Molecular and Photosynthetic Performance in the Yellow Leaf Mutant of Torreya grandis According to Transcriptome Sequencing, Chlorophyll a Fluorescence, and Modulated 820 nm Reflection
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Measurement of Leaf Pigment Content
2.3. Simultaneous Measurement of PF, DF, and MR Kinetics
2.4. Transcriptome Sequencing
2.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Validation
2.6. Statistical Analysis
3. Results
3.1. Analysis of Pigment Contents
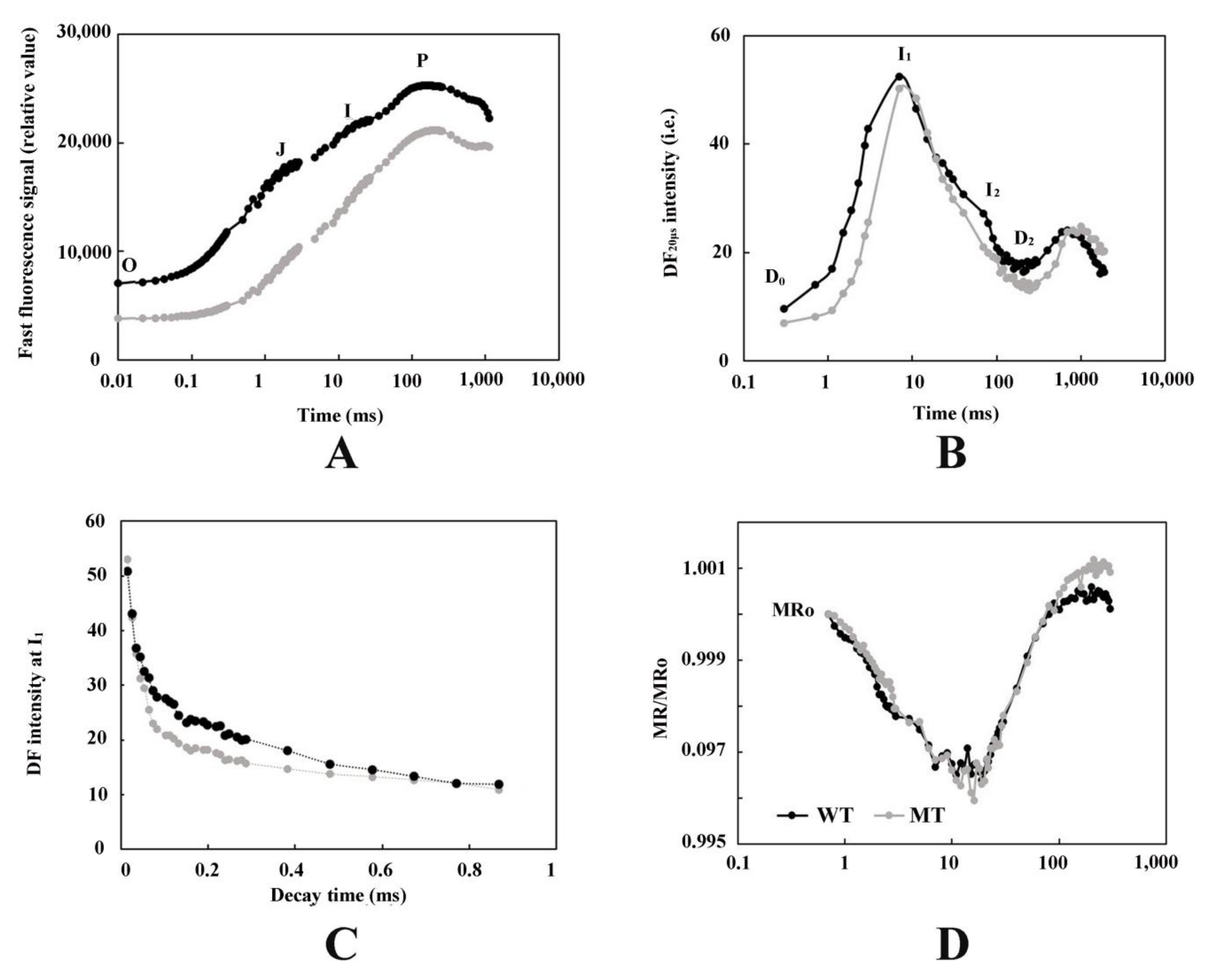
3.2. Analysis of PF, DF, and MR Kinetics
3.3. Overview of the Transcriptome Data by RNA-Seq Analysis
3.4. Pairwise Comparisons of the Transcriptomes between Wild Type and Mutant Type of T. grandis ‘Merrillii’
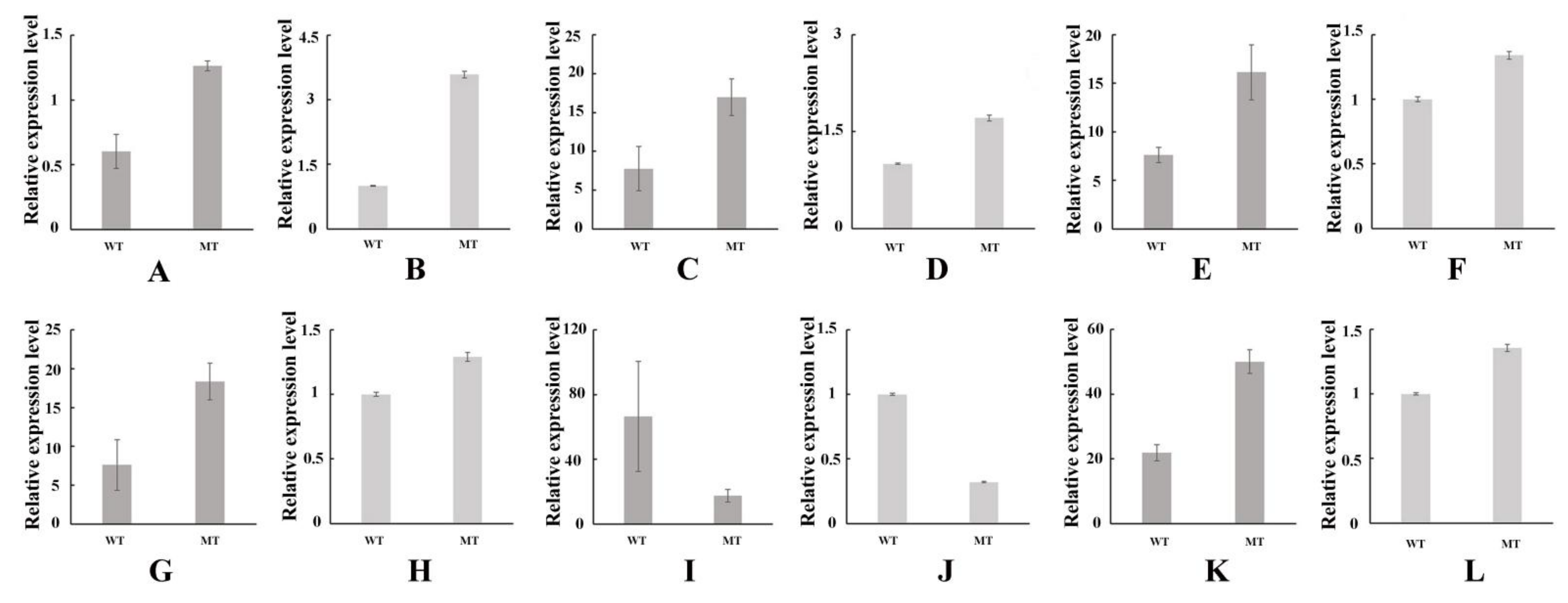
3.5. DEGs Involved in Photosynthesis and Photosynthetic Pigment Metabolism Associated Pathways
3.6. Validation of Transcription Data Using qRT-PCR
4. Discussion
4.1. Chlorophyll Deficiency Mutant
4.2. The Leaf Color Response of T. grandis ‘Merrillii’ Influences Chlorophyll Fluorescence
4.3. The Leaf Color Response of T. grandis ‘Merrillii’ Influences Chlorophyll Synthesis
4.4. The Leaf Color Response of T. grandis ‘Merrillii’ Influences Photosynthetic Electron Transfer
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, T.; Cui, J.J.; Xiao, Z.B.; Tian, H.X.; Yi, F.; Ma, X. Chemical composition of essential oil from the peel of Chinese Orreya grandis Fort. Org. Chem. Int. 2011, 3, 187372. [Google Scholar] [CrossRef]
- Chen, X.W.; Jin, H.B. Review of cultivation and development of Chinese torreya in China. For. Trees Livelihoods 2019, 28, 68–78. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Wang, R.; Ji, H.; Su, X. Chinese Torreya grandis cv. merrillii seed oil affects obesity through accumulation of sciadonic acid and altering the composition of gut microbiota. Food Sci. Hum. Wellness 2022, 11, 58–67. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X. Variation of fruit size and its frequency distribution in chinese torreya. Int. J. Fruit Sci. 2020, 20, S1445–S1457. [Google Scholar] [CrossRef]
- Ruppel, N.J.; Logsdon, C.A.; Whippo, C.W.; Kentaro, I.; Hangarter, R.P. A mutation in Arabidopsis seedling plastid development1 affects plastid differentiation in embryo-derived tissues during seedling growth. Plant Physiol. 2011, 155, 342–353. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, C.; Chang, A.; Du, C. Genetic, physiological and biochemical analysis of the formation of yellow-green leaf color of burley tobacco (Nicotiana tabacum). Int. J. Agric. Biol. 2016, 18, 192–197. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, Z.; Ma, X.; Wu, H.; Liu, Y.; Zhou, K.; Chen, Y.; Ma, W.; Bi, J.; Zhang, X.; et al. A knockdown mutation of yellow-green leaf2 blocks chlorophyll biosynthesis in rice. Plant Cell Rep. 2013, 32, 1855–1867. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Lee, J.-Y.; Cho, H.S.; Lee, S.S.; Ha, H.J.; Kim, S.; Choi, D.; Pai, H.-S. Inactivation of organellar Glutamyl- and seryl-tRNA synthetases leads to developmental arrest of chloroplasts and mitochondria in higher plants. J. Biol. Chem. 2005, 280, 37098–37106. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.H.; Yoo, E.S.; Lee, C.H.; Hirochika, H.G. An Differential regulation of chlorophyll a oxygenase genes in rice. Plant Mol. Biol. 2005, 57, 805–818. [Google Scholar] [CrossRef]
- Mochizuki, N.; Tanaka, R.; Grimm, B.; Masuda, T.; Moulin, M.; Smith, A.; Tanaka, A.; Terry, M. The cell biology of tetrapyrroles: A life and death struggle. Trends Plant Sci. 2010, 15, 488–498. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, M.L.; Zhang, Y.Z.; Du, L.F.; Pan, T. A chlorophyll-reduced seedling mutant in oilseed rape, Brassica napus, for utilization in F1 hybrid production. Plant Breed. 2010, 119, 131–135. [Google Scholar] [CrossRef]
- Jiang, W.B.; Zhuang, M.; Han, H.Z.; Dai, M.S.; Hua, G.P. Progress on color emerging mechanism and photosynthetic characteristics of colored-leaf plants. Acta Hortic. Sin. 2005, 32, 352–358. [Google Scholar] [CrossRef]
- Ishikawa, T.; Shigeoka, S. Recent advances in ascorbate biosynthesis and the physiological significance of ascorbate peroxidase in photosynthesizing organisms. Biosci. Biotechnol. Biochem. 2008, 72, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Y.; Yu, W.; Hänninen, H.; Song, L.; Du, X.; Zhang, R.; Wu, J. Novel insights into the influence of seed sarcotesta photosynthesis on accumulation of seed dry matter and oil content in Torreya grandis cv. “Merrillii”. Front. Plant Sci. 2018, 8, 2179. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Qiang, S.; Goltsev, V. Simultaneous in vivo recording of prompt and delayed fluorescence and 820 nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim. Biophys. Acta 2010, 1797, 1313–1326. [Google Scholar] [CrossRef]
- Goltsev, V.; Zaharieva, I.; Chernev, P.; Kouzmanova, M.; Kalaji, H.M.; Yordanov, I.; Krasteva, V.; Aleksandrov, V.; Stefanov, D.; Allakhverdiev, S.; et al. Drought induced modifications of photosynthetic electron transport in intact leaves: Analysis and use of neural networks as a tool for a rapid non-invasive estimation. Biochim. Biophys. Acta 2012, 1817, 1490–1498. [Google Scholar] [CrossRef]
