Antagonism of CGRP Receptor: Central and Peripheral Mechanisms and Mediators in an Animal Model of Chronic Migraine
Abstract
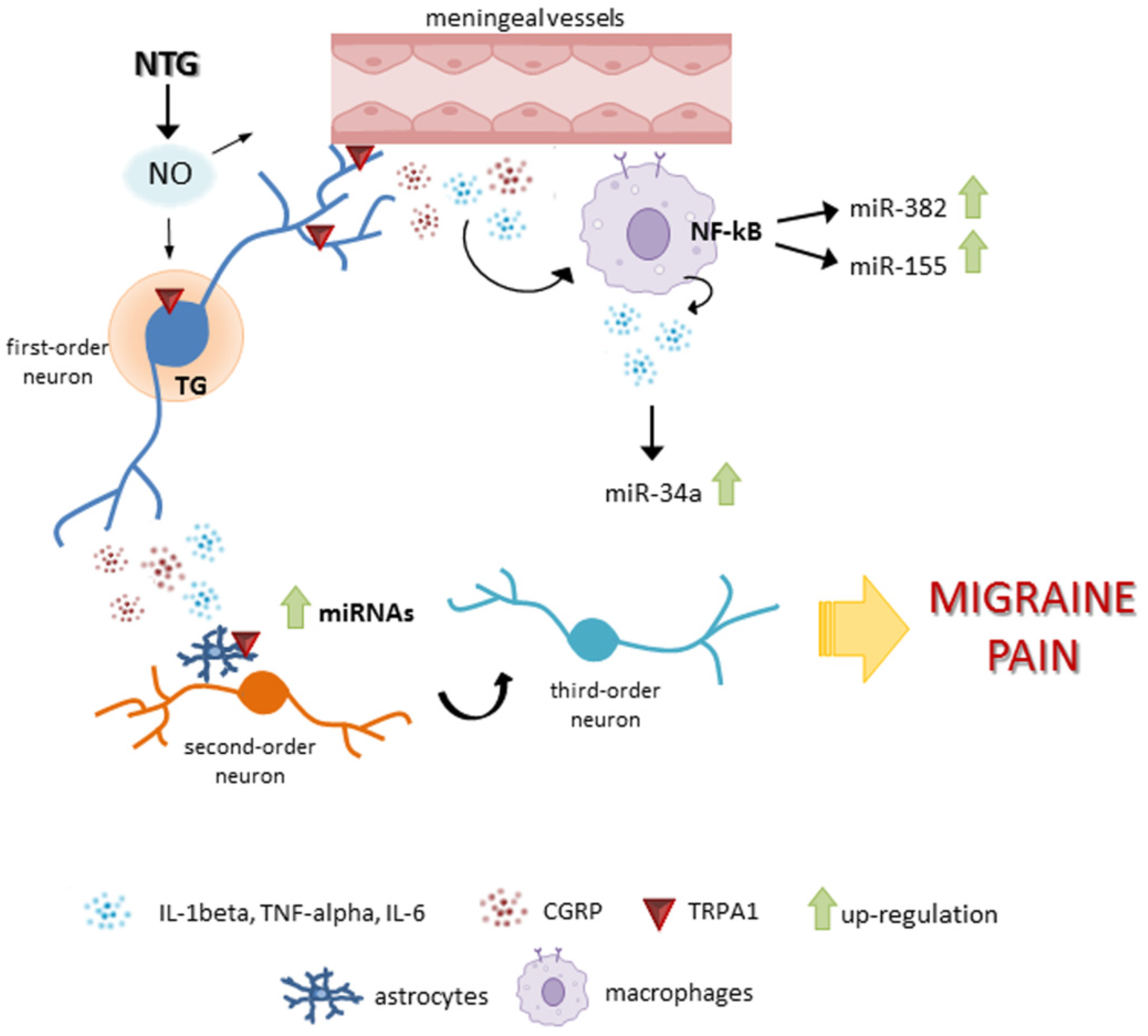
1. Introduction
2. Materials and Methods
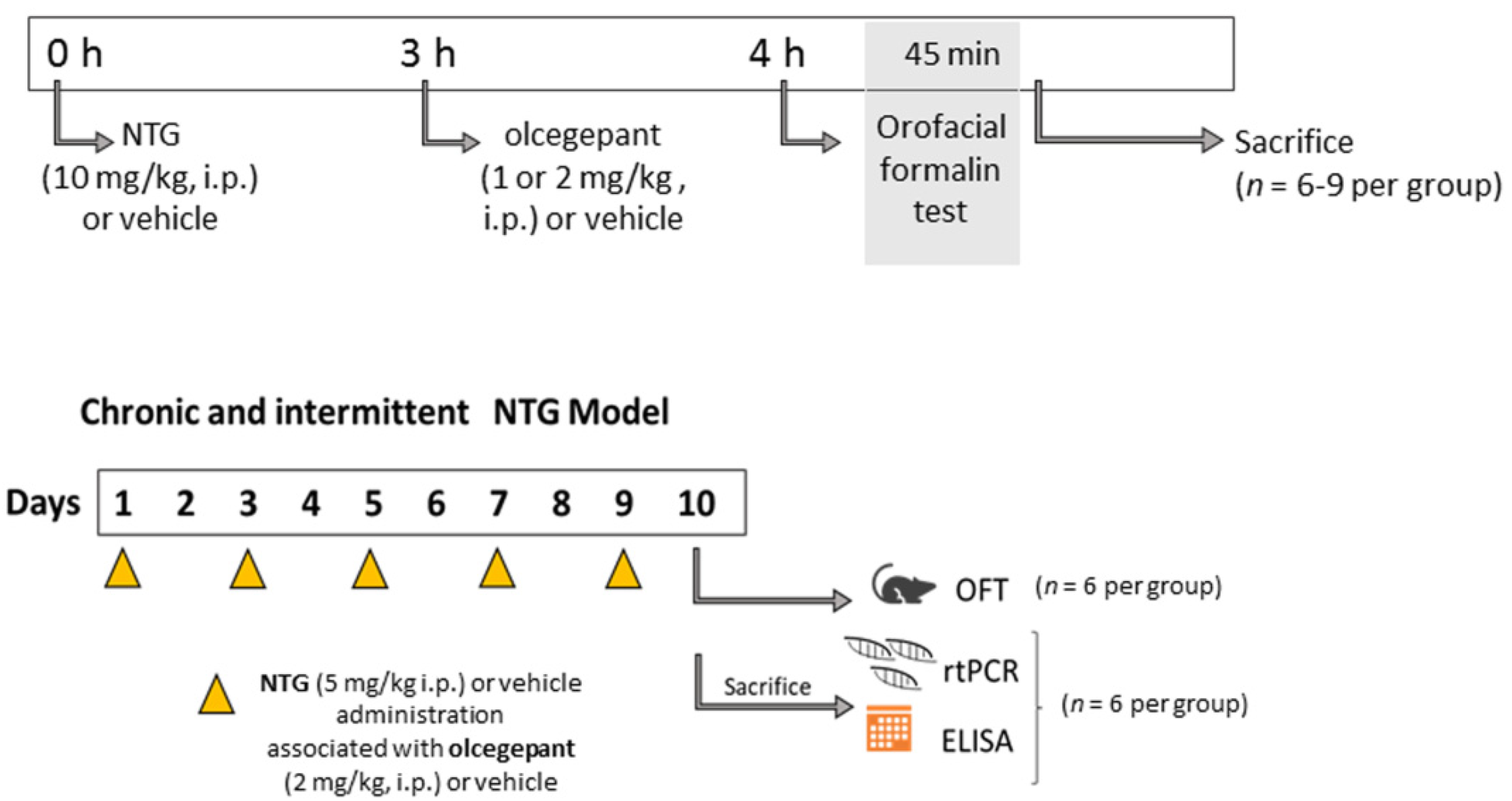
2.1. Animals and Experimental Design
2.2. Orofacial Formalin Test
2.3. Rt-PCR and Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Statistical Analysis
3. Results
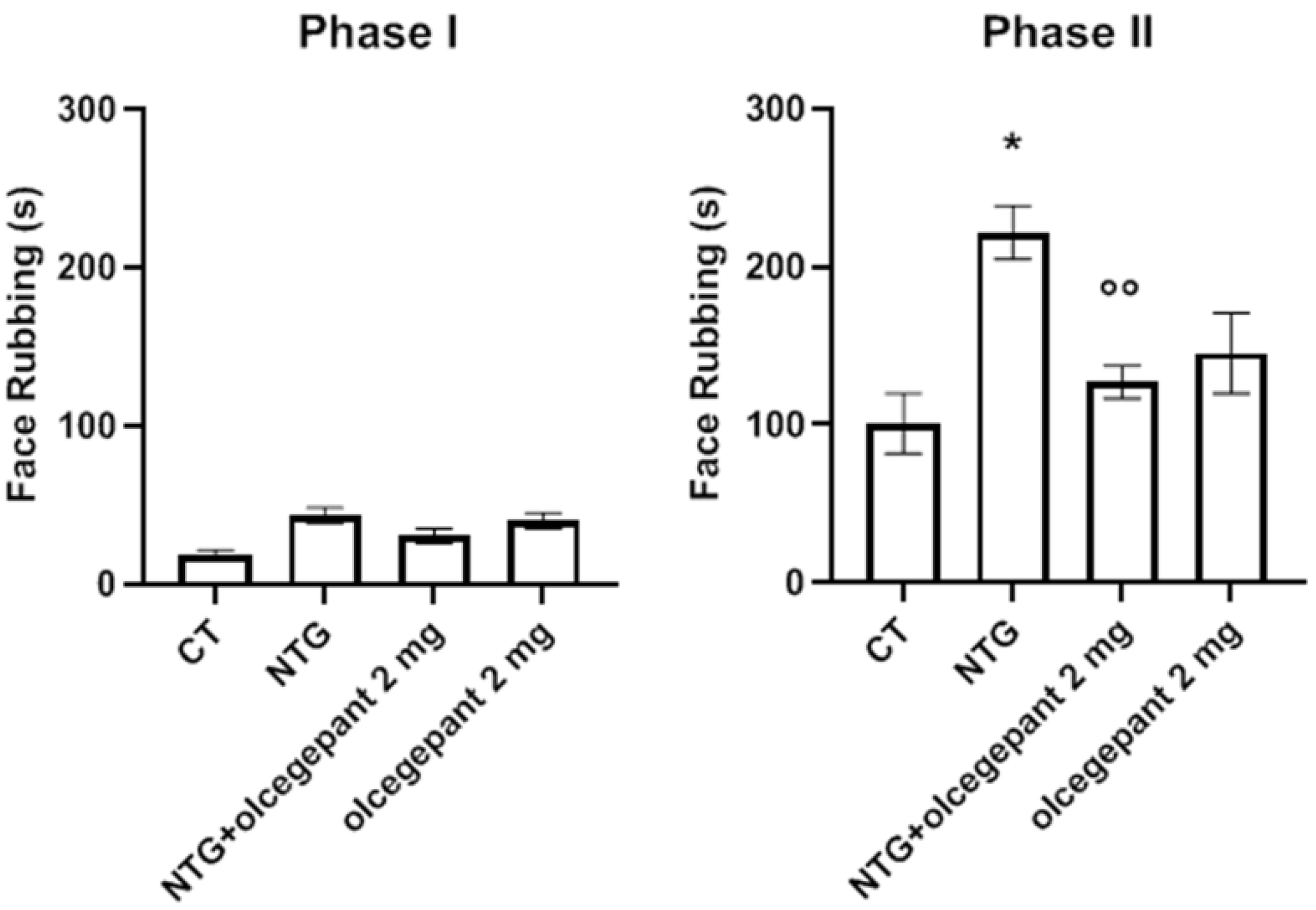
3.1. Acute Migraine Model
Orofacial Formalin Test
3.2. Chronic Migraine Model
3.2.1. Orofacial Formalin Test
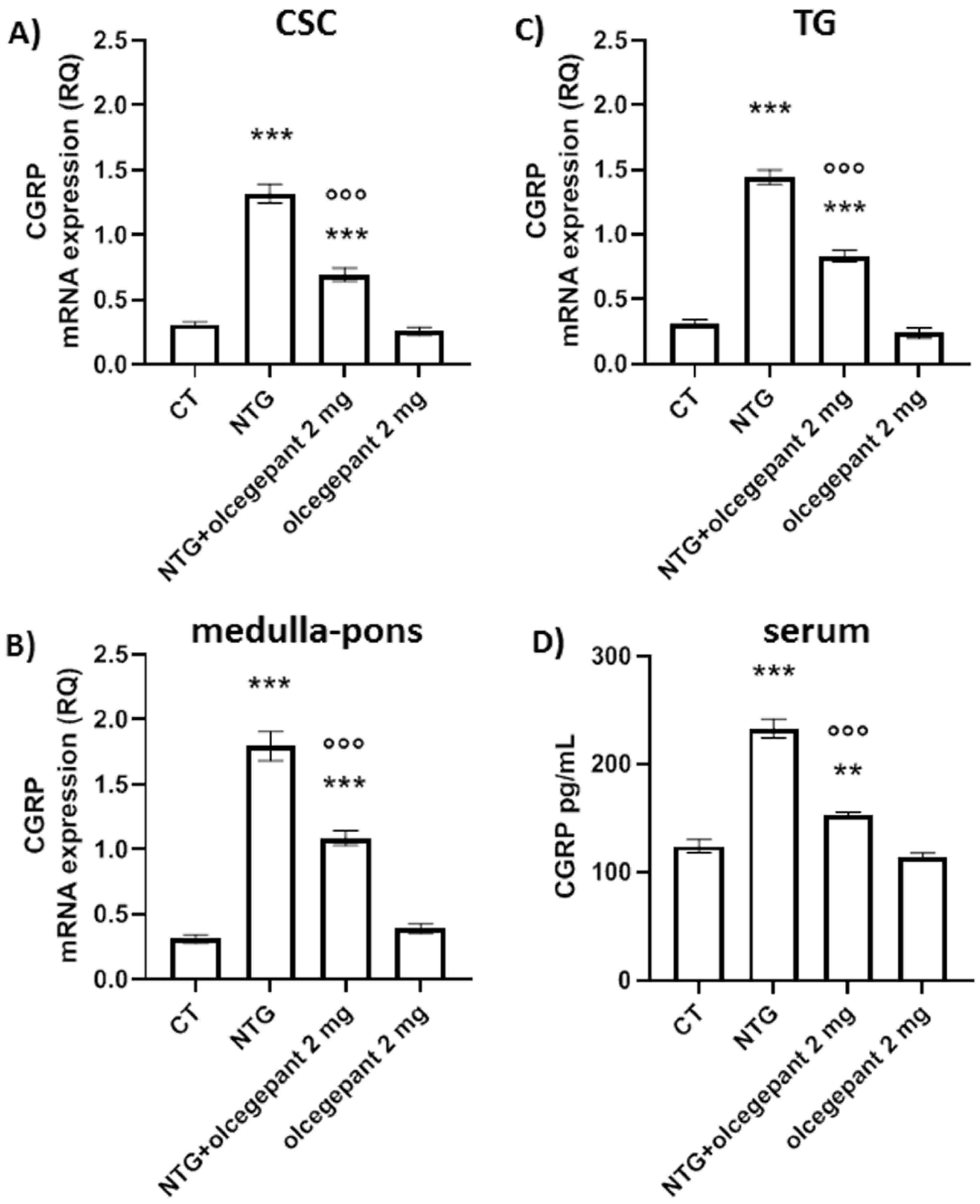
3.2.2. CGRP
3.2.3. Cytokines
3.2.4. microRNAs
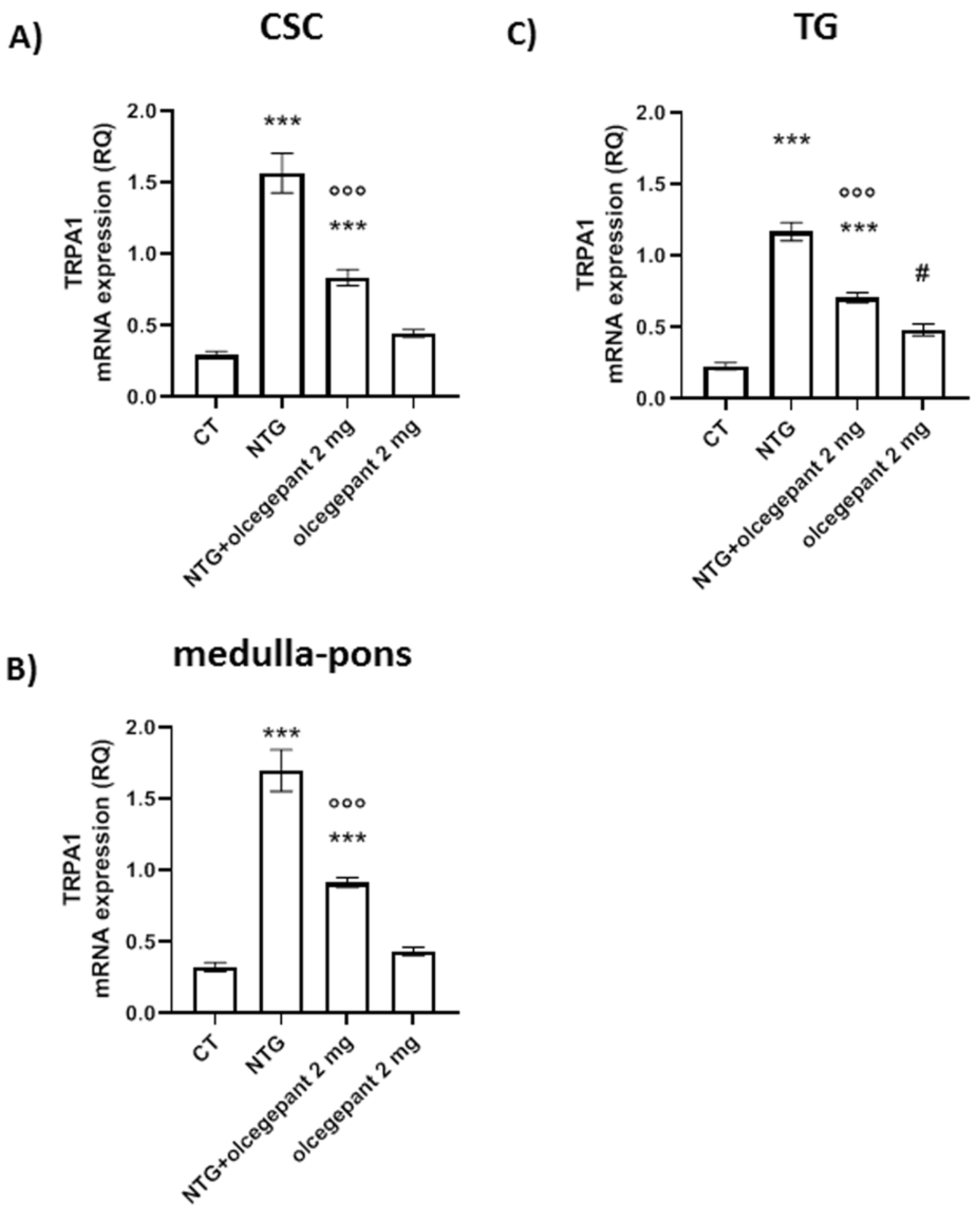
3.2.5. TRPA1 Gene Expression
4. Discussion
Possible Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Goadsby, P.J.; Holland, P.R.; Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol. Rev. 2017, 97, 553–622. [Google Scholar] [CrossRef]
- Edvinsson, L. CGRP and migraine: From bench to bedside. Rev. Neurol. 2021, 177, 785–790. [Google Scholar] [CrossRef]
- Mulderry, P.K.; Ghatei, M.A.; Spokes, R.A.; Jones, P.M.; Pierson, A.M.; Hamid, Q.A.; Kanse, S.; Amara, S.G.; Burrin, J.M.; Legon, S.; et al. Differential expression of alpha-CGRP and beta-CGRP by primary sensory neurons and enteric autonomic neurons of the rat. Neuroscience 1988, 25, 195–205. [Google Scholar] [CrossRef]
- Iyengar, S.; Johnson, K.W.; Ossipov, M.H.; Aurora, S.K. CGRP and the Trigeminal System in Migraine. Headache 2019, 59, 659–681. [Google Scholar] [CrossRef]
- Lennerz, J.K.; Rühle, V.; Ceppa, E.P.; Neuhuber, W.L.; Bunnett, N.W.; Grady, E.F.; Messlinger, K. Calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and calcitonin gene-related peptide (CGRP) immunoreactivity in the rat trigeminovascular system: Differences between peripheral and central CGRP receptor distribution. J. Comp. Neurol. 2008, 507, 1277–1299. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Donelan, J.; Kandere-Grzybowska, K.; Konstantinidou, A. The role of mast cells in migraine pathophysiology. Brain Res. Brain Res. Rev. 2005, 49, 65–76. [Google Scholar] [CrossRef]
- Afroz, S.; Arakaki, R.; Iwasa, T.; Oshima, M.; Hosoki, M.; Inoue, M.; Baba, O.; Okayama, Y.; Matsuka, Y. CGRP Induces Differential Regulation of Cytokines from Satellite Glial Cells in Trigeminal Ganglia and Orofacial Nociception. Int. J. Mol. Sci. 2019, 20, 711. [Google Scholar] [CrossRef]
- Cuesta, M.C.; Quintero, L.; Pons, H.; Suarez-Roca, H. Substance P and calcitonin gene-related peptide increase IL-1 beta, IL-6 and TNF alpha secretion from human peripheral blood mononuclear cells. Neurochem. Int. 2002, 40, 301–306. [Google Scholar] [CrossRef]
- Souza, G.R.; Talbot, J.; Lotufo, C.M.; Cunha, F.Q.; Cunha, T.M.; Ferreira, S.H. Fractalkine mediates inflammatory pain through activation of satellite glial cells. Proc. Natl. Acad. Sci. USA 2013, 110, 11193–11198. [Google Scholar] [CrossRef]
