MgrB Mutations and Altered Cell Permeability in Colistin Resistance in Klebsiella pneumoniae
Abstract
1. Introduction
2. Action of Colistin on Bacterial OM
Overview of Colistin Resistance in K. pneumoniae
| Resistance Mechanism | Genes Involved | References | |
|---|---|---|---|
| Chromosomal-mediated | Lipid A modification with L-Ara4N addition | arnBCADTEF operon | [9] |
| Lipid A modification with PEtn | pmrC | [17] | |
| LPS biosysnthesis | yciM * | [19] | |
| Activation of LPS-modifying operation in the two-component systems | pmrA/pmrBphoP/phoQqseB/qseC *crrA/crrB * | [9,18,23,36] | |
| Inactivation of negative feedback regulator of the PhoP/PhoQ system | mgrB | [8,9] | |
| Increased lipid A acylation | lpxM * | [21] | |
| Efflux pump | acrAB, kpnEF | [24,25] | |
| Plasmid-mediated | Lipid A modification with PEtn | mcr genes | [5] |
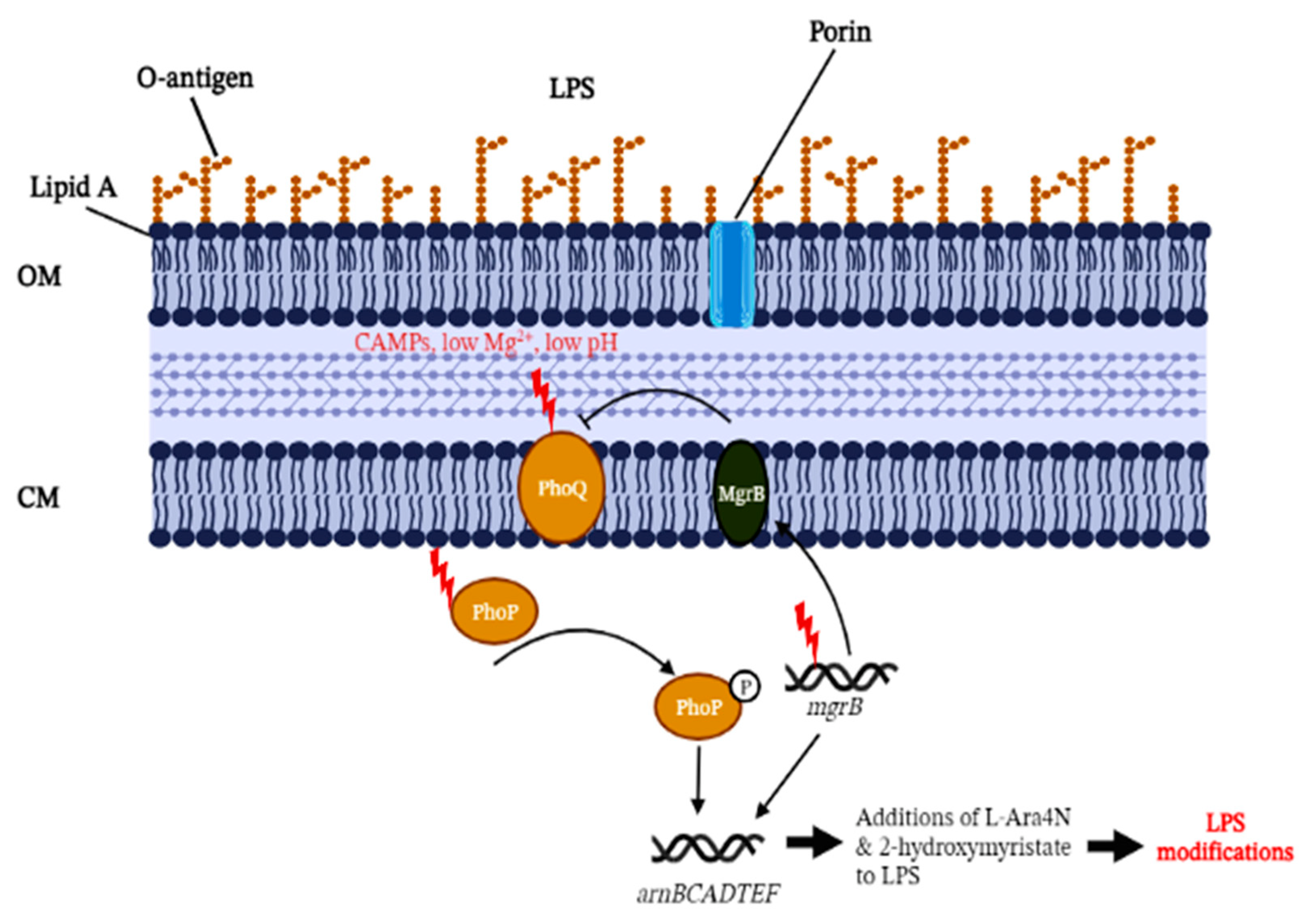
3. MgrB as a PhoP/PhoQ Regulator
3.1. MgrB-Dependent Lipid A Modifications
3.2. MgrB-Dependent Altered Cell Morphology
4. New OM-Targeting Antibiotics
4.1. Polymyxin Derivatives
4.2. Hydrophobic Membrane-Active Agents
4.3. Challenges and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wang, S.H.; Yang, K.Y.; Sheu, C.C.; Chen, W.C.; Chan, M.C.; Feng, J.Y.; Chen, C.M.; Wu, B.R.; Zheng, Z.R.; Chou, Y.C.; et al. The necessity of a loading dose when prescribing intravenous colistin in critically ill patients with CRGNB-associated pneumonia: A multi-center observational study. Crit. Care 2022, 26, 91. [Google Scholar] [CrossRef]
- May, K.L.; Grabowicz, M. The bacterial outer membrane is an evolving antibiotic barrier. Proc. Natl. Acad. Sci. USA 2018, 115, 8852–8854. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.I. Antibiotic Resistance and Regulation of the Gram-Negative Bacterial Outer Membrane Barrier by Host Innate Immune Molecules. mBio 2016, 7, e01541-16. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H. Antibiotic resistance caused by gram-negative multidrug efflux pumps. Clin. Infect. Dis. 1998, 27, S32–S41. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet. Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, Y.; Xiao, Y. Prevalence and transmission of mobilized colistin resistance (mcr) gene in bacteria common to animals and humans. Biosaf. Health 2020, 2, 71–78. [Google Scholar] [CrossRef]