- Oukarroum, A.; Goltsev, V.; Strasser, R.J. Temperature effects on pea plants probed by simultaneous measurements of the kinetics of prompt fluorescence, delayed fluorescence and modulated 820 nm reflection. PLoS ONE 2013, 8, e59433. [Google Scholar] [CrossRef]
- Gao, J.; Li, P.; Ma, F.; Goltsev, V. Photosynthetic performance during leaf expansion in Malus micromalus probed by chlorophyll a fluorescence and modulated 820 nm reflection. J. Photochem. Photobiol. B Biol. 2014, 137, 144–150. [Google Scholar] [CrossRef]
- Core, L.J.; Waterfall, J.J.; Lis, J.T. Nascent RNA sequencing reveals widespread pausing and divergent initiate at human promoters. Science 2008, 322, 1845–1848. [Google Scholar] [CrossRef]
- Wu, Q.J.; Chen, Z.D.; Sun, W.J.; Deng, T.T.; Chen, M.J. De novo sequencing of the leaf transcriptome reveals complex light-responsive regulatory networks in Camellia sinensis cv. Baijiguan. Front. Plant Sci. 2016, 7, 332. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Y.; Shen, J.-S.; Gu, M.; Cheng, T.-R.; Pan, H.-T.; Zhang, Q.-X. Leaf coloration and photosynthetic characteristics of hybrids between Forsythia ‘Courtaneur’ and Forsythia koreana ‘Suwon Gold’. Hortscience 2017, 52, 1661–1667. [Google Scholar] [CrossRef]
- Clark, S.M.; Vaitheeswaran, V.; Ambrose, S.J.; Purves, R.W.; Page, J.E. Transcriptome analysis of bitter acid biosynthesis and precursor pathways in hop (Humulus lupulus). BMC Plant Biol. 2013, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, P.; Li, Y.; Ma, L.; Li, L.; Yang, R.; Ma, Y.; Wang, S.; Wang, Q. Global transcriptome analysis and identi- fication of the flowering regulatory genes expressed in leaves of Lagerstroemia indica. DNA Cell Biol. 2014, 33, 680–688. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determination of total carotenoids and chlorophylls a and b of leaf in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Peng, J.G.; Jiang, X.R.; Jia, M.; Guo, L.; Gao, R.; Liu, Y. Contrasting patterns of sun-red and shade-green leaves of Buxus microphylla, in response to gradients of excess light during winter acclimation. Acta Physiol. Plant 2016, 38, 254. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of fluorescence transient. In Chlorophyll Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Ed.; Advances in Photosynthesis and Respiration; Springer: Berlin/Heidelberg, Germany, 2004; pp. 321–362. [Google Scholar]
