Secrets of Flavonoid Synthesis in Mushroom Cells
Abstract
1. Introduction
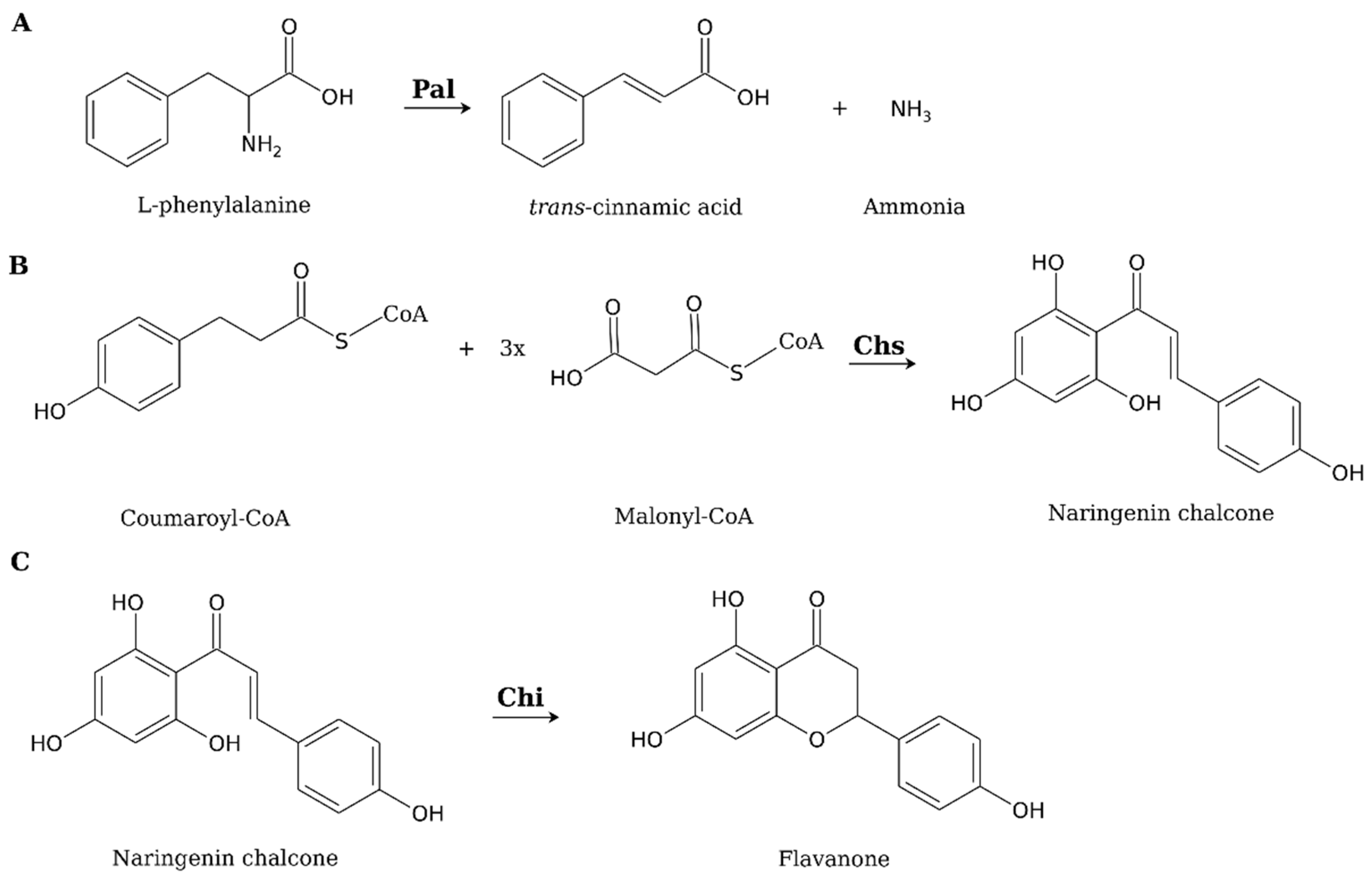
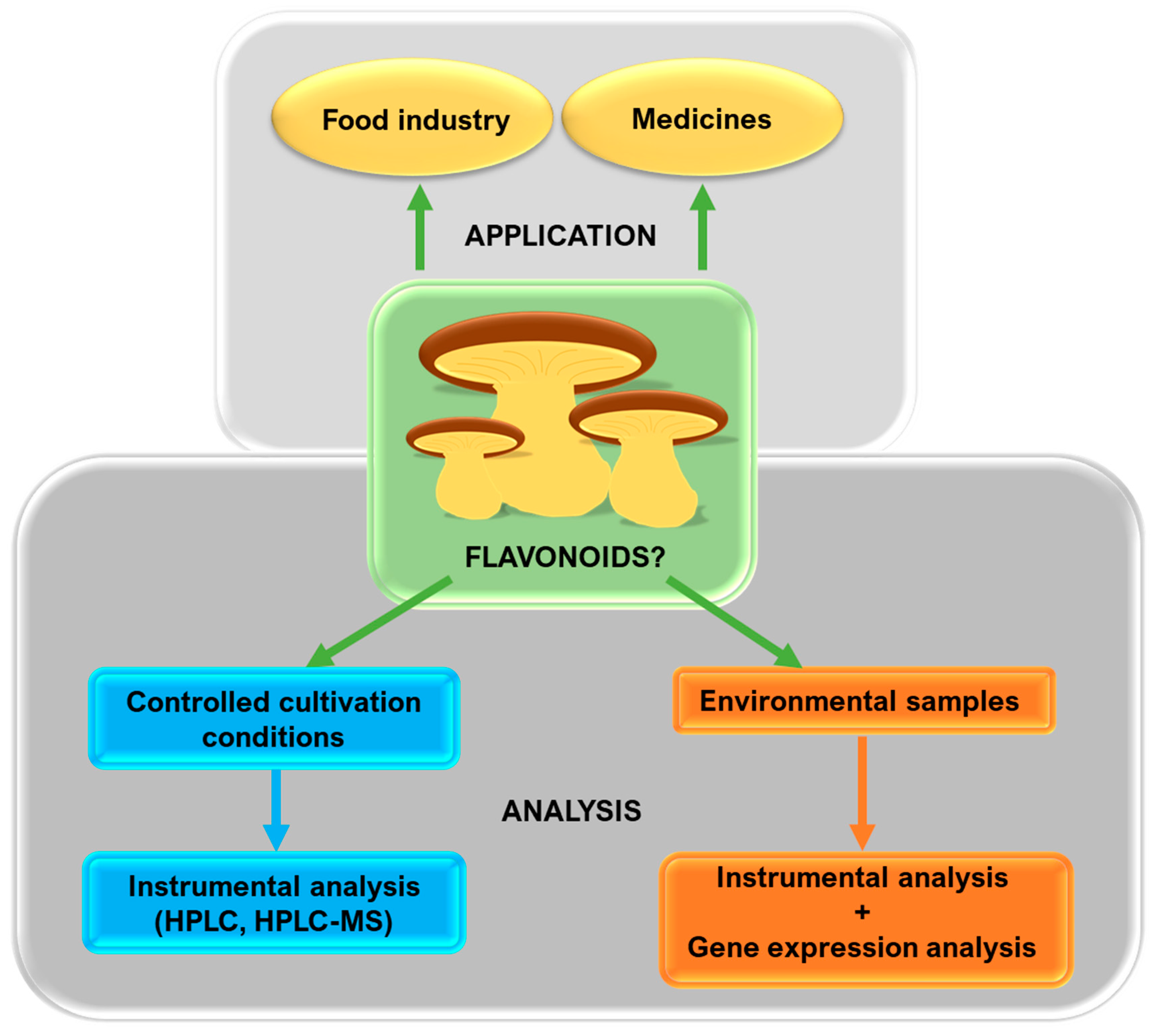
2. Are Mushrooms Capable of Producing Flavonoids?
3. Current Achievements and Future Prospects
4. Additional Suggestions
5. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Gao, J.; Radwan, M.M.; León, F.; Wang, X.; Jacob, M.R.; Tekwani, B.L.; Khan, S.I.; Lupien, S.; Hill, R.A.; Dugan, F.M.; et al. Antimicrobial and antiprotozoal activities of secondary metabolites from the fungus Eurotium repens. Med. Chem. Res. 2012, 21, 3080–3086. [Google Scholar] [CrossRef] [PubMed]
- Linnakoski, R.; Reshamwala, D.; Veteli, P.; Cortina-Escribano, M.; Vanhanen, H.; Marjomäki, V. Antiviral Agents from Fungi: Diversity, Mechanisms and Potential Applications. Front. Microbiol. 2018, 9, 2325. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Lin, R.; Yang, N.; Jia, J.; Wei, S.; Han, J.; Li, J.; Bi, H.; Xu, X. Antibacterial Secondary Metabolites from Marine-Derived Fungus Aspergillus sp. IMCASMF180035. Antibiotics 2021, 10, 377. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, A.; Cieniecka-Rosłonkiewicz, A.; Cholewińska, M. Plant endophytic fungi as a source of paclitaxel. Herba Pol. 2015, 60, 22–33. [Google Scholar] [CrossRef]
- Zaiyou, J.; Li, M.; Xiqiao, H. An endophytic fungus efficiently producing paclitaxel isolated from Taxus wallichiana var. Mairei. Medicine 2017, 96, e7406. [Google Scholar] [CrossRef]
- Daniel, J.; Haberman, M. Clinical potential of psilocybin as a treatment for mental health conditions. Ment. Health Clin. 2017, 7, 24–28. [Google Scholar] [CrossRef]
- Pukalski, J.; Marcol, N.; Wolan, N.; Płonka, P.M.; Ryszka, P.; Kowalski, T.; Latowski, D. Detection of a pheomelanin-like pigment by EPR spectroscopy in the mycelium of Plenodomus biglobosus. Acta Biochim. Pol. 2020, 67, 295–301. [Google Scholar] [CrossRef]
- Mattoon, R.E.; Cordero, R.J.B.; Casadevall, A. Fungal Melanins and Applications in Healthcare, Bioremediation and Industry. J. Fungi 2021, 7, 488. [Google Scholar] [CrossRef]
- Ghormade, V.; Pathan, E.K.; Deshpande, M.V. Can fungi compete with marine sources for chitosan production? Int. J. Biol. Macromol. 2017, 104, 1415–1421. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef]
- Cerimi, K.; Akkaya, K.C.; Pohl, C.; Schmidt, B.; Neubauer, P. Fungi as source for new bio-based materials: A patent review. Fungal Biol. Biotechnol. 2019, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, C.; Vitiello, G.; Adinolfi, B.; Silvestri, B.; Armanetti, P.; Manini, P.; Pezzella, A.; d’Ischia, M.; Luciani, G.; Menichetti, L. Melanin and Melanin-Like Hybrid Materials in Regenerative Medicine. Nanomaterials 2020, 10, 1518. [Google Scholar] [CrossRef] [PubMed]
