Photosynthesis in Response to Different Salinities and Immersions of Two Native Rhizophoraceae Mangroves
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials, Cultivation, and Salt Treatments
2.2. Measurement of Photosynthetic Capacity and Chlorophyll Fluorescence (ChlF) Parameters with a Fixed Light Source
2.3. Statistical Analysis
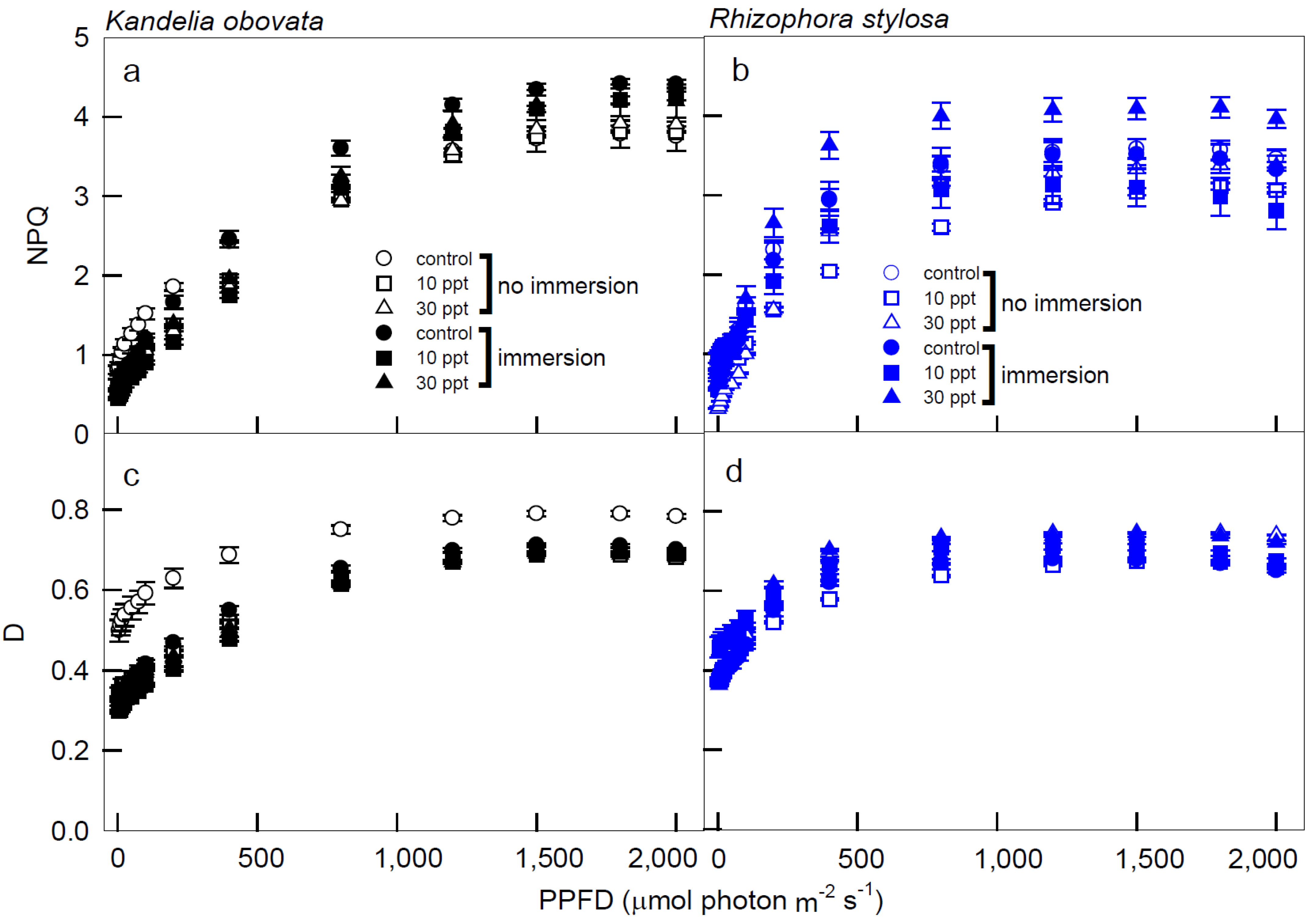
3. Results
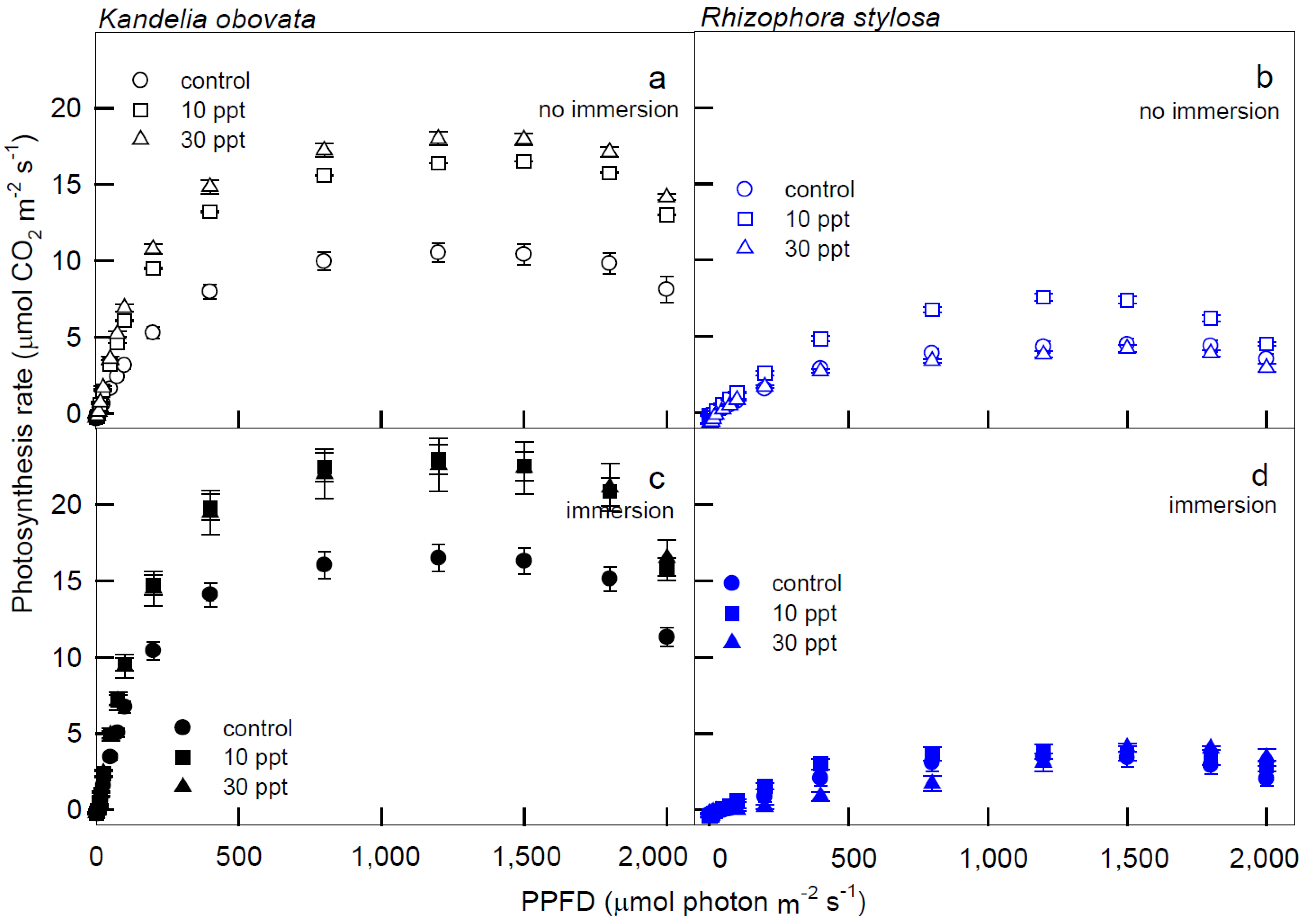
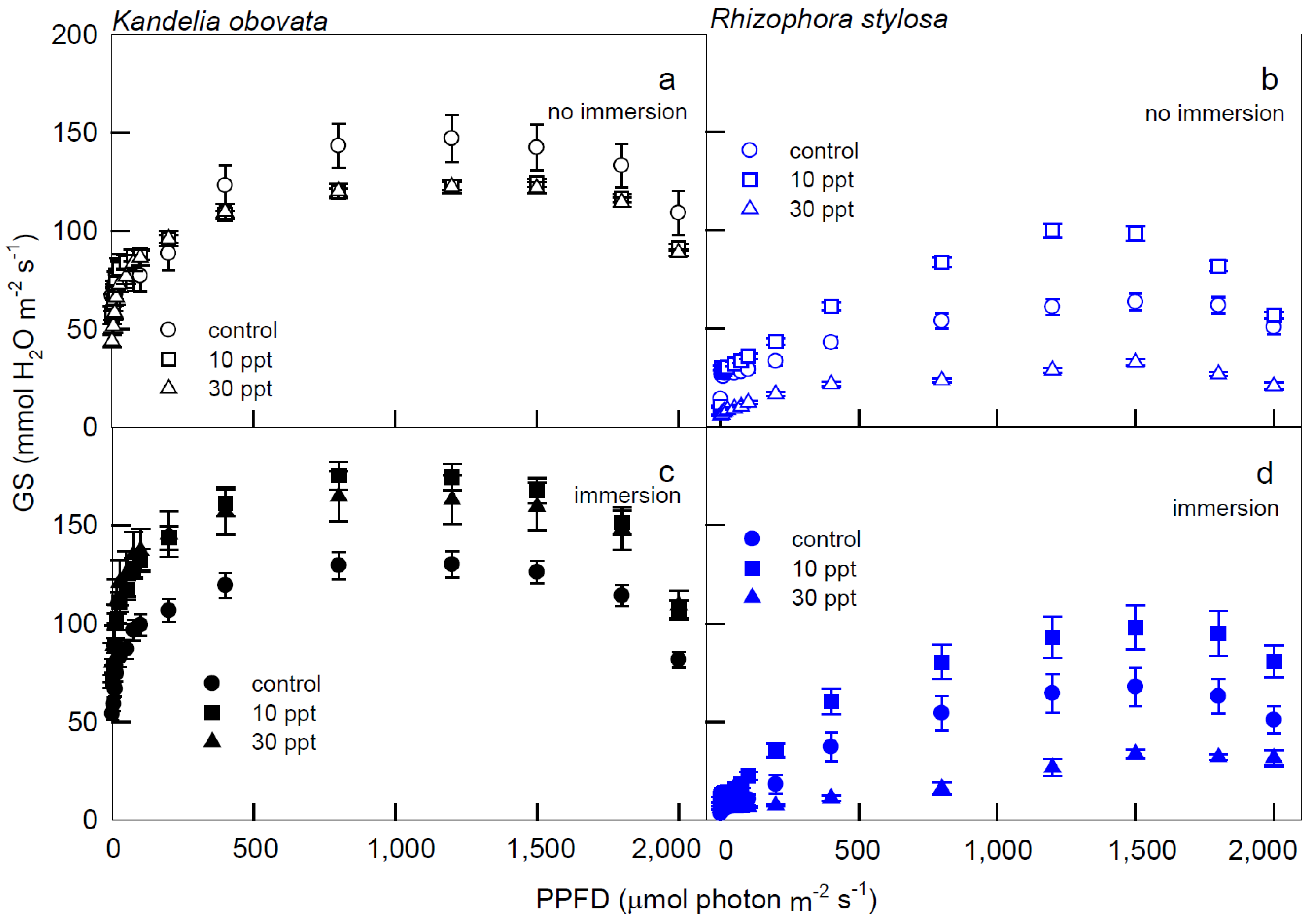
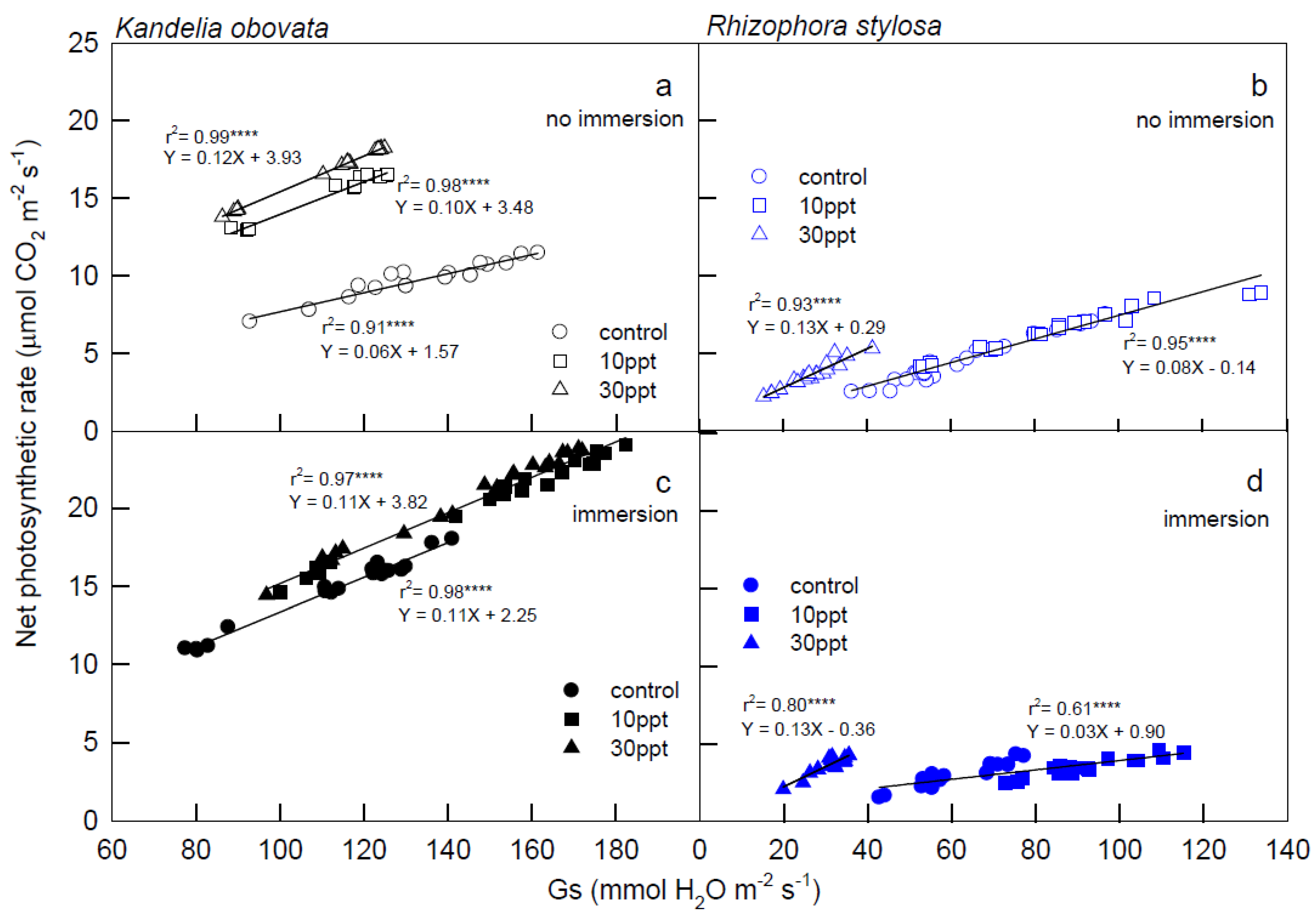
3.1. Photosynthesis Curves of CO2 Fixation and Stomatal Conductance
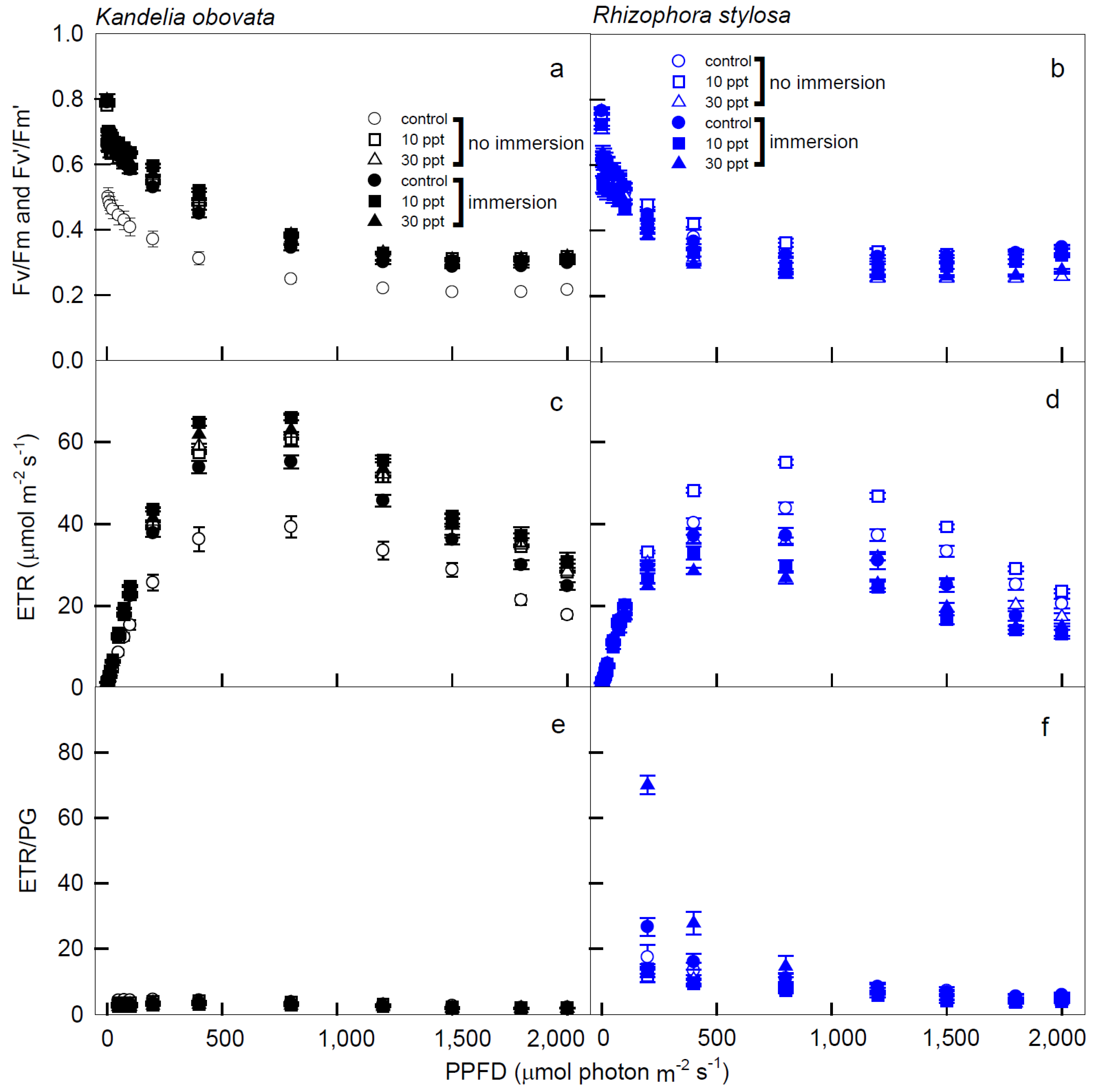
3.2. Chlorophyll Fluorescence Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nebula, M.; Harisankar, H.S.; Kumar, N.C. Metabolites and bioactivities of Rhizophoraceae mangroves. J. Nat. Prod. 2013, 3, 207–232. [Google Scholar] [CrossRef]
- Saenger, P.; Ragavan, P.; Sheue, C.R.; López-Portillo, J.; Yong, J.W.H.; Mageswaran, T. Mangrove Biogeography of the Indo-Pacific. Sabkha Ecosyst. 2019, 2019, 379–400. [Google Scholar]
- Giri, C.; Ochieng, E.; Tieszen, L.L.; Zhu, Z.; Singh, A.; Loveland, T.; Masek, J.; Duke, N. Status and distribution of mangrove forests of the world using earth observation satellite data. Glob. Ecol. Biogeog. 2010, 20, 154–159. [Google Scholar] [CrossRef]
- Bompy, F.; Lequeue, G.; Imbert, D.; Dulormne, M. Increasing fluctuations of soil salinity affect seedling growth performances and physiology in three Neotropical mangrove species. Plant Soil. 2014, 380, 399–413. [Google Scholar] [CrossRef]
- Ball, M.C. Salinity tolerance in the mangroves Aegiceras corniculatum and Avicennia marina. I. water use in relation to growth, carbon partitioning, and salt balance. Aust. J. Crop. Sci. 1988, 15, 447–464. [Google Scholar] [CrossRef]
