aliment. Pharmacol. Ther. 2005, 22, 495–512. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, D.J. Screening of intestinal microflora for effective probiotic bacteria. J. Agric. Food Chem. 2001, 49, 1751–1760. [Google Scholar] [CrossRef]
- Jiang, T.A.; Mustapha, A.; Savaiano, D.A. Improvement of lactose digestion in humans by ingestion of unfermented milk containing Bifidobacterium longum. J. Dairy Sci. 1996, 79, 750–757. [Google Scholar] [CrossRef]
- Ataie-Jafari, A.; Larijani, B.; Alavi Majd, H.; Tahbaz, F. Cholesterol-lowering effect of probiotic yogurt in comparison with ordinary yogurt in mildly to moderately hypercholesterolemic subjects. Ann. Nutr. Metab. 2009, 54, 22–27. [Google Scholar] [CrossRef]
- Federici, F.; Vitali, B.; Gotti, R.; Pasca, M.R.; Gobbi, S.; Peck, A.B.; Brigidi, P. Characterization and heterologous expression of the oxalyl coenzyme A decarboxylase gene from Bifidobacterium lactis. Appl. Environ. Microbiol. 2004, 70, 5066–5073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klimesova, K.; Whittamore, J.M.; Hatch, M. Bifidobacterium animalis subsp. lactis decreases urinary oxalate excretion in a mouse model of primary hyperoxaluria. Urolithiasis 2015, 43, 107–117. [Google Scholar] [CrossRef] [Green Version]
- Hatch, M.; Freel, R.W. A human strain of Oxalobacter (HC-1) promotes enteric oxalate secretion in the small intestine of mice and reduces urinary oxalate excretion. Urolithiasis 2013, 41, 379–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, H.; Miura, N.; Masai, M.; Yamamoto, K.; Hara, T. Reduction of oxalate content of foods by the oxalate degrading bacterium, Eubacterium lentum WYH-1. Int. J. Urol. 1996, 3, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Kotake, T.; Masai, M. In vitro degradation of oxalic acid by human faeces. Int. J. Urol. 1996, 3, 207–211. [Google Scholar] [CrossRef]
- Fiore, E.; Van Tyne, D.; Gilmore, M.S. Pathogenicity of Enterococci. Microbiol. Spectr. 2019, 7, 10. [Google Scholar] [CrossRef]
- Hokama, S.; Honma, Y.; Toma, C.; Ogawa, Y. Oxalate-degrading Enterococcus faecalis. Microbiol. Immunol. 2000, 44, 235–240. [Google Scholar] [CrossRef]
- Toyota, C.G.; Berthold, C.L.; Gruez, A.; Jonsson, S.; Lindqvist, Y.; Cambillau, C.; Richards, N.G. Differential substrate specificity and kinetic behavior of Escherichia coli YfdW and Oxalobacter formigenes formyl coenzyme A transferase. J. Bacteriol. 2008, 190, 2556–2564. [Google Scholar] [CrossRef] [Green Version]
- Toyota, C.G. Oxalate Metabolizing Enzymes of Oxalobacter Formigenes and Escherichia coli. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2008. [Google Scholar]
- Werther, T.; Zimmer, A.; Wille, G.; Golbik, R.; Weiss, M.S.; Konig, S. New insights into structure-function relationships of oxalyl CoA decarboxylase from Escherichia coli. FEBS J. 2010, 277, 2628–2640. [Google Scholar] [CrossRef] [PubMed]
- Abratt, V.R.; Reid, S.J. Oxalate-degrading bacteria of the human gut as probiotics in the management of kidney stone disease. Adv. Appl. Microbiol. 2010, 72, 63–87. [Google Scholar] [PubMed]
- Al, K.F.; Daisley, B.A.; Chanyi, R.M.; Bjazevic, J.; Razvi, H.; Reid, G.; Burton, J.P. Oxalate-Degrading Bacillus subtilis Mitigates Urolithiasis in a Drosophila melanogaster Model. mSphere 2020, 5, e00498-20. [Google Scholar] [CrossRef] [PubMed]
- Just, V.J.; Stevenson, C.E.M.; Bowater, L.; Tanner, A.; Lawson, D.M.; Bornemann, S. A closed conformation of Bacillus subtilis oxalate decarboxylase OxdC provides evidence for the true identity of the active site. J. Biol. Chem. 2004, 279, 19867–19874. [Google Scholar] [CrossRef] [Green Version]
- Grujic, D.; Salido, E.C.; Shenoy, B.C.; Langman, C.B.; McGrath, M.E.; Patel, R.J.; Rashid, A.; Mandapati, S.; Jung, C.W.; Margolin, A.L. Hyperoxaluria is reduced and nephrocalcinosis prevented with an oxalate-degrading enzyme in mice with hyperoxaluria. Am. J. Nephrol. 2009, 29, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Cowley, A.B.; Poage, D.W.; Dean, R.R.; Meschter, C.L.; Ghoddusi, M.; Li, Q.-S.; Sidhu, H. 14-day repeat-dose oral toxicity evaluation of oxazyme in rats and dogs. Int. J. Toxicol. 2010, 29, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Mufarrij, P.W.; Lange, J.N.; Knight, J.; Assimos, D.G.; Holmes, R.P. The effects of Oxazyme on oxalate degradation: Results and implications of in vitro experiments. J. Endourol. 2013, 27, 284–287. [Google Scholar] [CrossRef]
- Yadav, A.K.; Tyagi, A.; Kumar, A.; Saklani, A.C.; Grover, S.; Batish, V.K. Adhesion of indigenous Lactobacillus plantarum to gut extracellular matrix and its physicochemical characterization. Arch. Microbiol. 2014, 197, 155–164. [Google Scholar] [CrossRef]
- Kolandaswamy, A.; George, L.; Sadasivam, S. Heterologous expression of oxalate decarboxylase in Lactobacillus plantarum NC8. Curr. Microbiol. 2009, 58, 117–121. [Google Scholar] [CrossRef]
- Sørvig, E.; Grönqvist, S.; Naterstad, K.; Mathiesen, G.; Eijsink, V.G.H.; Axelsson, L. Construction of vectors for inducible gene expression in Lactobacillus sakei and L. plantarum. FEMS Microbiol. Lett. 2003, 229, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Sasikumar, P.; Gomathi, S.; Anbazhagan, K.; Abhishek, A.; Paul, E.; Vasudevan, V.; Sasikumar, S.; Selvam, G.S. Recombinant Lactobacillus plantarum expressing and secreting heterologous oxalate decarboxylase prevents renal calcium oxalate stone deposition in experimental rats. J. Biomed. Sci. 2014, 21, 86. [Google Scholar] [CrossRef] [Green Version]
