Assessment of the Impact of Post-Thaw Stress Pathway Modulation on Cell Recovery following Cryopreservation in a Hematopoietic Progenitor Cell Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cryopreservation
2.3. Modulation Studies
2.4. Cell Viability
2.4.1. Metabolic Activity
2.4.2. Fluorescence Microscopy
2.5. Data Analysis
3. Results
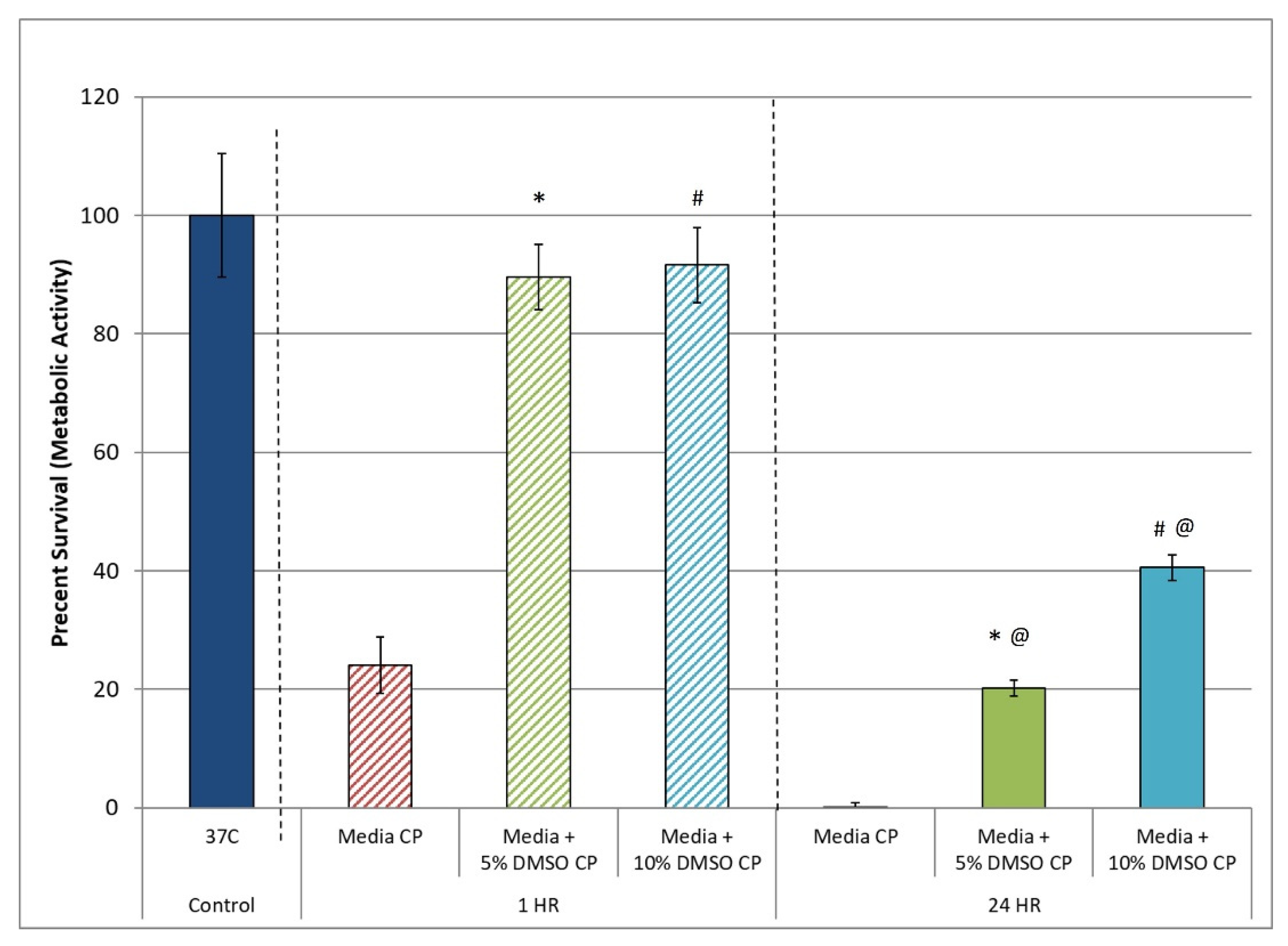
3.1. Assessment of the Post-Thaw Assessment Interval
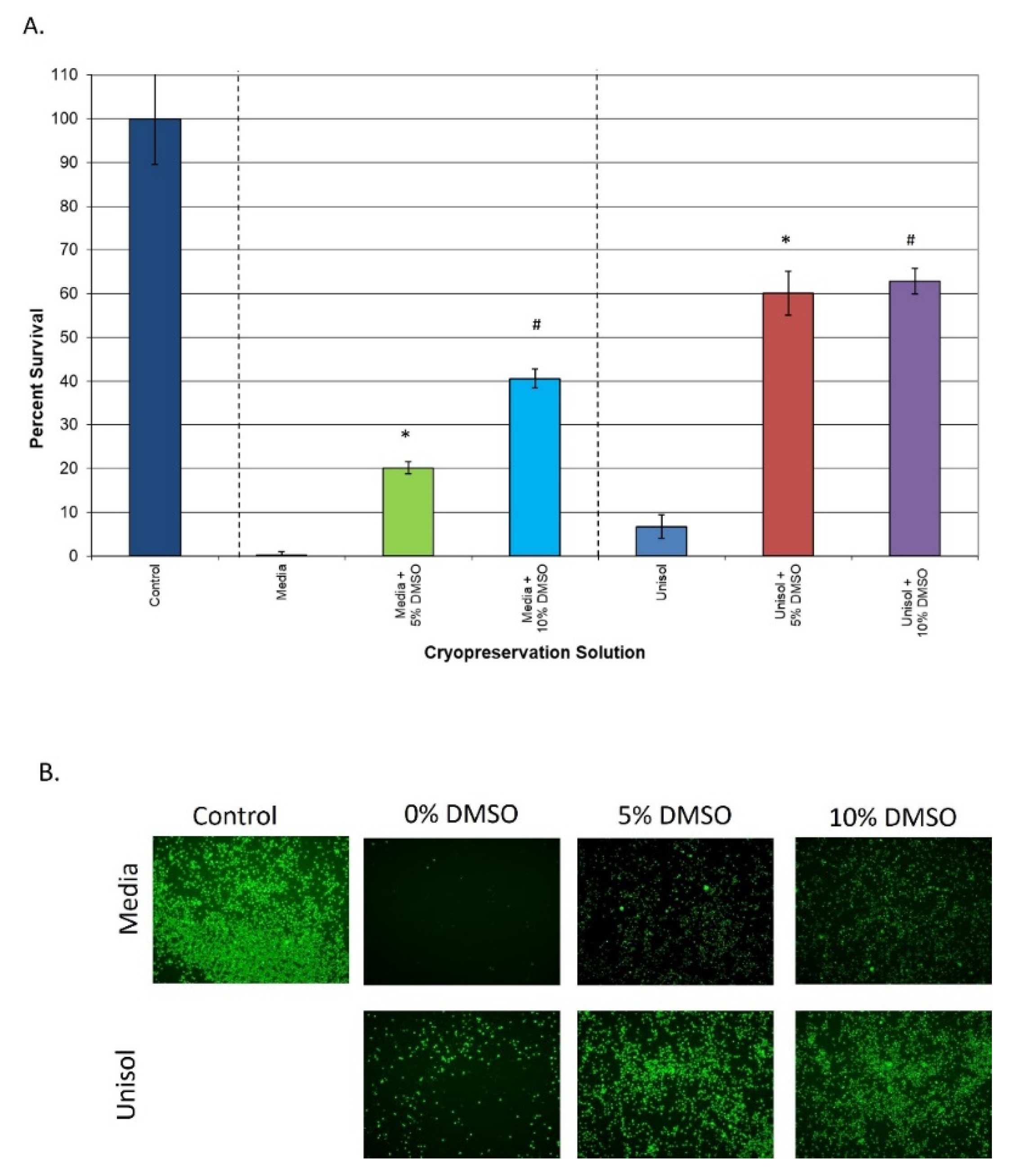
3.2. Comparison of Extracellular vs. Intracellular Freeze Media
3.3. Assessment of the Impact of Post-Thaw Cell Stress Modulation
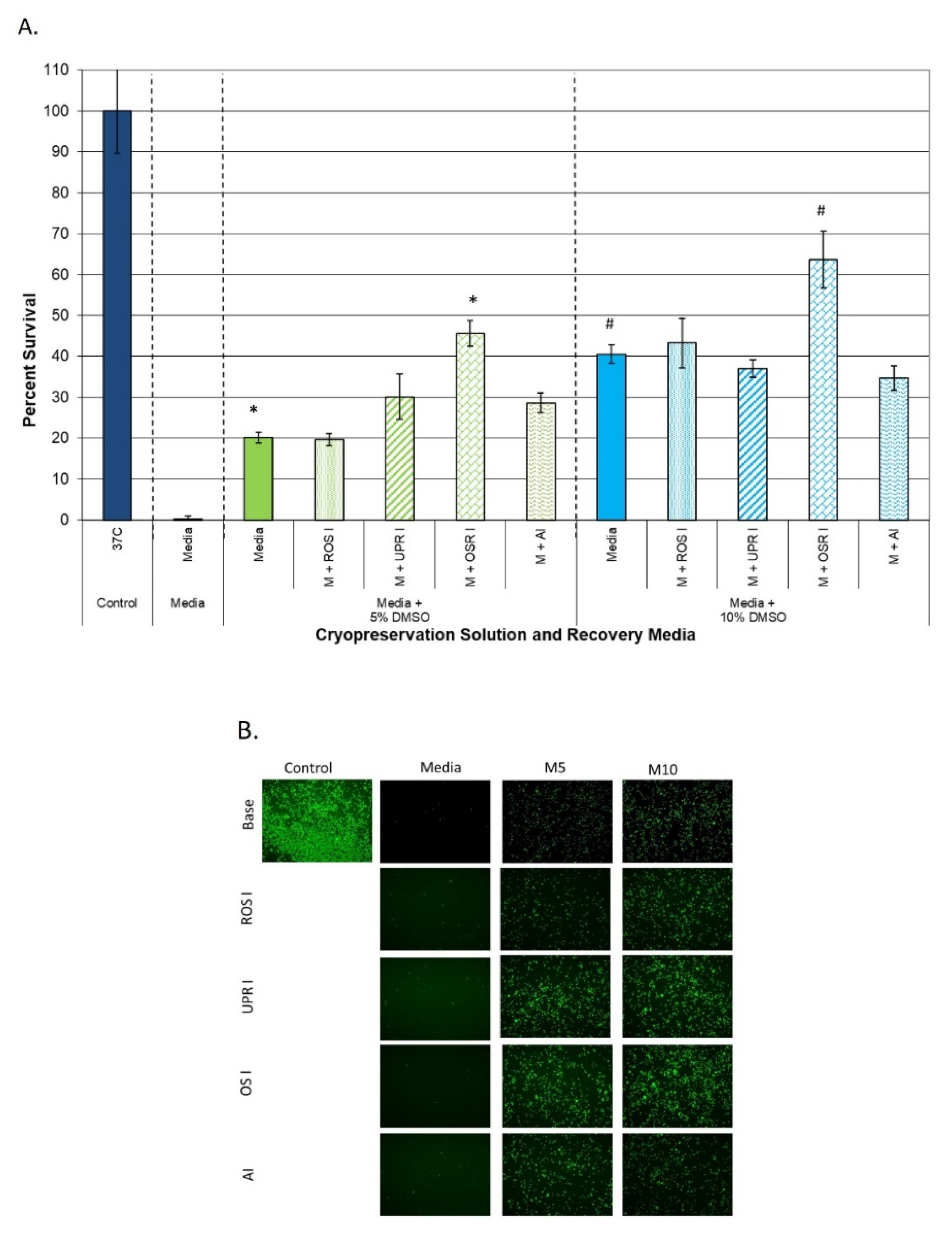
3.3.1. Impact on Samples Cryopreserved in Extracellular Freeze Media
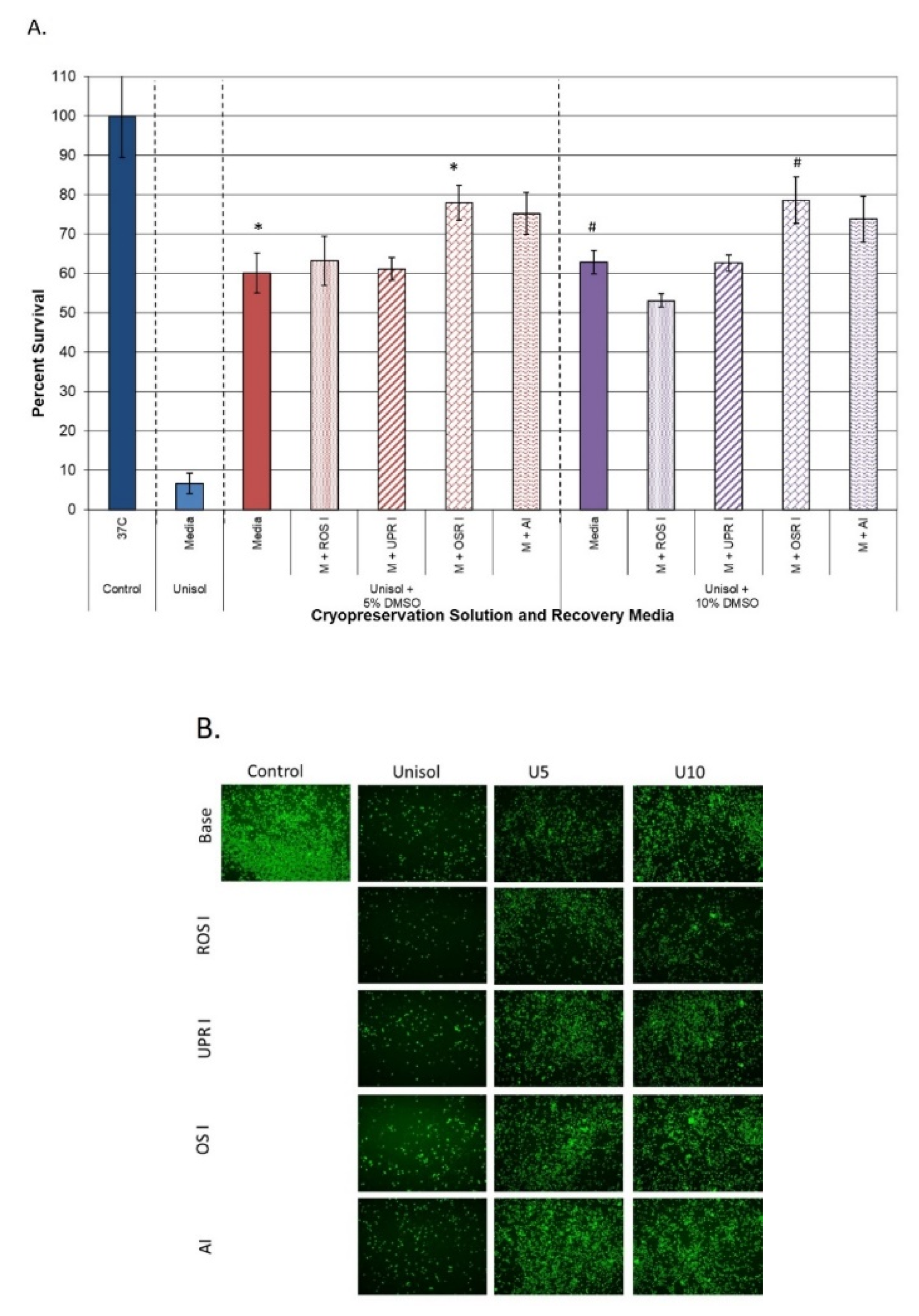
3.3.2. Impact on Samples Cryopreserved in Intracellular Freeze Media
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Baust, J.G. Concepts in Biopreservation. In Advances in Biopreservation; Baust, J.G., Baust, J.M., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 1–14. [Google Scholar]
- Lane, N. The future of cryobiology. In Life in the Frozen State; Fuller, B., Lane, N., Benson, E., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 645–657. [Google Scholar]
- Lovelock, J.E.; Bishop, M.W.H. Prevention of Freezing Damage to Living Cells by Dimethyl Sulphoxide. Nature 1959, 183, 1394. [Google Scholar] [CrossRef] [PubMed]
- Mazur, P.; Schmidt, J.J. Interactions of Cooling Velocity, Temperature, and Warming Velocity on the Survival of Frozen and Thawed Yeast. Cryobiology 1986, 5, 1–17. [Google Scholar] [CrossRef]
- Mazur, P. Freezing and Low Temperature Storage of Freezing of Living Cells: Mechanisms and implications. Amer. J. Physiol. 1984, 247, C125–C142. [Google Scholar] [CrossRef] [PubMed]
- Mazur, P. Equilibrium Quasi-Equilibrium, and Nonequilibrium Freezing of Mammalian Embryos. Cell BioPhys. 1990, 17, 53–92. [Google Scholar] [CrossRef] [PubMed]
- Meryman, H.T. The Exceeding of a Minimum Tolerable Cell Volume in Hypertonic Suspension as a Cause of Freezing Injury. The Frozen Cell; Wolstenholme, G.E.W., O’Connor, M., Eds.; Ciba Foundation Symposium: Churchill, MB, Canada, 1970; pp. 51–64. [Google Scholar]
- Toner, M.; Cravalho, E.; Karel, M. Cellular Response of Mouse Oocytes to Freezing Stress: Prediction of intracellular ice formation. J. Biomech. Eng. 1990, 115, 169–174. [Google Scholar] [CrossRef]
- Leibo, S.P.; Farrant, J.; Mazur, P.; Hanna, M.G.; Smith, L.H. Effects of Freezing on Marrow Stem Cell Suspensions: Interactions of cooling and warming rates in the presence of PVP, sucrose, or glycerol. Cryobiology 1970, 6, 315–332. [Google Scholar] [CrossRef]
- Steponkus, P.L.; Wiest, S.C. Freeze-Thaw Induced Lesions in the Plasma Membrane Low Temperature Stress in Crop Plants: The Role of the Membrane; Lyons, J.M., Graham, D.G., Raison, J.K., Eds.; Academic Press: Cambridge, MA, USA, 1979; pp. 231–253. [Google Scholar]
- Critzer, J.K.; Arneson, B.W.; Aaker, D.V.; Huse-Benda, A.R.; Ball, G.D. Factors Affecting The Survival of Mouse Two-Cell. Embryos. J. Reprod. Fertil. 1988, 82, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Farrant, J. Mechanisms of Injury and Protection in Living Cells and Tissues at Low Temperatures. Current Trends in Cryobiology; Smith, A.U., Ed.; Springer: Boston, MA, USA, 1970; pp. 139–152. [Google Scholar]
