The Role of the Dynamic Lung Extracellular Matrix Environment on Fibroblast Morphology and Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Fibroblast Cell Culture
2.2. Collagen-I 2D and 3D ECM Environments
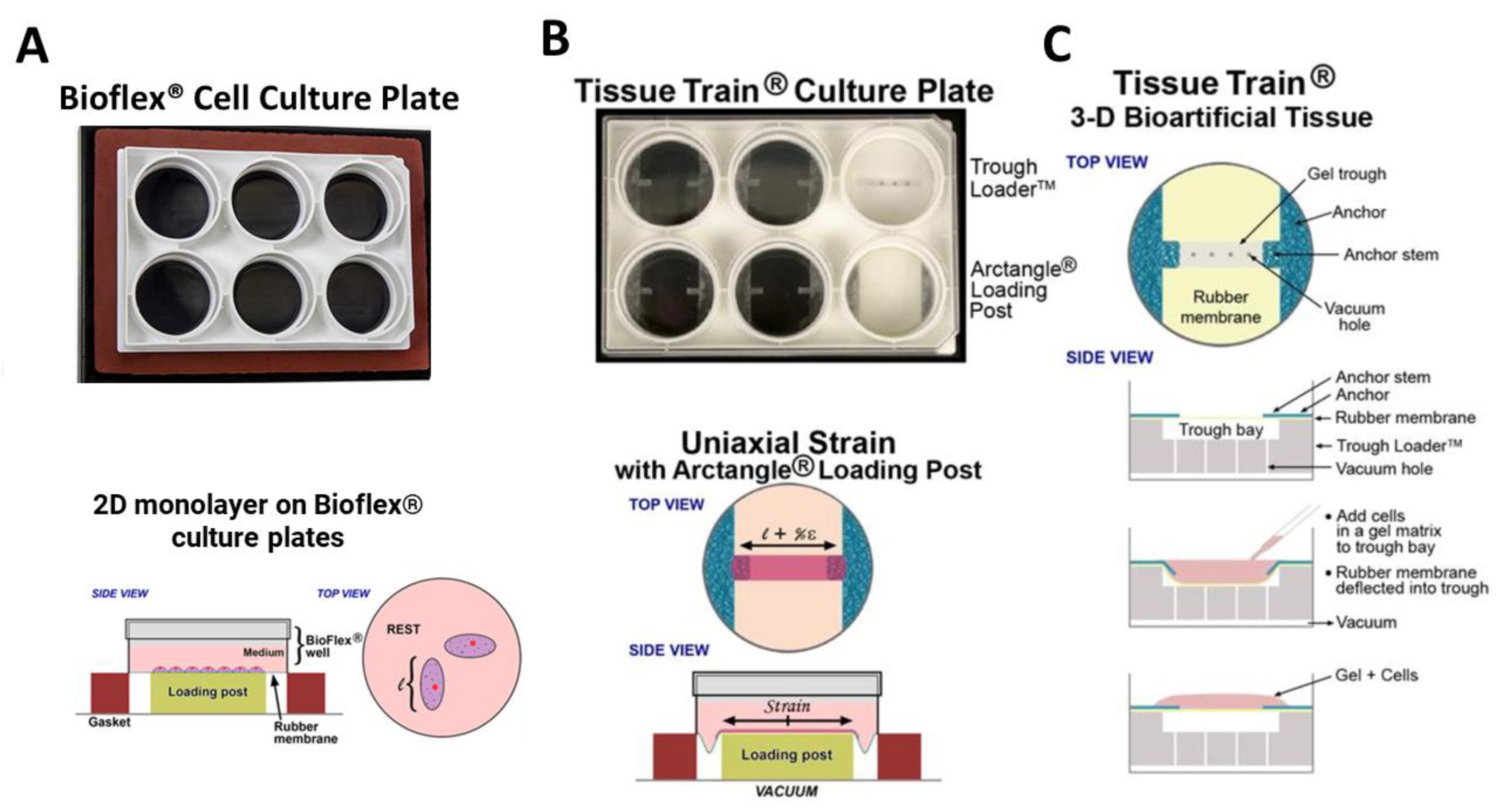
2.3. Mechanical Strain Experiments
2.4. Immunofluorescence Imaging
2.5. ELISA and Lactate Dehydrogenase Assays
2.6. Statistics
3. Results
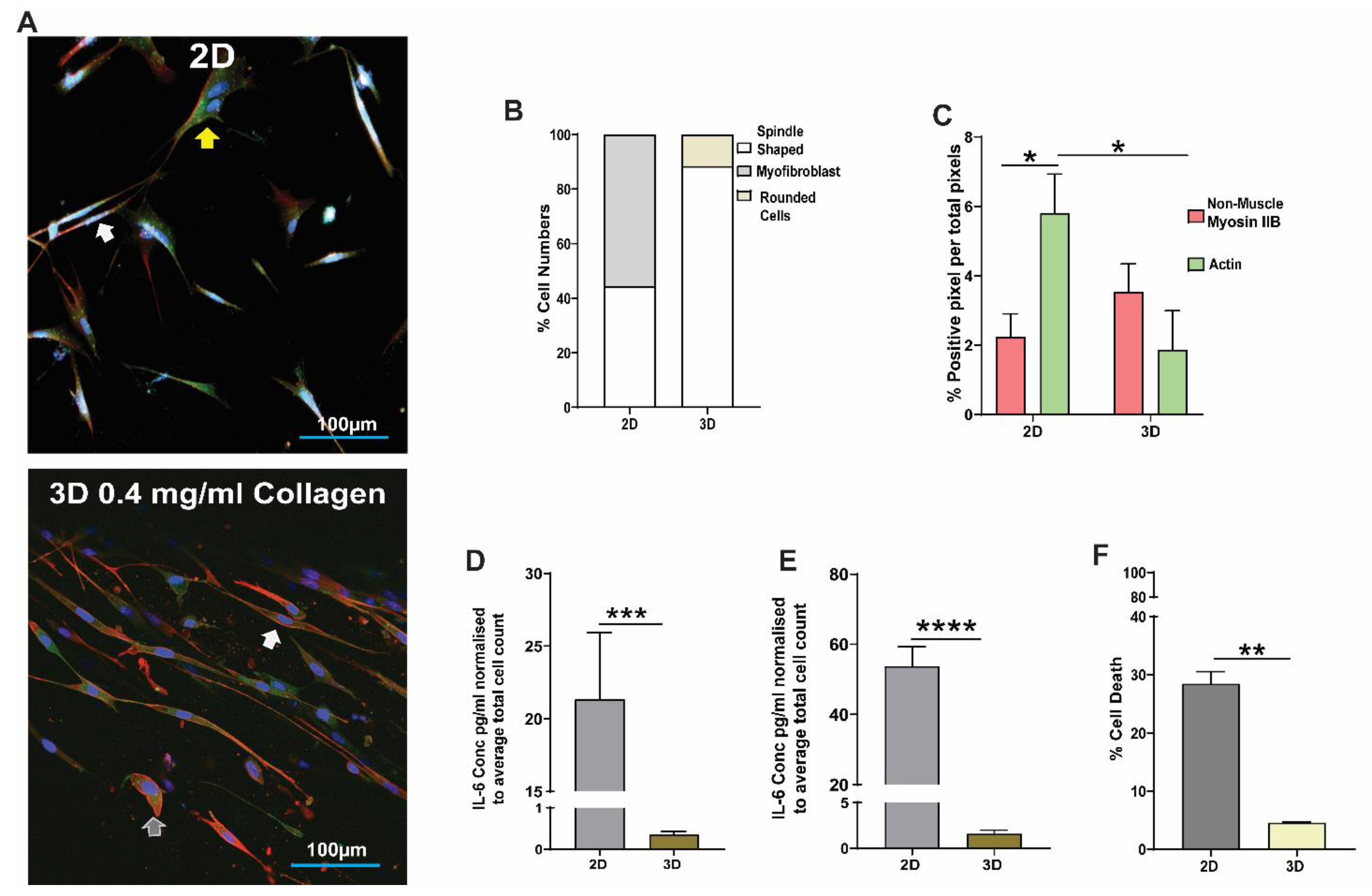
3.1. Effect of 2D and 3D Collagen-I Models on Lung Fibroblast Phenotype and Function
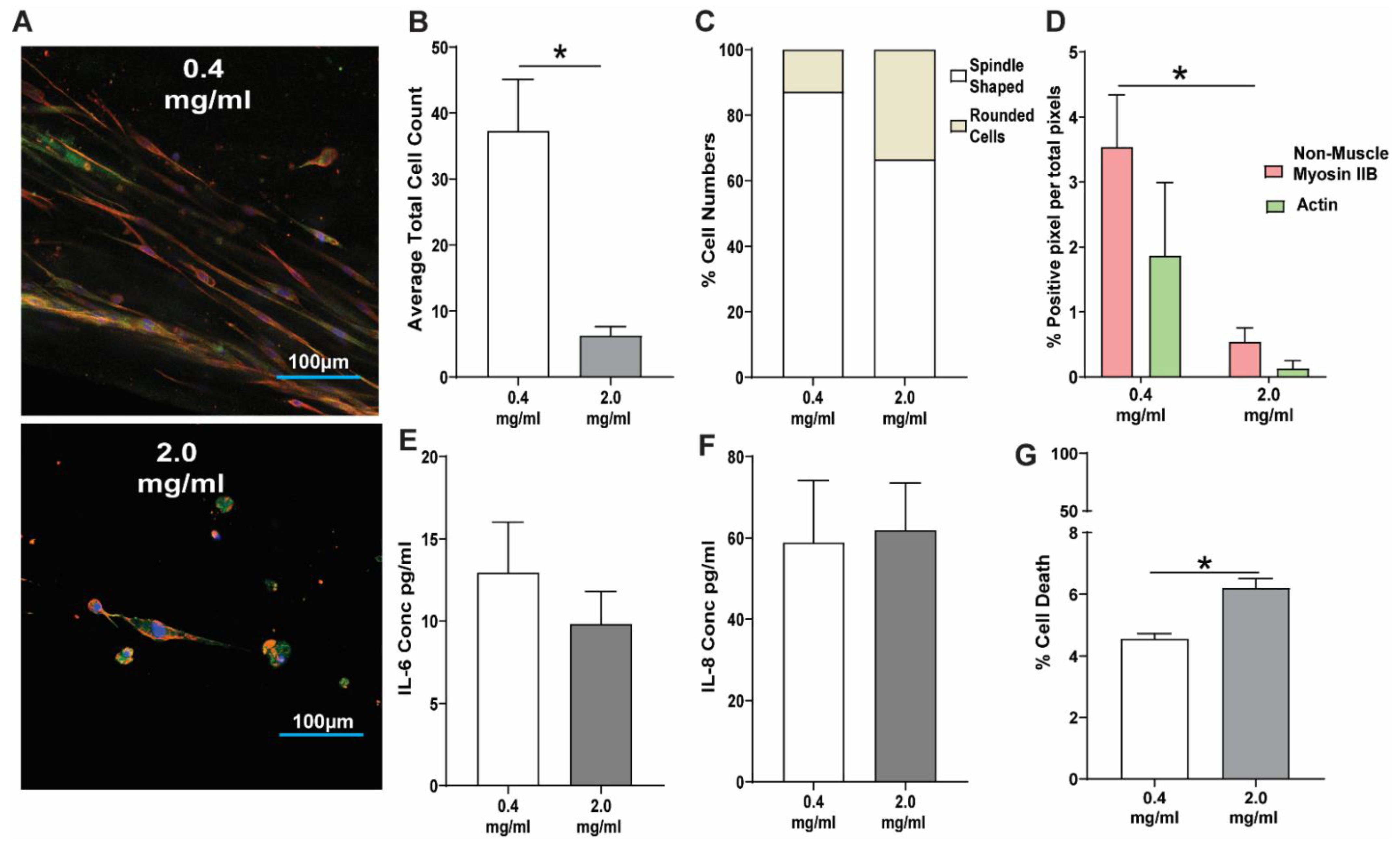
3.2. Response of Lung Fibroblasts to a High Concentration Collagen-I 3D Microenvironment
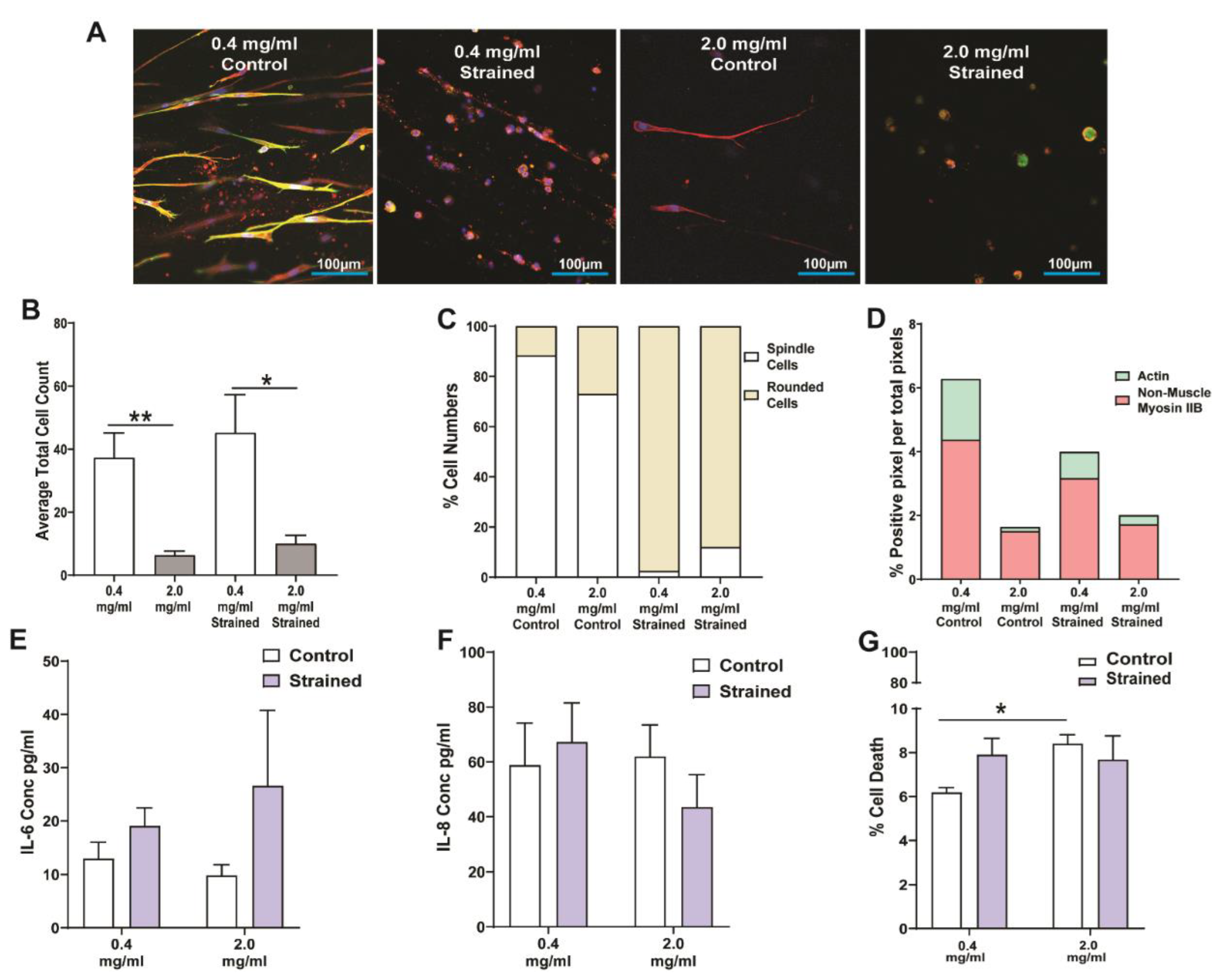
3.3. Response of Lung Fibroblasts to Mechanical Strain within a 3D Collagen-I Microenvironment
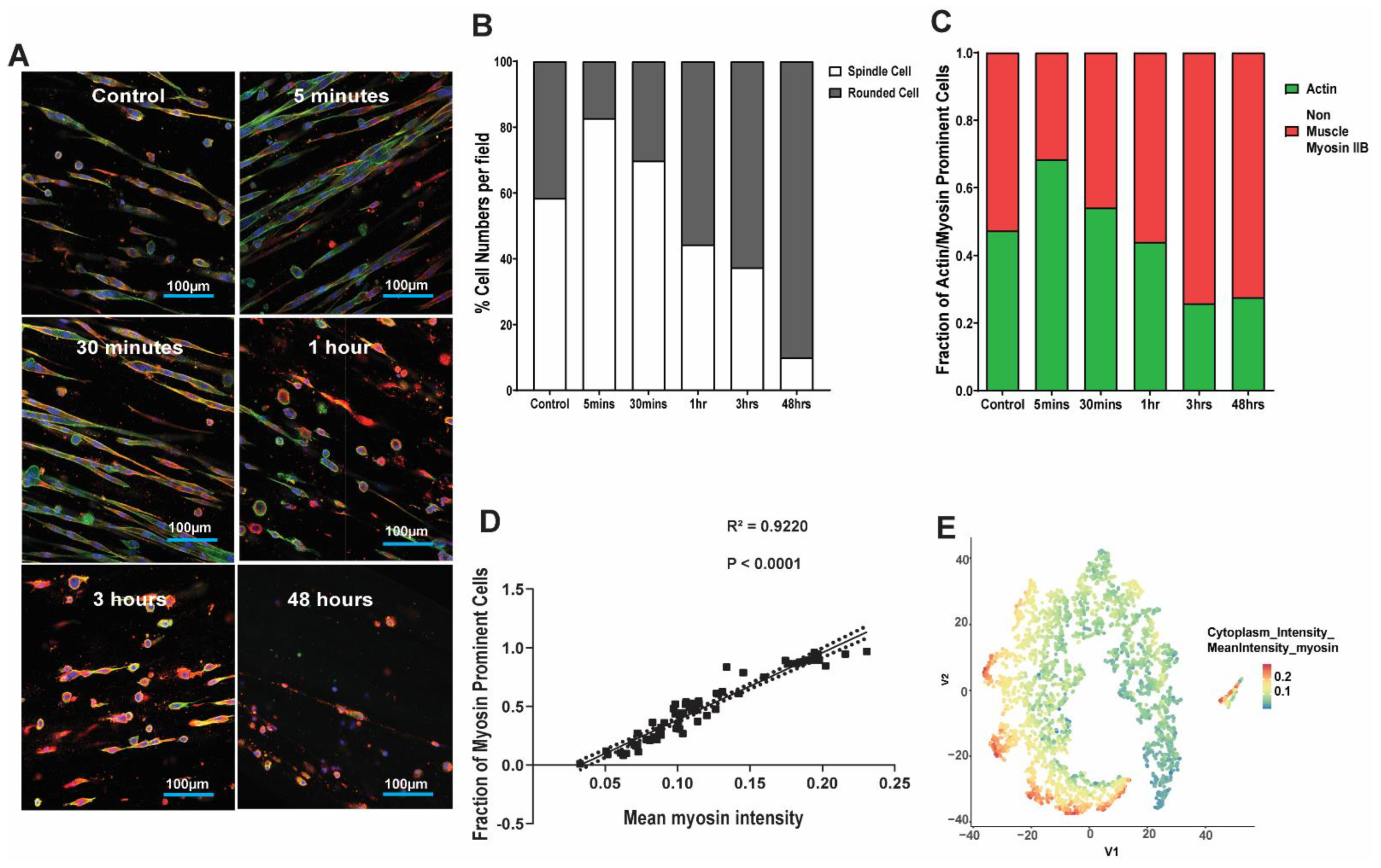
3.4. Kinetics of Lung Fibroblast Cytoskeleton and Morphological Alterations Following Mechanical Strain
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Senior, R.M.; Bielefeld, D.R.; Abensohn, M.K. The effects of proteolytic enzymes on the tensile strength of human lung. Am. Rev. Respir. Dis. 1975, 111, 184–188. [Google Scholar] [PubMed]
- Shifren, A.; Mecham, R.P. The stumbling block in lung repair of emphysema: Elastic fiber assembly. Proc. Am. Thorac. Soc. 2006, 3, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Dunsmore, S.E.; Rannels, D.E. Extracellular matrix biology in the lung. Am. J. Physiol. 1996, 270, L3–L27. [Google Scholar] [CrossRef]