- Mathiesen, G.; Sveen, A.; Brurberg, M.B.; Fredriksen, L.; Axelsson, L.; Eijsink, V.G. Genome-wide analysis of signal peptide functionality in Lactobacillus plantarum WCFS1. BMC Genom. 2009, 10, 425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathiesen, G.; Sveen, A.; Piard, J.-C.; Axelsson, L.; Eijsink, V.G.H. Heterologous protein secretion by Lactobacillus plantarum using homologous signal peptides. J. Appl. Microbiol. 2008, 105, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Anbazhagan, K.; Sasikumar, P.; Gomathi, S.; Priya, H.P.; Selvam, G.S. In vitro degradation of oxalate by recombinant Lactobacillus plantarum expressing heterologous oxalate decarboxylase. J. Appl. Microbiol. 2013, 115, 880–887. [Google Scholar] [CrossRef]
- Sasikumar, P.; Gomathi, S.; Anbazhagan, K.; Baby, A.E.; Sangeetha, J.; Selvam, G.S. Genetically engineered Lactobacillus plantarum WCFS1 constitutively secreting heterologous oxalate decarboxylase and degrading oxalate under in vitro. Curr. Microbiol. 2014, 69, 708–715. [Google Scholar] [CrossRef]
- Charteris, W.P.; Kelly, P.M.; Morelli, L.; Collins, J.K. Antibiotic susceptibility of potentially probiotic Lactobacillus species. J. Food Prot. 1998, 61, 1636–1643. [Google Scholar] [CrossRef]
- Moubareck, C.; Gavini, F.; Vaugien, L.; Butel, M.J.; Doucet-Populaire, F. Antimicrobial susceptibility of bifidobacteria. J. Antimicrob. Chemother. 2005, 55, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Maresca, D.; Zotta, T.; Mauriello, G. Adaptation to Aerobic Environment of Lactobacillus johnsonii/gasseri Strains. Front. Microbiol. 2018, 9, 157. [Google Scholar] [CrossRef]
- Fuochi, V.; Petronio, G.P.; Lissandrello, E.; Furneri, P.M. Evaluation of resistance to low pH and bile salts of human Lactobacillus spp. isolates. Int. J. Immunopathol. Pharmacol. 2015, 28, 426–433. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, B.; Champomier-Vergès, M.C.; Collado, M.; Anglade, P.; Baraige, F.; Sanz, Y.; de los Reyes-Gavilán, C.G.; Margolles, A.; Zagorec, M. Low-pH adaptation and the acid tolerance response of Bifidobacterium longum biotype longum. Appl. Environ. Microbiol. 2007, 73, 6450–6459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimamura, S.; Abe, F.; Ishibashi, N.; Miyakawa, H.; Yaeshima, T.; Araya, T.; Tomita, M. Relationship between oxygen sensitivity and oxygen metabolism of Bifidobacterium species. J. Dairy Sci. 1992, 75, 3296–3306. [Google Scholar] [CrossRef]
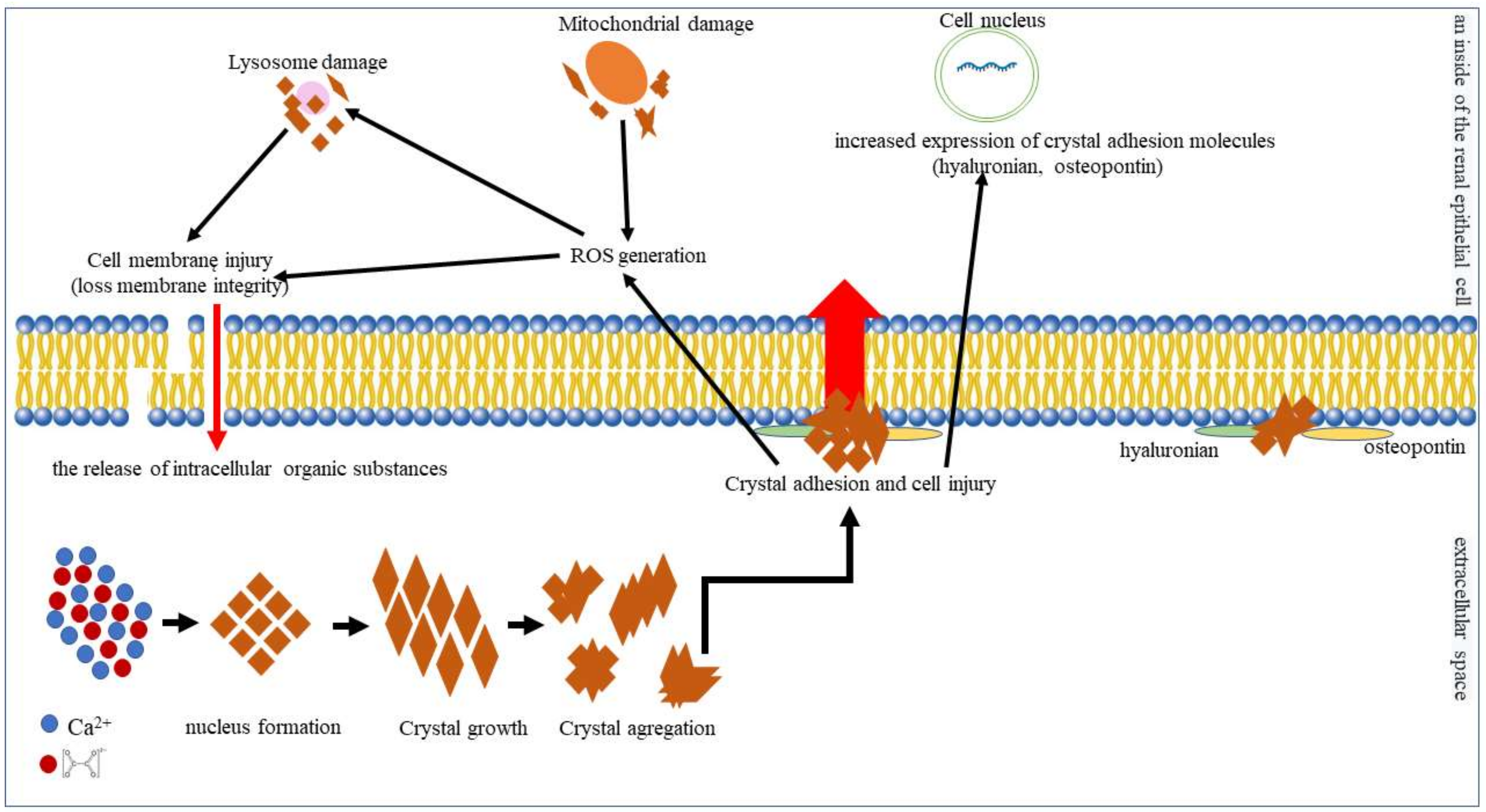
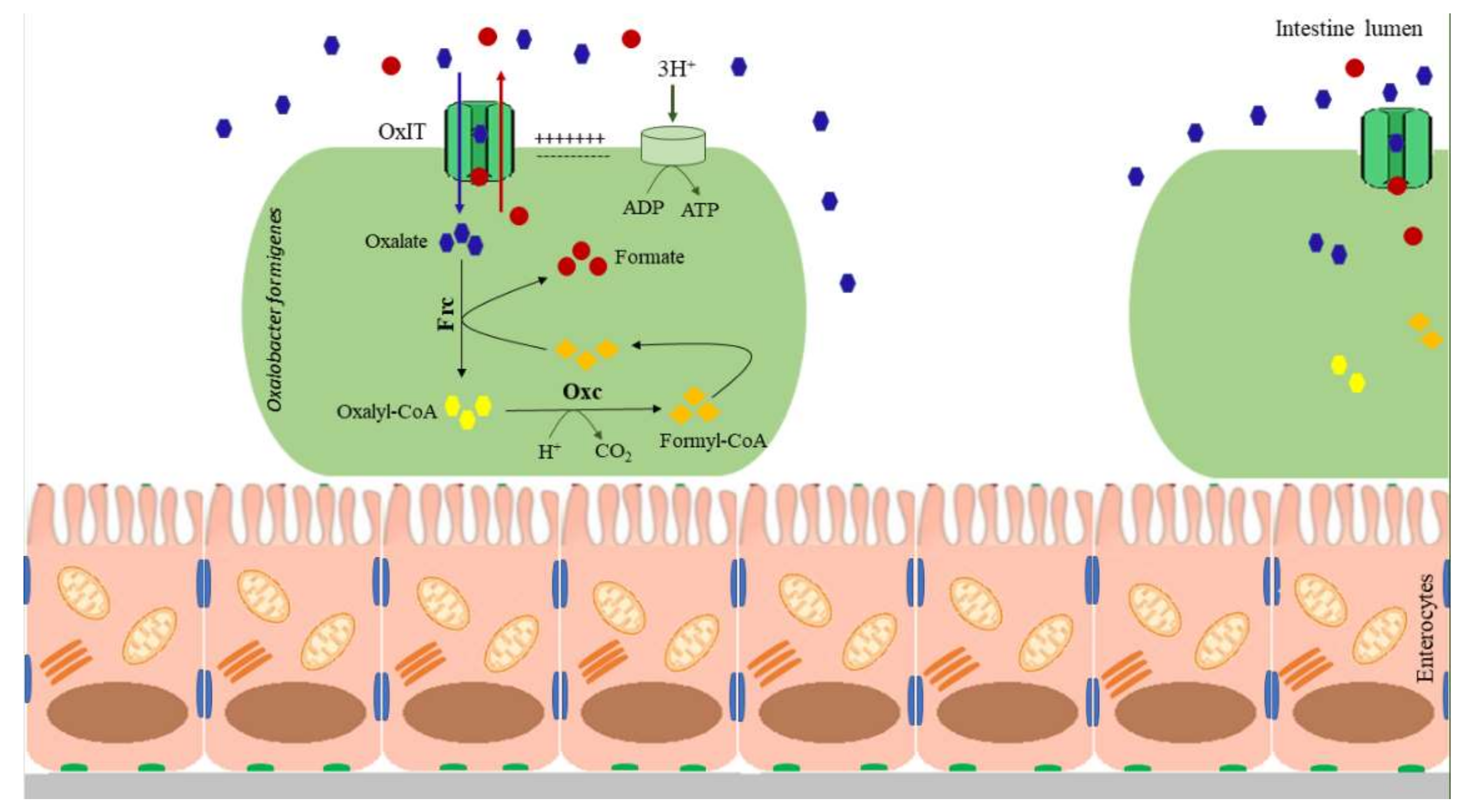
| Type of Risk Factors | Risk Factors | Description | References |
|---|---|---|---|
| Intrinsic factors | Gender | Deposits in the urinary tract were found to be 2–3 times more common in men than in women. | [6] |
| Age | The incidence of nephrolithiasis has increased dramatically over the past 30 years. | [1] | |
| Ethnic differences | Non-Hispanic white individuals were characterized by the highest stone risk (10.3%), followed by Hispanics (6.4%) and non-Hispanic African Americans (4.3%). | [17] | |
| Family or personal history | If someone in your family has had kidney stones, you are more likely to develop them than someone without a family history. | [18] | |
| Extrinsic factors | Environmental factors | Regions with higher average temperatures in the United States showed the highest risk of urinary tract stone occurrence. | [19,20] |
| “Western diets” | “Western diets” are characterized by the consumption of large amounts of animal protein, which leads to an increase in the excretion of calcium, oxalate, and uric acid in the urine, consequently predisposing individuals to kidney stones. | [1,2] | |
| Fluid intake | The reduction of fluid intake may contribute to increased urine saturation. | [21] | |
| Sodium intake | Increased sodium intake causes increased urine calcium and reduced citrate excretion. | [21] | |
| Calcium intake | Reduction in calcium intake causes an increase in urinary oxalate excretion. | [21] | |
| Meat intake | Increased meat intake causes a decrease in urine pH and an increase urinary calcium excretion. | [21] | |
| Fruits intake | Decreased fruit intake causes a decrease in urine pH and reduces in citrate excretion. | [21] | |
| Diet content in oxalate foods | Increased oxalate in food contributes to an increase in urinary oxalate extraction. | [21] | |
| BMI (body mass index) | An increase in BMI was positively correlated with increased risk of nephrolithiasis. | [1] | |
| Metabolic disorders | Diabetes mellitus, obesity and metabolic syndrome may increase the risk of kidney stones. | [21] | |
| Urinary tract infections | Patients who suffer from chronic urinary tract infections may form larger stones. | [21] |
| Oxalobacter formigenes | |
|---|---|
| Characteristic | Description |
| Morphology | Rod shaped |
| Gram staining | Gram-negative |
| Mobility | Nonmotile |
| Spore-forming | Nonspore-forming |
| Anaerobic/aerobic | Obligate anaerobes |
| Author | Type of Study | Study Design | Main Finding |
|---|---|---|---|
| Sidhu et al. [53] | Animal study | Laboratory rats known to be noncolonized were colonized with live bacteria or treated with a preparation of oxalate-degrading enzymes derived from O. formigenes to test for any subsequent increase in resistance to high oxalate challenge. | The absence of O. formigenes in the gut increases the risk for hyperoxaluria and recurrent kidney stone disease. Replacement therapy is an efficient procedure to prevent hyperoxaluria and its complications. |