- Alemu, D.; Tafesse, M.; Mondal, A.K. Mycelium-based composite: The future sustainable biomaterial. Int. J. Biomater. 2022, 2022, 8401528. [Google Scholar] [CrossRef]
- Dai, Q.; Zhang, F.-L.; Feng, T. Sesquiterpenoids Specially Produced by Fungi: Structures, Biological Activities, Chemical and Biosynthesis (2015–2020). J. Fungi 2021, 7, 1026. [Google Scholar] [CrossRef] [PubMed]
- Flynn, C.M.; Broz, K.; Jonkers, W.; Schmidt-Dannert, C.; Kistler, H.C. Expression of the Fusarium graminearum terpenome and involvement of the endoplasmic reticulum-derived toxisome. Fungal Genet. Biol. 2019, 124, 78–87. [Google Scholar] [CrossRef]
- Inamdar, A.A.; Morath, S.; Bennett, J.W. Fungal volatile organic compounds: More than just a funky smell? Annu. Rev. Microbiol. 2020, 74, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Jud, W.; Weikl, F.; Ghirardo, A.; Junker, R.R.; Polle, A.; Benz, J.P.; Pritsch, K.; Schnitzler, J.-P.; Rosenkranz, M. Volatile organic compound patterns predict fungal trophic mode and lifestyle. Commun. Biol. 2020, 4, 185–189. [Google Scholar] [CrossRef]
- Kalra, R.; Conlan, X.A.; Goel, M. Fungi as a Potential Source of Pigments: Harnessing Filamentous Fungi. Front. Chem. 2020, 8, 369. [Google Scholar] [CrossRef]
- Neri-Numa, I.A.; Arruda, H.S.; Geraldi, M.V.; Júnior, M.R.M.; Pastore, G.M. Natural prebiotic carbohydrates, carotenoids and flavonoids as ingredients in food systems. Curr. Opin. Food Sci. 2020, 33, 98–107. [Google Scholar] [CrossRef]
- Andarwulan, R.; Puspita, N.C.; Saraswati; Średnicka-Tober, D. Antioxidants Such as Flavonoids and Carotenoids in the Diet of Bogor, Indonesia Residents. Antioxidants 2021, 10, 587. [Google Scholar] [CrossRef]
- Barzee, T.J.; Cao, L.; Pan, Z.; Zhang, R. Fungi for future foods. J. Future Foods 2021, 1, 25–37. [Google Scholar] [CrossRef]
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Edible mushrooms: Improving human health and promoting quality life. Int. J. Microbiol. 2015, 2015, 376387. [Google Scholar] [CrossRef]
- González, A.; Cruz, M.; Losoya, C.; Nobre, C.; Loredo, A.; Rodríguez, R.; Contrerasa, J.; Belmares, R. Edible mushrooms as a novel protein source for functional foods. Food Funct. 2020, 11, 7400–7414. [Google Scholar] [CrossRef] [PubMed]