- Yeung, T.; Georges, P.C.; Flanagan, L.A.; Marg, B.; Ortiz, M.; Funaki, M.; Zahir, N.; Ming, W.; Weaver, V.; Janmey, P.A. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskelet. 2005, 60, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Gilpin, S.E.; Li, Q.; Evangelista-Leite, D.; Ren, X.; Reinhardt, D.P.; Frey, B.L.; Ott, H.C. Fibrillin-2 and Tenascin-C bridge the age gap in lung epithelial regeneration. Biomaterials 2017, 140, 212–219. [Google Scholar] [CrossRef]
- Balestrini, J.L.; Niklason, L.E. Extracellular matrix as a driver for lung regeneration. Ann. Biomed. Eng. 2015, 43, 568–576. [Google Scholar] [CrossRef]
- Bottaro, D.P.; Liebmann-Vinson, A.; Heidaran, M.A. Molecular signaling in bioengineered tissue microenvironments. Ann. N. Y. Acad. Sci. 2002, 961, 143–153. [Google Scholar] [CrossRef]
- Brown, B.N.; Badylak, S.F. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl. Res. 2014, 163, 268–285. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Tschumperlin, D.J. Micro-mechanical characterization of lung tissue using atomic force microscopy. J. Vis. Exp. 2011, 54, e2911. [Google Scholar] [CrossRef]
- Suki, B.; Stamenovic, D.; Hubmayr, R. Lung parenchymal mechanics. Compr. Physiol. 2011, 1, 1317–1351. [Google Scholar]
