[18F]NOS PET Brain Imaging Suggests Elevated Neuroinflammation in Idiopathic Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
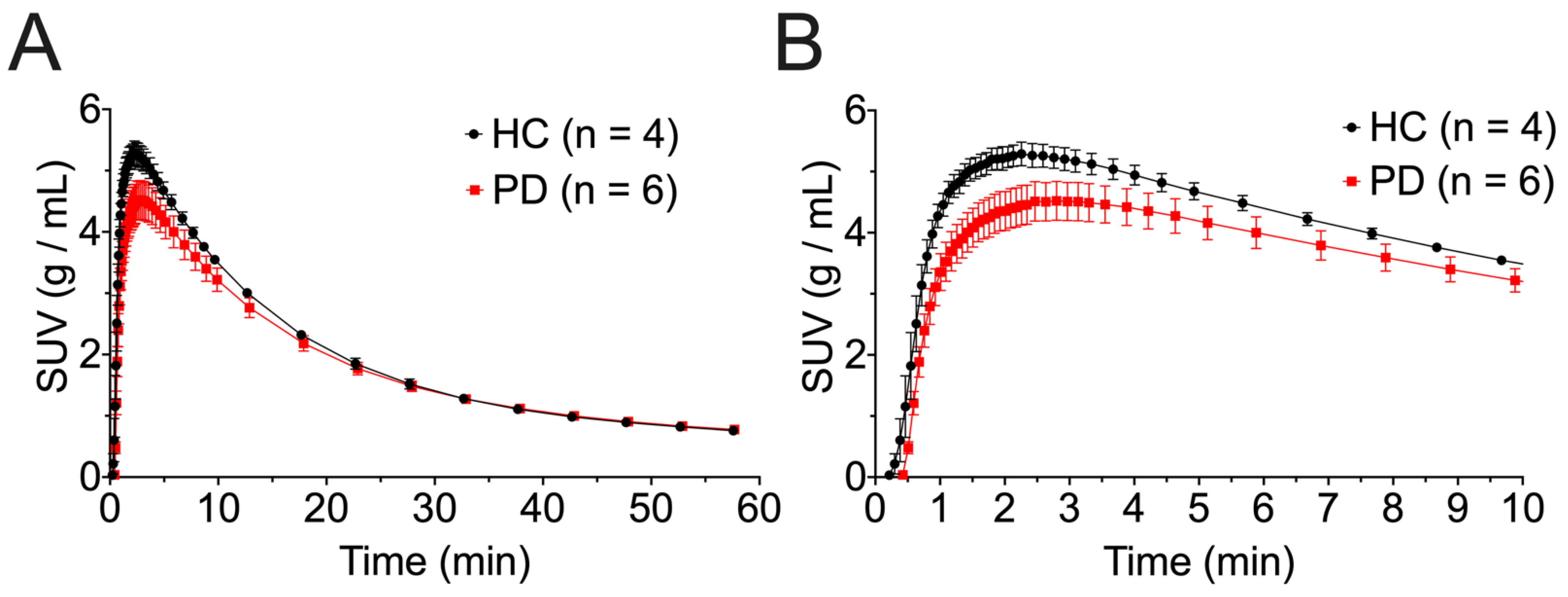
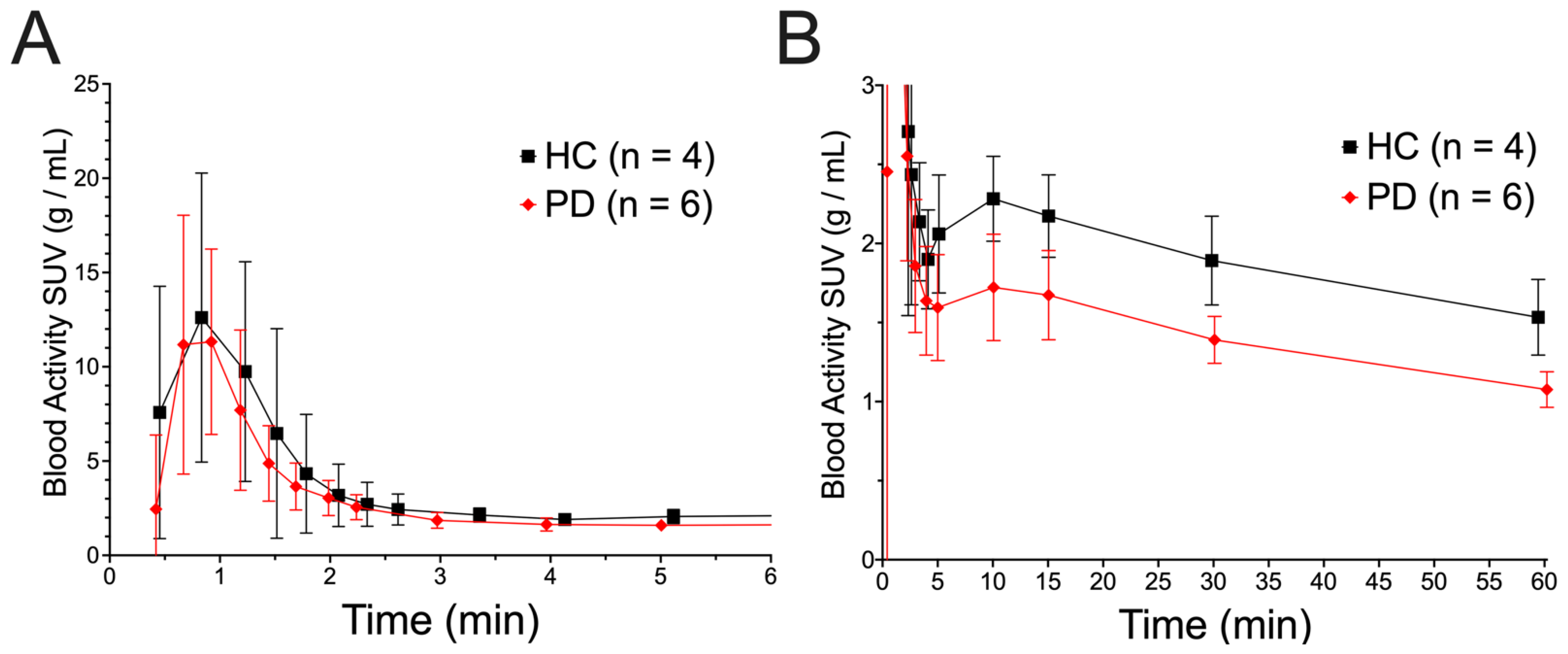
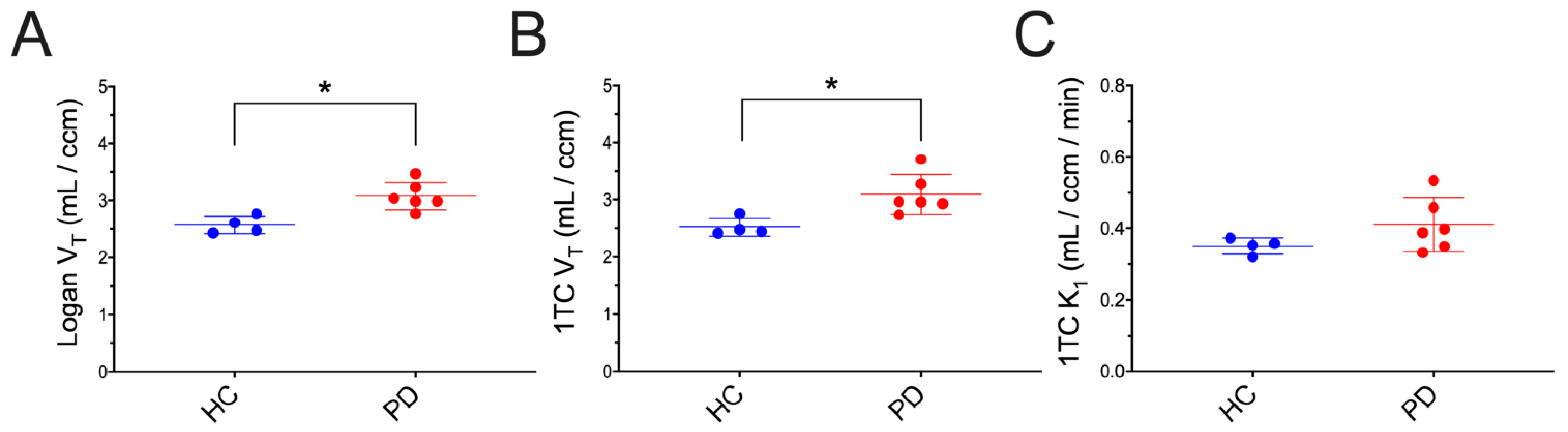
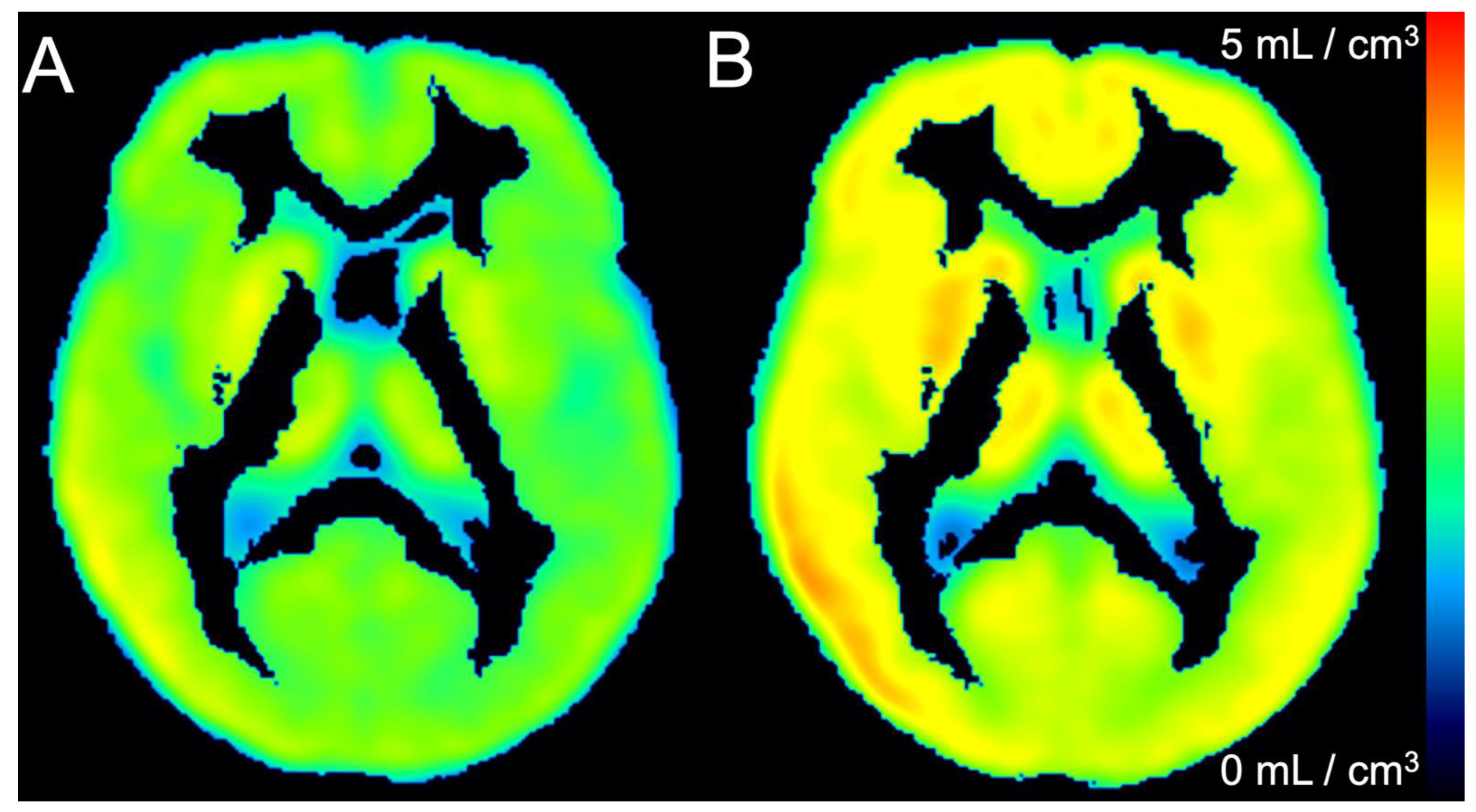
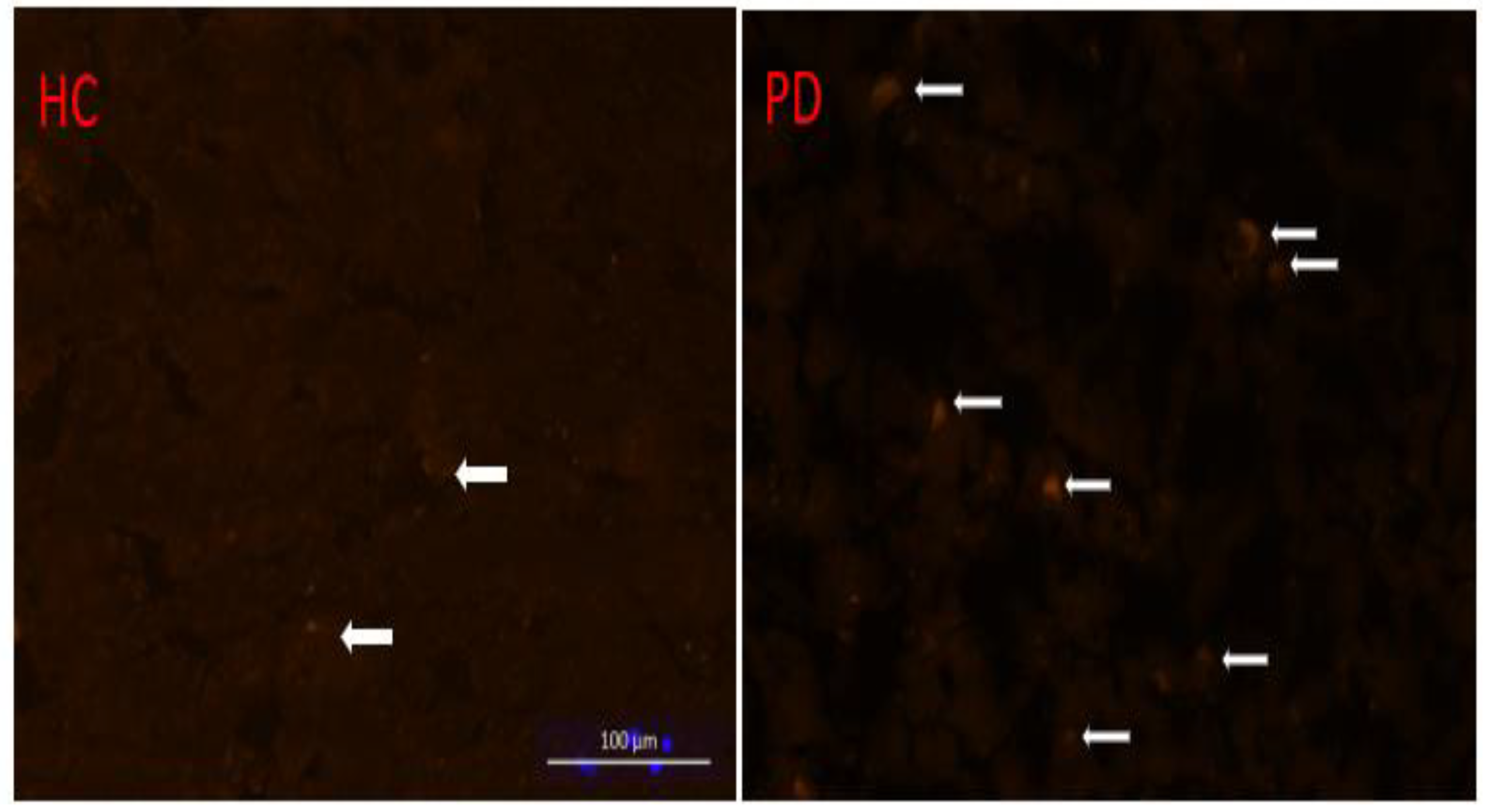
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bodini, B.; Poirion, E.; Tonietto, M.; Benoit, C.; Palladino, R.; Maillart, E.; Portera, E.; Battaglini, M.; Bera, G.; Kuhnast, B.; et al. Individual Mapping of Innate Immune Cell Activation Is a Candidate Marker of Patient-Specific Trajectories of Worsening Disability in Multiple Sclerosis. J. Nucl. Med. 2020, 61, 1043–1049. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Meyer, J.H.; Cervenka, S.; Kim, M.-J.; Kreisl, W.C.; Henter, I.D.; Innis, R.B. Neuroinflammation in psychiatric disorders: PET imaging and promising new targets. Lancet Psychiatry 2020, 7, 1064–1074. [Google Scholar] [CrossRef]
- Woodcock, E.A.; Hillmer, A.T.; Mason, G.F.; Cosgrove, K.P. Imaging Biomarkers of the Neuroimmune System among Substance Use Disorders: A Systematic Review. Mol. Neuropsychiatry 2019, 5, 125–146. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Constantinescu, R.; Thompson, J.P.; Biglan, K.M.; Holloway, R.G.; Kieburtz, K.; Marshall, F.J.; Ravina, B.M.; Schifitto, G.; Siderowf, A.; et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 2007, 68, 384–386. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.Y.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. α-synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef]
- Yamada, T.; McGeer, P.L.; McGeer, E.G. Lewy bodies in Parkinson’s disease are recognized by antibodies to complement proteins. Acta Neuropathol. 1992, 84, 100–104. [Google Scholar] [CrossRef]
- Theodore, S.; Cao, S.; McLean, P.; Standaert, D.G. Targeted Overexpression of Human α-Synuclein Triggers Microglial Activation and an Adaptive Immune Response in a Mouse Model of Parkinson Disease. J. Neuropathol. Exp. Neurol. 2008, 67, 1149–1158. [Google Scholar] [CrossRef]
- Zhang, W.; Dallas, S.; Zhang, D.; Guo, J.P.; Pang, H.; Wilson, B.; Miller, D.S.; Chen, B.; Zhang, W.; McGeer, P.L.; et al. Microglial PHOX and Mac-1 are essential to the enhanced dopaminergic neurodegeneration elicited by A30P and A53T mutant α-synuclein. Glia 2007, 55, 1178–1188. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, T.; Pei, Z.; Miller, D.S.; Wu, X.; Block, M.L.; Wilson, B.; Zhang, W.; Zhou, Y.; Hong, J.-S.; et al. Aggregated α-synuclein activates microglia: A process leading to disease progression in Parkinson’s disease. FASEB J 2005, 19, 533–542. [Google Scholar] [CrossRef]