- Fulgoni, V.L., III; Agarwal, S. Nutritional impact of adding a serving of mushrooms on usual intakes and nutrient adequacy using National Health and Nutrition Examination Survey 2011–2016 data. Food Sci. Nutr. 2021, 9, 1504–1511. [Google Scholar] [CrossRef] [PubMed]
- El-Ramady, H.; Abdalla, N.; Badgar, K.; Llanaj, X.; Törős, G.; Hajdú, P.; Eid, Y.; Prokisch, J. Edible Mushrooms for Sustainable and Healthy Human Food: Nutritional and Medicinal Attributes. Sustainability 2022, 14, 4941. [Google Scholar] [CrossRef]
- Dias, E.S.; de Brito, M.R. Mushrooms: Biology and life cycle. In Edible and Medicinal Mushrooms: Technology and Applications, 1st ed.; Diego, C.Z., Pardo-Giménez, A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; Chapter 3; pp. 15–33. [Google Scholar] [CrossRef]
- Chang, S.T.; Miles, P.G. Mushroom Biology: A New Discipline. Mycologist 1992, 6, 64–65. [Google Scholar] [CrossRef]
- Carvajal, A.E.S.S.; Koehnlein, E.A.; Soares, A.A.; Eler, G.J.; Nakashima, A.T.A.; Bracht, A.; Peralta, M.R. Bioactives of fruiting bodies and submerged culture mycelia of Agaricus brasiliensis (A. blazei) and their antioxidant properties. LWT-Food Sci. Technol. 2012, 46, 493–499. [Google Scholar] [CrossRef]
- Fijałkowska, A.; Muszyńska, B.; Sułkowska-Ziaja, K.; Kała, K.; Pawlik, A.; Stefaniuk, D.; Matuszewska, A.; Piska, K.; Pękala, E.; Kaczmarczyk, P.; et al. Medicinal potential of mycelium and fruiting bodies of an arboreal mushroom Fomitopsis officinalis in therapy of lifestyle diseases. Sci. Rep. 2020, 10, 20081. [Google Scholar] [CrossRef]
- Rapoport, A.; Guzhova, I.; Bernetti, L.; Buzzini, P.; Kieliszek, M.; Kot, A.N. Carotenoids and Some Other Pigments from Fungi and Yeasts. Metabolites 2021, 11, 92. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Vunduk, J.; Zizak, Z.; Niksic, M.; Jakovljevic, D.; Vrvic, M.M.; Van Griensven, L.J.L.D. Nutraceutical properties of the methanolic extract of edible mushroom Cantharellus cibarius (Fries): Primary mechanisms. Food Funct. 2015, 6, 1875–1886. [Google Scholar] [CrossRef]
- Yan, T.; Guo, Z.K.; Jiang, R.; Wei, W.; Wang, T.; Guo, Y.; Song, Y.C.; Jiao, R.H.; Tan, R.X.; Ge, H.M. New flavonol and diterpenoids from the endophytic fungus Aspergillus sp. Yxf3. Planta Med. 2013, 79, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Gil-Ramírez, A.; Pavo-Caballero, C.; Baeza, E.; Baenas, N.; Garcia-Viguera, C.; Marína, F.R.; Soler-Rivas, C. Mushrooms do not contain flavonoids. J. Funct. Foods 2016, 25, 1–13. [Google Scholar] [CrossRef]
- Mohanta, T.K. Fungi contain genes associated with flavonoid biosynthesis pathway. J. Funct. Foods 2020, 68, 103910. [Google Scholar] [CrossRef]