- Sabine, C.L.S.H.; Adamsa, J.B.; Rajkaran, A.; Bailey, D. The response of the red mangrove Rhizophora mucronata Lam. to salinity and inundation in South Africa. Aquat. Bot. 2011, 95, 71–76. [Google Scholar]
- Krauss, K.W.; Lovelock, C.E.; McKee, K.L.; López-Hoffman, L.; Ewe, S.M.L.; Sousa, W.P. Environmental drivers in mangrove establishment and early development: A review. Aquat. Bot. 2008, 89, 105–127. [Google Scholar] [CrossRef]
- Kanai, H.; Tajima, M.; Sakai, A. Effects of salinity on the growth and survival of the seedlings of mangrove, Rhizophora stylosa. Int. J. Plant Soil Sci. 2014, 3, 879–893. [Google Scholar] [CrossRef]
- Khator, K.; Mahawar, L.; Shekhawat, G.S. NaCl induced oxidative stress in legume crops of Indian Thar Desert: An insight in the cytoprotective role of HO 1, NO and antioxidants. Physiol. Mol. Biol. Plants 2020, 26, 51–62. [Google Scholar] [CrossRef]
- Khator, K.; Shekhawat, G.S. Nitric oxide improved salt stress tolerance by osmolyte accumulation and activation of antioxidant defense system in seedling of B. juncea (L.) Czern. Vegetos 2019, 32, 583–592. [Google Scholar] [CrossRef]
- Mahawar, L.; Shekhawat, G.S. EsHO1 mediated mitigation of NaCl induced oxidative stress and correlation between ROS, antioxidants and HO1 in seedlings of Eruca sativa: Underutilized oil yielding crop of arid region. Physiol. Mol. Biol. Plants 2019, 25, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Mahawar, L.; Shekhawat, G.S. Haem oxygenase: A functionally diverse enzyme of photosynthetic organisms and its role in Phytochrome chromophore biosynthesis, cellular signalling and defence mechanisms. Plant Cell Environ. 2018, 41, 483–500. [Google Scholar] [CrossRef] [PubMed]
