Komagataella phaffii Cue5 Piggybacks on Lipid Droplets for Its Vacuolar Degradation during Stationary Phase Lipophagy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Plasmids
2.2. Fluorescence Microscopy
2.3. Biochemical Studies
2.4. Statistical Analysis
3. Results
3.1. K. phaffii Cue5 Accumulates on LDs Specifically in the Stationary Phase
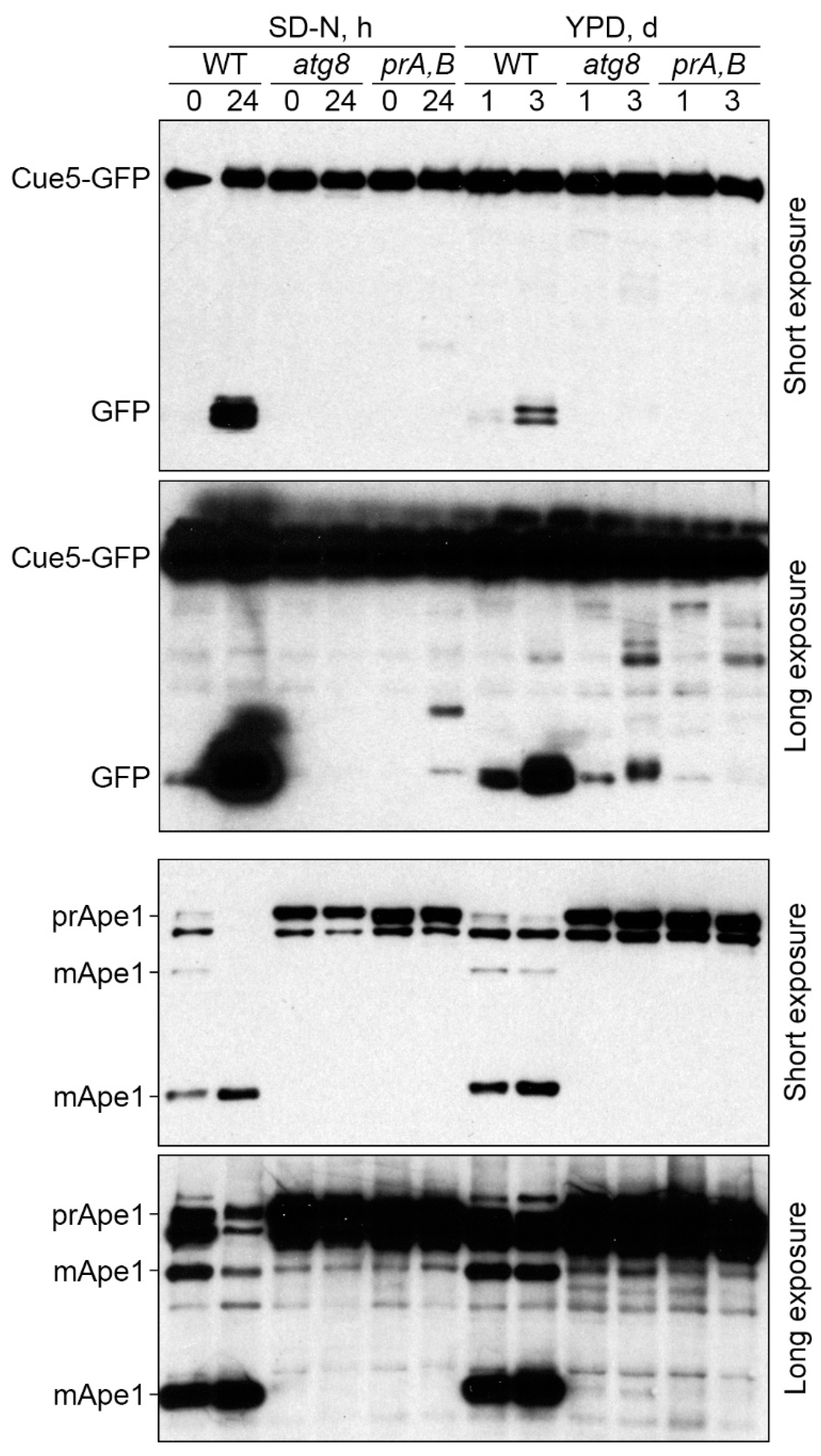
3.2. Cue5 Is Degraded by the Stationary Phase Lipophagy
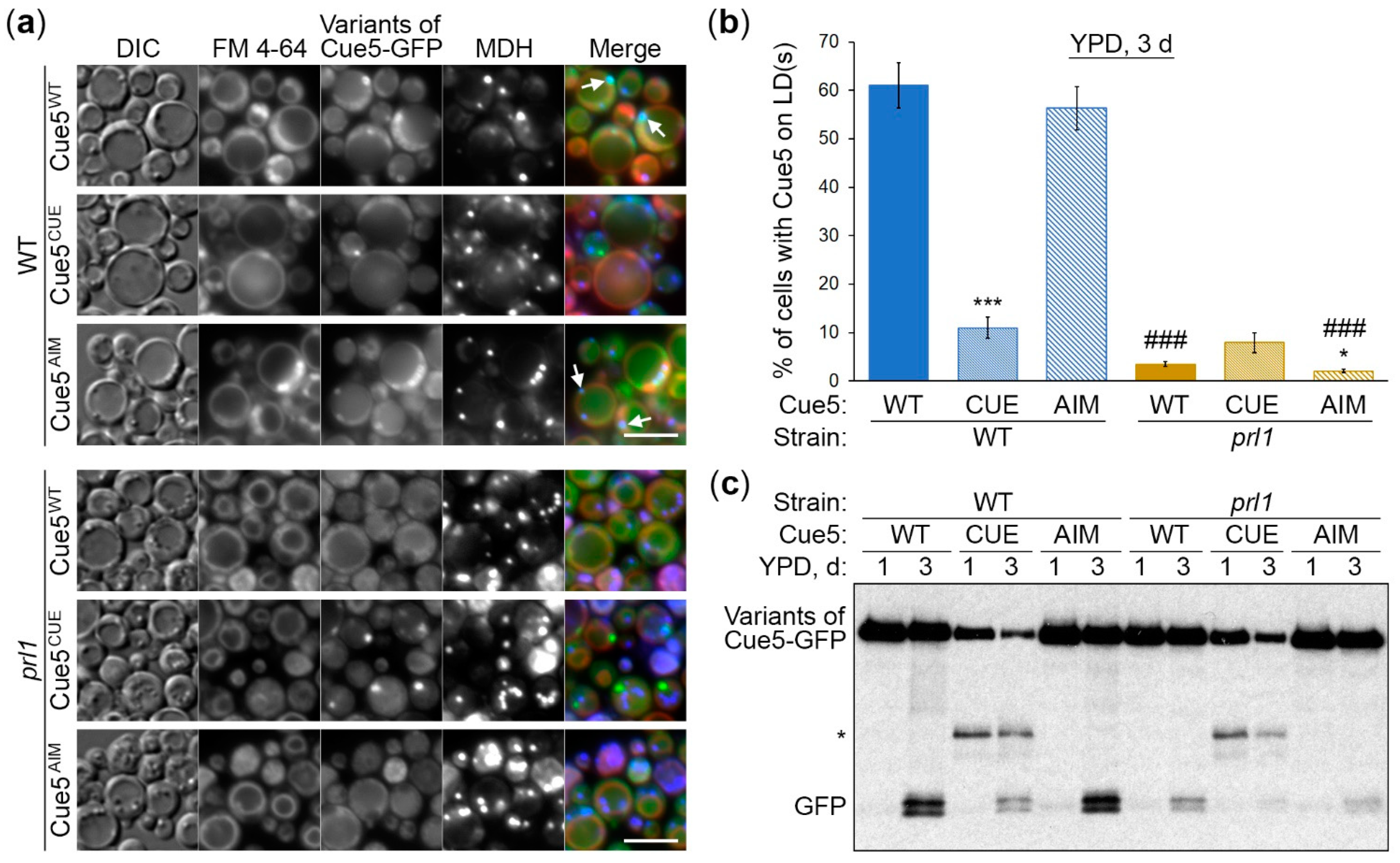
3.3. Cue5 Accumulation on LDs and Degradation by S-Phase Lipophagy Depend on CUE Domain
3.4. Cue5 Accumulation on LDs and Degradation by S-Phase Lipophagy Depend on Prl1
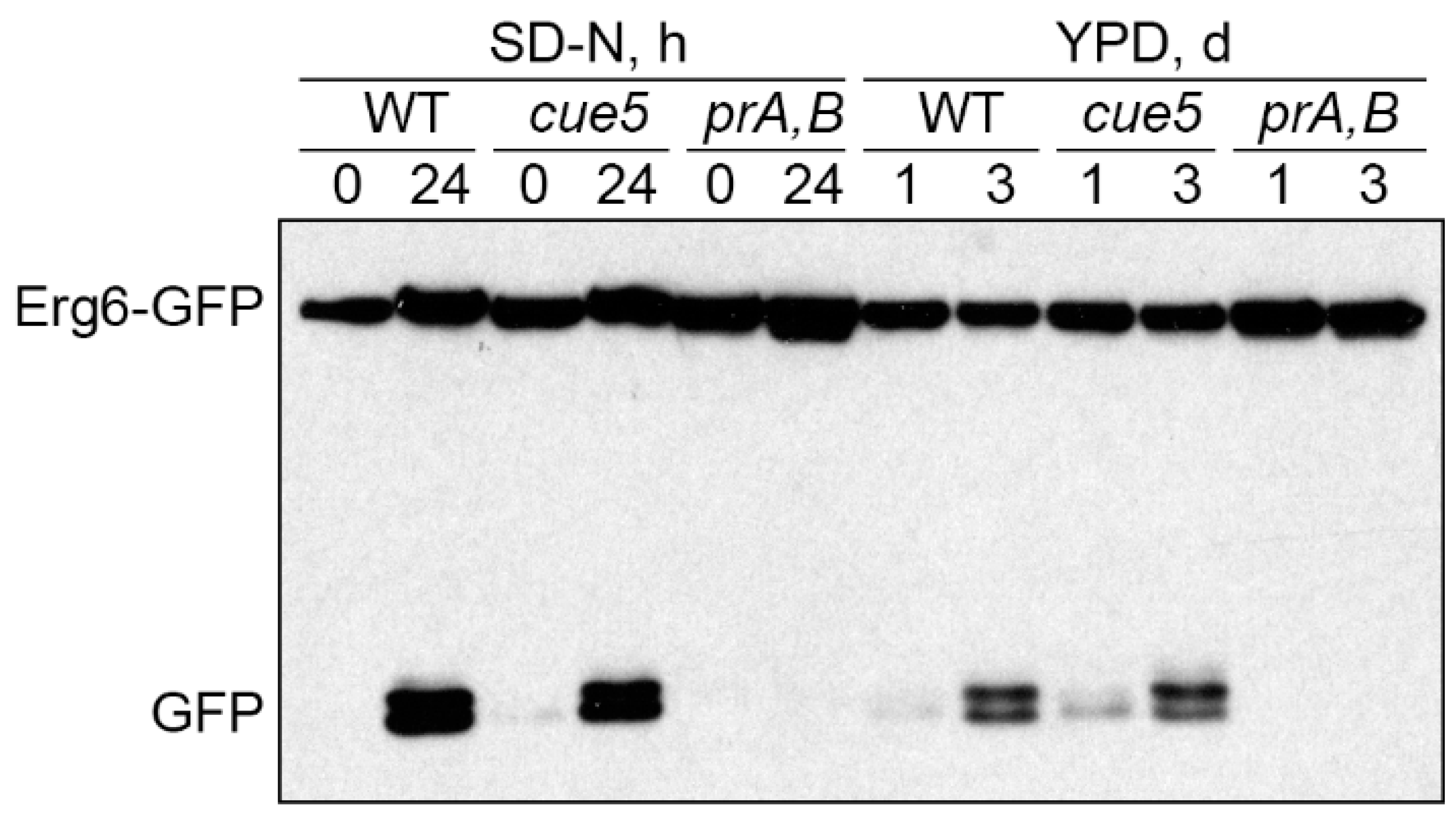
3.5. Cue5 Is Dispensable for S-Phase Lipophagy
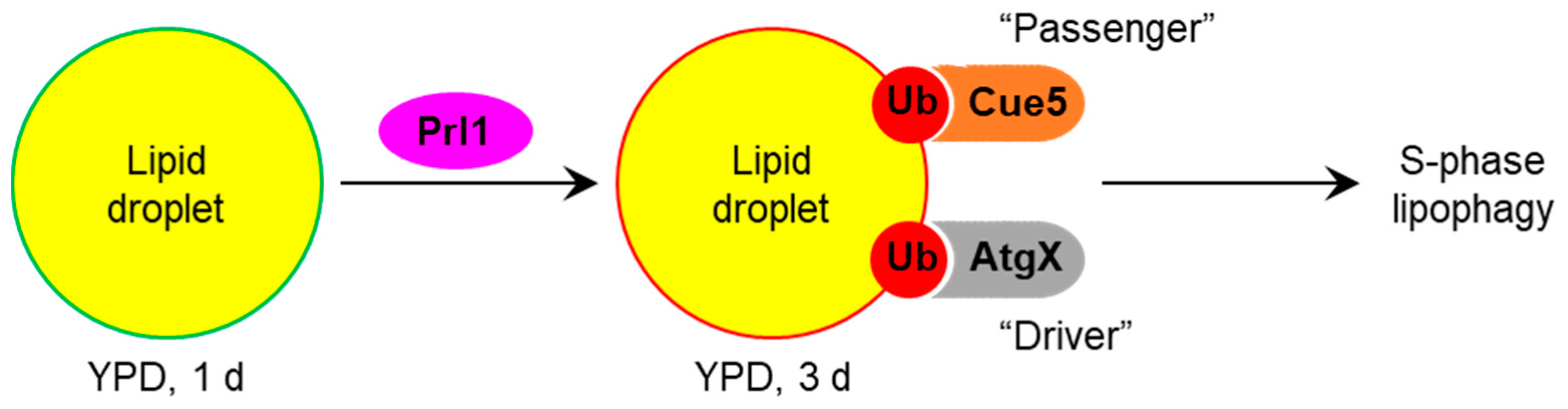
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lu, K.; Psakhye, I.; Jentsch, S. Autophagic clearance of polyQ proteins mediated by ubiquitin-Atg8 adaptors of the conserved CUET protein family. Cell 2014, 158, 549–563. [Google Scholar] [CrossRef] [Green Version]
- Lu, K.; Psakhye, I.; Jentsch, S. A new class of ubiquitin-Atg8 receptors involved in selective autophagy and polyQ protein clearance. Autophagy 2014, 10, 2381–2382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dikic, I.; Wakatsuki, S.; Walters, K.J. Ubiquitin-binding domains—From structures to functions. Nat. Rev. Mol. Cell Biol. 2009, 10, 659–671. [Google Scholar] [CrossRef]
- Shih, S.C.; Prag, G.; Francis, S.A.; Sutanto, M.A.; Hurley, J.H.; Hicke, L. A ubiquitin-binding motif required for intramolecular monoubiquitylation, the CUE domain. EMBO J. 2003, 22, 1273–1281. [Google Scholar] [CrossRef] [Green Version]