- Fung, Y.C. Mechanical Properties of Living Tissues 2; Springer: New York, NY, USA, 1981; 433p. [Google Scholar]
- Luque, T.; Melo, E.; Garreta, E.; Cortiella, J.; Nichols, J.; Farre, R.; Navajas, D. Local micromechanical properties of decellularized lung scaffolds measured with atomic force microscopy. Acta Biomater. 2013, 9, 6852–6859. [Google Scholar] [CrossRef] [PubMed]
- Hackett, T.L.; Osei, E.T. Modeling Extracellular Matrix-Cell Interactions in Lung Repair and Chronic Disease. Cells 2021, 10, 2145. [Google Scholar] [CrossRef]
- Kendall, R.T.; Feghali-Bostwick, C.A. Fibroblasts in fibrosis: Novel roles and mediators. Front. Pharmacol. 2014, 5, 123. [Google Scholar] [CrossRef]
- Burgess, J.K.; Mauad, T.; Tjin, G.; Karlsson, J.C.; Westergren-Thorsson, G. The extracellular matrix—The under-recognized element in lung disease? J. Pathol. 2016, 240, 397–409. [Google Scholar] [CrossRef]
- Bartemes, K.R.; Kita, H. Dynamic role of epithelium-derived cytokines in asthma. Clin. Immunol. 2012, 143, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Jordana, M.; Sarnstrand, B.; Sime, P.J.; Ramis, I. Immune-inflammatory functions of fibroblasts. Eur. Respir. J. 1994, 7, 2212–2222. [Google Scholar] [CrossRef]
- Meyer-Hoffert, U.; Lezcano-Meza, D.; Bartels, J.; Montes-Vizuet, A.R.; Schroder, J.M.; Teran, L.M. Th2- and to a lesser extent Th1-type cytokines upregulate the production of both CXC (IL-8 and gro-alpha) and CC (RANTES, eotaxin, eotaxin-2, MCP-3 and MCP-4) chemokines in human airway epithelial cells. Int. Arch. Allergy Immunol. 2003, 131, 264–271. [Google Scholar] [CrossRef]