- Han, Y.; Wan, H.; Cheng, T.; Wang, J.; Yang, W.; Pan, H.; Zhang, Q. Comparative RNA-seq analysis of transcriptome dynamics during petal development in Rosa chinensis. Sci. Rep. 2017, 7, 43382. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Shen, J.; Wu, Y.; Jiang, Z.; Xu, Y.; Zheng, T.; Wang, J.; Cheng, T.; Zhang, Q.; Pan, H. Selection and validation of appropriate reference genes for gene expression studies in Forsythia. Physiol. Mol. Biol. Plants 2020, 26, 173–188. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.D.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, P.; Gao, L.; Yang, R.; Li, L.; Zhang, E.; Wang, Q.; Li, Y.; Yin, Z. Characterization and complementation of a chlorophyll-Less dominant mutant GL1 in Lagerstroemia indica. DNA Cell Biol. 2017, 36, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Z.; Wang, P.; Wang, S.; Ma, L.; Li, L.; Yang, R.; Ma, Y.; Wang, Q. Comprehensive transcriptome analysis discovers novel candidate genes related to leaf color in a Lagerstroemia indica yellow leaf mutant. Genes Genom. 2015, 37, 851–863. [Google Scholar] [CrossRef]
- Li, B.Y. Study on the chlorophyll content in the leaves of Ligustrum vicaryi and L. quihoui. For. Sci. Tech. 2005, 30, 63–65. [Google Scholar]
- Ma, Z.H.; Wang, H.T.; Wei, W.Q.; Yan, Y.H. Change of photosynthetic pigments and enzyme activities of hot pepper (Capsicum annuum) CMS line leaves with chartreuse marker. China Cucurbits Veg. 2011, 24, 20–24. [Google Scholar]
- Zhou, Y.; Zhou, H.G.; Zhang, X.L. The Study on variegation and chimerism in ornamental plants. J. Guangxi Agric. Biol. 1999, 18, 68–73. [Google Scholar]
- Oster, U.; Tanaka, R.; Tanaka, A.; Rüdigger, W. Cloning and functional expression of the gene encoding the key enzyme for chlorophyll b biosynthesis (CAO) from Arabidopsis thaliana. Plant J. 2000, 21, 305–310. [Google Scholar] [CrossRef]
- Oukarroum, A.; Gharous, M.E.; Goltsev, V.; Strasser, R.J. Delayed fluorescence emission as a probe for the response of photosynthetic organisms to high temperature exposure: A comparative study. J. Lumin. 2016, 180, 321–327. [Google Scholar] [CrossRef]
- Momchil, P.; Lyubka, K.; Andon, V.; Jaco, V.; Vasilij, G. Effects of different metals on photosynthesis: Cadmium and zinc affect chlorophyll fluorescence in durum wheat. Int. J. Mol. Sci. 2018, 19, 787. [Google Scholar] [CrossRef]
- He, J.; Chee, C.W.; Goh, C.J. ‘Photoinhibition’ of Heliconia under natural tropical conditions: The importance of leaf orientation for light interception and leaf temperature. Plant Cell Environ. 1996, 19, 1238–1248. [Google Scholar] [CrossRef]