- Diogenes, A.; Akopian, A.N.; Hargreaves, K.M. NGF up-regulates TRPA1: Implications for orofacial pain. J. Dent. Res. 2007, 86, 550–555. [Google Scholar] [CrossRef]
- Hatano, N.; Itoh, Y.; Suzuki, H.; Muraki, Y.; Hayashi, H.; Onozaki, K.; Wood, I.C.; Beech, D.J.; Muraki, K. Hypoxia-inducible factor-1α (HIF1α) switches on transient receptor potential ankyrin repeat 1 (TRPA1) gene expression via a hypoxia response element-like motif to modulate cytokine release. J. Biol. Chem. 2012, 287, 31962–31972. [Google Scholar] [CrossRef]
- Eberhardt, M.; Dux, M.; Namer, B.; Miljkovic, J.; Cordasic, N.; Will, C.; Kichko, T.I.; de la Roche, J.; Fischer, M.; Suárez, S.A.; et al. H2S and NO cooperatively regulate vascular tone by activating a neuroendocrine HNO-TRPA1-CGRP signalling pathway. Nat. Commun. 2014, 5, 4381. [Google Scholar] [CrossRef]
- Kunkler, P.E.; Ballard, C.J.; Oxford, G.S.; Hurley, J.H. TRPA1 receptors mediate environmental irritant-induced meningeal vasodilatation. Pain 2011, 152, 38–44. [Google Scholar] [CrossRef]
- Edvinsson, L.; Haanes, K.A.; Warfvinge, K. Does inflammation have a role in migraine? Nat. Rev. Neurol. 2019, 15, 483–490. [Google Scholar] [CrossRef]
- Buzzi, M.G.; Pellegrino, M.G.; Bellantonio, P. Causes and mechanisms of primary headaches: Toward a bio-behavioral model. Ital. J. Neurol. Sci. 1995, 16, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Reuter, U.; Bolay, H.; Jansen-Olesen, I.; Chiarugi, A.; Sanchez del Rio, M.; Letourneau, R.; Theoharides, T.C.; Waeber, C.; Moskowitz, M.A. Delayed inflammation in rat meninges: Implications for migraine pathophysiology. Brain 2001, 124 Pt 12, 2490–2502. [Google Scholar] [CrossRef]
- Bolay, H.; Reuter, U.; Dunn, A.K.; Huang, Z.; Boas, D.A.; Moskowitz, M.A. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat. Med. 2002, 8, 136–142. [Google Scholar] [CrossRef]
- Parsons, A.A.; Strijbos, P.J. The neuronal versus vascular hypothesis of migraine and cortical spreading depression. Curr. Opin. Pharmacol. 2003, 3, 73–77. [Google Scholar] [CrossRef]
- Hong, P.; Tan, T.; Liu, Y.; Xiao, J. Gepants for abortive treatment of migraine: A network meta-analysis. Brain Behav. 2020, 10, e01701. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.C.; Schwedt, T.J. Calcitonin gene-related peptide (CGRP)-targeted therapies as preventive and acute treatments for migraine-The monoclonal antibodies and gepants. Prog. Brain Res. 2020, 255, 143–170. [Google Scholar] [CrossRef]
- Vandervorst, F.; Van Deun, L.; Van Dycke, A.; Paemeleire, K.; Reuter, U.; Schoenen, J.; Versijpt, J. CGRP monoclonal antibodies in migraine: An efficacy and tolerability comparison with standard prophylactic drugs. J. Headache Pain 2021, 22, 128. [Google Scholar] [CrossRef] [PubMed]
- Cavestro, C.; Ferrero, M.; Mandrino, S.; Di Tavi, M.; Rota, E. Novelty in Inflammation and Immunomodulation in Migraine. Curr. Pharm. Des. 2019, 25, 2919–2936. [Google Scholar] [CrossRef]