- Noda, N.N.; Ohsumi, Y.; Inagaki, F. Atg8-family interacting motif crucial for selective autophagy. FEBS Lett. 2010, 584, 1379–1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamark, T.; Johansen, T. Aggrephagy: Selective disposal of protein aggregates by macroautophagy. Int. J. Cell Biol. 2012, 2012, 736905. [Google Scholar] [CrossRef] [Green Version]
- Marshall, R.S.; McLoughlin, F.; Vierstra, R.D. Autophagic Turnover of Inactive 26S Proteasomes in Yeast Is Directed by the Ubiquitin Receptor Cue5 and the Hsp42 Chaperone. Cell Rep. 2016, 16, 1717–1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farre, J.C.; Subramani, S. Mechanistic insights into selective autophagy pathways: Lessons from yeast. Nat. Rev. Mol. Cell Biol. 2016, 17, 537–552. [Google Scholar] [CrossRef]
- Farre, J.C.; Manjithaya, R.; Mathewson, R.D.; Subramani, S. PpAtg30 tags peroxisomes for turnover by selective autophagy. Dev. Cell 2008, 14, 365–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazarko, T.Y.; Ozeki, K.; Till, A.; Ramakrishnan, G.; Lotfi, P.; Yan, M.; Subramani, S. Peroxisomal Atg37 binds Atg30 or palmitoyl-CoA to regulate phagophore formation during pexophagy. J. Cell Biol. 2014, 204, 541–557. [Google Scholar] [CrossRef] [Green Version]
- Nazarko, T.Y. Atg37 regulates the assembly of the pexophagic receptor protein complex. Autophagy 2014, 10, 1348–1349. [Google Scholar] [CrossRef] [Green Version]
- Kanki, T.; Wang, K.; Cao, Y.; Baba, M.; Klionsky, D.J. Atg32 is a mitochondrial protein that confers selectivity during mitophagy. Dev. Cell 2009, 17, 98–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamoto, K.; Kondo-Okamoto, N.; Ohsumi, Y. Mitochondria-anchored