- Tliba, O.; Amrani, Y.; Panettieri, R.A., Jr. Is airway smooth muscle the “missing link” modulating airway inflammation in asthma? Chest 2008, 133, 236–242. [Google Scholar] [CrossRef]
- Bell, E.; Ivarsson, B.; Merrill, C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc. Natl. Acad. Sci. USA 1979, 76, 1274–1278. [Google Scholar] [CrossRef] [PubMed]
- Elsdale, T.; Bard, J. Collagen substrata for studies on cell behavior. J. Cell Biol. 1972, 54, 626–637. [Google Scholar] [CrossRef]
- Mostaco-Guidolin, L.B.; Osei, E.T.; Ullah, J.; Hajimohammadi, S.; Fouadi, M.; Li, X.; Li, V.; Shaheen, F.; Yang, C.X.; Chu, F.; et al. Defective Fibrillar Collagen Organization by Fibroblasts Contributes to Airway Remodeling in Asthma. Am. J. Respir. Crit. Care Med. 2019, 200, 431–443. [Google Scholar] [CrossRef]
- Suki, B.; Bates, J.H. Extracellular matrix mechanics in lung parenchymal diseases. Respir. Physiol. Neurobiol. 2008, 163, 33–43. [Google Scholar] [CrossRef]
- Garvin, J.; Qi, J.; Maloney, M.; Banes, A.J. Novel system for engineering bioartificial tendons and application of mechanical load. Tissue Eng. 2003, 9, 967–979. [Google Scholar] [CrossRef]