- Mahawar, L.; Khator, K.; Shekhawat, G.S. Role of Proline in mitigating NaCl induced oxidative stress in Eruca sativa Miller: An important oil yielding crop of Indian Thar Desert. Vegetos 2018, 31, 55–63. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.; Wu, Q.; Fang, B. The growth of Kandelia candel seedlings in mangrove habitats of the Zhangjiang estuary in Fujian, China. Acta Ecol. Sin. 2006, 26, 1648–1655. [Google Scholar] [CrossRef]
- Chen, L.Z.; Wang, W.Q.; Lin, P. Photosynthetic and physiological responses of Kandelia candel L. Druce seedlings to duration of tidal immersion in artificial seawater. Environ. Exp. Bot. 2005, 54, 256–266. [Google Scholar]
- Li, S.; Meng, X.; Ge, Z.; Zhang, L. Vulnerability assessment of the coastal mangrove ecosystems in Guangxi, China, to sea-level rise. Reg. Environ. Chang. 2015, 15, 265–275. [Google Scholar] [CrossRef]
- Jiang, Z.; Guan, W.; Xiong, Y.; Li, M.; Chen, Y.; Liao, B. Interactive effects of intertidal elevation and light level on early growth of five mangrove species under Sonneratia apetala Buch. Hamplantation canopy: Turning monocultures to mixed forests. Forests 2019, 10, 83. [Google Scholar] [CrossRef]
- Wu, K.L.; Peng, Y.I.; Zheng, K.; Li, X. Responses of aerenchyma in five mangrove species on artificial-non-tidal habitats. Acta Ecol. Sin. 2010, 30, 6927–6934. [Google Scholar]
- Luna, M.M.A.; Bernini, E.; Lage-Pinto, F. Photochemical responses by seedlings of two mangrove species grown under different light levels. Pan-Am. J. Aquat. Sci. 2019, 14, 86–99. [Google Scholar]
- Attiwill, P.M.; Clough, P.M. Carbon dioxide and water vapour exchange in the white mangrove. Photosynthetica 2018, 14, 40–47. [Google Scholar]