- Reish, H.E.A.; Standaert, D.G. Role of α-Synuclein in Inducing Innate and Adaptive Immunity in Parkinson Disease. J. Park. Dis. 2015, 5, 1–19. [Google Scholar] [CrossRef]
- Liberatore, G.T.; Jackson-Lewis, V.; Vukosavic, S.; Mandir, A.S.; Vila, M.; McAuliffe, W.G.; Dawson, V.L.; Dawson, T.M.; Przedborski, S. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat. Med. 1999, 5, 1403–1409. [Google Scholar] [CrossRef]
- Zhang, Q.-S.; Heng, Y.; Yuan, Y.-H.; Chen, N.-H. Pathological α-synuclein exacerbates the progression of Parkinson’s disease through microglial activation. Toxicol. Lett. 2016, 265, 30–37. [Google Scholar] [CrossRef]
- Molina, J.A.; Jiménez-Jiménez, F.J.; Navarro, J.A.; Vargas, C.; Gomez, P.; Benito-León, J.; Ortí-Pareja, M.; Cisneros, E.; Arenas, J. Cerebrospinal fluid nitrate levels in patients with Parkinson’s disease. Acta Neurol. Scand. 1996, 93, 123–126. [Google Scholar] [CrossRef]
- Shukla, R.; Rajani, M.; Srivastava, N.; Barthwal, M.K.; Dikshit, M. Nitrite and malondialdehyde content in cerebrospinal fluid of patients with parkinson’s disease. Int. J. Neurosci. 2006, 116, 1391–1402. [Google Scholar] [CrossRef]
- Kavya, R.; Saluja, R.; Singh, S.; Dikshit, M. Nitric oxide synthase regulation and diversity: Implications in Parkinson’s disease. Nitric Oxide 2006, 15, 280–294. [Google Scholar] [CrossRef]
- Zhang, L.; Dawson, V.L.; Dawson, T.M. Role of nitric oxide in Parkinson’s disease. Pharmacol. Ther. 2006, 109, 33–41. [Google Scholar] [CrossRef]