- Biscetti, L.; De Vanna, G.; Cresta, E.; Bellotti, A.; Corbelli, I.; Cupini, M.L.; Calabresi, P.; Sarchielli, P. Immunological findings in patients with migraine and other primary headaches: A narrative review. Clin. Exp. Immunol. 2022, 207, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Sayed, D.; Abdellatif, M. MicroRNAs in development and disease. Physiol. Rev. 2011, 91, 827–887. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, L.; Demartini, C.; Corrado, M.; Vaghi, G.; Piella, E.M.; Allena, M.; Zanaboni, A.M.; Greco, R.; Tassorelli, C.; De Icco, R. Expression of Selected microRNAs in Migraine: A New Class of Possible Biomarkers of Disease? Processes 2021, 9, 2199. [Google Scholar] [CrossRef]
- Greco, R.; De Icco, R.; Demartini, C.; Zanaboni, A.M.; Tumelero, E.; Sances, G.; Allena, M.; Tassorelli, C. Plasma levels of CGRP and expression of specific microRNAs in blood cells of episodic and chronic migraine subjects: Towards the identification of a panel of peripheral biomarkers of migraine? J. Headache Pain 2020, 21, 122. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Yang, L.; Zheng, Z.; Li, Y.; Ren, W.; Wu, C.; Guo, L. Calcitonin gene-related peptide induces IL-6 expression in RAW264.7 macrophages mediated by mmu_circRNA_007893. Mol. Med. Rep. 2017, 16, 9367–9374. [Google Scholar] [CrossRef]
- Greco, R.; Ferrigno, A.; Demartini, C.; Zanaboni, A.; Mangione, A.S.; Blandini, F.; Nappi, G.; Vairetti, M.; Tassorelli, C. Evaluation of ADMA-DDAH-NOS axis in specific brain areas following nitroglycerin administration: Study in an animal model of migraine. J. Headache Pain 2015, 16, 560. [Google Scholar] [CrossRef]
- Greco, R.; Demartini, C.; Zanaboni, A.; Casini, I.; De Icco, R.; Reggiani, A.; Misto, A.; Piomelli, D.; Tassorelli, C. Characterization of the peripheral FAAH inhibitor, URB937, in animal models of acute and chronic migraine. Neurobiol. Dis. 2021, 147, 105157. [Google Scholar] [CrossRef]
- Harriott, A.M.; Strother, L.C.; Vila-Pueyo, M.; Holland, P.R. Animal models of migraine and experimental techniques used to examine trigeminal sensory processing. J. Headache Pain 2019, 20, 91. [Google Scholar] [CrossRef]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Ramachandran, R.; Bhatt, D.K.; Ploug, K.B.; Hay-Schmidt, A.; Jansen-Olesen, I.; Gupta, S.; Olesen, J. Nitric oxide synthase, calcitonin gene-related peptide and NK-1 receptor mechanisms are involved in GTN-induced neuronal activation. Cephalalgia 2014, 34, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Munro, G.; Petersen, S.; Jansen-Olesen, I.; Olesen, J. A unique inbred rat strain with sustained cephalic hypersensitivity as a model of chronic migraine-like pain. Sci. Rep. 2018, 8, 1836. [Google Scholar] [CrossRef] [PubMed]