- Farrant, J. Water Transport and Cell Survival in Cryobiological Procedures. Philos. Trans. R. Soc. Lond. B 1977, 278, 294–306. [Google Scholar]
- Pegg, D.E.; Daiper, M.P. The Mechanism of Injury to Slowly- Frozen Erythrocytes. Biophys. J. 1988, 54, 471–488. [Google Scholar] [CrossRef]
- Paynter, S.J.; Fuller, B.J.; McGrath, J.J.; Shaw, R.W. The Effects of Cryoprotectant Permeability on Mouse Oocytes. Cryo Lett. 1995, 16, 321–324. [Google Scholar]
- Coger, R.; Toner, M. Preservation Techniques for Biomaterials. The Biomedical Engineering Handbook; Park, J., Bronzino, J., Eds.; CRC Press: Boca Raton, FL, USA, 1995; pp. 1567–1578. [Google Scholar]
- Polge, C.; Smith, A.U.; Parkes, A.S. Retrieval of Spermatozoa after Vitrification and Dehydration at Low Temperatures. Nature 1949, 164, 666–667. [Google Scholar] [CrossRef]
- Hubel, A. Parameters of cell freezing: Implications for the cryopreservation. Transfus. Med. Rev. 1997, 11, 224–233. [Google Scholar] [CrossRef]
- Brockbank, K.G. Effects of cryopreservation upon vein function in vivo. Cryobiology 1994, 31, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Fuller, B.J. Cryoprotectants: The essential antifreezes to protect life in the frozen state. Cryo Lett. 2004, 25, 375–388. [Google Scholar]
- Baust, J.M.; Van Buskirk, R.; Baust, J.G. Cryopreservation Outcome is Enhanced by Intracellular-Type Medium and Inhibition of Apoptosis. Cryobiology 1998, 37, 410–411. [Google Scholar]
- Baust, J.M.; Van Buskirk, R.G.; Baust, J.G. Cell Viability Improves Following Inhibition of Cryopreservation- Induced Apoptosis. In Vitro Cellular and Developmental Biology Animal; Springer: Cham, Switzerland, 2000; Volume 36, pp. 262–270. [Google Scholar]
- Baust, J.M.; Vogel, M.J.; Van Buskirk, R.; Baust, J.G. A Molecular Basis of Cryopreservation Failure and its Modulation to Improve Cell Survival. Cell Transplant. 2001, 10, 561–571. [Google Scholar] [CrossRef]
- Baust, J.M. Molecular mechanisms of cellular demise associated with cryopreservation failure. Cell Preserv. Technol. 2002, 1, 17–31. [Google Scholar] [CrossRef]
- Baust, J.M.; Van Buskirk, R.G.; Baust, J.G. Gene activation of the apoptotic Caspase cascade following cryogenic storage. Cell Preserv. Technol. 2002, 1, 63. [Google Scholar] [CrossRef]
- Fu, T.; Guo, D.; Huang, X.; O’Gorman, M.R.; Huang, L.; Crawford, S.E.; Soriano, H.E. Apoptosis occurs in isolated and banked primary mouse hepatocytes. Cell Transplant. 2001, 10, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Yagi, T.; Hardin, J.A.; Valenzuela, Y.M.; Miyoshi, H.; Gores, G.J.; Nyberg, S.L. Caspase Inhibition Reduces Apoptotic Death of Cryopreserved Porcine Hepatocytes. Hepatology 2001, 33, 1432–1440. [Google Scholar] [CrossRef]
- Cosentino, L.M.; Corwin, W.; Baust, J.M.; Diaz-Mayoral, N.; Cooley, H.; Shao, W.; Van Buskirk, R.; Baust, J.G. Preliminary report: Evaluation of storage conditions and cryococktails during peripheral blood mononuclear cell cryopreservation. Cell Preserv. Technol. 2007, 5, 189–204. [Google Scholar] [CrossRef]
- Robilotto, A.T.; Baust, J.M.; Van Buskirk, R.G.; Baust, J.G. Involvement of the Cysteine Protease Calpain Family in Cell Death Following Cryopreservation. Cell Preserv. Technol. 2007, 4, 17–30. [Google Scholar] [CrossRef]