- Holiman, P.C.H.; Hertog, M.G.L.; Katan, M.B. Analysis and health effects of flavonoids. Food Chem. 1996, 57, 43–46. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Ferreyra, M.L.F.; Serra, P.; Casati, P. Recent advances on the roles of flavonoids as plant protective molecules after UV and high light exposure. Physiol. Plant. 2021, 173, 736–749. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Mathesius, U. Flavonoid Functions in Plants and Their Interactions with Other Organisms. Plants 2018, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Malterud, K.E.; Bremnes, T.E.; Faegri, A.; Moe, T.; Sandanger Dugstad, E.K.; Anthonsen, T.; Henriksen, L.M. Flavonoids from the wood of salix caprea as inhibitors of wood-destroying fungi. J. Nat. Prod. 1985, 48, 559–563. [Google Scholar] [CrossRef]
- Weidenbörner, M.; Hindorf, H.; Jha, H.C.; Tsotsonos, P. Antifungal activity of flavonoids against storage fungi of the genus Aspergillus. Phytochemistry 1990, 29, 1103–1105. [Google Scholar] [CrossRef]
- Afifi, F.Ü.; Al-Khalil, S.; Abdul-Haq, B.K.; Mahasneh, A.; Al-Eisawi, D.M.; Sharaf, M.; Wong, L.K.; Schiff, P.L., Jr. Antifungal flavonoids from Varthemia iphionoides. Phytother. Res. 1991, 5, 173–175. [Google Scholar] [CrossRef]
- Örner, M.W.; Jha, H.C. Antifungal activity of flavonoids and their mixtures against different fungi occurring on grain. Pestic. Sci. 1993, 38, 347–351. [Google Scholar] [CrossRef]
- Çitoğlu, G.S.; Sever, B.; Antus, S.; Baitz-Gács, E.; Altanlar, N. Antifungal flavonoids from Ballota glandulosissima. Pharm. Biol. 2003, 41, 483–486. [Google Scholar] [CrossRef]
- Meragelman, T.L.; Tucker, K.D.; McCloud, T.G.; Cardellina, J.H.; Shoemaker, R.H. Antifungal flavonoids from Hildegardia barteri. J. Nat. Prod. 2005, 68, 1790–1792. [Google Scholar] [CrossRef]
- Galeotti, F.; Barile, E.; Curir, P.; Dolci, M.; Lanzotti, V. Flavonoids from carnation (Dianthus caryophyllus) and their antifungal activity. Phytochem. Lett. 2008, 1, 44–48. [Google Scholar] [CrossRef]
- Kanwal, Q.; Hussain, I.; Siddiqui, H.L.; Javaid, A. Antifungal activity of flavonoids isolated from mango (Mangifera indica L.) leaves. Nat. Prod. Res. 2010, 24, 1907–1914. [Google Scholar] [CrossRef] [PubMed]
- Ammar, M.I.; Nenaah, G.E.; Mohamed, A.H.H. Antifungal activity of prenylated flavonoids isolated from Tephrosia apollinea L. against four phytopathogenic fungi. Crop Protect. 2013, 49, 21–25. [Google Scholar] [CrossRef]