- Hunot, S.; Boissiere, F.; Faucheux, B.; Brugg, B.; Mouatt-Prigent, A.; Agid, Y.; Hirsch, E.C. Nitric oxide synthase and neuronal vulnerability in Parkinson’s disease. Neuroscience 1996, 72, 355–363. [Google Scholar] [CrossRef]
- Knott, C.; Stern, G.; Wilkin, G.P. Inflammatory regulators in Parkinson’s disease: iNOS, lipocortin-1, and cyclooxygenases-1 and -2. Mol. Cell. Neurosci. 2000, 16, 724–739. [Google Scholar] [CrossRef]
- Gouilly, D.; Saint-Aubert, L.; Ribeiro, M.J.; Salabert, A.S.; Tauber, C.; Peran, P.; Arlicot, N.; Pariente, J.; Payoux, P. Neuroinflammation PET imaging of the translocator protein (TSPO) in Alzheimer’s disease: An update. Eur. J. Neurosci. 2022, 55, 1322–1343. [Google Scholar] [CrossRef]
- Tansey, M.G.; Wallings, R.L.; Houser, M.C.; Herrick, M.K.; Keating, C.E.; Joers, V. Inflammation and immune dysfunction in Parkinson disease. Nat. Rev. Immunol. 2022, 2022, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.F.; Gao, F. Neuroinflammation in Parkinson’s disease: A meta-analysis of PET imaging studies. J. Neurol. 2022, 269, 2304–2314. [Google Scholar] [CrossRef] [PubMed]
- Lavisse, S.; Goutal, S.; Wimberley, C.; Tonietto, M.; Bottlaender, M.; Gervais, P.; Kuhnast, B.; Peyronneau, M.-A.; Barret, O.; Lagarde, J.; et al. Increased microglial activation in patients with Parkinson disease using [18F]-DPA714 TSPO PET imaging. Park. Relat. Disord. 