- Khondker, A.; Rheinstadter, M.C. How do bacterial membranes resist polymyxin antibiotics? Commun. Biol. 2020, 3, 77. [Google Scholar] [CrossRef] [PubMed]
- Olaitan, A.O.; Morand, S.; Rolain, J.M. Mechanisms of polymyxin resistance: Acquired and intrinsic resistance in bacteria. Front. Microbiol. 2014, 5, 643. [Google Scholar] [CrossRef]
- Cannatelli, A.; Giani, T.; D’Andrea, M.M.; Di Pilato, V.; Arena, F.; Conte, V.; Tryfinopoulou, K.; Vatopoulos, A.; Rossolini, G.M.; COLGRIT Study Group. MgrB inactivation is a common mechanism of colistin resistance in KPC-producing Klebsiella pneumoniae of clinical origin. Antimicrob. Agents Chemother. 2014, 58, 5696–5703. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, K.E.; Thi Nguyen, T.N.; Boinett, C.J.; Baker, S.; Simionatto, S. Molecular and epidemiological surveillance of polymyxin-resistant Klebsiella pneumoniae strains isolated from Brazil with multiple mgrB gene mutations. Int. J. Med. Microbiol. 2020, 310, 151448. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Jayol, A.; Bontron, S.; Villegas, M.V.; Ozdamar, M.; Turkoglu, S.; Nordmann, P. The mgrB gene as a key target for acquired resistance to colistin in Klebsiella pneumoniae. J. Antimicrob. Chemother. 2015, 70, 75–80. [Google Scholar] [CrossRef]
- Nwabor, O.F.; Terbtothakun, P.; Voravuthikunchai, S.P.; Chusri, S. A Bibliometric Meta-Analysis of Colistin Resistance in Klebsiella pneumoniae. Diseases 2021, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.F.; Silva, D.; Rodrigues, A.; Pina-Vaz, C. Colistin Update on Its Mechanism of Action and Resistance, Present and Future Challenges. Microorganisms 2020, 8, 1716. [Google Scholar] [CrossRef] [PubMed]
- Teuber, M.; Bader, J. Action of polymyxin B on bacterial membranes. Arch. Microbiol. 1976, 109, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Mularski, A.; Wilksch, J.; Hanssen, E.; Li, J.; Tomita, T.; Pidot, S.J.; Stinear, T.; Separovic, F.; Strugnell, D. A nanomechanical study of the effects of colistin on the Klebsiella pneumoniae AJ218 capsule. Eur. Biophys. J. 2017, 46, 351–361. [Google Scholar] [CrossRef]