- Heng, B.C.; Clement, M.V.; Cao, T. Caspase inhibitor Z-VAD-FMK enhances the freeze-thaw survival rate of human embryonic stem cells. Biosci. Rep. 2007, 27, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Peter, A.T.; Linde-Forsberg, C. Efficacy of the anticaspase agent zVAD-fmk on post-thaw viability of canine spermatozoa. Theriogenology 2003, 59, 1525–1532. [Google Scholar] [CrossRef]
- Heng, B.C. Effect of Rho-associated kinase (ROCK) inhibitor Y-27632 on the post-thaw viability of cryopreserved human bone marrow-derived mesenchymal stem cells. Tissue Cell 2009, 41, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Martin-Ibanez, R.; Unger, C.; Stromberg, A.; Baker, D.; Canals, J.M.; Hovatta, O. Novel cryopreservation method for dissociated human embryonic stem cells in the presence of a ROCK inhibitor. Hum. Reprod. 2008, 23, 2744–2754. [Google Scholar] [CrossRef]
- Li, X.; Krawetz, R.; Liu, S.; Meng, G.; Rancourt, D.E. ROCK inhibitor improves survival of cryopreserved serum/feeder-free single human embryonic stem cells. Hum Reprod. 2009, 24, 580–589. [Google Scholar] [CrossRef]
- Corwin, W.; Baust, J.M.; Van Buskirk, R.G.; Baust, J.G. Post-thaw caspase inhibition improves the CP outcome for bovine corneal endothelial cells. Cryobiology 2007, 55, 369–370. [Google Scholar] [CrossRef]
- Berens, C.; Heine, A.; Muller, J.; Held, S.A.; Mayer, K.; Brossart, P.; Oldenburg, J.; Potzsch, B.; Wolf, D.; Ruhl, H. Variable resistance to freezing and thawing of CD34-positive stem cells and lymphocyte subpopulations in leukapheresis products. Cytotherapy 2016, 18, 1325–1331. [Google Scholar] [CrossRef]
- Baust, J.M.; Van Buskirk, R.; Baust, J.G. Modulation of the Cryopreservation “Cap”: Elevated survival with reduced dimethyl sulfoxide concentration. Cryobiology 2002, 45, 97–108. [Google Scholar] [CrossRef]
- Baust, J.M.; Van Buskirk, R.G.; Baust, J.G. Cryopreservation of an Engineered Skin Equivalent: The apoptosis paradigm. In ASME International Mechanical Engineering Congress and Exposition; ASME: New York, NY, USA, 1999; pp. 71–76. [Google Scholar]
- Baust, J.M.; Snyder, K.K.; Van Buskirk, R.G.; Baust, J.G. Changing Paradigms in Biopreservation. Biopreserv. Biobank. 2009, 7, 3–12. [Google Scholar] [CrossRef]
- Baust, J.M.; Corwin, W.; Van Buskirk, R.G.; Baust, J.G. Biobanking: The future of cell preservation strategies. Biobank. 21st Century 2015, 864, 37–53. [Google Scholar]
- Baust, J.M.; Baust, J.G. Paradigm Shift in CP: Molecular-based advances to improving outcome. In Cryogenic Engineering: Fifty Years of Progress; Timmerhaus, K., Reed, R., Eds.; Springer Publishing: New York, NY, USA, 2006. [Google Scholar]
- Baust, J.M.; Campbell, L.H.; Harbel, J.W. Best Practices for Cryopreserving, Thawing, Recovering and Assessing Cells. Vitro Cell. Dev. Biol. Anim. 2017, 53, 855. [Google Scholar] [CrossRef]
- Taylor, M.J. Biology of Cell Survival in the Cold: The Basis for Biopreservation of Tissues and Organs; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Baicu, S.C.; Taylor, M.J. Acid-base buffering in organ preservation solutions as a function of temperature: New parameters for comparing buffer capacity and efficiency. Cryobiology 2002, 45, 33–48. [Google Scholar] [CrossRef]
- Baust, J.M. Advances in media for cryopreservation and hypothermic storage. Bioprocess. Int. 2005, 3, 46–56. [Google Scholar]
- Taylor, M.J.; Campbell, L.H.; Rutledge, R.N.; Brockbank, K.G. Comparison of Unisol with Euro-Collins solution as a vehicle solution for cryoprotectants. Transplant Proc. 2001, 33, 677–679. [Google Scholar] [CrossRef]