2021, 82, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Varnäs, K.; Cselényi, Z.; Jucaite, A.; Halldin, C.; Svenningsson, P.; Farde, L.; Varrone, A. PET imaging of [11C]PBR28 in Parkinson’s disease patients does not indicate increased binding to TSPO despite reduced dopamine transporter binding. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 367–375. [Google Scholar] [CrossRef]
- Owen, D.R.; Yeo, A.J.; Gunn, R.N.; Song, K.; Wadsworth, G.; Lewis, A.; Rhodes, C.; Pulford, D.J.; Bennacef, I.; Parker, C.A.; et al. An 18-kDa Translocator Protein (TSPO) Polymorphism Explains Differences in Binding Affinity of the PET Radioligand PBR28. J. Cereb. Blood Flow Metab. 2012, 32, 1–5. [Google Scholar] [CrossRef]
- Wimberley, C.; Lavisse, S.; Hillmer, A.; Hinz, R.; Turkheimer, F.; Zanotti-Fregonara, P. Kinetic modeling and parameter estimation of TSPO PET imaging in the human brain. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 246–256. [Google Scholar] [CrossRef]
- Mach, R.H. New Targets for the Development of PET Tracers for Imaging Neurodegeneration in Alzheimer Disease. J. Nucl. Med. 2014, 55, 1221–1224. [Google Scholar] [CrossRef][Green Version]