- Akerman, S.; Romero-Reyes, M.; Karsan, N.; Bose, P.; Hoffmann, J.R.; Holland, P.R.; Goadsby, P.J. Therapeutic targeting of nitroglycerin-mediated trigeminovascular neuronal hypersensitivity predicts clinical outcomes of migraine abortives. Pain 2021, 162, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
- Greco, R.; Demartini, C.; Zanaboni, A.M.; Tumelero, E.; Reggiani, A.; Misto, A.; Piomelli, D.; Tassorelli, C. FAAH inhibition as a preventive treatment for migraine: A pre-clinical study. Neurobiol. Dis. 2020, 134, 104624. [Google Scholar] [CrossRef] [PubMed]
- Tajti, J.; Uddman, R.; Edvinsson, L. Neuropeptide localization in the “migraine generator” region of the human brainstem. Cephalalgia 2001, 21, 96–101. [Google Scholar] [CrossRef]
- Vila-Pueyo, M.; Strother, L.C.; Kefel, M.; Goadsby, P.J.; Holland, P.R. Divergent influences of the locus coeruleus on migraine pathophysiology. Pain 2019, 160, 385–394. [Google Scholar] [CrossRef]
- Greco, R.; Demartini, C.; Zanaboni, A.M.; Redavide, E.; Pampalone, S.; Toldi, J.; Fülöp, F.; Blandini, F.; Nappi, G.; Sandrini, G.; et al. Effects of kynurenic acid analogue 1 (KYNA-A1) in nitroglycerin-induced hyperalgesia: Targets and anti-migraine mechanisms. Cephalalgia 2017, 37, 1272–1284. [Google Scholar] [CrossRef]
- Chen, H.; Tang, X.; Li, J.; Hu, B.; Yang, W.; Zhan, M.; Ma, T.; Xu, S. IL-17 crosses the blood-brain barrier to trigger neuroinflammation: A novel mechanism in nitroglycerin-induced chronic migraine. J. Headache Pain 2022, 23, 1. [Google Scholar] [CrossRef]
- Yao, G.; Han, X.; Hao, T.; Huang, Q.; Yu, T. Effects of rizatriptan on the expression of calcitonin gene-related peptide and cholecystokinin in the periaqueductal gray of a rat migraine model. Neurosci. Lett. 2015, 587, 29–34. [Google Scholar] [CrossRef]
- Samuels, E.R.; Szabadi, E. Functional neuroanatomy of the noradrenergic locus coeruleus: Its roles in the regulation of arousal and autonomic function part II: Physiological and pharmacological manipulations and pathological alterations of locus coeruleus activity in humans. Curr. Neuropharmacol. 2008, 6, 254–285. [Google Scholar] [CrossRef] [PubMed]
- McCall, J.G.; Al-Hasani, R.; Siuda, E.R.; Hong, D.Y.; Norris, A.J.; Ford, C.P.; Bruchas, M.R. CRH Engagement of the Locus Coeruleus Noradrenergic System Mediates Stress-Induced Anxiety. Neuron 2015, 87, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Llorca-Torralba, M.; Borges, G.; Neto, F.; Mico, J.A.; Berrocoso, E. Noradrenergic Locus Coeruleus pathways in pain modulation. Neuroscience 2016, 338, 93–113. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Edvinsson, L.; Ekman, R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann. Neurol. 1990, 28, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, G.; Zsombok, T.; Modos, E.A.; Olajos, S.; Jakab, B.; Nemeth, J.; Szolcsanyi, J.; Vitrai, J.; Bagdy, G. NO-induced migraine attack: Strong increase in plasma calcitonin gene-related peptide (CGRP) concentration and negative correlation with platelet serotonin release. Pain 2003, 106, 461–470. [Google Scholar] [CrossRef]