receptor Atg32 mediates degradation of mitochondria via selective autophagy. Dev. Cell 2009, 17, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Zutphen, T.; Todde, V.; de Boer, R.; Kreim, M.; Hofbauer, H.F.; Wolinski, H.; Veenhuis, M.; van der Klei, I.J.; Kohlwein, S.D. Lipid droplet autophagy in the yeast Saccharomyces cerevisiae. Mol. Biol. Cell 2014, 25, 290–301. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.W.; Miao, Y.H.; Chang, Y.S. A sterol-enriched vacuolar microdomain mediates stationary phase lipophagy in budding yeast. J. Cell Biol. 2014, 206, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Seo, A.Y.; Lau, P.W.; Feliciano, D.; Sengupta, P.; Gros, M.A.L.; Cinquin, B.; Larabell, C.A.; Lippincott-Schwartz, J. AMPK and vacuole-associated Atg14p orchestrate mu-lipophagy for energy production and long-term survival under glucose starvation. Elife 2017, 6, e21690. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Rahman, M.A.; Nazarko, T.Y. Nitrogen Starvation and Stationary Phase Lipophagy Have Distinct Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 94. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Kumar, R.; Sanchez, E.; Nazarko, T.Y. Lipid Droplets and Their Autophagic Turnover via the Raft-Like Vacuolar Microdomains. Int. J. Mol. Sci. 2021, 22, 8144. [Google Scholar] [CrossRef]
- Sears, I.B.; O’Connor, J.; Rossanese, O.W.; Glick, B.S. A versatile set of vectors for constitutive and regulated gene expression in Pichia pastoris. Yeast 1998, 14, 783–790. [Google Scholar] [CrossRef]