| Sidhu et al. [54] | Animal model of severe hyperoxaluria | Male Sprague-Dawley rats were divided into six subgroups: group 1 was given a normal diet, group 2 was given an oxalate-rich diet; group 3 was given an oxalate-rich diet and an esophageal gavage of 1 × 103 O. formigenes each feeding for a two-week period; group 4 was given an oxalate-rich diet and an esophageal gavage of 1 × 105 O. formigenes each feeding for a two week period; group 5 was given an oxalate-rich diet and an esophageal gavage of 1 × 107 O. formigenes each feeding for a two week period; and group 6 was given an oxalate-rich diet and an esophageal gavage of 1 × 109 O. formigenes each feeding for a two week period. Urinary samples were also collected to assess the level of oxalate extraction. | Urine oxalate levels were lower in the group that received an oxalate-rich diet and O. formigenes compared to the group that received only an oxalate-rich diet. The amount of the decrease proved to be directly proportional to the dose of bacteria. |
| Hatch et al. [48] | Animal study | Male Sprague–Dawley rats were divided into two groups: group 1 was colonized by esophageal gavage of a 1.5 mL inoculum of 20 × 108 bacteria from a 24-h culture of a wild rat strain of O. formigenes, while group 2 was noncolonized. Both groups of rats were fed the same diet. Urinary samples were also collected to assess the level of oxalate extraction. | Rats colonized by O. formigenes were characterized by decreased urinary oxalate levels as compared with noncolonized rats on the same diet |
| Duncan et al. [51] | Clinical study (Four healthy volunteers) | Adult volunteers lacking detectable oxalate-degrading activity in feces were subjected to an oxalate loading test and then administered 500 mg wet weight containing approximately 108 viable cells in 1 mg of O. formigenes strain HC−1. Finally, stool samples were collected to assess the presence of O. formigenes. Determination of O. formigenes was carried out by culture as well as PCR. Urinary samples were also collected to assess the level of oxalate extraction. | O. formigenes intake may reduce urinary oxalate levels in patients on a diet high in calcium oxalate. |
| Hoppe et al. [52] | Clinical study (16 patients with urolithiasis) | Patients were divided into two groups. The first group included nine patients to whom O. formigenes was administered as a frozen cell paste containing 1 g live cells equivalent to >1010 CFU. The second group included seven patients to whom O. formigenes was administered as two enteric-coated capsules per dosing (137 mg of lyophilized bulk powder of freeze-dried live cells equivalent to ~107 CFU). Urine and plasma samples were collected to assess the level of oxalate extraction. | O. formigenes intake can reduce the urinary oxalate levels in patients with urolithiasis. |
| Siener et al. [55] | Clinical study (37 patients with idiopathic calcium oxalate stone) | The presence of O. formigenes in the feces was determined to classify patients into colonized and noncolonized groups. Determination of O. formigenes was carried out by culture as well as PCR. Venous blood samples were obtained in the morning after an overnight fasting period, while 24-h urine samples were collected to assess baseline oxalate urinary excretions. The patients’ diets were not controlled. | Plasma oxalate concentrations were significantly higher in noncolonized (5.79 μmol/L) than colonized stone formers (1.70 μmol/L). Colonization with O. formigenes was significantly inversely associated with the number of stone episodes. O. formigenes colonization was shown to decrease urinary oxalate excretion. |
| Kaufman et al. [56] | Clinical study (247 patients with recurrent calcium oxalate stones and 259 controls) | Stool samples were collected to assess the presence of O. formigenes. Determination of O. formigenes occurrence was carried out by culture as well as PCR. Urinary samples were also collected to assess the level of oxalate extraction. During the study, patients were on whatever diet they reported in the survey. | The prevalence of O. formigenes was 17% among case-patients and 38% among control subjects. Moreover, the colonization with O. formigenes was associated with a 70% reduction in the risk of recurrent calcium oxalate stone formation. |
| Troxel et al. [49] | Clinical study (five first time calcium stone formers and10 control participants) | Stool samples were collected for culture and detection of O. formigenes by PCR. Urinary samples were also collected to assess the level of oxalate extraction. Additionally, all participants underwent standard metabolic testing to evaluate their risk for recurrent stone formation. | Urine oxalate levels were lower in O. formigenes-positive patients compared with O. formigenes-negative patients (29.4 mg/day vs. 41.7 mg/day). |
| Kwak et al. [57] | Clinical study (30 healthy volunteers and 38 patients with urolithiasis) | Determination of O. formigenes presence in stool samples was carried out by PCR. Patients’ diets were not controlled. | The colonization rate of O. formigenes in patients with urolithiasis was significantly lower than in healthy volunteers known to be free from urolithiasis. |