- Notter, T.; Coughlin, J.; Gschwind, T.; Weber-Stadlbauer, U.; Wang, Y.; Kassiou, M.; Vernon, A.; Benke, D.; Pomper, M.G.; Sawa, A.; et al. Translational evaluation of translocator protein as a marker of neuroinflammation in schizophrenia. Mol. Psychiatry 2018, 23, 323–334. [Google Scholar] [CrossRef]
- Zhou, D.; Chu, W.; Rothfuss, J.; Zeng, C.; Xu, J.; Jones, L.; Welch, M.J.; Mach, R.H. Synthesis, radiolabeling, and in vivo evaluation of an 18F-labeled isatin analog for imaging caspase-3 activation in apoptosis. Bioorg. Med. Chem. Lett. 2006, 16, 5041–5046. [Google Scholar] [CrossRef]
- Zhou, D.; Lee, H.; Rothfuss, J.M.; Chen, D.L.; Ponde, D.E.; Welch, M.J.; Mach, R.H. Design and Synthesis of 2-Amino-4-methylpyridine Analogues as Inhibitors for Inducible Nitric Oxide Synthase and in Vivo Evaluation of [18F]6-(2-Fluoropropyl)-4-methyl-pyridin-2-amine as a Potential PET Tracer for Inducible Nitric Oxide Synthase. J. Med. Chem. 2009, 52, 2443–2453. [Google Scholar] [CrossRef]
- Herrero, P.; Laforest, R.; Shoghi, K.; Zhou, D.; Ewald, G.; Pfeifer, J.; Duncavage, E.; Krupp, K.; Mach, R.; Gropler, R. Feasibility and Dosimetry Studies for 18F-NOS as a Potential PET Radiopharmaceutical for Inducible Nitric Oxide Synthase in Humans. J. Nucl. Med. 2012, 53, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.J.; Isakow, W.; Byers, D.E.; Engle, J.T.; Griffin, E.A.; Kemp, D.; Brody, S.L.; Gropler, R.J.; Miller, J.P.; Chu, W.; et al. Imaging Pulmonary Inducible Nitric Oxide Synthase Expression with PET. J. Nucl. Med. 2015, 56, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef] [PubMed]