- Cregg, J.M.; Russell, K.A. Transformation. Methods Mol. Biol. 1998, 103, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R. Simplified protocol for faster transformation of (a large number of) Pichia pastoris strains. Yeast 2019, 36, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Cregg, J.M.; Barringer, K.J.; Hessler, A.Y.; Madden, K.R. Pichia pastoris as a host system for transformations. Mol. Cell Biol. 1985, 5, 3376–3385. [Google Scholar] [CrossRef]
- Gould, S.J.; McCollum, D.; Spong, A.P.; Heyman, J.A.; Subramani, S. Development of the yeast Pichia pastoris as a model organism for a genetic and molecular analysis of peroxisome assembly. Yeast 1992, 8, 613–628. [Google Scholar] [CrossRef]
- Nazarko, T.Y.; Farre, J.C.; Subramani, S. Peroxisome size provides insights into the function of autophagy-related proteins. Mol. Biol. Cell 2009, 20, 3828–3839. [Google Scholar] [CrossRef] [Green Version]
- Tuttle, D.L.; Dunn, W.A., Jr. Divergent modes of autophagy in the methylotrophic yeast Pichia pastoris. J. Cell Sci. 1995, 108 Pt 1, 25–35. [Google Scholar] [CrossRef]
- Baerends, R.J.; Faber, K.N.; Kram, A.M.; Kiel, J.A.; van der Klei, I.J.; Veenhuis, M. A stretch of positively charged amino acids at the N terminus of Hansenula polymorpha Pex3p is involved in incorporation of the protein into the peroxisomal membrane. J. Biol. Chem. 2000, 275, 9986–9995. [Google Scholar] [CrossRef] [Green Version]
- Farre, J.C.; Vidal, J.; Subramani, S. A cytoplasm to vacuole targeting pathway in P. pastoris. Autophagy 2007, 3, 230–234. [Google Scholar] [CrossRef] [Green Version]
- Lu, K.; den Brave, F.; Jentsch, S. Receptor oligomerization guides pathway choice between proteasomal and autophagic degradation. Nat. Cell Biol. 2017, 19, 732–739. [Google Scholar] [CrossRef]
