Immunogenic Cell Death: An Emerging Target in Gastrointestinal Cancers
Abstract
1. Introduction
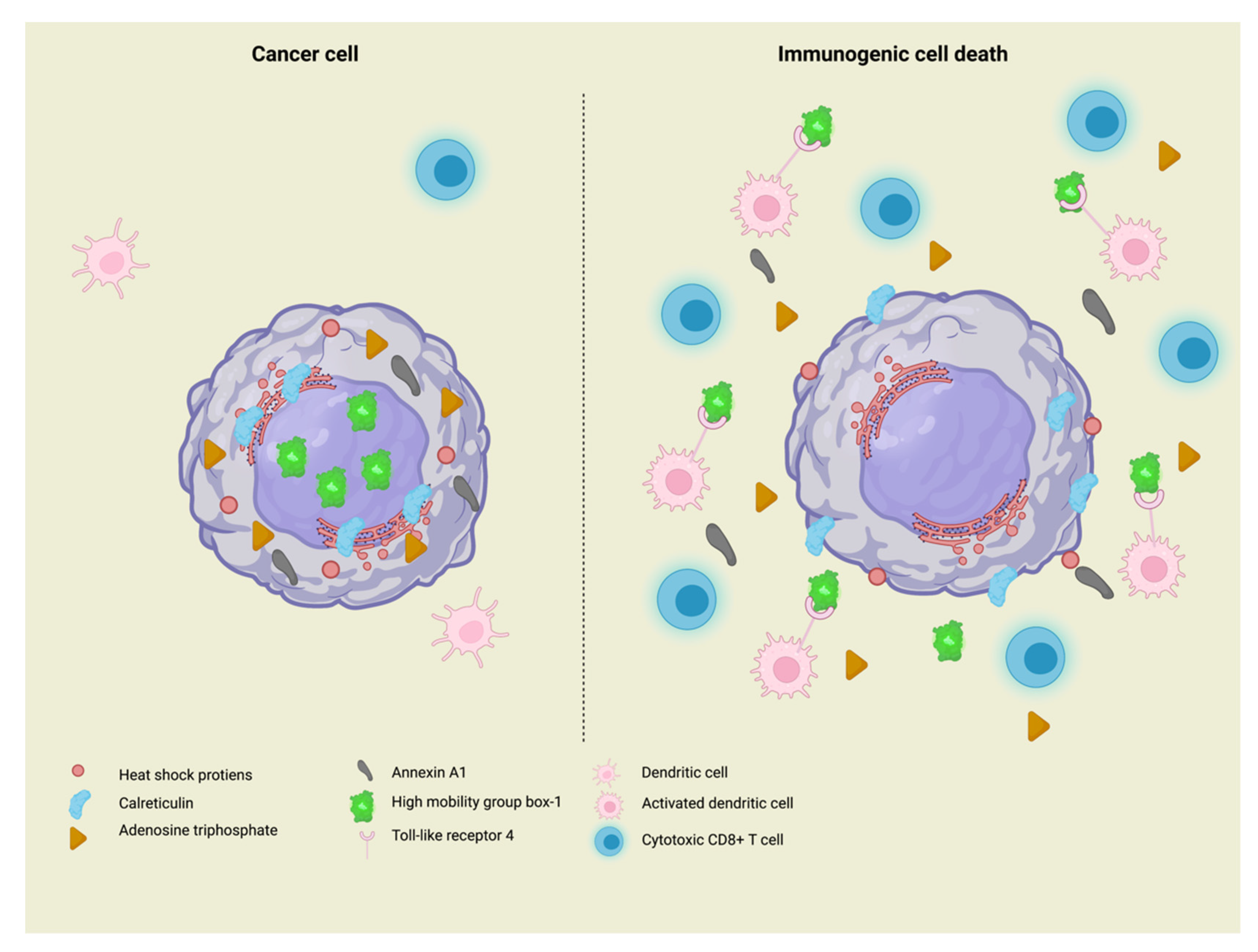
2. Immunogenic Cell Death
3. ICD in Colorectal Cancer
4. ICD in Gastric Cancer
5. ICD in Pancreatic Cancer
6. ICD in Liver Cancer
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Green, D.R.; Ferguson, T.; Zitvogel, L.; Kroemer, G. Immunogenic and Tolerogenic Cell Death. Nat. Rev. Immunol. 2009, 9, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Casares, N.; Pequignot, M.O.; Tesniere, A.; Ghiringhelli, F.; Roux, S.; Chaput, N.; Schmitt, E.; Hamai, A.; Hervas-Stubbs, S.; Obeid, M.; et al. Caspase-Dependent Immunogenicity of Doxorubicin-Induced Tumor Cell Death. J. Exp. Med. 2005, 202, 1691–1701. [Google Scholar] [CrossRef]
- Tesniere, A.; Schlemmer, F.; Boige, V.; Kepp, O.; Martins, I.; Ghiringhelli, F.; Aymeric, L.; Michaud, M.; Apetoh, L.; Barault, L.; et al. Immunogenic Death of Colon Cancer Cells Treated with Oxaliplatin. Oncogene 2010, 29, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, C.; Cuomo, A.; Spadoni, I.; Magni, E.; Silvola, A.; Conte, A.; Sigismund, S.; Ravenda, P.S.; Bonaldi, T.; Zampino, M.G.; et al. The EGFR-Specific Antibody Cetuximab Combined with Chemotherapy Triggers Immunogenic Cell Death. Nat. Med. 2016, 22, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Spisek, R.; Charalambous, A.; Mazumder, A.; Vesole, D.H.; Jagannath, S.; Dhodapkar, M.V. Bortezomib Enhances Dendritic Cell (DC)–Mediated Induction of Immunity to Human Myeloma via Exposure of Cell Surface Heat Shock Protein 90 on Dying Tumor Cells: Therapeutic Implications. Blood 2007, 109, 4839–4845. [Google Scholar] [CrossRef] [PubMed]
- Golden, E.B.; Frances, D.; Pellicciotta, I.; Demaria, S.; Barcellos-Hoff, M.H.; Formenti, S.C. Radiation Fosters Dose-Dependent and Chemotherapy-Induced Immunogenic Cell Death. OncoImmunology 2014, 3, e28518. [Google Scholar] [CrossRef] [PubMed]
- Shekarian, T.; Sivado, E.; Jallas, A.-C.; Depil, S.; Kielbassa, J.; Janoueix-Lerosey, I.; Hutter, G.; Goutagny, N.; Bergeron, C.; Viari, A.; et al. Repurposing Rotavirus Vaccines for Intratumoral Immunotherapy Can Overcome Resistance to Immune Checkpoint Blockade. Sci. Transl. Med. 2019, 11, eaat5025. [Google Scholar] [CrossRef]
- Garg, A.D.; Dudek, A.M.; Ferreira, G.B.; Verfaillie, T.; Vandenabeele, P.; Krysko, D.V.; Mathieu, C.; Agostinis, P. ROS-Induced Autophagy in Cancer Cells Assists in Evasion from Determinants of Immunogenic Cell Death. Autophagy 2013, 9, 1292–1307. [Google Scholar] [CrossRef]