| Kodama et al. [58] | Clinical study (55 male and 37 female healthy volunteers) | Determination of the presence of O. formigenes in stool samples was carried out by PCR and culture-based method. Participants’ diets were not controlled. | Female subjects showed a 15% lower rate of O. formigenes occurrence than males. |
| Kumar et al. [59] | Clinical study (63 patients with calcium oxalate stone formers and 40 controls from North India) | Stool samples were collected to assess the presence of O. formigenes. Determination of O. formigenes was carried out by PCR and Southern blotting. Urinary samples were also collected to assess the level of oxalate extraction. | O. formigenes was present in 65% of normal individuals and 30% of calcium oxalate stone formers. Colonies were present in only 5.6% of patients with three or more stone episodes. Oxalate excretion was lower in patients colonized with O. formigenes compared to those with no colonization. |
| Kharlamb et al. [60] | Clinical study (patients with confirmed O. formigenes colonization) | The impact of antibiotics (amoxicillin and clarithromycin) on O. formigenes colonization was compared in two groups: group 1 received antibiotics for gastric infection with Helicobacter pylori, while in group 2 without H. pylori, subjects were not receiving antibiotics. O. formigenes colonization in stool was detected by oxalate degradation at baseline and after one and six months. | Among the 12 patients who were positive for O. formigenes who did not receive antibiotics, 11 (92%) had O. formigenes, as evaluated through stool tests at one and six months. Of the 19 participants who were positive for O. formigenes and who received antibiotics for H. pylori, only seven (36.8%) continued to be colonized by O. formigenes on follow-up stool testing at one and six months. |
| Nazzal et al. [62] | Clinical study (23 patients with a positive test of H. pylori and 46 controls) | Patients with confirmed H. pylori infection received antibiotics (amoxicillin and clarithromycin), while control groups did not. Fecal samples were examined for the presence of O. formigenes and for microbiota characteristics. Determination of O. formigenes was carried out by PCR. Urine, collected after serially fasting and following a standard meal, was tested for oxalate and electrolyte concentrations. | O. formigenes colonization was significantly suppressed in antibiotic-exposed subjects but remained stable in controls. |
| Jiang et al. [63] | Clinical study (11 O. formigenes positive and 11 O.formigenes negative nonstone forming adults) | The study was divided into two, three-week dietary phases. For the first phase, dietary oxalate intake was varied, including 50 mg daily for the first week, 250 mg for the second week, and 750 mg for the third week. For the second phase, dietary calcium intake was varied, i.e., 400 mg daily for the first week, 1000 mg for the second week, and 2000 mg for the third week. Finally, urine and stool samples were collected and used to determine stone risk parameters and O. formigenes levels. | O. formigenes levels increased 10-fold as dietary oxalate increased 15-fold, while there was a decrease in O. formigenes content with increasing calcium intake. |
| Hoppe et al. [69] | Phase I/II clinical trial (28 patients randomized to the treatment group (OC5) or the placebo group | There was no significant difference in the change in urinary oxalate excretion and plasma oxalate excretion between the studied groups after eight weeks of OC5 treatment. However, the group which received OC5 treatment was characterized by increased urinary oxalate excretion compared to urinary creatinine excretion. | Treatment with OC5 preparation did not significantly reduce urinary or plasma oxalate extraction; however, this therapy was well tolerated and successfully delivered to the gastrointestinal tract. |
| Milliner et al. [68] | Phase II/III clinical trial (26 patients randomized to the treatment group (OC3) or the placebo group | There were no significant differences in the change in urinary oxalate excretion, urinary oxalate/urinary creatinine ratio or plasma oxalate excretion among the studied groups after 24 weeks of OC3 treatment; however, this treatment was well tolerated. | OC3 treatment was well tolerated but was not found to reduce urinary oxalate excretion. |
| Lactobacillus spp. | |
|---|---|
| Characteristic | Description |
| Morphology | Rod shaped |
| Gram staining | Gram-positive |
| Mobility | Nonmotile |
| Spore-forming | Nonspore-forming |
| Anaerobic/aerobic | Facultative anaerobic |
| Catalase | Catalase-negative |
| Author | Type of Study | Study Design | Main Finding |
|---|---|---|---|
| Turroni et al. [81] | In vitro | Among the 60 Lactobacillus strains analyzed, 32 belonged to the L. acidophilus group, 6 to L. gasseri, 7 to L. plantarum, 3 to L. casei, 2 to L. rhamnosus, 1 to L. salivarius, 1 to L. johnsonii, 2 to L. paracasei, 1 to L. delbrueckii subsp. Lactis, 1 to L. delbrueckii subsp. Bulgaricus, 2 to L. brevis, 1 to L. reuteri, and 1 to L. helveticus. All strains were grown in an oxalate-supplemented media to evaluate the ability of the bacteria to degrade oxalate. The identification of oxc and fcr was performed with PCR. | High oxalate degradation levels were obtained with L. casei LC11 (48%) and L. rhamnosus PB41 (47%). All the other tested strains exhibited a markedly lower oxalate-degrading activity, ranging from 0% to 20%. The homologues of the oxc and fcr were identified in L. acidophilus LA14. |