- Zhu, Y.K.; Umino, T.; Liu, X.D.; Wang, H.J.; Romberger, D.J.; Spurzem, J.R.; Rennard, S.I. Contraction of fibroblast-containing collagen gels: Initial collagen concentration regulates the degree of contraction and cell survival. In Vitro Cell. Dev. Biol. Anim. 2001, 37, 10–16. [Google Scholar] [CrossRef]
- Van Spreeuwel, A.C.C.; Bax, N.A.M.; van Nierop, B.J.; Aartsma-Rus, A.; Goumans, M.T.H.; Bouten, C.V.C. Mimicking Cardiac Fibrosis in a Dish: Fibroblast Density Rather than Collagen Density Weakens Cardiomyocyte Function. J. Cardiovasc. Transl. Res. 2017, 10, 116–127. [Google Scholar] [CrossRef][Green Version]
- Copland, I.B.; Reynaud, D.; Pace-Asciak, C.; Post, M. Mechanotransduction of stretch-induced prostanoid release by fetal lung epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 291, L487–L495. [Google Scholar] [CrossRef]
- Abercrombie, M.F.M.; James, D.W. Wound contraction in relation to collagen formation in scorbutic guinea-pigs. J. Embryol. Exp. Morphol. 1956, 4, 167–175. [Google Scholar] [CrossRef]
- Gabbiani, G.; Ryan, G.B.; Majne, G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia 1971, 27, 549–550. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.K.; Stopak, D.; Wild, P. Fibroblast traction as a mechanism for collagen morphogenesis. Nature 1981, 290, 249–251. [Google Scholar] [CrossRef]