- Tatsuno, K.; Yamazaki, T.; Hanlon, D.; Han, P.; Robinson, E.; Sobolev, O.; Yurter, A.; Rivera-Molina, F.; Arshad, N.; Edelson, R.L.; et al. Extracorporeal Photochemotherapy Induces Bona Fide Immunogenic Cell Death. Cell Death Dis. 2019, 10, 578. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Long-Term Outcomes with Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients with Advanced Melanoma. J. Clin. Oncol. 2022, 40, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez–Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non–Small-Cell Lung Cancer with PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 2019, 37, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Ciuleanu, T.-E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. First-Line Nivolumab plus Ipilimumab Combined with Two Cycles of Chemotherapy in Patients with Non-Small-Cell Lung Cancer (CheckMate 9LA): An International, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2021, 22, 198–211. [Google Scholar] [CrossRef]
- Motzer, R.J.; Rini, B.I.; McDermott, D.F.; Arén Frontera, O.; Hammers, H.J.; Carducci, M.A.; Salman, P.; Escudier, B.; Beuselinck, B.; Amin, A.; et al. Nivolumab plus Ipilimumab versus Sunitinib in First-Line Treatment for Advanced Renal Cell Carcinoma: Extended Follow-up of Efficacy and Safety Results from a Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2019, 20, 1370–1385. [Google Scholar] [CrossRef]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Tomczak, P.; Park, S.H.; Venugopal, B.; Ferguson, T.; Symeonides, S.N.; Hajek, J.; Gurney, H.; Chang, Y.-H.; Lee, J.L.; et al. Pembrolizumab versus Placebo as Post-Nephrectomy Adjuvant Therapy for Clear Cell Renal Cell Carcinoma (KEYNOTE-564): 30-Month Follow-up Analysis of a Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2022, 23, 1133–1144. [Google Scholar] [CrossRef]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef]
- Royal, R.E.; Levy, C.; Turner, K.; Mathur, A.; Hughes, M.; Kammula, U.S.; Sherry, R.M.; Topalian, S.L.; Yang, J.C.; Lowy, I.; et al. Phase 2 Trial of Single Agent Ipilimumab (Anti-CTLA-4) for Locally Advanced or Metastatic Pancreatic Adenocarcinoma. J. Immunother. 2010, 33, 828–833. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2022. [Google Scholar]