- Sosef, M.N.; Baust, J.M.; Sugimachi, K.; Fowler, A.; Tompkins, R.G.; Toner, M. Cryopreservation of isolated primary rat hepatocytes: Enhanced survival and long-term hepatospecific function. Ann. Surg. 2005, 241, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.H.; Brockbank, K.G.M. Serum free solutions for the cryopreservation of cells. Vitro Cell Dev. Biol. 2007, 43, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Brockbank, K.; Taylor, M. Tissue Preservation. In Advances in Biopreservation; Baust, J.G., Baust, J.M., Eds.; CRC Press-Taylor and Francis Publishing: New York, NY, USA, 2007. [Google Scholar]
- Baust, J.M.; Vogel, M.J.; Snyder, K.K.; VanBuskirk, R.G.; Baust, J.G. Activation of Mitochondrial-Induced Apoptosis Contributes to Cryopreservation Failure. Cell Preserv. Technol. 2007, 5, 155–164. [Google Scholar] [CrossRef]
- Stylianou, J.; Vowels, M.; Hadfield, K. CryoStor™ Significantly Improves Cryopreservation of Hematopoietic Stem Cells (HSC). Cytotherapy 2005, 7 (Suppl. 1), 117. [Google Scholar]
- Sugimachi, K.; Sosef, M.N.; Baust, J.M.; Fowler, A.; Tompkins, R.G.; Toner, M. Long-term function of cryopreserved rat hepatocytes in a coculture system. Cell Transplant. 2004, 13, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Orellana, M.D.; de Santis, G.C.; Abraham, K.J.; Fontes, A.M.; Magalhães, D.A.R.; Oliveira, V.d.; Costa, E.d.O.; Palma, P.V.B.; Covasab, D.T. Efficient recovery of undifferentiated human embryonic stem cell cryopreserved with hydroxyethyl startch, dimethyl sulphoxide and serum replacement. Cryobiology 2015, 71, 151–160. [Google Scholar] [CrossRef]
- Karimi-Busheri, F.; Rasouli-Nia, A.; Weinfeld, M. Key Issues Related to Cryopreservation and Storage of Stem Cells and Cancer Stem Cells: Protecting Biological Integrity. Adv. Exp. Med. Biol. 2016, 951, 1–12. [Google Scholar]
- Wagey, R.; Elliott, M.; Sampaio, A.V.; Szilvassy, S.; Thomas, T.E.; Eaves, A.; Louis, S.A. Characterization of mesenchymal progenitor cells derived from human pluripotent stem cells and cryopreserved in defined cryopreservation medium. Cytotherapy 2017, 19, S196–S197. [Google Scholar] [CrossRef]
- Valyi-Nagy, K.; Betsou, F.; Susma, A.; Valyi-Nagy, T. Optimization of Viable Glioblastoma Cryopreservation for Establishment of Primary Tumor Cell Cultures. Biopreserv. Biobank. 2021, 19, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Ginis, I.; Grinblat, B.; Shirvan, M. Evaluation of Bone Marrow-Derived Mesenchymal Stem Cells After Cryopreservation and Hypothermic Storage in Clinically Safe Medium. Tissue Eng. Part C Methods 2012, 18, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.W.; Choi, J.R.; Wan Safwani, W.K. Biobanking of human mesenchymal stem cells: Future strategy to facilitate clinical applications. Adv. Exp. Med. Biol. 2016, 951, 99–110. [Google Scholar] [PubMed]
- Briard, J.G.; Jahan, S.; Chandran, P.; Allan, D.; Pineault, N.; Ben, R.N. Small-molecule ice recrystallization inhibitors improve the post-thaw function of hematopoietic stem and progenitor cells. ACS Omega 2016, 1, 1010–1018. [Google Scholar] [CrossRef]
- Eroglu, A.; Russo, M.J.; Bieganski, R.; Fowler, A.; Cheley, S.; Bayley, H.; Toner, M. Intracellular Trehalose Improves the Survival of Cryopreserved Mammalian Cells. Nat. Biotechnol. 2000, 18, 163–167. [Google Scholar] [CrossRef]