- Cheeseman, J.M. Depressions of photosynthesis in mangrove canopies. In Photoinhibition of Photosynthesis—From Molecular Mechanisms to the Field; Baker, N.R., Bowyer, J.R., Eds.; Bios Scientific Publishers: Oxford, UK, 1994; pp. 377–390. [Google Scholar]
- Andrews, T.J.; Clough, B.F.; Muller, G.J. Photosynthetic gas exchange properties and carbon isotope ratios of some mangroves in north Queensland. In Physiology and Management of Mangroves; Teas, H.J., Ed.; Junk: Hague, The Netherlands, 1984; Volume 9, pp. 15–23. [Google Scholar]
- Carter, D.R.; Cheeseman, J.M.; Clough, B.F.; Lovelock, C.E.; Sim, R.G.; Ong, J.E. Photosynthetic characteristics of the mangrove, Bruguiera parviflora (Roxb.) Wright and Arn., under natural conditions. In Current Research in Photosynthesis; Baltscheffsky, M., Ed.; Kluwer: Dordrecht, The Netherlands, 1990; Volume IV, pp. 859–862. [Google Scholar]
- Cheeseman, J.M.; Herendeen, L.B.; Cheeseman, A.T.; Clough, B.F. Photosynthesis and photoprotection in mangroves under field conditions. Plant Cell Environ. 1997, 20, 579–588. [Google Scholar] [CrossRef]
- Youssef, T.; Saenger, P. Mangrove zonation in Mobbs Bay-Australia. Estuarine. Coast. Shelf Sci. 1990, 49, 43–50. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Clough, B.F. Influence of solar radiation and leaf angle on leaf xanthophyll concentrations in mangroves. Oecologia 1922, 91, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Tounekti, T.; Mahdhi, M.; Al-Turki, T.A.; Khemira, H. Water relations and photo-protection mechanisms during drought stress in four coffee (Coffea arabica) cultivars from southwestern Saudi Arabia. S. Afr. J. Bot. 2008, 117, 17–25. [Google Scholar] [CrossRef]