- Kroemer, G.; Galassi, C.; Zitvogel, L.; Galluzzi, L. Immunogenic Cell Stress and Death. Nat. Immunol. 2022, 23, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Perreault, C.; Finn, O.J.; Kroemer, G. Beneficial Autoimmunity Improves Cancer Prognosis. Nat. Rev. Clin. Oncol. 2021, 18, 591–602. [Google Scholar] [CrossRef]
- Jhunjhunwala, S.; Hammer, C.; Delamarre, L. Antigen Presentation in Cancer: Insights into Tumour Immunogenicity and Immune Evasion. Nat. Rev. Cancer 2021, 21, 298–312. [Google Scholar] [CrossRef]
- Sprooten, J.; Garg, A.D. Type I interferons and endoplasmic reticulum stress in health and disease. In International Review of Cell and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 350, pp. 63–118. ISBN 978-0-12-819744-8. [Google Scholar]
- Kepp, O.; Bezu, L.; Yamazaki, T.; Di Virgilio, F.; Smyth, M.J.; Kroemer, G.; Galluzzi, L. ATP and Cancer Immunosurveillance. EMBO J. 2021, 40, e108130. [Google Scholar] [CrossRef]
- Vacchelli, E.; Ma, Y.; Baracco, E.E.; Sistigu, A.; Enot, D.P.; Pietrocola, F.; Yang, H.; Adjemian, S.; Chaba, K.; Semeraro, M.; et al. Chemotherapy-Induced Antitumor Immunity Requires Formyl Peptide Receptor 1. Science 2015, 350, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Apetoh, L.; Ghiringhelli, F.; Tesniere, A.; Obeid, M.; Ortiz, C.; Criollo, A.; Mignot, G.; Maiuri, M.C.; Ullrich, E.; Saulnier, P.; et al. Toll-like Receptor 4–Dependent Contribution of the Immune System to Anticancer Chemotherapy and Radiotherapy. Nat. Med. 2007, 13, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Hopfner, K.-P.; Hornung, V. Molecular Mechanisms and Cellular Functions of CGAS–STING Signalling. Nat. Rev. Mol. Cell Biol. 2020, 21, 501–521. [Google Scholar] [CrossRef] [PubMed]
- Bezu, L.; Sauvat, A.; Humeau, J.; Gomes-da-Silva, L.C.; Iribarren, K.; Forveille, S.; Garcia, P.; Zhao, L.; Liu, P.; Zitvogel, L.; et al. EIF2α Phosphorylation Is Pathognomonic for Immunogenic Cell Death. Cell Death Differ. 2018, 25, 1375–1393. [Google Scholar] [CrossRef] [PubMed]
- Fucikova, J.; Spisek, R.; Kroemer, G.; Galluzzi, L. Calreticulin and Cancer. Cell Res. 2021, 31, 5–16. [Google Scholar] [CrossRef]
- Togashi, Y.; Shitara, K.; Nishikawa, H. Regulatory T Cells in Cancer Immunosuppression—Implications for Anticancer Therapy. Nat. Rev. Clin. Oncol. 2019, 16, 356–371. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Chan, T.A.; Kroemer, G.; Wolchok, J.D.; López-Soto, A. The Hallmarks of Successful Anticancer Immunotherapy. Sci. Transl. Med. 2018, 10, eaat7807. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Xiao, Y.; Loupakis, F.; Kawanishi, N.; Wang, J.; Battaglin, F.; Soni, S.; Zhang, W.; Mancao, C.; Salhia, B.; et al. Immunogenic Cell Death Pathway Polymorphisms for Predicting Oxaliplatin Efficacy in Metastatic Colorectal Cancer. J. Immunother. Cancer 2020, 8, e001714. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Shen, P.; Ma, Y.; Ma, W.; Wu, H.; Liu, H.; An, Q. Bullatacin Triggers Immunogenic Cell Death of Colon Cancer Cells by Activating Endoplasmic Reticulum Chaperones. J. Inflamm. 2021, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.C.-Y.; Chiang, S.-F.; Yang, P.-C.; Ke, T.-W.; Chen, T.-W.; Hu, C.-H.; Huang, Y.-W.; Chang, H.-Y.; Chen, W.T.-L.; Chao, K.S.C. Immunogenic Cell Death by the Novel Topoisomerase I Inhibitor TLC388 Enhances the Therapeutic Efficacy of Radiotherapy. Cancers 2021, 13, 1218. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability–High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.A.; Shiu, K.-K.; Kim, T.-W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab versus Chemotherapy for Microsatellite Instability-High or Mismatch Repair-Deficient Metastatic Colorectal Cancer (KEYNOTE-177): Final Analysis of a Randomised, Open-Label, Phase 3 Study. Lancet Oncol. 2022, 23, 659–670. [Google Scholar] [CrossRef]
- Antoniotti, C.; Rossini, D.; Pietrantonio, F.; Catteau, A.; Salvatore, L.; Lonardi, S.; Boquet, I.; Tamberi, S.; Marmorino, F.; Moretto, R.; et al. Upfront FOLFOXIRI plus Bevacizumab with or without Atezolizumab in the Treatment of Patients with Metastatic Colorectal Cancer (AtezoTRIBE): A Multicentre, Open-Label, Randomised, Controlled, Phase 2 Trial. Lancet Oncol. 2022, 23, 876–887. [Google Scholar] [CrossRef]
- Salvatore, L.; Bensi, M.; Corallo, S.; Bergamo, F.; Pellegrini, I.; Rasola, C.; Borelli, B.; Tamburini, E.; Randon, G.; Galuppo, S.; et al. Phase II Study of Preoperative (PREOP) Chemoradiotherapy (CTRT) plus Avelumab (AVE) in Patients (PTS) with Locally Advanced Rectal Cancer (LARC): The AVANA Study. J. Clin. Oncol. 2021, 39 (Suppl. 15), 3511. [Google Scholar] [CrossRef]
- Laengle, J.; Kuehrer, I.; Pils, D.; Stift, A.; Teleky, B.; Herbst, F.; Dauser, B.; Monschein, M.; Razek, P.; Haegele, S.; et al. Interim Analysis of Neoadjuvant Chemoradiotherapy with Sequential Ipilimumab and Nivolumab in Rectal Cancer (CHINOREC): A Prospective Randomized, Open-Label, Multicenter, Phase II Clinical Trial. J. Clin. Oncol. 2022, 40 (Suppl. 16), e15604. [Google Scholar] [CrossRef]