- Chiou, Y.W.; Lin, H.K.; Tang, M.J.; Lin, H.H.; Yeh, M.L. The influence of physical and physiological cues on atomic force microscopy-based cell stiffness assessment. PLoS ONE 2013, 8, e77384. [Google Scholar] [CrossRef] [PubMed]
- Grinnell, F. Fibroblasts, myofibroblasts, and wound contraction. J. Cell Biol. 1994, 124, 401–404. [Google Scholar] [CrossRef]
- Desmouliere, A.; Redard, M.; Darby, I.; Gabbiani, G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am. J. Pathol. 1995, 146, 56–66. [Google Scholar]
- Plant, A.L.; Bhadriraju, K.; Spurlin, T.A.; Elliott, J.T. Cell response to matrix mechanics: Focus on collagen. Biochim. Biophys. Acta 2009, 1793, 893–902. [Google Scholar] [CrossRef]
- Grace, L.H.Y.; Wah, T.Y. Effect of Collagen gel structure on fibroblast phenotype. J. Emerg. Investig. 2012, 1–9. [Google Scholar]
- Hakkinen, K.M.; Harunaga, J.S.; Doyle, A.D.; Yamada, K.M. Direct comparisons of the morphology, migration, cell adhesions, and actin cytoskeleton of fibroblasts in four different three-dimensional extracellular matrices. Tissue Eng. Part A 2011, 17, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Htwe, S.S.; Harrington, H.; Knox, A.; Rose, F.; Aylott, J.; Haycock, J.W.; Ghaemmaghami, A.M. Investigating NF-kappaB signaling in lung fibroblasts in 2D and 3D culture systems. Respir. Res. 2015, 16, 144. [Google Scholar] [CrossRef] [PubMed]