- Wong, S.L.; Huang, M.Y.; Chen, C.W.; Weng, J.H. Light induction of nonphotochemical quenching, CO2 fixation, and photoinhibition in woody and fern species adapted to different light regimes. Photosynthetica 2014, 52, 272–280. [Google Scholar] [CrossRef]
- Ellison, J.C. Vulnerability assessment of mangroves to climate change and sea-level rise impacts. Wetlands Ecol. Manag. 2015, 23, 115–137. [Google Scholar] [CrossRef]
- Bang, J.H.; Bae, M.; Lee, E.J. Plant distribution along an elevational gradient in a macrotidal salt marsh on the west coast of Korea. Aqua Bot. 2018, 147, 52–60. [Google Scholar] [CrossRef]
- Chen, L.Z.; Wang, W.Q. Ecophysiological responses of viviparous mangrove Rhizophora stylosa seedlings to simulated sea-level rise. J. Coast. Res. 2017, 33, 1333–1340. [Google Scholar] [CrossRef]
- Li, H.; Li, Z.; Shen, Z.J.; Luo, M.R.; Liu, Y.L.; Wei, M.Y.; Wang, W.H.; Qin, Y.Y.; Gao, C.H.; Li, K.K.; et al. Physiological and proteomic responses of mangrove plant Avicennia marina seedlings to simulated periodical inundation. Plant Soil 2020, 450, 231–254. [Google Scholar] [CrossRef]
- Wang, C.W.; Wong, S.L.; Liao, T.S.; Weng, J.H.; Chen, M.N.; Huang, M.Y.; Chen, C.I. Photosynthesis in response to salinity and submergence in two Rhizophoraceae mangroves adapted to different tidal elevations. Tree Physiol. 2021, 42, 1016–1028. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Li, X.P.; Niyogi, K.K. Non-photochemical quenching: A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.P.; Ruban, A.V. Restoration of rapidly reversible photoprotective energy dissipation in the absence of PsbS protein by enhanced △pH. J. Biol. Chem. 2011, 286, 19973–19981. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.H.; Chen, L.F.; Jiang, C.Y.; Shih, F.C.; Chen, H.Y. A comparison between yellow-green and green cultivars of four vegetable species in pigments, ascorbate, photosynthesis, energy dissipation, and photoinhibition. Photosynthetica 2011, 49, 361–370. [Google Scholar] [CrossRef]