- Jin, Y.S. Recent advances in natural antifungal flavonoids and their derivatives. Bioorg. Med. Chem. Lett. 2019, 29, 126589. [Google Scholar] [CrossRef]
- Siqueira, J.O.; Safir, G.R.; Nair, M.G. Stimulation of vesicular-arbuscular mycorrhiza formation and growth of white clover by flavonoid compounds. New Phytol. 1991, 118, 87–93. [Google Scholar] [CrossRef]
- Tian, B.; Pei, Y.; Huang, W.; Ding, J.; Siemann, E. Increasing flavonoid concentrations in root exudates enhance associations between arbuscular mycorrhizal fungi and an invasive plant. ISME J. 2021, 15, 1919–1930. [Google Scholar] [CrossRef]
- Butkhup, L.; Samappito, W.; Jorjong, S. Evaluation of bioactivities and phenolic contents of wild edible mushrooms from northeastern Thailand. Food Sci. Biotechnol. 2018, 27, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Podkowa, A.; Kryczyk-Poprawa, A.; Opoka, W.; Muszyńska, B. Culinary–medicinal mushrooms: A review of organic compounds and bioelements with antioxidant activity. Eur. Food Res. Technol. 2020, 247, 513–533. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Serafini, M.; Peluso, I.; Raguzzini, A. Flavonoids as anti-inflammatory agents. Proc. Nutr. Soc. 2010, 69, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Curr. Med. Chem. 2015, 22, 132–149. [Google Scholar] [CrossRef]
- Choy, K.W.; Murugan, D.; Leong, X.F.; Abas, R.; Alias, A.; Mustafa, M.R. Flavonoids as Natural Anti-Inflammatory Agents Targeting Nuclear Factor-kappa B (NFκB) Signaling in Cardiovascular Diseases: A Mini Review. Front. Pharmacol. 2019, 10, 1295. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Wu, S.C.; Yang, Z.Q.; Liu, F.; Peng, W.J.; Qu, S.Q.; Li, Q.; Song, X.B.; Zhu, K.; Shen, J.Z. Antibacterial Effect and Mode of Action of Flavonoids from Licorice Against Methicillin-Rresistant Staphylococcus aureus. Front. Microbiol. 2019, 10, 2489. [Google Scholar] [CrossRef]
- Janabi, A.H.W.; Kamboh, A.A.; Saeed, M.; Xiaoyu, L.; BiBi, J.; Majeed, F.; Naveed, M.; Mughal, M.J.; Korejo, N.A.; Kamboh, R.; et al. Flavonoid-rich foods (FRF): A promising nutraceutical approach against lifespan-shortening diseases. Iran. J. Basic Med. Sci. 2020, 23, 140–153. [Google Scholar] [CrossRef]
- Wang, X.; Ding, G.; Liu, B.; Wang, Q. Flavonoids and antioxidant activity of rare and endangered fern: Isoetes sinensis. PLoS ONE 2020, 15, e0232185. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Guan, Y.; Yi, H.; Lai, S.; Sun, Y.; Cao, S. Antibacterial activity and mechanism of plant flavonoids to gram-positive bacteria predicted from their lipophilicities. Sci. Rep. 2021, 11, 10471. [Google Scholar] [CrossRef] [PubMed]