- Manuyakorn, W.; Smart, D.E.; Noto, A.; Bucchieri, F.; Haitchi, H.M.; Holgate, S.T.; Howarth, P.H.; Davies, D.E. Mechanical Strain Causes Adaptive Change in Bronchial Fibroblasts Enhancing Profibrotic and Inflammatory Responses. PLoS ONE 2016, 11, e0153926. [Google Scholar] [CrossRef] [PubMed]
- Grymes, R.A.; Sawyer, C. A novel culture morphology resulting from applied mechanical strain. In Vitro Cell. Dev. Biol. Anim. 1997, 33, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Wilgus, T.A. Growth Factor-Extracellular Matrix Interactions Regulate Wound Repair. Adv. Wound Care 2012, 1, 249–254. [Google Scholar] [CrossRef]
- Booth, A.J.; Hadley, R.; Cornett, A.M.; Dreffs, A.A.; Matthes, S.A.; Tsui, J.L.; Weiss, K.; Horowitz, J.C.; Fiore, V.F.; Barker, T.H.; et al. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am. J. Respir. Crit. Care Med. 2012, 186, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Dabaghi, M.; Saraei, N.; Carpio, M.B.; Nanduri, V.; Ungureanu, J.; Babi, M.; Chandiramohan, A.; Noble, A.; Revill, S.D.; Zhang, B.; et al. A Robust Protocol for Decellularized Human Lung Bioink Generation Amenable to 2D and 3D Lung Cell Culture. Cells 2021, 10, 1538. [Google Scholar] [CrossRef]
| Reagent | Volume (µL) Ratios for 6-Well Tissue Train® (200 µL/well) |
|---|---|
| A (5× MEM) | 288 µL |
| B (Fetal Bovine Serum) | 144 µL |
| C (1M Hepes) | 36 µL |
| Collagel® (Type 1 Collagen, 3 mg/mL in 0.01 MHCl) | 1260 µL |