- Pokorska, B.; Romanowska, E. Photoinhibition and D1 protein degradation in mesophyll and agranal bundle sheath thylakoids of maize. Funct. Plant Biol. 2007, 34, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.H.; Zhang, X.H.; Camberato, J.J.; Xue, J.Q. Photosynthetic performance of maize hybrids to drought stress. Russ. J. Plant Physiol. 2015, 62, 788–796. [Google Scholar] [CrossRef]
- Liu, M. Response of photosynthesis and chlorophyll fluorescence to drought stress in two maize cultivars. Afr. J. Agric. Res. 2012, 7, 4751–4760. [Google Scholar] [CrossRef]
- Mathur, S.; Allakhverdiev, S.I.; Jajoo, A. Analysis of high temperature stress on the dynamics of antenna size and reducing side heterogeneity of photosystem ii in wheat leaves (Triticum aestivum). Biochim. Biophys. Acta 2011, 1807, 22–29. [Google Scholar] [CrossRef]
- Chen, S.G.; Yang, J.; Zhang, M.S.; Strasser, R.J.; Sheng, Q. Classification and characteristics of heat tolerance in ageratina adenophora populations using fast chlorophyll a fluorescence rise o-j-i-p. Environ. Exp. Bot. 2016, 122, 126–140. [Google Scholar] [CrossRef]
- Schansker, G.; Srivastava, A.; Govindjee, S.R.J. Characterization of the 820-nm transmission signal paralleling the chlorophyll a fluorescence rise (OJIP) in pea leaves. Funct. Plant Biol. 2003, 30, 785–796. [Google Scholar] [CrossRef]
- Schraser, S.M.; Wase, R.R.; Wacholtz, W.F.; Ort, D.R.; Sharkey, T.D. Thylakoid membrane responses to moderately high leaf temperature in Pima cotton. Plant Cell Environ. 2004, 27, 725–735. [Google Scholar] [CrossRef]
- Zong, Y.Z.; Wang, W.F.; Xue, Q.W.; Shangguan, Z.P. Interactive effects of elevated co and drought on photosynthetic capacity and PSⅡ performance in maize. Photosynthetica 2014, 52, 63–70. [Google Scholar] [CrossRef]
- Noguchi, T. Dual role of triplet localization on the accessory chlorophyll in the photosystem II reaction center: Photoprotection and photodamage of the D1 protein. Plant Cell Physiol. 2002, 43, 1112–1116. [Google Scholar] [CrossRef]
- Anderson, I.C.; Robertson, D.S. Role of carotenoids in protecting chlorophyll from photodestruction. Plant Cell 1960, 35, 531–534. [Google Scholar] [CrossRef]
- Wang, C.; Liu, G.; Zhang, C.; He, F.; Li, Y.; Wu, J.; Shi, H. Changes in leaf photosynthetic characteristics and chloroplast ultrastructure during tobacco leaf growth and development. J. Jiangxi Agric. Univ. 2010, 32, 254–259. [Google Scholar] [CrossRef]