- Petersen, S.H.; Kua, L.F.; Nakajima, S.; Yong, W.P.; Kono, K. Chemoradiation Induces Upregulation of Immunogenic Cell Death-Related Molecules Together with Increased Expression of PD-L1 and Galectin-9 in Gastric Cancer. Sci. Rep. 2021, 11, 12264. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Kawazoe, A.; Yañez, P.; Li, N.; Lonardi, S.; Kolesnik, O.; Barajas, O.; Bai, Y.; Shen, L.; Tang, Y.; et al. The KEYNOTE-811 Trial of Dual PD-1 and HER2 Blockade in HER2-Positive Gastric Cancer. Nature 2021, 600, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Campos Bragagnoli, A.; et al. First-Line Nivolumab plus Chemotherapy versus Chemotherapy Alone for Advanced Gastric, Gastro-Oesophageal Junction, and Oesophageal Adenocarcinoma (CheckMate 649): A Randomised, Open-Label, Phase 3 Trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef]
- Xing, X.; Shi, J.; Jia, Y.; Dou, Y.; Li, Z.; Dong, B.; Guo, T.; Cheng, X.; Li, X.; Du, H.; et al. Effect of Neoadjuvant Chemotherapy on the Immune Microenvironment in Gastric Cancer as Determined by Multiplex Immunofluorescence and T Cell Receptor Repertoire Analysis. J. Immunother. Cancer 2022, 10, e003984. [Google Scholar] [CrossRef] [PubMed]
- Verschoor, Y.L.; Kodach, L.; van den Berg, J.; van Sandick, J.W.; van Dieren, J.; Balduzzi, S.; Grootscholten, C.; Veenhof, X.; Hartemink, K.; Vollebergh, M.A.; et al. Neoadjuvant Atezolizumab plus Docetaxel/Oxaliplatin/Capecitabine in Non-Metastatic Gastric and Gastroesophageal Junction Adenocarcinoma: The PANDA Trial. J. Clin. Oncol. 2022, 40 (Suppl. 16), 4059. [Google Scholar] [CrossRef]
- Al-Batran, S.-E.; Lorenzen, S.; Thuss-Patience, P.C.; Homann, N.; Schenk, M.; Lindig, U.; Heuer, V.; Kretzschmar, A.; Goekkurt, E.; Haag, G.M.; et al. Surgical and Pathological Outcome, and Pathological Regression, in Patients Receiving Perioperative Atezolizumab in Combination with FLOT Chemotherapy versus FLOT Alone for Resectable Esophagogastric Adenocarcinoma: Interim Results from DANTE, a Randomized, Multicenter, Phase IIb Trial of the FLOT-AIO German Gastric Cancer Group and Swiss SAKK. J. Clin. Oncol. 2022, 40 (Suppl. 16), 4003. [Google Scholar] [CrossRef]
- Tougeron, D.; Dahan, L.; El Hajbi, F.; Le Malicot, K.; Evesque, L.; Aparicio, T.; Bouché, O.; Bonichon-Lamichhane, N.; Chibaudel, B.; Angelergues, A.; et al. The PRODIGE 59-DURIGAST Trial: A Randomized Phase II Study Evaluating FOLFIRI plus Durvalumab and FOLFIRI plus Durvalumab plus Tremelimumab in Second-Line Treatment of Patients with Advanced Gastric or Gastro-Esophageal Junction Adenocarcinoma. J. Clin. Oncol. 2022, 40 (Suppl. 16), 4036. [Google Scholar] [CrossRef]