- Juhasz, G.; Zsombok, T.; Jakab, B.; Nemeth, J.; Szolcsanyi, J.; Bagdy, G. Sumatriptan causes parallel decrease in plasma calcitonin gene-related peptide (CGRP) concentration and migraine headache during nitroglycerin induced migraine attack. Cephalalgia 2005, 25, 179–183. [Google Scholar] [CrossRef]
- O’Connor, T.P.; van der Kooy, D. Enrichment of a vasoactive neuropeptide (calcitonin gene related peptide) in the trigeminal sensory projection to the intracranial arteries. J. Neurosci. 1988, 8, 2468–2476. [Google Scholar] [CrossRef]
- Raddant, A.C.; Russo, A.F. Calcitonin gene-related peptide in migraine: Intersection of peripheral inflammation and central modulation. Expert Rev. Mol. Med. 2011, 13, e36. [Google Scholar] [CrossRef]
- Demartini, C.; Tassorelli, C.; Zanaboni, A.M.; Tonsi, G.; Francesconi, O.; Nativi, C.; Greco, R. The role of the transient receptor potential ankyrin type-1 (TRPA1) channel in migraine pain: Evaluation in an animal model. J. Headache Pain 2017, 18, 94. [Google Scholar] [CrossRef]
- Holzmann, B. Modulation of immune responses by the neuropeptide CGRP. Amino. Acids 2013, 45, 1–7. [Google Scholar] [CrossRef]
- Han, D. Association of Serum Levels of Calcitonin Gene-related Peptide and Cytokines during Migraine Attacks. Ann. Indian Acad. Neurol. 2019, 22, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; D’Ovidio, C.; Conti, C.; Gallenga, C.E.; Lauritano, D.; Caraffa, A.; Kritas, S.K.; Ronconi, G. Progression in migraine: Role of mast cells and pro-inflammatory and anti-inflammatory cytokines. Eur. J. Pharmacol. 2019, 844, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Cady, R.J.; Glenn, J.R.; Smith, K.M.; Durham, P.L. Calcitonin gene-related peptide promotes cellular changes in trigeminal neurons and glia implicated in peripheral and central sensitization. Mol. Pain 2011, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Vause, C.V.; Durham, P.L. Calcitonin gene-related peptide differentially regulates gene and protein expression in trigeminal glia cells: Findings from array analysis. Neurosci. Lett. 2010, 473, 163–167. [Google Scholar] [CrossRef]
- Ceruti, S.; Villa, G.; Fumagalli, M.; Colombo, L.; Magni, G.; Zanardelli, M.; Fabbretti, E.; Verderio, C.; van den Maagdenberg, A.M.; Nistri, A.; et al. Calcitonin gene-related peptide-mediated enhancement of purinergic neuron/glia communication by the algogenic factor bradykinin in mouse trigeminal ganglia from wild-type and R192Q Cav2.1 Knock-in mice: Implications for basic mechanisms of migraine pain. J. Neurosci. 2011, 31, 3638–3649. [Google Scholar] [CrossRef]
- Hansted, A.K.; Bhatt, D.K.; Olesen, J.; Jensen, L.J.; Jansen-Olesen, I. Effect of TRPA1 activator allyl isothiocyanate (AITC) on rat dural and pial arteries. Pharmacol. Rep. 2019, 71, 565–572. [Google Scholar] [CrossRef]