- Kolthammer, J.A.; Su, K.-H.; Grover, A.; Narayanan, M.; Jordan, D.W.; Muzic, R.F. Performance evaluation of the Ingenuity TF PET/CT scanner with a focus on high count-rate conditions. Phys. Med. Biol. 2014, 59, 3843–3859. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Wu, D.; Ceritoglu, C.; Li, Y.; Kolasny, A.; Vaillant, M.A.; Faria, A.V.; Oishi, K.; Miller, M.I. MRICloud: Delivering High-Throughput MRI Neuroinformatics as Cloud-Based Software as a Service. Comput. Sci. Eng. 2016, 18, 21–35. [Google Scholar] [CrossRef]
- Kuwabara, H.M.K.; Bauwer, W.; Nemoto, E.; Haut, M.W.; Wong, D.F. An integrative data analysis environment (IDAE) for PET neuroimaging research. In 10th International Symposium on Functional NeuroReceptor Mapping of the Living Brain Abstract Book; Mapping NeuroReceptors at Work (NRM): Egmond aan Zee, The Netherlands, 2014; p. 131. [Google Scholar]
- Logan, J.; Fowler, J.S.; Volkow, N.D.; Wolf, A.P.; Dewey, S.L.; Schlyer, D.J.; MacGregor, R.R.; Hitzemann, R.; Bendriem, B.; Gatley, S.J.; et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(-)-cocaine PET studies in human subjects. J. Cereb. Blood Flow Metab. 1990, 10, 740–747. [Google Scholar] [CrossRef]
- User’s Guide PMOD Kinetic Modeling (PKIN), Version 4.2; PMOD Techologies Ltd.: Zurich, Switzerland, 2020.