- Agostini, A.; Orlacchio, A.; Carbone, C.; Guerriero, I. Understanding Tricky Cellular and Molecular Interactions in Pancreatic Tumor Microenvironment: New Food for Thought. Front. Immunol. 2022, 13, 876291. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, K.; Zhao, R.; Ji, T.; Wang, X.; Yang, X.; Zhang, Y.; Cheng, K.; Liu, S.; Hao, J.; et al. Inducing Enhanced Immunogenic Cell Death with Nanocarrier-Based Drug Delivery Systems for Pancreatic Cancer Therapy. Biomaterials 2016, 102, 187–197. [Google Scholar] [CrossRef]
- Cebrián, M.J.G.; Bauden, M.; Andersson, R.; Holdenrieder, S.; Ansari, D. Paradoxical Role of HMGB1 in Pancreatic Cancer: Tumor Suppressor or Tumor Promoter? Anticancer Res. 2016, 36, 4381–4390. [Google Scholar] [CrossRef]
- Wittwer, C.; Boeck, S.; Heinemann, V.; Haas, M.; Stieber, P.; Nagel, D.; Holdenrieder, S. Circulating Nucleosomes and Immunogenic Cell Death Markers HMGB1, SRAGE and DNAse in Patients with Advanced Pancreatic Cancer Undergoing Chemotherapy: HMGB1, SRAGE, DNAse and Circulating Nucleosomes in PC. Int. J. Cancer 2013, 133, 2619–2630. [Google Scholar] [CrossRef]
- Lu, J.; Liu, X.; Liao, Y.-P.; Salazar, F.; Sun, B.; Jiang, W.; Chang, C.H.; Jiang, J.; Wang, X.; Wu, A.M.; et al. Nano-Enabled Pancreas Cancer Immunotherapy Using Immunogenic Cell Death and Reversing Immunosuppression. Nat. Commun. 2017, 8, 1811. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.L.; Yogaratnam, Y.; Samad, M.; Kasow, S.; Dalgleish, A.G. Effect of Gemcitabine Based Chemotherapy on the Immunogenicity of Pancreatic Tumour Cells and T-Cells. Clin. Transl. Oncol. 2021, 23, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Padrón, L.J.; Maurer, D.M.; O’Hara, M.H.; O’Reilly, E.M.; Wolff, R.A.; Wainberg, Z.A.; Ko, A.H.; Fisher, G.; Rahma, O.; Lyman, J.P.; et al. Sotigalimab and/or Nivolumab with Chemotherapy in First-Line Metastatic Pancreatic Cancer: Clinical and Immunologic Analyses from the Randomized Phase 2 PRINCE Trial. Nat. Med. 2022, 28, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Mills, B.N.; Zhao, T.; Han, B.J.; Murphy, J.D.; Patel, A.P.; Johnston, C.J.; Lord, E.M.; Belt, B.A.; Linehan, D.C.; et al. Assessing the Magnitude of Immunogenic Cell Death Following Chemotherapy and Irradiation Reveals a New Strategy to Treat Pancreatic Cancer. Cancer Immunol. Res. 2020, 8, 94–107. [Google Scholar] [CrossRef]