| D (0.1 M NaOH in 5× MEM) | 72 µL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hackett, T.-L.; Vriesde, N.R.T.F.; AL-Fouadi, M.; Mostaco-Guidolin, L.; Maftoun, D.; Hsieh, A.; Coxson, N.; Usman, K.; Sin, D.D.; Booth, S.; et al. The Role of the Dynamic Lung Extracellular Matrix Environment on Fibroblast Morphology and Inflammation. Cells 2022, 11, 185. https://doi.org/10.3390/cells11020185
Hackett T-L, Vriesde NRTF, AL-Fouadi M, Mostaco-Guidolin L, Maftoun D, Hsieh A, Coxson N, Usman K, Sin DD, Booth S, et al. The Role of the Dynamic Lung Extracellular Matrix Environment on Fibroblast Morphology and Inflammation. Cells. 2022; 11(2):185. https://doi.org/10.3390/cells11020185
Chicago/Turabian StyleHackett, Tillie-Louise, Noamie R. T. F. Vriesde, May AL-Fouadi, Leila Mostaco-Guidolin, Delaram Maftoun, Aileen Hsieh, Nicole Coxson, Kauna Usman, Don D. Sin, Steve Booth, and et al. 2022. "The Role of the Dynamic Lung Extracellular Matrix Environment on Fibroblast Morphology and Inflammation" Cells 11, no. 2: 185. https://doi.org/10.3390/cells11020185
APA StyleHackett, T.-L., Vriesde, N. R. T. F., AL-Fouadi, M., Mostaco-Guidolin, L., Maftoun, D., Hsieh, A., Coxson, N., Usman, K., Sin, D. D., Booth, S., & Osei, E. T. (2022). The Role of the Dynamic Lung Extracellular Matrix Environment on Fibroblast Morphology and Inflammation. Cells, 11(2), 185. https://doi.org/10.3390/cells11020185