- Acker, J. The Use of Intracellular Protectants in Cell Biopreservation. In Advances in Biopreservation; Baust, J.G., Baust, J.M., Eds.; CRC Press-Taylor and Francis Publishing: New York, NY, USA, 2007. [Google Scholar]
- Shivakumar, S.B.; Bharti, D.; Subbarao, R.B.; Jang, S.-J.; Park, J.-S.; Ullah, I.; Park, J.-K.; Byun, J.-H.; Park, B.-W.; Rho, G.-J. DMSO- and Serum-Free Cryopreservation of Wharton’s Jelly Tissue Isolated from Human Umbilical Cord. J. Cell. Biochem. 2016, 117, 2397–2412. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yu, G.; Azarin, S.; Hubel, A. Freezing Responses in DMSO-Based Cryopreservation of Human iPS Cells: Aggregates Versus Single Cells. Tissue Eng. Part C Methods 2018, 24, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Gilfanova, R.; Callegari, A.; Childs, A.; Yang, G.; Luarca, M.; Gutierrez, A.G.; Medina, K.I.; Mai, J.; Hui, A.; Kline, M.; et al. A bioinspired and chemically defined alternative to dimethyl sulfoxide for the cryopreservation of human hematopoietic stem cells. Bone Marrow Transplant. 2021, 56, 2644–2650. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Hornberger, K.; Dutton, J.R.; Hubel, A. Cryopreservation of Human iPS Cell Aggregates in a DMSO-Free Solution—An Optimization and Comparative Study. Front. Bioeng. Biotechnol. 2020, 8, 1. [Google Scholar] [CrossRef]
- Lin, S.-L.; Chang, W.-J.; Lin, C.-Y.; Hsieh, S.-C.; Lee, S.-Y.; Fan, K.-H.; Lin, C.-T.; Huang, H.-M. Static magnetic field increases survival rate of dental pulp stem cells during DMSO-free cryopreservation. Electromagn. Biol. Med. 2015, 34, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, R.; Jahan, S.; McGregor, C.; Pineault, N. Dimethyl sulfoxide-free cryopreservation solutions for hematopoietic stem cell grafts. Cytotherapy 2021, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, S.; Gross, S.; Acker, J.; Toner, M.; Carpenter, J.; Pyatt, D. Cryopreservation of Stem Cells Using Trehalose: Evaluation of the Method Using a Human Hematopoietic Cell Line. Stem Cells Dev. 2004, 13, 295–305. [Google Scholar] [CrossRef]
- Baust, J.M.; Corwin, W.L.; Snyder, K.K.; Baust, J.G.; Van Buskirk, R.G. Development of a novel device for the controlled dry thawing of cryopreserved cell products. Bioprocess. J. 2016, 15, 30–41. [Google Scholar] [CrossRef]
- Baust, J.M. Enhancing Cryopreservation Outcome through Molecular and Device-Based Strategies. Critical Capabilities for Biologic Production; International Society for Bioprocessing Technology: Norfolk, VA, USA, 2021. [Google Scholar]
- Gao, D.; Yu, J.; Luo, D. Thermal Instruments and Devices in Cryobiological Research and Applications. In Advances in Biopreservation; Baust, J.G., Baust, J.M., Eds.; CRC Press-Taylor and Francis Publishing: New York, NY, USA, 2007. [Google Scholar]
- Linkermann, A.; Brasen, J.H.; Himmerkus, N.; Liu, S.; Huber, T.B.; Kunzendorf, U.; Krautwald, S. Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int. 2012, 81, 751–761. [Google Scholar] [CrossRef]
- Jaeschke, H.; Lemasters, J.J. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology 2003, 125, 1246–1257. [Google Scholar] [CrossRef]
- Koenigsmann, M.P.; Koenigsmann, M.; Notter, M.; Neuloh, M.; Mucke, C.; Thiel, E.; Berdel, W.E. Adhesion molecules on peripheral blood-derived CD34+ cells: Effects of cryopreservation and short-term ex vivo incubation with serum and cytokines. Bone Marrow Transplant. 1998, 22, 1077–1085. [Google Scholar] [CrossRef][Green Version]
- Sarkar, S.; Kalia, V.; Montelaro, R.C. Caspase-mediated apoptosis and cell death of rhesus macaque CD4+ T-cells due to cryopreservation of peripheral blood monoculcear cells can be rescued by cytokine treatment after thawing. Cryobiology 2003, 47, 44–58. [Google Scholar] [CrossRef]
- Rauen, U.; de Groot, H. New insights into the cellular and molecular mechanisms of cold storage injury. J. Investig. Med. 2004, 52, 299–309. [Google Scholar] [CrossRef]
- Zhang, J.-M.; Wang, H.-C.; Wang, H.-X.; Ruan, L.-H.; Zhang, Y.-M.; Li, J.-T.; Tian, S.; Zhang, Y.-C. Oxidative stress and activities of caspase-8, -9, and -3 are involved in cryopreservation-induced apoptosis in granulosa cells. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 166, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Corwin, W.L.; Baust, J.M.; Baust, J.G.; Van Buskirk, R.G. The unfolded protein response in human corneal endothelial cells following hypothermic storage: Implications of a novel stress pathway. Cryobiology 2011, 63, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Matosevic, S.; Zylberberg, C. Cryopreservation of stem cells for the future: Looking beyond DMSO. Cytotherapy 2014, 16, S99. [Google Scholar] [CrossRef]
- Duchez, P.; Rodriguez, L.; Chevaleyre, J.; De La Grange, P.B.; Ivanovic, Z. Clinical-scale validation of a new efficient procedure for cryopreservation of ex vivo expanded cord blood hematopoietic stem and progenitor cells. Cytotherapy 2016, 18, 1543–1547. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Suemori, H. Slow cooling cryopreservation optimized to human pluripotent stem cells. Adv. Exp. Med. Biol. 2016, 951, 57–65. [Google Scholar]
- Yong, K.W.; Choi, J.R.; Saifullah, D.; Wan, K.Z.; Safwani, W.K. Biosafety and bioefficacy assessment of human mesenchymal stem cells: What do we know so far? Regen. Med. 2018, 13, 219–232. [Google Scholar] [CrossRef]
- Lecchi, L.; Giovanelli, S.; Gagliardi, B.; Pezzali, I.; Ratti, I.; Marconi, M. An update on methods for cryopreservation and thawing of hemopoietic stem cells. Transfus. Apher. Sci. 2016, 54, 324–336. [Google Scholar] [CrossRef]
- Gaudet, I. The Cold Truth: Cryopreservation of final product in cell therapy manufacturing; Hitachi, Chemical Advanced Therapeutics Solutions, LLC.: Allendale, NJ, USA, 2018. [Google Scholar]
- Golab, K.; Leveson-Gower, D.; Wang, X.-J.; Grzanka, J.; Marek-Trzonkowska, H.; Krzystyniak, A.; Millis, J.M.; Trzonkowski, P.; Witkowski, P. Challenges in cryopreservation of regulatory T cells (Trefs) for clinical therapeutic applications. Int. Immunopharmacol. 2013, 16, 371–375. [Google Scholar] [CrossRef]
- Hanley, P.J. Fresh versus frozen: Effects of cryopreservation on CAR-T cells. Mol. Ther. 2019, 27, 1213–1214. [Google Scholar] [CrossRef]
- Xu, H.; Cao, W.; Huang, L.; Xia, M.; Cao, Y.; Zhao, L.; Wang, N.; Zhou, J. Effects of cryopreservation on chimeric antigen receptor T cell functions. Cryobiology 2018, 83, 4047. [Google Scholar] [CrossRef]
- Yang, H.; Acker, J.P.; Cabuhat, M.; Letcher, B.; Larratt, L.; McGann, L.E. Association of post-thaw viable CD34+ cells and CFU-GM with time to hematopoietic engraftment. Bone Marrow Transplant. 2005, 35, 881–887. [Google Scholar] [CrossRef]
- De Boer, F.; Drager, A.M.; Pinedo, H.M.; Kessler, F.L.; Monnee-van Muijen, M.; Weijers, G.; Westra, G.; van der Wall, E.; Netelenbos, T.; Oberink, J.; et al. Early apoptosis largely accounts for functional impairment of CD34+ cells in frozen-thawed stem cell grafts. J. Hematother. Stem Cell Res. 2002, 11, 951–963. [Google Scholar] [CrossRef]
- Van Buskirk, R.G.; Baust, J.G.; Baust, J.M. Navigating the Post-Preservation Viability Fog. Genet. Eng. News 2006, 26, 38–39. [Google Scholar]
- Van Buskirk, R.G. Viability and Functional Assays used to Assess Preservation Efficacy: The Multiple Endpoint/Tier Approach. In Advances in Biopreservation; Baust, J.G., Baust, J.M., Eds.; CRC Press-Taylor and Francis Publishing: New York, NY, USA, 2007. [Google Scholar]
- Baust, J.M. Properties of cells and tissues influencing preservation outcome: Molecular basis of preservation-induced cell death. In Advances in Biopreservation; Baust, J.G., Baust, J.M., Eds.; CRC Press -Taylor and Francis Publishing: New York, NY, USA, 2007. [Google Scholar]
- Bissoyi, A.; Pramanik, K. Role of the Apoptosis Pathway in Cryopreservation-Induced Cell Death in Mesenchymal Stem Cells Derived from Umbilical Cord Blood. Biopreserv. Biobank. 2014, 12, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Hubel, A. Preservation of Cellular Therapies: Gene Therapy, Cellular, and Metabolic Engineering. In Advances in Biopreservation; Baust, J.G., Baust, J.M., Eds.; CRC Press-Taylor and Francis Publishing: New York, NY, USA, 2007. [Google Scholar]
- D’Rozario, J.; Parisotto, R.; Stapleton, J.; Gidley, A.; Owen, D. Pre infusion, post thaw CD34+peripheral blood stem cell enumeration as a predictor of hematopoietic engraftment in autologous hematopoietic cell transplantation. Transf. Apheres. Sci. 2014, 50, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Erol, O.D.; Pervin, B.; Seker, M.E.; Aerts-Kaya, F. Effects of storage media, supplements and cryopreservation methods on quality of stem cells. World J. Stem. Cells 2021, 13, 1197–1214. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Yue, A.; Ruan, Z.; Yin, Y.; Wang, R.; Ren, Y.; Zhu, L. Comparison of the Effects of Different Cryoprotectants on Stem Cells from Umbilical Cord Blood. Stem Cells Int. 2016, 2016, 1396783. [Google Scholar] [CrossRef]
- Gilfanova, R.; Auclair, K.M.; Hui, A.; Norris, P.J.; Muench, M.O. Reduced dimethyl sulfoxide concentrations successfully cryopreserve human hematopoietic stem cells with multi-lineage long-term engraftment ability in mice. Cytotherapy 2021, 23, 1053–1059. [Google Scholar] [CrossRef]
- Hornberger, K.; Yu, G.; McKenna, D.; Hubel, A. Cryopreservation of Hematopoietic Stem Cells: Emerging Assays, Cryoprotectant Agents, and Technology to Improve Outcomes. Transfus. Med. Hemother. 2019, 46, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Kilbride, P.; Meneghel, J.; Creasey, G.; Masoudzadeh, F.; Drew, T.; Creasey, H.; Bloxham, D.; Morris, G.J.; Jestice, K. Automated dry thawing of cryopreserved hematopoietic cells is not adversely influenced by cryostorage time, patient age or gender. PLoS ONE 2020, 15, e0240310. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.M.; Yadock, D.J.; Nicoud, I.B.; Mathew, A.J.; Heimfeld, S. Improved post-thaw recovery of peripheral blood stem/progenitor cells using a novel intracellular-like cryopreservation solution. Cytotherapy 2009, 11, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.J.; Hollister, W.R.; Addona, T.; Baust, J.G.; Van Buskirk, R.G. Vitamin E and EDTA Improve the Efficacy of Hypothermosol-Implication of Apoptosis. In Vitro Mol. Toxicol. 1999, 12, 163–172. [Google Scholar]
- Corwin, W.L.; Baust, J.M.; Van Buskirk, R.G.; Baust, J.G. Implications of differential stress response activation following non-frozen hepatocellular storage. Biopreserv. Biobank. 2013, 11, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Corwin, W.L.; Baust, J.M.; Van Buskirk, R.G.; Baust, J.G. In Vitro assessment of apoptosis and necrosis following cold storage in a human airway cell model. Biopreserv. Biobank. 2009, 7, 19–27. [Google Scholar] [CrossRef]
- Anderson, C.D.; Belous, A.; Pierce, J.; Nicoud, I.B.; Knox, C.; Wakata, A.; Pinson, C.W.; Chari, R.S. Mitochondrial calcium uptake regulates cold preservation-induced Bax translocation and early reperfusion apoptosis. Am. J. Transplant. 2004, 4, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.V.; Schneeberger, S.; Seiler, R.; Brandacher, G.; Mark, W.; Steurer, W.; Skas, V.; Usson, Y.; Margreiter, R.; Gnaiger, E. Mitochondrial defects and heterogeneous cytochrome c releaseafter cardiac cold ischemia and reperfusion. Am. J. Physiol.-Heart Circ. Physiol. 2004, 286, H1633–H1641. [Google Scholar] [CrossRef]
- Rauen, U.; Elling, B.; Gizewski, E.R.; Korth, H.G.; Sustmann, R.; de Groot, H. Involvement of Reactive Oxygen Species in the Preservation Injury to Cultured Liver Endothelial Cells. Free Radic. Biol. Med. 1997, 22, 17–24. [Google Scholar] [CrossRef]
- Snyder, K.K.; Baust, J.M.; VanBuskirk, R.G.; Baust, J.G. Enhanced Hypothermic Storage of Neonatal Cardiomyocytes. Cell Preserv. Technol. 2005, 3, 61–74. [Google Scholar] [CrossRef]
- Mathew, A.J.; Van Buskirk, R.G.; Baust, J.G. Improved Hypothermic Preservation of Human Renal Cells through Suppression of Both Apoptosis and Necrosis. Cell Preserv. Technol. 2002, 1, 239–253. [Google Scholar] [CrossRef]
- Corwin, W.C.; Baust, J.M.; Baust, J.G.; Van Buskirk, R.G. Biopreservation of biologics: Translation from stem cells to cancer: Differential activation of stress pathways in human mesenchymal stem cells following biopreservation. Cryobiology 2014, 69, 186. [Google Scholar] [CrossRef]
- Baust, J.M. The Impact of the Molecular-Based Cell Death Continuum on Preservation Outcome. Cryobiology 2019, 91, 182. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baust, J.M.; Snyder, K.K.; Van Buskirk, R.G.; Baust, J.G. Assessment of the Impact of Post-Thaw Stress Pathway Modulation on Cell Recovery following Cryopreservation in a Hematopoietic Progenitor Cell Model. Cells 2022, 11, 278. https://doi.org/10.3390/cells11020278
Baust JM, Snyder KK, Van Buskirk RG, Baust JG. Assessment of the Impact of Post-Thaw Stress Pathway Modulation on Cell Recovery following Cryopreservation in a Hematopoietic Progenitor Cell Model. Cells. 2022; 11(2):278. https://doi.org/10.3390/cells11020278
Chicago/Turabian StyleBaust, John M., Kristi K. Snyder, Robert G. Van Buskirk, and John G. Baust. 2022. "Assessment of the Impact of Post-Thaw Stress Pathway Modulation on Cell Recovery following Cryopreservation in a Hematopoietic Progenitor Cell Model" Cells 11, no. 2: 278. https://doi.org/10.3390/cells11020278
APA StyleBaust, J. M., Snyder, K. K., Van Buskirk, R. G., & Baust, J. G. (2022). Assessment of the Impact of Post-Thaw Stress Pathway Modulation on Cell Recovery following Cryopreservation in a Hematopoietic Progenitor Cell Model. Cells, 11(2), 278. https://doi.org/10.3390/cells11020278