- Van Loenhout, J.; Flieswasser, T.; Boullosa, L.F.; De Waele, J.; Van Audenaerde, J.; Marcq, E.; Jacobs, J.; Lin, A.; Lion, E.; Dewitte, H.; et al. Cold Atmospheric Plasma-Treated PBS Eliminates Immunosuppressive Pancreatic Stellate Cells and Induces Immunogenic Cell Death of Pancreatic Cancer Cells. Cancers 2019, 11, 1597. [Google Scholar] [CrossRef]
- Duewell, P.; Steger, A.; Lohr, H.; Bourhis, H.; Hoelz, H.; Kirchleitner, S.V.; Stieg, M.R.; Grassmann, S.; Kobold, S.; Siveke, J.T.; et al. RIG-I-like Helicases Induce Immunogenic Cell Death of Pancreatic Cancer Cells and Sensitize Tumors toward Killing by CD8+ T Cells. Cell Death Differ. 2014, 21, 1825–1837. [Google Scholar] [CrossRef]
- Carbone, C.; Piro, G.; Agostini, A.; Delfino, P.; De Sanctis, F.; Nasca, V.; Spallotta, F.; Sette, C.; Martini, M.; Ugel, S.; et al. Intratumoral Injection of TLR9 Agonist Promotes an Immunopermissive Microenvironment Transition and Causes Cooperative Antitumor Activity in Combination with Anti-PD1 in Pancreatic Cancer. J. Immunother. Cancer 2021, 9, e002876. [Google Scholar] [CrossRef]
- Seery, T.E.; Nangia, C.S.; McKean, H.A.; Bhar, P.; Sender, L.S.; Reddy, S.K.; Soon-Shiong, P. Phase 2 Quilt 88 Trial of DAMP Inducers Combined with IL15 Superagonist, N-803, and Anti–PD-L1 NK Cell Therapy More than Doubles Historical Overall Survival in Patients with Third- to Sixth-Line Advanced Pancreatic Cancer. J. Clin. Oncol. 2022, 40 (Suppl. 16), 4147. [Google Scholar] [CrossRef]
- Pinato, D.J.; Murray, S.M.; Forner, A.; Kaneko, T.; Fessas, P.; Toniutto, P.; Mínguez, B.; Cacciato, V.; Avellini, C.; Diaz, A.; et al. Trans-Arterial Chemoembolization as a Loco-Regional Inducer of Immunogenic Cell Death in Hepatocellular Carcinoma: Implications for Immunotherapy. J. Immunother. Cancer 2021, 9, e003311. [Google Scholar] [CrossRef]
- Saborowski, A.; Waldschmidt, D.; Hinrichs, J.; Ettrich, T.J.; Martens, U.M.; Mekolli, A.; De Toni, E.N.; Berg, T.; Geißler, M.; Hausner, G.; et al. IMMUTACE: A Biomarker-Orientated Phase II, Single-Arm, Open-Label AIO Study of Transarterial Chemoembolization (TACE) in Combination with Nivolumab Performed for Intermediate-Stage Hepatocellular Carcinoma (HCC.; AIO-HEP-0217)—Updated Efficacy Results. J. Clin. Oncol. 2022, 40 (Suppl. 16), 4116. [Google Scholar] [CrossRef]