- Wong, S.L.; Chen, C.W.; Huang, H.W.; Weng, J.H. Using combined measurements for comparison of light induction of stomatal conductance, electron transport rate and CO2 fixation in woody and fern species adapted to different light regimes. Tree Physiol. 2012, 32, 535–544. [Google Scholar] [CrossRef]
- Coe, R.A.; Lin, H.C. Light-response curves in land plants. In Photosynthesis: Methods and Protocols; Covshoff, S., Ed.; Springer Science + Business Media: New York, NY, USA, 2018; pp. 83–93. [Google Scholar]
- Eyland, D.; van Wesemael, J.; Lawson, T.; Carpentier, S. The impact of slow stomatal kinetics on photosynthesis and water use efficiency under fluctuating light. Plant Phys. 2021, 186, 998–1012. [Google Scholar] [CrossRef]
- Reef, R.; Lovelock, C.E. Regulation of water balance in mangroves. Ann. Bot. 2014, 115, 327–331. [Google Scholar] [CrossRef]
- Deans, R.M.; Brodribb, T.J.; Busch, F.A.; Farquhar, G.D. Plant water-use strategy mediates stomatal effects on the light induction of photosynthesis. New Phytol. 2019, 222, 382–395. [Google Scholar] [CrossRef]
- Hsueh, M.L.; Lee, H.H. Diversity and distribution of the mangrove forests in Taiwan. Wetl. Ecol. Manag. 2000, 8, 233–342. [Google Scholar] [CrossRef]
- Chen, Y.P.; Ye, Y. Growth and physiological responses of saplings of two mangrove species to intertidal elevation. Marine Ecol. Prog. Ser. 2013, 482, 107–118. [Google Scholar] [CrossRef]