| Turroni et al. [82] | In vitro | The oxalate-degrading activities of 14 Bifidobacterium strains (B. adolescentis ATCC 15703, B. animalis subsp. Lactis DSM 10140, BA30, Bb12, BI07, and L15, B. bifidum S16, B. breve ATCC 15700 and BBSF, B. catenulatum B665, B. longum biotype longum S123, ATCC 15707, and W11, and B. longum biotype suis ATCC 27533), cultured in oxalate-rich media, was measured by a capillary electrophoresis technique. The identification of the oxc gene was performed with PCR | Only the five B. animalis subsp. Lactis strains (DSM 10140, BA30, Bb12, BI07, and L15) showed oxalate-degrading activity with 100% of oxalate consumption after 5 days of incubation, whereas no degrading activity was exhibited by all the other bifidobacterial strains tested. |
| Mogna et al. [83] | In vitro | Thirteen strains of Lactobacillus(L. paracasei, L. gasseri LGS01, LGS02, L. acidophilus LA07, LA02, L. plantarum LP01, L. reuteri LRE03, LRE02, L. rhamnosus GG, LR06, L. reuteri LRE04, LR06, L. delbrueckii subsp. Delbrueckii LDD01) and five Bifidobacterium (B. breve BR03, B. animalis DSM 20104, B. longum BL02, BL03, B. lactis BA05) were cultured in an ammonium oxalate-supplemented medium and their oxalate-degrading activity was tested by a novel HPLC method. | Screening of different Bifidobacterium strains for their in vitro ability to metabolize oxalates showed that B. breve BR03 degraded 28.2% of oxalate, B. animalis—27.7%, B. longum BL03—25.3%, B. lactis BA05—15.5%. |
| Lewanika et al. [71] | In vitro | L. gasseri AM63 was grown anaerobically in a medium supplemented with sodium oxalate. The oxalate-degrading capacity of L. gasseri was measured by commercial oxalate enzymatic kit assay. The identification of oxc and frc orthologs was performed by PCR. | L. gasseri AM63T ability to degrade oxalate was confirmed. Molecular analysis confirmed the presence of orthologs of the oxc and fcr genes in the genome of L. gasseri AM63T. |
| Azcarate-Peril et al. [84] | In vitro | Strains of L. acidophilus were grown in a medium supplemented with sodium oxalate. The identification of oxc and frc was performed by PCR. | Oxc and frc expression were induced as an operon in the presence of oxalate under acid conditions. |
| Gomathi et al. [86] | In vitro | Six hundred and seventy-three bacterial isolates were isolated from stool samples collected from 30 patients. A strain analysis showed that 251 of the isolates were lactic acid bacteria, but only 17 were capable of metabolizing oxalate. The bacteria selected in this way were analyzed to assess their ability to adhere to epithelial cells. | Obtained results suggest that L. fermentum TY5, L. fermentum AB1, and L. salivarius AB11 may degrade an oxalate and are characterized by significant adhesion to epithelial cells and strong antimicrobial activity. Therefore, these strains may serve as good probiotic candidates for preventing hyperoxaluria. |
| Giardina et al. [87] | In vitro | Eleven strains of lactic acid bacteria (Lactobacillus and Bifidobacterium), already included in the list of bacteria which are safe for human use, were investigated for their ability to degrade oxalate, by means of the RP-HPLC-UV method, and modulate inflammation in an in vitro model system based on peripheral blood mononuclear cells. | L. plantarum PBS067, L. acidophilus LA-14, B. breve PBS077, and B. longum PBS078 were able to degrade an oxalate in conditions of hyperoxaluria and the inflammatory events associated with the oxalate accumulation. |
| Campieri et al. [80] | In vitro and animal | L. acidophilus, L. plantarum, L. brevis, S. thermophilus, B. infantis were grown under anaerobic conditions in an oxalate-supplemented media to evaluate their ability to degrade oxalate. Next, the mixture of freeze-dried bacteria was administered to six patients with idiopathic calcium oxalate urolithiasis and mild hyperoxaluria for a period of four weeks. Finally, urinary samples were collected to assess the level of oxalate extraction. | All the tested bacteria showed an oxalate degradation capacity of 1–11%. The mixed probiotic treatment resulted in a great reduction of the excretion of oxalate in all six patients. |
| Wei et al. [90] | In vivo (ethylene glycol induced-animal model of kidney stones) | Male rats were given 1% ethylene glycol dissolved in their drinking water for four weeks to develop hyperoxaluria, and half of them received an additional LPN1 for four weeks prior to treatment with ethylene glycol as a preventive intervention. | LPN1 probiotic can prevent ethylene glycol induced hyperoxaluria by regulating the gut microflora and improving gut barrier function. |
| Lieske et al. [92] | Clinical (10 patients with chronic fat malabsorption, calcium oxalate stones, and hyperoxaluria) | Patients took 1 (4 g), 2 (8 g), and 3 (12 g) packets of Oxadrop® daily for three four-week periods. The preparation was mixed in a glass of cold beverage including water, orange juice, or tea, but no milk. The preparation was taken 1 to 2 h after the major meal of the day. Finally, urine samples were collected and it has been determined urinary concentrations of oxalate. | 70% of patients were characterized by decreased urinary oxalate extraction. Moreover, taking 4 g of Oxadrop® per day reduced urinary oxalate excretion by 19%, and this increased to 24% when 8 g per day were administered. |