- Christ, B.; Müller, K.H. Zur serienmäßigen bestimmung des gehaltes an flavonol-derivaten in drogen. Arch. Pharm. 1960, 293, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Pękal, A.; Pyrzynska, K. Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Nakayama, T.; Takahashi, S.; Waki, T. Formation of Flavonoid Metabolons: Functional Significance of Protein-Protein Interactions and Impact on Flavonoid Chemodiversity. Front. Plant Sci. 2019, 10, 821. [Google Scholar] [CrossRef]
- Ramírez-Anguiano, A.C.; Santoyo, S.; Reglero, G.; Soler-Rivas, C. Radical scavenging activities, endogenous oxidative enzymes and total phenols in edible mushrooms commonly consumed in Europe. J. Sci. Food Agric. 2007, 87, 2272–2278. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, S.M.; Chun, J.; Lee, H.B.; Lee, J. Influence of heat treatment on the antioxidant activities and polyphenolic compounds of Shiitake (Lentinus edodes) mushroom. Food Chem. 2006, 99, 381–387. [Google Scholar] [CrossRef]
- Meir, Z.; Osherov, N. Vitamin Biosynthesis as an Antifungal Target. J. Fungi 2018, 4, 72. [Google Scholar] [CrossRef]
- Perli, T.; Wronska, A.K.; Ortiz-Merino, R.A.; Pronk, J.T.; Daran, J.-M. Vitamin requirements and biosynthesis in Saccharomyces cerevisiae. Yeast 2020, 37, 283–304. [Google Scholar] [CrossRef]
- Slana, M.; Žigon, D.; Makovec, T.; Lenasi, H. The response of filamentous fungus Rhizopus nigricans to flavonoids. J. Basic Microbiol. 2011, 51, 433–441. [Google Scholar] [CrossRef]
- Gonzales, G.B.; Smagghe, G.; Wittevrongel, J.; Huynh, N.T.; Camp, J.V.; Raes, K. Metabolism of quercetin and naringenin by food-grade fungal inoculum, Rhizopus azygosporus Yuan et Jong (ATCC 48108). J. Agric. Food Chem. 2016, 64, 9263–9267. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Chan, P.L.; Xie, Y.; Ma, K.L.; Cheung, M.K.; Kwan, H.S. Modified recipe to inhibit fruiting body formation for living fungal sylocybin manufacture. PLoS ONE 2019, 14, e0209812. [Google Scholar] [CrossRef]
- Frings, R.A.; Maciá-Vicente, J.G.; Buße, S.; Čmoková, A.; Kellner, A.; Hofrichter, M.; Hennicke, F. Multilocus phylogeny- and fruiting feature-assisted delimitation of european Cyclocybe aegerita from a new asian species complex and related species. Mycol. Prog. 2020, 19, 1001–1016. [Google Scholar] [CrossRef] [PubMed]
- Hasnat, M.A.; Pervin, M.; Lim, B.O. Acetylcholinesterase Inhibition and in Vitro and in Vivo Antioxidant Activities of Ganoderma lucidum Grown on Germinated Brown Rice. Molecules 2013, 18, 6663–6678. [Google Scholar] [CrossRef]