- Kitao, M.; Utsugi, H.; Kuramoto, S.; Tabuchi, R. Light-dependent photosynthetic characteristics indicated by chlorophyll fluorescence in five mangrove species native to Pohnpei Island, Micronesia. Physiol. Plant 2003, 117, 376–382. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, N.; Arena, C.; Virzo De Santo, A. Temperature response of photosynthesis, excitation energy dissipation and alternative electron sinks to carbonassimilation in Beta vulgaris L. Environ. Exp. Bot. 2006, 55, 248–257. [Google Scholar] [CrossRef]
- Murchie, E.H.; Niyogi, K.K. Manipulation of photoprotection to improve plant photosynthesis. Plant Physiol. 2011, 155, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Esteban, R.; Fernández-Marín, B.; Hernandez, A.; Jiménez, E.T.; León, A.; García-Mauriño, S.; Silva, C.D.; Dolmus, J.R.; Dolmus, C.M.; Molina, M.J.; et al. Salt crystal deposition as a reversible mechanism to enhance photoprotection in black mangrove. Trees 2013, 27, 229–237. [Google Scholar] [CrossRef]
- La Porta, N.; Bertamini, M.; Nedunchezhian, N.; Raddi, P.; Muthuchelian, K. Photoinhibition of photosynthesis in needles of two cypress (Cupressus sempervirens) clones. Tree Physiol. 2005, 25, 1033–1039. [Google Scholar] [CrossRef]
- Leakey, A.D.B.; Press, M.C.; Scholes, J.D. Hightemperature inhibition of photosynthesis is greater under sunflecks than uniform irradiance in a tropical rain forest tree seedling. Plant Cell Environ. 2003, 26, 1681–1690. [Google Scholar] [CrossRef]
- Adams, W.W.; Zarter, C.R.; Ebbert, V.; Demmig-Adams, B. Photoprotective strategies of overwintering evergreens. Bioscience 2004, 54, 41–49. [Google Scholar] [CrossRef]
| K. obovata | R. stylosa | |||
|---|---|---|---|---|
| ETR × NPQ | ETR × NPQ | |||
| PPFD | Immersion | Salinity | R2 with Positive (+) or Negative (-) Correlation | |
| Low (0–400) | no immersion | control | 0.99 **** (+) | 0.98 **** (+) |
| 10 ppt | 0.99 **** (+) | 0.99 **** (+) | ||