| Lieske et al. [93] | Clinical (40 patients with nephrolithiasis and mild hyperoxaluria of unknown etiologist) | Patients were divided into three study groups that received placebo, Agri-King Synbiotic Preparation, and Oxadrop®, respectively. Finally, urinary samples were collected to assess the level of oxalate extraction. | Tested probiotic preparation did not influence urinary oxalate levels in patients on a restricted oxalate diet. |
| Goldfarb et al. [94] | Clinical (20 stone formers with idiopathic and enteric hyperoxaluria) | Patients were divided into two studied groups that received placebo and Oxadrop®, respectively. Finally, urinary samples were also collected to assess the level of oxalate extraction. | Oxadrop® did not reduce urinary oxalate excretion in participants with idiopathic hyperoxaluria as compared with the placebo group. |
| Siener et al. [95] | Clinical (20 healthy subjects) | Healthy volunteers who were initially on a normal diet changed to a supplemented diet with calcium oxalate for six weeks and received lactic acid bacteria preparation for five weeks. After this time, they returned to their original diet. Urine samples were collected weekly throughout the study period. Moreover, blood samples were analyzed before and at the end of treatment. | The study preparation neither reduced urinary oxalate excretion nor plasma oxalate concentration. |
| Ferraz et al. [96] | Clinical (14 stone-forming patients without hyperoxaluria) | Patients consumed a diet supplemented with oxalate for four weeks and a lyophilized L. casei and B. breve preparation was given after meals during the last two weeks. Finally, urinary samples were collected to assess the level of oxalate extraction. | Seven out of 14 patients presented a reduction in oxaluria after probiotic preparation as compared before treatment, being the reduction higher than 25% in four participants and up to 50% in two participants. |
| Okombo et al. [88] | Clinical (11 healthy volunteers) | Participants took VSL#3® for four weeks followed by a four-week washout period. Oxalate load tests, providing a total of 80 mg oxalate, were conducted at baseline (pre-probiotic), and after the probiotic and washout periods. Fecal samples were collected before the initiation of the study to assess the presence of O. formigenes. Finally, urinary samples were collected to assess the level of oxalate extraction. | The average total 22 h oxalate absorption at baseline (30.8%) was higher compared to after the probiotic (11.6%) and washout (11.5%) periods. |
| Al-Wahsh et al. [97] | Clinical (11 healthy nonstone formers) | Healthy nonstone formers divided into three groups: (i) oral ingestion of sodium oxalate (176 mg), (ii) 2 packets of VSL#3® preparation with a 176 mg oxalate sodium, (iii) 1 packet of VSL#3® preparation with a 176 mg oxalate sodium. Finally, urinary samples were collected to assess the level of oxalate extraction. | Both the doses of VSL#3® were effective in reducing urinary oxalate and estimated oxalate absorption with no significant difference between the two probiotic doses. |
| Kwak et al. [89] | Stone-forming animal model using selective cyclo-oxygenase 2 inhibitor | Male Sprague–Dawley rats divided into seven groups: (i) rats were maintained on the powdered regular diet for the whole study, (ii) rats received the powdered regular diet supplemented with 3% (w/v) sodium oxalate, (iii) rats were maintained on the powdered regular diet supplemented with 3% sodium oxalate for the whole study with each rat receiving 1 mL of celecoxib (100 mg/kg) for the first eight days, (iv) rats were maintained on the powdered regular diet supplemented with 3% sodium oxalate for the whole study with each rat receiving 1 mL of celecoxib (100 mg/kg) for the first eight days and 10% (w/v) skim milk, (v) rats were maintained on the powdered regular diet supplemented with 3% sodium oxalate for the whole study with each rat receiving 1 mL of celecoxib (100 mg/kg) for the first eight days and L. casei HY2743 (5 × 108 CFU/mL diet), (vi) rats were maintained on the powdered regular diet supplemented with 3% sodium oxalate for the whole study with each rat receiving 1 mL of celecoxib (100 mg/kg) for the first eight days and L. casei HY7201 (5 × 108 CFU/mL diet), (vii) rats were maintained on the powdered regular diet supplemented with 3% sodium oxalate for the whole study with each rat receiving 1 mL of celecoxib (100 mg/kg) for the first eight days and Lactobacillus casei HY2743, L. casei HY7201 and 10% (w/v) skim milk. Finally, urinary samples were also collected to assess the level of oxalate extraction and all rats were sacrificed and morphologic examination involving crystal formation was observed under a microscope. | In both groups of co-treatment and previous treatment with L. casei HY2743 and L. casei HY7201, urine oxalate excretion decreased compared to the group without Lactobacillus. The dissecting microscope examination of kidneys in the rats in two previous treatment groups and the co-treatment group with L. casei HY7201 showed less abundant crystals than control groups. |
| Bifidobacterium spp. | |
|---|---|
| Characteristic | Description |
| Morphology | Bifid or irregular V- or Y-shaped rods resembling branches |
| Gram staining | Gram-positive |
| Mobility | Nonmotile |
| Spore-forming | Nonspore-forming |
| Anaerobic/aerobic | Anaerobic |
| Catalase | Catalase-negative |
| Author | Type of Study | Study Design | Main Finding |
|---|---|---|---|