- Ma, Y.T.; Kirkham, A.; Curbishley, S.; Rowe, A.; Blahova, M.; Mehrzad, H.; Karkhanis, S.; Punia, P.; James, M.; Rao, A.R.; et al. A Randomised Phase II Clinical Trial of Low-Dose Cyclophosphamide and Transarterial Chemoembolization (TACE) with or without Vaccination with Dendritic Cells (DC) Pulsed with HepG2 Lysate Ex Vivo in Patients with Hepatocellular Carcinoma (HCC): The ImmunoTACE Trial. J. Clin. Oncol. 2022, 40 (Suppl. 16), 4012. [Google Scholar]
- Chen, Y.; Yang, P.; Du, S.; Zhou, J.; Huang, C.; Zhu, W.; Hu, Y.; Yu, Y.; Liu, T.; Zeng, Z. A Phase II Study of Stereotactic Body Radiotherapy (SBRT) Combined with Sintilimab in Patients with Recurrent or Oligometastatic Hepatocellular Carcinoma (HCC). J. Clin. Oncol. 2022, 40 (Suppl. 16), 4071. [Google Scholar] [CrossRef]
- Zhu, H.; Shan, Y.; Ge, K.; Lu, J.; Kong, W.; Jia, C. Oxaliplatin Induces Immunogenic Cell Death in Hepatocellular Carcinoma Cells and Synergizes with Immune Checkpoint Blockade Therapy. Cell Oncol. 2020, 43, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
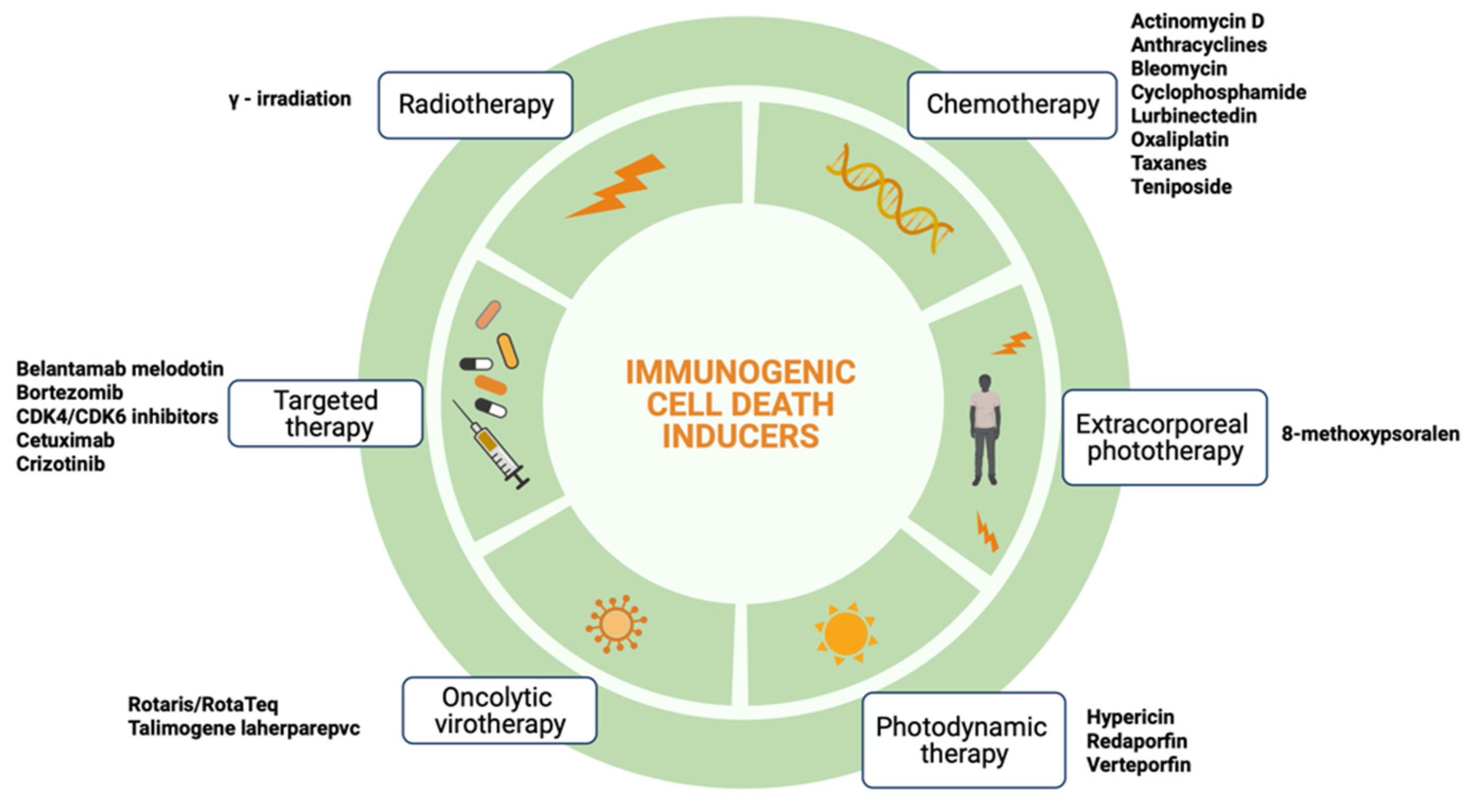
| ICD Inducers | CALR Exposure | HMGB-1 Release | eIF2α Phosphorylation | ANXA1 Release | ATP Release | HPS70 Exposure | IFN-1 Release |
|---|---|---|---|---|---|---|---|
| Oxaliplatin | x | x | x | x | x | x | |
| Anthracyclines | x | x | x | x | x | x | |
| Bleomycin | x | x | x | x | |||
| Radiotherapy | x | x | x | x | |||
| Photodynamic therapy | x | x | x | ||||
| Oncolytic virotherapy | x | x | x | x | |||
| Extracorporeal phototherapy | x | x | x |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiaravalli, M.; Spring, A.; Agostini, A.; Piro, G.; Carbone, C.; Tortora, G. Immunogenic Cell Death: An Emerging Target in Gastrointestinal Cancers. Cells 2022, 11, 3033. https://doi.org/10.3390/cells11193033
Chiaravalli M, Spring A, Agostini A, Piro G, Carbone C, Tortora G. Immunogenic Cell Death: An Emerging Target in Gastrointestinal Cancers. Cells. 2022; 11(19):3033. https://doi.org/10.3390/cells11193033
Chicago/Turabian StyleChiaravalli, Marta, Alexia Spring, Antonio Agostini, Geny Piro, Carmine Carbone, and Giampaolo Tortora. 2022. "Immunogenic Cell Death: An Emerging Target in Gastrointestinal Cancers" Cells 11, no. 19: 3033. https://doi.org/10.3390/cells11193033
APA StyleChiaravalli, M., Spring, A., Agostini, A., Piro, G., Carbone, C., & Tortora, G. (2022). Immunogenic Cell Death: An Emerging Target in Gastrointestinal Cancers. Cells, 11(19), 3033. https://doi.org/10.3390/cells11193033