- Nassini, R.; Materazzi, S.; Vriens, J.; Prenen, J.; Benemei, S.; De Siena, G.; la Marca, G.; Andrè, E.; Preti, D.; Avonto, C.; et al. The ‘headache tree’ via umbellulone and TRPA1 activates the trigeminovascular system. Brain 2012, 135 Pt 2, 376–390. [Google Scholar] [CrossRef]
- Imai, S.; Saeki, M.; Yanase, M.; Horiuchi, H.; Abe, M.; Narita, M.; Kuzumaki, N.; Suzuki, T. Change in microRNAs associated with neuronal adaptive responses in the nucleus accumbens under neuropathic pain. J. Neurosci. 2011, 31, 15294–15299. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Chen, S.P.; Liao, Y.C.; Fuh, J.L.; Wang, Y.F.; Wang, S.J. Elevated circulating endothelial-specific microRNAs in migraine patients: A pilot study. Cephalalgia 2018, 38, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Wang, Y.; Pan, Q.; Tian, R.; Zhang, D.; Qin, G.; Zhou, J.; Chen, L. MicroRNA-155-5p promotes neuroinflammation and central sensitization via inhibiting SIRT1 in a nitroglycerin-induced chronic migraine mouse model. J. Neuroinflammation 2021, 18, 287. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.H.; Duroux, M.; Gazerani, P. Serum MicroRNA Signatures in Migraineurs During Attacks and in Pain-Free Periods. Mol. Neurobiol. 2016, 53, 1494–1500. [Google Scholar] [CrossRef]
- Reuter, U.; Chiarugi, A.; Bolay, H.; Moskowitz, M.A. Nuclear factor-kappaB as a molecular target for migraine therapy. Ann. Neurol. 2002, 51, 507–516. [Google Scholar] [CrossRef]
- Greco, R.; Tassorelli, C.; Cappelletti, D.; Sandrini, G.; Nappi, G. Activation of the transcription factor NF-kappaB in the nucleus trigeminalis caudalis in an animal model of migraine. Neurotoxicology 2005, 26, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Sarchielli, P.; Floridi, A.; Mancini, M.L.; Rossi, C.; Coppola, F.; Baldi, A.; Pini, L.A.; Calabresi, P. NF-κB activity and iNOS expression in monocytes from internal jugular blood of migraine without aura patients during attacks. Cephalalgia 2006, 26, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Zhu, Y.Y. MiR-30a relieves migraine by degrading CALCA. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2022–2028. [Google Scholar] [CrossRef]
- Tafuri, E.; Santovito, D.; De Nardis, V.; Marcantonio, P.; Paganelli, C.; Affaitati, G.; Bucci, M.; Mezzetti, A.; Giamberardino, M.A.; Cipollone, F. MicroRNA profiling in migraine without aura: Pilot study. Ann. Med. 2015, 47, 468–473. [Google Scholar] [CrossRef] [PubMed]
- De Icco, R.; Fiamingo, G.; Greco, R.; Bottiroli, S.; Demartini, C.; Zanaboni, A.M.; Allena, M.; Guaschino, E.; Martinelli, D.; Putortì, A.; et al. Neurophysiological and biomolecular effects of erenumab in chronic migraine: An open label study. Cephalalgia 2020, 40, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Kurowska-Stolarska, M.; Alivernini, S.; Ballantine, L.E.; Asquith, D.L.; Millar, N.L.; Gilchrist, D.S.; Reilly, J.; Ierna, M.; Fraser, A.R.; Stolarski, B.; et al. MicroRNA-155 as a proinflammatory regulator in clinical and experimental arthritis. Proc. Natl. Acad. Sci. USA 2011, 108, 11193–11198. [Google Scholar] [CrossRef]