| Federici et al. [103] | In vitro | Twelve strains of Bifidobavterium were grown under anaerobic conditions in an oxalate-supplemented media to evaluate the ability of the bacteria to degrade oxalate. The identification of oxc was also performed through PCR and Western Blotting. The activity of the oxalyl-CoA decarboxylase was measured capillary electrophoresis. | Oxc was identified in the Bifidobacterium lactis DSM 1014, B. dentium, B. gallicum, B. pseudocatenulatum, and B. pseudolongum. Among the 12 studied bacterial strains, Bifidobacterium lactis DSM 10140 showed the highest oxalate-degrading activity in a preliminary screening. Moreover, the oxalate-degrading ability of certain Bifidobacterium species was also shown to be strain-specific. |
| Giardina et al. [87] | In vitro | Eleven strains of lactic acid bacteria (Lactobacillus and Bifidobacterium), already included in the list of bacteria which are safe for the human use, were investigated for their ability to degrade oxalate by means of the RP-HPLC-UV method and modulate inflammation in an in vitro model system based on peripheral blood mononuclear cells. | L. plantarum PBS067, L. acidophilus LA-14, B. breve PBS077, and B. longum PBS078 were able to degrade oxalate in conditions of hyperoxaluria and reduce the incidence of inflammatory events associated with oxalate accumulation. |
| Campieri et al. [80] | In vitro and animal | L. acidophilus, L. plantarum, L. brevis, S. thermophilus, and B. infantis were grown under anaerobic conditions in an oxalate-supplemented media to evaluate the ability of the bacteria to degrade oxalate. In the next step, a mixture of freeze-dried bacteria was administered to six patients with idiopathic calcium oxalate urolithiasis and mild hyperoxaluria for a period of four weeks. Finally, urinary samples were collected to assess the level of oxalate extraction. | All of the tested bacteria showed an oxalate degradation capacity of 1–11%. The mixed probiotics treatment resulted in a significant reduction in the excretion of oxalate in all six patients. |
| Mogna et al. [83] | In vitro | Thirteen Lactobacillus strains (L. paracasei, L. gasseri LGS01, LGS02, L. acidophilus LA07, LA02, L. plantarum LP01, L. reuteri LRE03, LRE02, L. rhamnosus GG, LR06, L. reuteri LRE04, LR06, L. delbrueckii subsp. Delbrueckii LDD01) and five Bifidobacteria (B. breve BR03, B. animalis DSM 20104, B. longum BL02, BL03, B. lactis BA05) were cultured in ammonium oxalate-supplemented medium, and then their oxalate-degrading activity was tested by a novel HPLC method. | Screening of different Bifidobacterium strains for their in vitro ability to metabolize oxalates showed that B. breve BR03 degraded 28.2% of oxalate, B. animalis degraded 27.7%, B. longum BL03 degraded 25.3%, and B. lactis BA05 degraded 15.5%. |
| Klimesova et l. [104] | Mouse model of primary hyperoxaluria | B. animalis subsp. Lactis DSM 10140 and B. adolescentis ATCC 15703 were administered to wild-type mice and to mice deficient in the hepatic enzyme, alanine-glyoxylate aminotransferase, that were fed an oxalate-supplemented diet. Finally, urine samples were collected to assess the level of oxalate extraction, and fecal samples were collected and the colonization status of mice with Bifidobacterium was monitored by PCR on weekly basis. | The administration of B. animalis subsp. Lactis led to a significant decrease in urinary oxalate excretion in wild-type and Agxt−/− mice compared to treatment with B. adolescentis. The wild-type mice were characterized by a higher level of B. animalis subsp. Lactis colonization than Agxt−/− mice. |
| Hatch [105] | Male and female C57BL/6 mice with Agxt knockout | Agxt knockout mice were fed a diet supplemented with oxalate during the study period. In the course of the study, a gavage inoculum containing both HC-1 and B. animalis was given to one of the groups and HC-1 alone was given to the other. Twelve days following the gavage procedure, when mice were confirmed as colonized, urine was collected to assess the level of oxalate extraction. | The combined administration of the human Oxalobacter strain, HC-1, and B. animalis reduced urinary oxalate excretion in knockout mice compared with the same mice before therapy. The mice that received the combined inoculum were characterized by 16% less urinary oxalate excretion than those that received HC-1 alone. |
| Characteristic | O. formigenes | Lactobacillus | Bifidobacterium |
|---|---|---|---|
| Oxalate degradability | The presence of two enzymes (formyl-CoA-transferase and oxalyl-CoA-decarboxylase) ensures the ability of bacteria to break down oxalate [46]. | Lactobacillus strains have oxc-like genes which promote oxalate degradability; thus, these strains showed highly variable oxalate degrading capacity. The highest ability to degrade oxalates was demonstrated in L. acidophilus NCFM and L. gasseri AM63T; however, this capacity was lower than that of O. formigenes [71,81,82,83,84]. | Bifidobacterium species, including B. animalis subsp. Lactis, B. dentium, B. gallicum, B. pseudocatenulatum, and B. pseudolongum, were characterized by the presence of oxc and frc homologues, suggesting that these bacteria may be capable of breaking down calcium oxalate. The best ability to degrade oxalates was demonstrated in B. lactis DSM 10140; however, this capacity was lower than that of O. formigenes [103]. |