- Alpert, N.M.; Eriksson, L.; Chang, J.Y.; Bergstrom, M.; Litton, J.E.; Correia, J.A.; Bohm, C.; Ackerman, R.H.; Taveras, J.M. Strategy for the Measurement of Regional Cerebral Blood Flow Using Short-Lived Tracers and Emission Tomography. J. Cereb. Blood Flow Metab. 1984, 4, 28–34. [Google Scholar] [CrossRef]
- McGregor, M.M.; Nelson, A.B. Circuit Mechanisms of Parkinson’s Disease. Neuron 2019, 101, 1042–1056. [Google Scholar] [CrossRef]
- Wetherill, R.R.; Doot, R.K.; Young, A.J.; Lee, H.; Schubert, E.K.; Wiers, C.E.; Leone, F.T.; Mach, R.H.; Kranzler, H.R.; Dubroff, J.G. Using 18F-NOS PET Imaging to Measure Pulmonary Inflammation in Electronic and Combustible Cigarette Users: A Pilot Study. medRxiv 2022. [Google Scholar] [CrossRef]

| Patient ID * | M/F † | Age | Ht ‡ | Wt § | IA ‖ | UPDRS ¶ | UPDRS ¶ | UPDRS ¶ | UPDRS ¶ | UPDRS ¶ | Anti-Parkinson’s |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (y) | (cm) | (kg) | (MBq) | NM-EDL # | M-EDL ** | ME ∆ | MC √ | Total | Medications | ||
| Parkinson’s Disease Patients Cohort | |||||||||||
| 833589-02 | F | 65 | 150 | 52 | 224 | 1 | 0 | 15 | 2 | 18 | carbidopa/levodopa |
| rasagaline | |||||||||||
| 833589-05 | F | 54 | 165 | 52 | 184 | 18 | 11 | 26 | 4 | 59 | carbidopa/levodopa |
| selegiline | |||||||||||
| 833589-06 | M | 67 | 179 | 93 | 228 | 12 | 2 | 27 | 4 | 45 | carbidopa/levodopa |
| amantadine | |||||||||||
| 833589-07 | M | 63 | 170 | 74 | 218 | 5 | 4 | 25 | 3 | 37 | carbidopa/levodopa |
| selegiline | |||||||||||
| 833589-09 | M | 63 | 193 | 111 | 193 | 9 | 1 | 20 | 9 | 39 | carbidopa/levodopa |
| rasagaline, rotigotine | |||||||||||
| 833589-11 | M | 70 | 186 | 82 | 165 | 2 | 8 | 33 | 0 | 43 | carbidopa/levodopa |
| rasagaline | |||||||||||
| Healthy controls cohort | |||||||||||
| 842717-6D | M | 54 | 179 | 73 | 223 | N/A | N/A | N/A | N/A | N/A | N/A |
| 834090-04 | M | 74 | 177 | 127 | 227 | N/A | N/A | N/A | N/A | N/A | N/A |
| 833589-10 | F | 62 | 157 | 84 | 217 | N/A | N/A | N/A | N/A | N/A | N/A |
| 834090-01 | M | 83 | 170 | 73 | 213 | N/A | N/A | N/A | N/A | N/A | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doot, R.K.; Young, A.J.; Nasrallah, I.M.; Wetherill, R.R.; Siderowf, A.; Mach, R.H.; Dubroff, J.G. [18F]NOS PET Brain Imaging Suggests Elevated Neuroinflammation in Idiopathic Parkinson’s Disease. Cells 2022, 11, 3081. https://doi.org/10.3390/cells11193081
Doot RK, Young AJ, Nasrallah IM, Wetherill RR, Siderowf A, Mach RH, Dubroff JG. [18F]NOS PET Brain Imaging Suggests Elevated Neuroinflammation in Idiopathic Parkinson’s Disease. Cells. 2022; 11(19):3081. https://doi.org/10.3390/cells11193081
Chicago/Turabian StyleDoot, Robert K., Anthony J. Young, Ilya M. Nasrallah, Reagan R. Wetherill, Andrew Siderowf, Robert H. Mach, and Jacob G. Dubroff. 2022. "[18F]NOS PET Brain Imaging Suggests Elevated Neuroinflammation in Idiopathic Parkinson’s Disease" Cells 11, no. 19: 3081. https://doi.org/10.3390/cells11193081
APA StyleDoot, R. K., Young, A. J., Nasrallah, I. M., Wetherill, R. R., Siderowf, A., Mach, R. H., & Dubroff, J. G. (2022). [18F]NOS PET Brain Imaging Suggests Elevated Neuroinflammation in Idiopathic Parkinson’s Disease. Cells, 11(19), 3081. https://doi.org/10.3390/cells11193081