- Heerden, P.V.; Strasser, R.J.; Kruger, G.H.J. Reduction of dark chilling stress in N-2-fixing soybean by nitrate as indicated by chlorophyll a fluorescence kinetics. Physiol. Plant. 2010, 121, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Júlvez, M.; Hurley, J.K.; Tollin, G.; Gómez-Moreno, C.; Fillat, M.F. Overexpression in E. coli, of the complete pet H gene product from anabaena: Purification and properties of a 49 kDA ferredoxin-NADP+ reductase. Biochim. Biophys. Acta 1996, 1297, 200–206. [Google Scholar] [CrossRef]




| Torreya grandis ‘Merrillii’ | Chl a (mg/g) | Chl b (mg/g) | Chl (a+b) (mg/g) | Chl a/b | Carotenoid |
|---|---|---|---|---|---|
| WT 1 | 0.38 ± 0.02 | 0.09 ± 0.01 | 0.46 ± 0.03 | 4.36 ± 0.19 | 0.14 ± 0.01 |
| MT 2 | 0.07 ± 0.00 ** 3 | 0.03 ± 0.00 ** | 0.11 ± 0.00 ** | 2.17 ± 0.33 ** | 0.15 ± 0.01 |
| Torreya grandis | VPSⅠ 3 (0.7–20 ms) | VPSⅡ-PSⅠ 4 (20–300 ms) | VPSⅡ 5 |
|---|---|---|---|
| WT 1 | 0.193 ± 0.01 | 0.015 ± 0.001 | 0.208 ± 0.014 |
| MT 2 | 0.207 ± 0.003 ** | 0.017 ± 0.001 ** | 0.224 ± 0.004 ** 6 |
| Torreya grandis | Fv/Fm 3 | Ψo 4 | δRo 5 | γRC 6 | φEo 7 | φRo 8 | φDo 9 | RC/CS0 10 | RC/CSM |
|---|---|---|---|---|---|---|---|---|---|
| WT 1 | 0.82 ± 0.01 | 0.70 ± 0.04 | 0.35 ± 0.02 | 0.50 ± 0.00 | 0.57 ± 0.04 | 0.20 ± 0.03 | 0.18 ± 0.01 ** | 3804 ± 240 | 21,305 ± 2004 |
| MT 2 | 0.73 ± 0.06 ** | 0.43 ± 0.09 ** | 0.41 ± 0.09 | 0.32 ± 0.06 ** | 0.32 ± 0.09 ** | 0.12 ± 0.02 ** | 0.27 ± 0.06 | 3149 ± 254 | 12,146 ± 2875 ** 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, J.; Li, X.; Zhu, X.; Ding, Z.; Huang, X.; Chen, X.; Jin, S. Molecular and Photosynthetic Performance in the Yellow Leaf Mutant of Torreya grandis According to Transcriptome Sequencing, Chlorophyll a Fluorescence, and Modulated 820 nm Reflection. Cells 2022, 11, 431. https://doi.org/10.3390/cells11030431
Shen J, Li X, Zhu X, Ding Z, Huang X, Chen X, Jin S. Molecular and Photosynthetic Performance in the Yellow Leaf Mutant of Torreya grandis According to Transcriptome Sequencing, Chlorophyll a Fluorescence, and Modulated 820 nm Reflection. Cells. 2022; 11(3):431. https://doi.org/10.3390/cells11030431
Chicago/Turabian StyleShen, Jianshuang, Xueqin Li, Xiangtao Zhu, Zhicheng Ding, Xiaoling Huang, Xia Chen, and Songheng Jin. 2022. "Molecular and Photosynthetic Performance in the Yellow Leaf Mutant of Torreya grandis According to Transcriptome Sequencing, Chlorophyll a Fluorescence, and Modulated 820 nm Reflection" Cells 11, no. 3: 431. https://doi.org/10.3390/cells11030431
APA StyleShen, J., Li, X., Zhu, X., Ding, Z., Huang, X., Chen, X., & Jin, S. (2022). Molecular and Photosynthetic Performance in the Yellow Leaf Mutant of Torreya grandis According to Transcriptome Sequencing, Chlorophyll a Fluorescence, and Modulated 820 nm Reflection. Cells, 11(3), 431. https://doi.org/10.3390/cells11030431