- Robichaud, S.; Fairman, G.; Vijithakumar, V.; Mak, E.; Cook, D.P.; Pelletier, A.R.; Huard, S.; Vanderhyden, B.C.; Figeys, D.; Lavallee-Adam, M.; et al. Identification of novel lipid droplet factors that regulate lipophagy and cholesterol efflux in macrophage foam cells. Autophagy 2021, 17, 3671–3689. [Google Scholar] [CrossRef] [PubMed]
- Vevea, J.D.; Garcia, E.J.; Chan, R.B.; Zhou, B.; Schultz, M.; Di Paolo, G.; McCaffery, J.M.; Pon, L.A. Role for Lipid Droplet Biogenesis and Microlipophagy in Adaptation to Lipid Imbalance in Yeast. Dev. Cell 2015, 35, 584–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, E.J.; Liao, P.C.; Tan, G.; Vevea, J.D.; Sing, C.N.; Tsang, C.A.; McCaffery, J.M.; Boldogh, I.R.; Pon, L.A. Membrane dynamics and protein targets of lipid droplet microautophagy during ER stress-induced proteostasis in the budding yeast, Saccharomyces cerevisiae. Autophagy 2021, 17, 2363–2383. [Google Scholar] [CrossRef] [PubMed]


| Mutant | Strain | Background | Genotype and Plasmid | Source |
|---|---|---|---|---|
| WT | GS115 | GS115 | his4 | [22] |
| WT | SRK69 | GS115 | his4::pRK10 (PCUE5-CUE5-GFP, HIS4) | This study |
| WT | SRK160 | GS115 | his4::pRK24 (PCUE5-cue5CUE-GFP, HIS4) | This study |
| WT | SRK162 | GS115 | his4::pRK25 (PCUE5-cue5AIM-GFP, HIS4) | This study |
| WT | PPY12h | PPY12h | arg4 his4 | [23] |
| WT | SRK8 | PPY12h | his4::pRK2 (PERG6-ERG6-GFP, HIS4) | [17] |
| atg8 | SJCF925 | PPY12h | ∆atg8::GeneticinR arg4 his4 | [24] |
| atg8 | SRK87 | SJCF925 | his4::pRK10 (PCUE5-CUE5-GFP, HIS4) | This study |
| cue5 | SRK196 | PPY12h | ∆cue5::ZeocinR (pRK6) arg4 his4 | This study |
| cue5 | SRK201 | SRK196 | his4::pRK2 (PERG6-ERG6-GFP, HIS4) | This study |