- Lin, J.-T.; Liu, C.-W.; Chen, Y.-C.; Hu, C.-C.; Juang, L.-D.; Shiesh, C.-C.; Yang, D.-J. Chemical composition, antioxidant and anti-inflammatory properties for ethanolic extracts from Pleurotus eryngii fruiting bodies harvested at different time. LWT-Food Sci. Technol. 2014, 55, 374–382. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Baghdadi, A.; Jaafar, H.Z.E.; Swamy, M.K.; Wahab, P.E.M. Optimization of Flavonoid Extraction from Red and Brown Rice Bran and Evaluation of the Antioxidant Properties. Molecules 2018, 23, 1863. [Google Scholar] [CrossRef]
- Du, S.; Huang, X.; Cai, Y.; Hao, Y.; Qiu, S.; Liu, L.; Cui, M.; Luo, L. Differential Antioxidant Compounds and Activities in Seedlings of Two Rice Cultivars Under Chilling Treatment. Front. Plant Sci. 2021, 12, 631421. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, S.; Bai, X.; Li, X.; Wang, Q.; Wang, L.; Wang, W.; Li, Z. Transcriptomic and Non-Targeted Metabolomic Analyses Reveal the Flavonoid Biosynthesis Pathway in Auricularia cornea. Molecules 2022, 27, 2334. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Z.; Wang, X.; Liu, R.; Zou, L. Mushrooms Do Produce Flavonoids: Metabolite Profiling and Transcriptome Analysis of Flavonoid Synthesis in the Medicinal Mushroom Sanghuangporus baumii. J. Fungi 2022, 8, 582. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Ma, L.-T.; Lee, Y.-R.; Shaw, J.-F.; Wang, S.-Y.; Chu, F.-H. Differential gene expression network in terpenoid synthesis of Antrodia cinnamomea in mycelia and fruiting bodies. J. Agric. Food Chem. 2017, 65, 1874–1886. [Google Scholar] [CrossRef]
- Song, H.-Y.; Kim, D.-H.; Kim, J.-M. Comparative transcriptome analysis of dikaryotic mycelia and mature fruiting bodies in the edible mushroom Lentinula edodes. Sci. Rep. 2018, 8, 8983. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, X.; Gao, W.; Zhao, M.; Zhang, J.; Huang, C. Differential Expression Patterns of Pleurotus ostreatus Catalase Genes during Developmental Stages and under Heat Stress. Genes 2017, 8, 335. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Dai, Y.; Yang, C.; Wei, P.; Song, B.; Yang, Y.; Sun, L.; Zhang, Z.-W.; Li, Y. Comparative transcriptome analysis identified candidate genes related to Bailinggu mushroom formation and genetic markers for genetic analyses and breeding. Sci. Rep. 2017, 7, 9266. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Xiao, X.; Wang, J.; Chen, C.-Y.O.; Hu, H. Polyphenolic composition and antioxidant, antiproliferative, and antimicrobial activities of mushroom Inonotus sanghuang. LWT-Food Sci. Technol. 2017, 82, 154–161. [Google Scholar] [CrossRef]