- Elmesmari, A.; Fraser, A.R.; Wood, C.; Gilchrist, D.; Vaughan, D.; Stewart, L.; McSharry, C.; McInnes, I.B.; Kurowska-Stolarska, M. MicroRNA-155 regulates monocyte chemokine and chemokine receptor expression in Rheumatoid Arthritis. Rheumatology 2016, 55, 2056–2065. [Google Scholar] [CrossRef] [PubMed]
- Thorlund, K.; Sun-Edelstein, C.; Druyts, E.; Kanters, S.; Ebrahim, S.; Bhambri, R.; Ramos, E.; Mills, E.J.; Lanteri-Minet, M.; Tepper, S. Risk of medication overuse headache across classes of treatments for acute migraine. J. Headache Pain 2016, 17, 107. [Google Scholar] [CrossRef]
- Saengjaroentham, C.; Strother, L.C.; Dripps, I.; Sultan Jabir, M.R.; Pradhan, A.; Goadsby, P.J.; Holland, P.R. Differential medication overuse risk of novel anti-migraine therapeutics. Brain 2020, 143, 2681–2688. [Google Scholar] [CrossRef]
- Navratilova, E.; Behravesh, S.; Oyarzo, J.; Dodick, D.W.; Banerjee, P.; Porreca, F. Ubrogepant does not induce latent sensitization in a preclinical model of medication overuse headache. Cephalalgia 2020, 40, 892–902. [Google Scholar] [CrossRef]
- Tepper, S.J.; Diener, H.C.; Ashina, M.; Brandes, J.L.; Friedman, D.I.; Reuter, U.; Cheng, S.; Nilsen, J.; Leonardi, D.K.; Lenz, R.A.; et al. Erenumab in chronic migraine with medication overuse: Subgroup analysis of a randomized trial. Neurology 2019, 92, e2309–e2320. [Google Scholar] [CrossRef]
- Moreno-Ajona, D.; Villar-Martínez, M.D.; Goadsby, P.J. New Generation Gepants: Migraine Acute and Preventive Medications. J. Clin. Med. 2022, 11, 1656. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J.; Diener, H.C.; Husstedt, I.W.; Goadsby, P.J.; Hall, D.; Meier, U.; Pollentier, S.; Lesko, L.M.; BIBN 4096 BS Clinical Proof of Concept Study Group. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N. Engl. J. Med. 2004, 350, 1104–1110. [Google Scholar] [CrossRef]
- Ernstsen, C.; Christensen, S.L.; Olesen, J.; Kristensen, D.M. No additive effect of combining sumatriptan and olcegepant in the GTN mouse model of migraine. Cephalalgia 2021, 41, 329–339. [Google Scholar] [CrossRef]
- Tvedskov, J.F.; Tfelt-Hansen, P.; Petersen, K.A.; Jensen, L.T.; Olesen, J. CGRP receptor antagonist olcegepant (BIBN4096BS) does not prevent glyceryl trinitrate-induced migraine. Cephalalgia 2010, 30, 1346–1353. [Google Scholar] [CrossRef]
- Edvinsson, L.; Warfvinge, K. Recognizing the role of CGRP and CGRP receptors in migraine and its treatment. Cephalalgia 2019, 39, 366–373. [Google Scholar] [CrossRef]
- Christensen, S.L.; Ernstsen, C.; Olesen, J.; Kristensen, D.M. No central action of CGRP antagonising drugs in the GTN mouse model of migraine. Cephalalgia 2020, 40, 924–934. [Google Scholar] [CrossRef]