| cue5 | SRK223 | SRK196 | his4::pRK10 (PCUE5-CUE5-GFP, HIS4) | This study |
| cue5 | SRK225 | SRK196 | his4::pRK24 (PCUE5-cue5CUE-GFP, HIS4) | This study |
| cue5 | SRK227 | SRK196 | his4::pRK25 (PCUE5-cue5AIM-GFP, HIS4) | This study |
| pep4 prb1 | SMD1163 | GS115 | pep4 prb1 his4 | [25] |
| pep4 prb1 | SRK35 | SMD1163 | his4::pRK2 (PERG6-ERG6-GFP, HIS4) | [17] |
| pep4 prb1 | SRK71 | SMD1163 | his4::pRK10 (PCUE5-CUE5-GFP, HIS4) | This study |
| prl1 | SRK3 | PPY12h | prl1::ZeocinR (pRK6) arg4 his4 | [17] |
| prl1 | SRK166 | SRK3 | his4::pRK10 (PCUE5-CUE5-GFP, HIS4) | This study |
| prl1 | SRK168 | SRK3 | his4::pRK24 (PCUE5-cue5CUE-GFP, HIS4) | This study |
| prl1 | SRK170 | SRK3 | his4::pRK25 (PCUE5-cue5AIM-GFP, HIS4) | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, R.; Shroff, A.; Nazarko, T.Y. Komagataella phaffii Cue5 Piggybacks on Lipid Droplets for Its Vacuolar Degradation during Stationary Phase Lipophagy. Cells 2022, 11, 215. https://doi.org/10.3390/cells11020215
Kumar R, Shroff A, Nazarko TY. Komagataella phaffii Cue5 Piggybacks on Lipid Droplets for Its Vacuolar Degradation during Stationary Phase Lipophagy. Cells. 2022; 11(2):215. https://doi.org/10.3390/cells11020215
Chicago/Turabian StyleKumar, Ravinder, Ankit Shroff, and Taras Y. Nazarko. 2022. "Komagataella phaffii Cue5 Piggybacks on Lipid Droplets for Its Vacuolar Degradation during Stationary Phase Lipophagy" Cells 11, no. 2: 215. https://doi.org/10.3390/cells11020215
APA StyleKumar, R., Shroff, A., & Nazarko, T. Y. (2022). Komagataella phaffii Cue5 Piggybacks on Lipid Droplets for Its Vacuolar Degradation during Stationary Phase Lipophagy. Cells, 11(2), 215. https://doi.org/10.3390/cells11020215