- Shao, Y.; Guo, H.; Zhang, J.; Liu, H.; Wang, K.; Zuo, S.; Xu, P.; Xia, Z.; Zhou, Q.; Zhang, H.; et al. The Genome of the Medicinal Macrofungus Sanghuang Provides Insights Into the Synthesis of Diverse Secondary Metabolites. Front. Microbiol. 2020, 10, 3035. [Google Scholar] [CrossRef] [PubMed]
- Mapari, S.A.S.; Meyer, A.S.; Thrane, U.; Frisvad, J.C. Identification of potentially safe promising fungal cell factories for the production of polyketide natural food colorants using chemotaxonomic rationale. Microb. Cell Fact. 2009, 8, 24. [Google Scholar] [CrossRef]
- Brilhante, R.S.N.; da Rocha, M.G.; de Oliveira, J.S.; Pereira-Neto, W.A.; de Melo Guedes, G.M.; de Aguiar Cordeiro, R.; Sidrim, J.J.C.; Rocha, M.F.G.; Castelo-Branco, D.d.S.C.M. Cryptococcus neoformans/Cryptococcus gattii species complex melanized by epinephrine: Increased yeast survival after amphotericin B exposure. Microb. Pathog. 2020, 143, 104123. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Q.; Zhang, J.; Liu, K.; Li, K.; Liu, G.; Dong, C. Comparative Transcriptome Analysis Between a Spontaneous Albino Mutant and its Sibling Strain of Cordyceps militaris in Response to Light Stress. Front. Microbiol. 2018, 9, 1237. [Google Scholar] [CrossRef]
- Fasciana, T.; Gargano, M.L.; Serra, N.; Galia, E.; Arrigo, I.; Tricoli, M.R.; Diquattro, O.; Graceffa, G.; Vieni, S.; Venturella, G.; et al. Potential Activity of Albino Grifola frondosa Mushroom Extract against Biofilm of Meticillin-Resistant Staphylococcus aureus. J. Fungi 2021, 7, 551. [Google Scholar] [CrossRef]
- Alam, N.; Yoon, K.N.; Lee, T.S. Evaluation of the antioxidant and antityrosinase activities of three extracts from Pleurotus nebrodensis fruiting bodies. Afr. J. Biotechnol. 2011, 10, 2978–2986. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pukalski, J.; Latowski, D. Secrets of Flavonoid Synthesis in Mushroom Cells. Cells 2022, 11, 3052. https://doi.org/10.3390/cells11193052
Pukalski J, Latowski D. Secrets of Flavonoid Synthesis in Mushroom Cells. Cells. 2022; 11(19):3052. https://doi.org/10.3390/cells11193052
Chicago/Turabian StylePukalski, Jan, and Dariusz Latowski. 2022. "Secrets of Flavonoid Synthesis in Mushroom Cells" Cells 11, no. 19: 3052. https://doi.org/10.3390/cells11193052
APA StylePukalski, J., & Latowski, D. (2022). Secrets of Flavonoid Synthesis in Mushroom Cells. Cells, 11(19), 3052. https://doi.org/10.3390/cells11193052