| 30 ppt | 0.98 **** (+) | 0.90 **** (+) | ||
| immersion | control | 0.99 **** (+) | 0.92 **** (+) | |
| 10 ppt | 0.98 **** (+) | 0.91 **** (+) | ||
| 30 ppt | 0.98 **** (+) | 0.89 **** (+) | ||
| High (800–2000) | no immersion | control | 0.75 (-) | 0.14 (-) |
| 10 ppt | 0.85 * (-) | 0.76 (-) | ||
| 30 ppt | 0.87 * (-) | 0.66 (-) | ||
| immersion | control | 0.85 * (-) | 0.13 (+) | |
| 10 ppt | 0.90 * (-) | 0.41 (+) | ||
| 30 ppt | 0.85 * (-) | 0.003 (-) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-I.; Lin, K.-H.; Huang, M.-Y.; Wong, S.-L.; Liao, T.-S.; Chen, M.-N.; Weng, J.-H.; Hsueh, M.-L.; Lai, Y.-H.; Wang, C.-W. Photosynthesis in Response to Different Salinities and Immersions of Two Native Rhizophoraceae Mangroves. Cells 2022, 11, 3054. https://doi.org/10.3390/cells11193054
Chen C-I, Lin K-H, Huang M-Y, Wong S-L, Liao T-S, Chen M-N, Weng J-H, Hsueh M-L, Lai Y-H, Wang C-W. Photosynthesis in Response to Different Salinities and Immersions of Two Native Rhizophoraceae Mangroves. Cells. 2022; 11(19):3054. https://doi.org/10.3390/cells11193054
Chicago/Turabian StyleChen, Chung-I, Kuan-Hung Lin, Meng-Yuan Huang, Shau-Lian Wong, Tien-Szu Liao, Ming-Nan Chen, Jen-Hsien Weng, Mei-Li Hsueh, Yu-Hsiang Lai, and Ching-Wen Wang. 2022. "Photosynthesis in Response to Different Salinities and Immersions of Two Native Rhizophoraceae Mangroves" Cells 11, no. 19: 3054. https://doi.org/10.3390/cells11193054
APA StyleChen, C.-I., Lin, K.-H., Huang, M.-Y., Wong, S.-L., Liao, T.-S., Chen, M.-N., Weng, J.-H., Hsueh, M.-L., Lai, Y.-H., & Wang, C.-W. (2022). Photosynthesis in Response to Different Salinities and Immersions of Two Native Rhizophoraceae Mangroves. Cells, 11(19), 3054. https://doi.org/10.3390/cells11193054