- Vaara, M. Polymyxins and Their Potential Next Generation as Therapeutic Antibiotics. Front. Microbiol. 2019, 10, 1689. [Google Scholar] [CrossRef]
- Kim, S.Y.; Choi, H.J.; Ko, K.S. Differential expression of two-component systems, pmrAB and phoPQ, with different growth phases of Klebsiella pneumoniae in the presence or absence of colistin. Curr. Microbiol. 2014, 69, 37–41. [Google Scholar] [CrossRef]
- Guckes, K.R.; Kostakioti, M.; Breland, E.J.; Gu, A.P.; Shaffer, C.L.; Martinez, C.R., 3rd; Hultgren, S.J.; Hadjifrangiskou, M. Strong cross-system interactions drive the activation of the QseB response regulator in the absence of its cognate sensor. Proc. Natl. Acad. Sci. USA 2013, 110, 16592–16597. [Google Scholar] [CrossRef]
- Pitt, M.E.; Cao, M.D.; Butler, M.S.; Ramu, S.; Ganesamoorthy, D.; Blaskovich, M.A.T.; Coin, L.J.M.; Cooper, M.A. Octapeptin C4 and polymyxin resistance occur via distinct pathways in an epidemic XDR Klebsiella pneumoniae ST258 isolate. J. Antimicrob. Chemother. 2019, 74, 582–593. [Google Scholar] [CrossRef]
- Kieffer, N.; Royer, G.; Decousser, J.W.; Bourrel, A.S.; Palmieri, M.; Ortiz De La Rosa, J.M.; Jacquier, H.; Denamur, E.; Nordmann, P.; Poirel, L. mcr-9, an Inducible Gene Encoding an Acquired Phosphoethanolamine Transferase in Escherichia coli, and Its Origin. Antimicrob. Agents Chemother. 2019, 63, e00965-19. [Google Scholar] [CrossRef]
- Halaby, T.; Kucukkose, E.; Janssen, A.B.; Rogers, M.R.; Doorduijn, D.J.; van der Zanden, A.G.; Al Naiemi, N.; Vandenbroucke-Grauls, C.M.; van Schaik, W. Genomic Characterization of Colistin Heteroresistance in Klebsiella pneumoniae during a Nosocomial Outbreak. Antimicrob. Agents Chemother. 2016, 60, 6837–6843. [Google Scholar] [CrossRef] [PubMed]
- Mahalakshmi, S.; Sunayana, M.R.; SaiSree, L.; Reddy, M. yciM is an essential gene required for regulation of lipopolysaccharide synthesis in Escherichia coli. Mol. Microbiol. 2014, 91, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.H.; Lin, T.L.; Lin, Y.T.; Wang, J.T. Amino Acid Substitutions of CrrB Responsible for Resistance to Colistin through CrrC in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2016, 60, 3709–3716. [Google Scholar] [CrossRef]
- Padilla, E.; Llobet, E.; Domenech-Sanchez, A.; Martinez-Martinez, L.; Bengoechea, J.A.; