Regulation of Leaf Angle Protects Photosystem I under Fluctuating Light in Tobacco Young Leaves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Condition
2.2. Experimental Design
2.3. Chlorophyll Fluorescence and P700 Measurements
2.4. Statistical Analysis
3. Results
3.1. Photoprotection under Constant High Light Is Not Affected by Leaf Angle
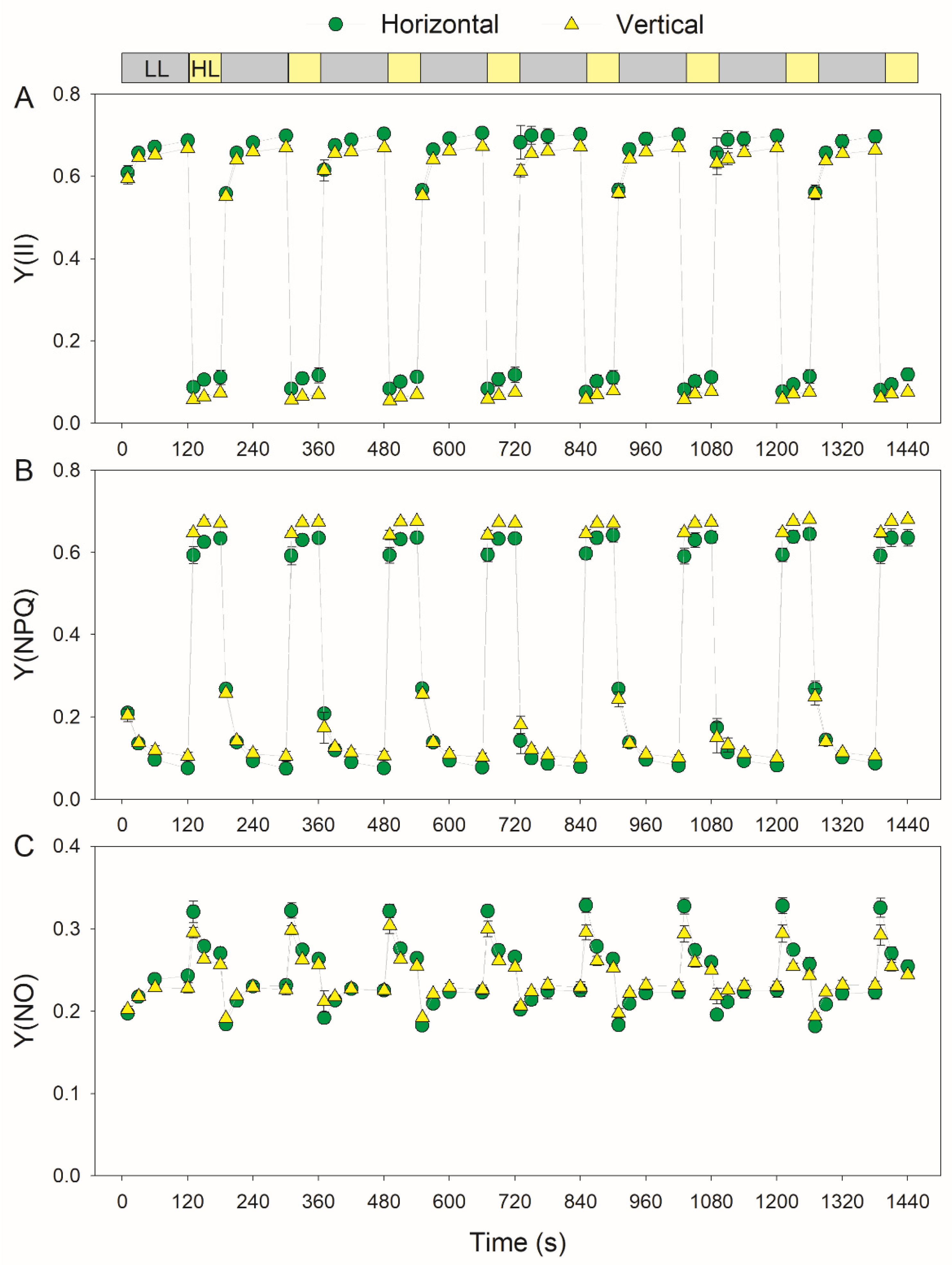
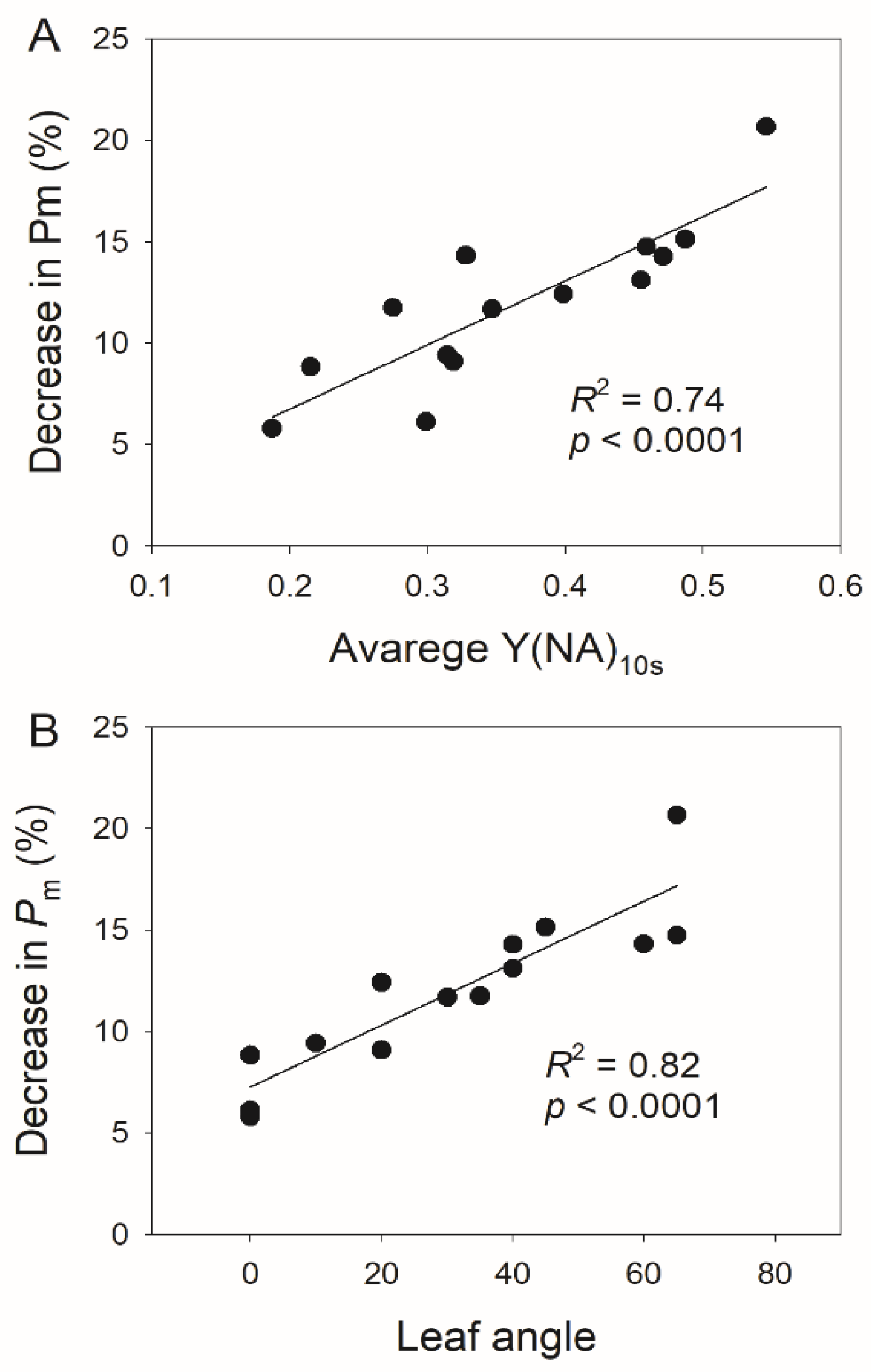
3.2. Photosynthetic Responses to Fluctuating Light Is Affected by Leaf Angle
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Pearcy, R.W. Sunflecks and photosynthesis in plant canopies. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1990, 41, 421–453. [Google Scholar] [CrossRef]
- Slattery, R.A.; Walker, B.J.; Weber, A.P.M.; Ort, D.R. The impacts of fluctuating light on crop performance. Plant Physiol. 2018, 176, 990–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamori, W.; Makino, A.; Shikanai, T. A physiological role of cyclic electron transport around photosystem I in sustaining photosynthesis under fluctuating light in rice. Sci. Rep. 2016, 6, 20147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, M.-Y.; Zhang, Y.-J.; Liu, L.-A.; Shi, L.; Ma, Q.-H.; Chow, W.S.; Jiang, C.-D. Do rapid photosynthetic responses protect maize leaves against photoinhibition under fluctuating light? Photosynth. Res. 2020, 149, 57–68. [Google Scholar] [CrossRef]
- Yamamoto, H.; Takahashi, S.; Badger, M.R.; Shikanai, T. Artificial remodelling of alternative electron flow by flavodiiron proteins in Arabidopsis. Nat. Plants 2016, 2, 16012. [Google Scholar] [CrossRef]
- Yamamoto, H.; Shikanai, T. PGR5-dependent cyclic electron flow protects photosystem I under fluctuating light at donor and acceptor sides. Plant Physiol. 2019, 179, 588–600. [Google Scholar] [CrossRef] [Green Version]
- Wada, S.; Yamamoto, H.; Suzuki, Y.; Yamori, W.; Shikanai, T.; Makino, A. Flavodiiron protein substitutes for cyclic electron flow without competing CO2 assimilation in rice. Plant Physiol. 2018, 176, 1509–1518. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Hu, H.; Zhang, S.B. Photosynthetic regulation under fluctuating light at chilling temperature in evergreen and deciduous tree species. J. Photochem. Photobiol. B Biol. 2021, 219, 112203. [Google Scholar] [CrossRef]
- Suorsa, M.; Jarvi, S.; Grieco, M.; Nurmi, M.; Pietrzykowska, M.; Rantala, M.; Kangasjarvi, S.; Paakkarinen, V.; Tikkanen, M.; Jansson, S.; et al. Proton Gradient Regulation5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant Cell 2012, 24, 2934–2948. [Google Scholar] [CrossRef] [Green Version]
- Takagi, D.; Takumi, S.; Hashiguchi, M.; Sejima, T.; Miyake, C. Superoxide and singlet oxygen produced within the thylakoid membranes both cause photosystem I photoinhibition. Plant Physiol. 2016, 171, 1626–1634. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Yang, Y.-J.; Zhang, S.-B. The role of water-water cycle in regulating the redox state of photosystem I under fluctuating light. Biochim. Biophys. Acta-Bioenerg. 2019, 1860, 383–390. [Google Scholar] [CrossRef]
- Huang, W.; Yang, Y.-J.; Zhang, S.-B. Photoinhibition of photosystem I under fluctuating light is linked to the insufficient ΔpH upon a sudden transition from low to high light. Environ. Exp. Bot. 2019, 160, 112–119. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Zhang, S.-B.; Huang, W. Photosynthetic regulation under fluctuating light in young and mature leaves of the CAM plant Bryophyllum pinnatum. Biochim. Biophys. Acta-Bioenerg. 2019, 1860, 469–477. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Zhang, S.-B.; Wang, J.-H.; Huang, W. Photosynthetic regulation under fluctuating light in field-grown Cerasus cerasoides: A comparison of young and mature leaves. Biochim. Biophys. Acta-Bioenerg. 2019, 1860, 148073. [Google Scholar] [CrossRef]
- Zhang, S.; Scheller, H.V. Photoinhibition of photosystem I at chilling temperature and subsequent recovery in Arabidopsis thaliana. Plant Cell Physiol. 2004, 45, 1595–1602. [Google Scholar] [CrossRef]
- Zivcak, M.; Brestic, M.; Kunderlikova, K.; Sytar, O.; Allakhverdiev, S.I. Repetitive light pulse-induced photoinhibition of photosystem I severely affects CO2 assimilation and photoprotection in wheat leaves. Photosynth. Res. 2015, 126, 449–463. [Google Scholar] [CrossRef]
- Lima-Melo, Y.; Gollan, P.J.; Tikkanen, M.; Silveira, J.A.G.; Aro, E.M. Consequences of photosystem-I damage and repair on photosynthesis and carbon use in Arabidopsis thaliana. Plant J. 2019, 97, 1061–1072. [Google Scholar] [CrossRef]
- Sejima, T.; Takagi, D.; Fukayama, H.; Makino, A.; Miyake, C. Repetitive short-pulse light mainly inactivates photosystem I in sunflower leaves. Plant Cell Physiol. 2014, 55, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Shimakawa, G.; Miyake, C. What quantity of photosystem I is optimum for safe photosynthesis? Plant Physiol. 2019, 179, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Tikkanen, M.; Mekala, N.R.; Aro, E.-M. Photosystem II photoinhibition-repair cycle protects Photosystem I from irreversible damage. Biochim. Biophys. Acta-Bioenerg. 2014, 1837, 210–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armbruster, U.; Correa Galvis, V.; Kunz, H.H.; Strand, D.D. The regulation of the chloroplast proton motive force plays a key role for photosynthesis in fluctuating light. Curr. Opin. Plant Biol. 2017, 37, 56–62. [Google Scholar] [CrossRef]
- Suorsa, M.; Rossi, F.; Tadini, L.; Labs, M.; Colombo, M.; Jahns, P.; Kater, M.M.; Leister, D.; Finazzi, G.; Aro, E.-M.; et al. PGR5-PGRL1-dependent cyclic electron transport modulates linear electron transport rate in Arabidopsis thaliana. Mol. Plant 2016, 9, 271–288. [Google Scholar] [CrossRef] [Green Version]
- Jokel, M.; Johnson, X.; Peltier, G.; Aro, E.M.; Allahverdiyeva, Y. Hunting the main player enabling Chlamydomonas reinhardtii growth under fluctuating light. Plant J. 2018, 94, 822–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allahverdiyeva, Y.; Suorsa, M.; Tikkanen, M.; Aro, E.M. Photoprotection of photosystems in fluctuating light intensities. J. Exp. Bot. 2015, 66, 2427–2436. [Google Scholar] [CrossRef] [PubMed]
- Alboresi, A.; Storti, M.; Morosinotto, T. Balancing protection and efficiency in the regulation of photosynthetic electron transport across plant evolution. New Phytol. 2019, 221, 105–109. [Google Scholar] [CrossRef] [Green Version]
- Takagi, D.; Amako, K.; Hashiguchi, M.; Fukaki, H.; Ishizaki, K.; Goh, T.; Fukao, Y.; Sano, R.; Kurata, T.; Demura, T.; et al. Chloroplastic ATP synthase builds up a proton motive force preventing production of reactive oxygen species in photosystem I. Plant J. 2017, 91, 306–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanazawa, A.; Ostendorf, E.; Kohzuma, K.; Hoh, D.; Strand, D.D.; Sato-Cruz, M.; Savage, L.; Cruz, J.A.; Fisher, N.; Froehlich, J.E.; et al. Chloroplast ATP Synthase Modulation of the Thylakoid Proton Motive Force: Implications for Photosystem I and Photosystem II Photoprotection. Front. Plant Sci. 2017, 8, 719. [Google Scholar] [CrossRef] [Green Version]
- Ilík, P.; Pavlovič, A.; Kouřil, R.; Alboresi, A.; Morosinotto, T.; Allahverdiyeva, Y.; Aro, E.M.; Yamamoto, H.; Shikanai, T. Alternative electron transport mediated by flavodiiron proteins is operational in organisms from cyanobacteria up to gymnosperms. New Phytol. 2017, 214, 967–972. [Google Scholar] [CrossRef] [Green Version]
- Shikanai, T.; Yamamoto, H. Contribution of cyclic and pseudo-cyclic electron transport to the formation of proton motive force in chloroplasts. Mol. Plant 2017, 10, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Allahverdiyeva, Y.; Mustila, H.; Ermakova, M.; Bersanini, L.; Richaud, P.; Ajlani, G.; Battchikova, N.; Cournac, L.; Aro, E.-M. Flavodiiron proteins Flv1 and Flv3 enable cyanobacterial growth and photosynthesis under fluctuating light. Proc. Natl. Acad. Sci. USA 2013, 110, 4111–4116. [Google Scholar] [CrossRef] [Green Version]
- Gerotto, C.; Alboresi, A.; Meneghesso, A.; Jokel, M.; Suorsa, M.; Aro, E.-M.; Morosinotto, T. Flavodiiron proteins act as safety valve for electrons in Physcomitrella patens. Proc. Natl. Acad. Sci. USA 2016, 113, 12322–12327. [Google Scholar] [CrossRef] [Green Version]
- Shimakawa, G.; Ishizaki, K.; Tsukamoto, S.; Tanaka, M.; Sejima, T.; Miyake, C. The liverwort, Marchantia, drives alternative electron flow using a flavodiiron protein to protect PSI. Plant Physiol. 2017, 173, 1636–1647. [Google Scholar] [CrossRef] [Green Version]
- Chaux, F.; Burlacot, A.; Mekhalfi, M.; Auroy, P.; Blangy, S.; Richaud, P.; Peltier, G. Flavodiiron proteins promote fast and transient O2 photoreduction in Chlamydomonas. Plant Physiol. 2017, 174, 1825–1836. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.-J.; Sun, H.; Zhang, S.-B.; Huang, W. Roles of alternative electron flows in response to excess light in Ginkgo biloba. Plant Sci. 2021, 312, 111030. [Google Scholar] [CrossRef] [PubMed]
- Storti, M.; Segalla, A.; Mellon, M.; Alboresi, A.; Morosinotto, T. Regulation of electron transport is essential for photosystem I stability and plant growth. New Phytol. 2020, 228, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-J.; Ding, X.-X.; Huang, W. Stimulation of cyclic electron flow around photosystem I upon a sudden transition from low to high light in two angiosperms Arabidopsis thaliana and Bletilla striata. Plant Sci. 2019, 287, 110166. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.-L.; Yang, Y.-J.; Huang, W. Moderate heat stress accelerates photoinhibition of photosystem I under fluctuating light in tobacco young leaves. Photosynth. Res. 2020, 144, 373–382. [Google Scholar] [CrossRef]
- Tan, S.-L.; Yang, Y.-J.; Liu, T.; Zhang, S.-B.; Huang, W. Responses of photosystem I compared with photosystem II to combination of heat stress and fluctuating light in tobacco leaves. Plant Sci. 2020, 292, 110371. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Tan, S.-L.; Huang, J.-L.; Zhang, S.-B.; Huang, W. The water-water cycle facilitates photosynthetic regulation under fluctuating light in the epiphytic orchid Dendrobium officinale. Environ. Exp. Bot. 2020, 180, 104238. [Google Scholar] [CrossRef]
- Tikkanen, M.; Aro, E.M. Integrative regulatory network of plant thylakoid energy transduction. Trends Plant Sci. 2014, 19, 10–17. [Google Scholar] [CrossRef]
- Tazoe, Y.; Ishikawa, N.; Shikanai, T.; Ishiyama, K.; Takagi, D.; Makino, A.; Sato, F.; Endo, T. Overproduction of PGR5 enhances the electron sink downstream of photosystem I in a C 4 plant, Flaveria bidentis. Plant J. 2020, 103, 814–823. [Google Scholar] [CrossRef]
- Pastenes, C.; Pimentel, P.; Lillo, J. Leaf movements and photoinhibition in relation to water stress in field-grown beans. J. Exp. Bot. 2005, 56, 425–433. [Google Scholar] [CrossRef] [Green Version]
- Kao, W.-Y.; Forseth, I.N. Dirunal leaf movement, chlorophyll fluorescence and carbon assimilation in soybean grown under different nitrogen and water availabilities. Plant Cell Environ. 1992, 15, 703–710. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, S.B.; Cao, K.F. Evidence for leaf fold to remedy the deficiency of physiological photoprotection for photosystem II. Photosynth. Res. 2012, 110, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, J.L.; Zhang, S.B.; Hu, H. Evidence for the regulation of leaf movement by photosystem II activity. Environ. Exp. Bot. 2014, 107, 167–172. [Google Scholar] [CrossRef]
- Tan, S.-L.; Huang, J.-L.; Zhang, F.-P.; Zhang, S.-B.; Huang, W. Photosystem I photoinhibition induced by fluctuating light depends on background low light irradiance. Environ. Exp. Bot. 2021, 181, 104298. [Google Scholar] [CrossRef]
- Huang, W.; Suorsa, M.; Zhang, S.B. In vivo regulation of thylakoid proton motive force in immature leaves. Photosynth. Res. 2018, 138, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Tikkanen, M.; Cai, Y.-F.; Wang, J.-H.; Zhang, S.-B. Chloroplastic ATP synthase optimizes the trade-off between photosynthetic CO2 assimilation and photoprotection during leaf maturation. Biochim. Biophys. Acta-Bioenerg. 2018, 1859, 1067–1074. [Google Scholar] [CrossRef]
- Barth, C.; Krause, G.H.; Winter, K. Responses of photosystem I compared with photosystem II to high-light stress in tropical shade and sun leaves. Plant Cell Environ. 2001, 24, 163–176. [Google Scholar] [CrossRef]
- Schreiber, U.; Klughammer, C. Non-photochemical fluorescence quenching and quantum yields in PS I and PS II: Analysis of heat-induced limitations using Maxi-Imaging-PAM and Dual-PAM-100. PAM Appl. Notes 2008, 1, 15–18. [Google Scholar] [CrossRef]
- Kimura, H.; Hashimoto-Sugimoto, M.; Iba, K.; Terashima, I.; Yamori, W. Improved stomatal opening enhances photosynthetic rate and biomass production in fluctuating light. J. Exp. Bot. 2020, 71, 2339–2350. [Google Scholar] [CrossRef] [PubMed]
- Yamori, W.; Kusumi, K.; Iba, K.; Terashima, I. Increased stomatal conductance induces rapid changes to photosynthetic rate in response to naturally fluctuating light conditions in rice. Plant. Cell Environ. 2020, 43, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Kono, M.; Noguchi, K.; Terashima, I. Roles of the cyclic electron flow around PSI (CEF-PSI) and O2-dependent alternative pathways in regulation of the photosynthetic electron flow in short-term fluctuating light in Arabidopsis thaliana. Plant Cell Physiol. 2014, 55, 990–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Yang, Y.-J.; Huang, W. The water-water cycle is more effective in regulating redox state of photosystem I under fluctuating light than cyclic electron transport. Biochim. Biophys. Acta-Bioenerg. 2020, 1861, 148235. [Google Scholar] [CrossRef]
- Tan, S.-L.; Liu, T.; Zhang, S.-B.; Huang, W. Balancing light use efficiency and photoprotection in tobacco leaves grown at different light regimes. Environ. Exp. Bot. 2020, 175, 104046. [Google Scholar] [CrossRef]
- Huang, W.; Sun, H.; Tan, S.-L.; Zhang, S.-B. The water-water cycle is not a major alternative sink in fluctuating light at chilling temperature. Plant Sci. 2021, 305, 110828. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, S.-B.; Liu, T.; Huang, W. Decreased photosystem II activity facilitates acclimation to fluctuating light in the understory plant Paris polyphylla. Biochim. Biophys. Acta-Bioenerg. 2020, 1861, 148135. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Tan, S.-L.; Sun, H.; Huang, J.-L.; Huang, W.; Zhang, S.-B. Photosystem I is tolerant to fluctuating light under moderate heat stress in two orchids Dendrobium officinale and Bletilla striata. Plant Sci. 2021, 303, 110795. [Google Scholar] [CrossRef]
- Storti, M.; Alboresi, A.; Gerotto, C.; Aro, E.; Finazzi, G.; Morosinotto, T. Role of cyclic and pseudo-cyclic electron transport in response to dynamic light changes in Physcomitrella patens. Plant. Cell Environ. 2019, 42, 1590–1602. [Google Scholar] [CrossRef]
- Sonoike, K. Photoinhibition of photosystem I. Physiol. Plant. 2011, 142, 56–64. [Google Scholar] [CrossRef]
- Tan, S.-L.; Huang, X.; Li, W.-Q.; Zhang, S.-B.; Huang, W. Elevated CO2 Concentration Alters Photosynthetic Performances under Fluctuating Light in Arabidopsis thaliana. Cells 2021, 10, 2329. [Google Scholar] [CrossRef]
- Munekage, Y.; Hojo, M.; Meurer, J.; Endo, T.; Tasaka, M.; Shikanai, T. PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 2002, 110, 361–371. [Google Scholar] [CrossRef] [Green Version]
- Shi, Q.-; Zhang, S.-B.; Wang, J.-H.; Huang, W. Pre-illumination at high light significantly alleviates the over-reduction of photosystem I under fluctuating light. Plant Sci. 2021, 312, 111053. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.-B.; Xia, H.-X.; Chen, K.; Plenković-Moraj, A.; Huang, W.; Sun, G. Photosynthetic regulation in response to fluctuating light conditions under temperature stress in three mosses with different light requirements. Plant Sci. 2021, 311, 111020. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Shi, Q.; Zhang, S.-B.; Huang, W. Coordination of Cyclic Electron Flow and Water–Water Cycle Facilitates Photoprotection under Fluctuating Light and Temperature Stress in the Epiphytic Orchid Dendrobium officinale. Plants 2021, 10, 606. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Z.-L.; Sun, H.; Wang, X.-Q.; Zhang, S.-B.; Huang, W. Regulation of Leaf Angle Protects Photosystem I under Fluctuating Light in Tobacco Young Leaves. Cells 2022, 11, 252. https://doi.org/10.3390/cells11020252
Zeng Z-L, Sun H, Wang X-Q, Zhang S-B, Huang W. Regulation of Leaf Angle Protects Photosystem I under Fluctuating Light in Tobacco Young Leaves. Cells. 2022; 11(2):252. https://doi.org/10.3390/cells11020252
Chicago/Turabian StyleZeng, Zhi-Lan, Hu Sun, Xiao-Qian Wang, Shi-Bao Zhang, and Wei Huang. 2022. "Regulation of Leaf Angle Protects Photosystem I under Fluctuating Light in Tobacco Young Leaves" Cells 11, no. 2: 252. https://doi.org/10.3390/cells11020252
APA StyleZeng, Z.-L., Sun, H., Wang, X.-Q., Zhang, S.-B., & Huang, W. (2022). Regulation of Leaf Angle Protects Photosystem I under Fluctuating Light in Tobacco Young Leaves. Cells, 11(2), 252. https://doi.org/10.3390/cells11020252