| Ability to grow in the presence of oxalate | Oxalate use as the sole source of carbon and energy causes these bacteria to show unlimited growth in conditions of high oxalate concentrations [41,42]. | Under conditions of limited access to other energy sources, they are able to grow using oxalate as an energy source [70,71]. | In the case of Bifidobacterium strains, it was shown that bacteria with a high capacity to degrade oxalate are also characterized by growth restriction at high oxalate concentrations and vice versa. The only bacteria that showed a relatively high ability to degrade oxalate and grow indefinitely at high concentrations was B. infantis [80]. |
| Sensitivity to antibiotics | O. formigenes shows high sensitivity [44,45]. | Susceptibility analyses of Lactobacillus bacteria showed that the bacteria were resistant to a group of 14 antibiotics, which included inhibitors of cell wall synthesis, protein synthesis, nucleic acid synthesis and cytoplasmic membrane function [127]. | All strains of Bifidobacterium were sensitive to penicillins: penicillin G, amoxicillin piperacillin, ticarcillin, imipenem and common anti-Gram-positive antibiotics (macrolides, clindamycin, pristinamycin, vancomycin and teicoplanin) [128]. |
| Sensitivity to low pH and the presence of oxygen | Sensitivity to low pH strongly limits the use of oral probiotics containing O. formingens due to transport through the acidic gastric-intestinal juice. However, the individual strains of O. formigenes may differ in their resistance to pH and the presence of oxygen. The persistence of unfavorable conditions favors the development of the “stationary growth advantage” (GASP) phenotype of selected strains. Moreover, due to the presence of superoxide dismutase, some O. formigenes strains show the ability to temporarily acclimatize to aerobic conditions [49,50]. | Lactobacillus strains are capable of shifting from fermentative to respiratory metabolism; this was associated with an increase in biomass, long-term survival, and production of antioxidant enzymes. In turn, the sensitivity of Lactobacillus to low pH showed high variability: L. gasseri and L. fermentum were the most resistant to low pH, while L. gasseri showed the best resistance to bile acid salts [129,130]. | In general, it can be considered that Bifidobacterium, with the exception of B. animalis, have a weak acid tolerance. However, there are some mechanisms that allow adaptation to acidic conditions, and thus ensure the survival of bacteria in gastrointestinal juice. Bifidobacterium shows reduced NAD-oxidase and -peroxidase activity, and reduced NAD-oxidase and -peroxidase activity was inversely correlated with their sensitivity to oxygen [131,132]. |
| Possibility of lyophilization and production in the form of conventional probiotics | Most strains are sensitive to the conditions of lyophilization and the formulation of conventional probiotics. Moreover, the administration of O. formigenes in yoghurt is also very limited due to the low pH [49,50]. | Most Lactobacillus strains are FDA GRAS, and are administered in the form of encapsulated probiotics. Therefore, these bacteria tolerate the lyophilization and formulation processes well [72]. | Most Bifidobacterium strains are FDA GRAS and are administered in fermented dairy products. Therefore, these bacteria tolerate production processes well [131]. |
| The optimal mode of administration | Successful and long-lasting colonization has been observed in healthy adults where O. formigenes was formulated as a spread on a turkey sandwich with a sodium oxalate load, while colonization of the intestines of O. formigenes, which was provided either in lyophilized form or as a frozen cell paste to patients with primary hyperoxaluria, was unsuccessful [51,52]. | Oral preparations in the form of drops, capsules, sachets and lozenges [72]. | Usually administered in fermented dairy products [131]. |
| FDA approved as GRAS | Not certified GRAS by the FDA [46]. | Certified as GRAS by the FDA [72]. | Certified as GRAS by the FDA [99]. |
| Clinical trials | Clinical trials of Oxabact® OC5 (I/II phase) and Oxabact® OC3 (II/III phase) did not confirm the ability of the preparations to lower urine oxalate excretion [67,68,69]. | Studies on an Oxadrop® probiotic preparation containing L. acidophilus, B. infantis, S. thermophilus, and L. brevis yielded conflicting results. A VSL#3® supplement containing Streptococcus thermophilus, three Bifidobacterium species (B. breve, B. longum, and B. infantis), and four Lactobacillus species (L. acidophilus, L. plantarum, L. paracasei, and L. delbrueckii subsp. Bulgaricus) reduced urinary oxalate and increased oxalate absorption [92,93,94,95,96,97,98]. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wigner, P.; Bijak, M.; Saluk-Bijak, J. Probiotics in the Prevention of the Calcium Oxalate Urolithiasis. Cells 2022, 11, 284. https://doi.org/10.3390/cells11020284
Wigner P, Bijak M, Saluk-Bijak J. Probiotics in the Prevention of the Calcium Oxalate Urolithiasis. Cells. 2022; 11(2):284. https://doi.org/10.3390/cells11020284
Chicago/Turabian StyleWigner, Paulina, Michał Bijak, and Joanna Saluk-Bijak. 2022. "Probiotics in the Prevention of the Calcium Oxalate Urolithiasis" Cells 11, no. 2: 284. https://doi.org/10.3390/cells11020284
APA StyleWigner, P., Bijak, M., & Saluk-Bijak, J. (2022). Probiotics in the Prevention of the Calcium Oxalate Urolithiasis. Cells, 11(2), 284. https://doi.org/10.3390/cells11020284