- Sixt, M.L.; Messlinger, K.; Fischer, M.J. Calcitonin gene-related peptide receptor antagonist olcegepant acts in the spinal trigeminal nucleus. Brain 2009, 132 Pt 11, 3134–3341. [Google Scholar] [CrossRef]
- Long, T.; He, W.; Pan, Q.; Zhang, S.; Zhang, D.; Qin, G.; Chen, L.; Zhou, J. Microglia P2X4R-BDNF signalling contributes to central sensitization in a recurrent nitroglycerin-induced chronic migraine model. J. Headache Pain 2020, 21, 4. [Google Scholar] [CrossRef] [PubMed]




| Experimental Groups | I SET: OFT (n) | II SET: rt-PCR; ELISA (n) | |
|---|---|---|---|
| Acute migraine model | CT (NTG vehicle + olcegepant vehicle) | 8 | - |
| NTG (NTG 10 mg/kg + olcegepant vehicle) | 9 | - | |
| NTG + olcegepant 1 mg (NTG 10 mg/kg + olcegepant 1 mg/kg) | 6 | - | |
| olcegepant 1 mg (NTG vehicle + olcegepant 1 mg/kg) | 6 | - | |
| NTG + olcegepant 2 mg (NTG 10 mg/kg + olcegepant 2 mg/kg) | 6 | - | |
| olcegepant 2 mg (NTG vehicle + olcegepant 2 mg/kg) | 6 | - | |
| Chronic migraine model | CT (NTG vehicle + olcegepant vehicle) | 6 | 6 |
| NTG (NTG 5 mg/kg + olcegepant vehicle) | 6 | 6 | |
| NTG + olcegepant 2 mg (NTG 5 mg/kg + olcegepant 2 mg/kg) | 6 | 6 | |
| olcegepant 2 mg (NTG vehicle + olcegepant 2 mg/kg) | 6 | 6 |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| GAPDH | AACCTGCCAAGTATGATGAC | GGAGTTGCTGTTGAAGTCA |
| TNF-alpha | CCTCACACTCAGATCATCTTCTC | CGCTTGGTGGTTTGCTAC |
| IL-1beta | TCTTCCTTGTGCAAGTGTCTG | CAGGTCATTCTCCTCACTGTC |
| Calca (α-CGRP) | CAGTCTCAGCTCCAAGTCATC | TTCCAAGGTTGACCTCAAAG |
| TRPA1 | CTCCCCGAGTGCATGAAAGT | TGCATATACGCGGGGATGTC |
| U6 | TGCGGGTGCTCGCTTCGGCAGC | CCAGTGCAGGGTCCGAGGT |
| miR-155-5p | TTGAATTCTAACACCTTCGTGGCTACAGAG | TTAGATCTCATTTATCGAGGGAAGGATTG |
| miR-382-5p | GGCTGTGAGTAATTCTTTGGCAG | GGCAGTATACTTGCTGATTGCT |
| miR-34a-5p | GCAGTGTCTTAGCTGGTTGTTG | TGCAGCACTTCTAGGGCAGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greco, R.; Demartini, C.; Francavilla, M.; Zanaboni, A.M.; Tassorelli, C. Antagonism of CGRP Receptor: Central and Peripheral Mechanisms and Mediators in an Animal Model of Chronic Migraine. Cells 2022, 11, 3092. https://doi.org/10.3390/cells11193092
Greco R, Demartini C, Francavilla M, Zanaboni AM, Tassorelli C. Antagonism of CGRP Receptor: Central and Peripheral Mechanisms and Mediators in an Animal Model of Chronic Migraine. Cells. 2022; 11(19):3092. https://doi.org/10.3390/cells11193092
Chicago/Turabian StyleGreco, Rosaria, Chiara Demartini, Miriam Francavilla, Anna Maria Zanaboni, and Cristina Tassorelli. 2022. "Antagonism of CGRP Receptor: Central and Peripheral Mechanisms and Mediators in an Animal Model of Chronic Migraine" Cells 11, no. 19: 3092. https://doi.org/10.3390/cells11193092
APA StyleGreco, R., Demartini, C., Francavilla, M., Zanaboni, A. M., & Tassorelli, C. (2022). Antagonism of CGRP Receptor: Central and Peripheral Mechanisms and Mediators in an Animal Model of Chronic Migraine. Cells, 11(19), 3092. https://doi.org/10.3390/cells11193092






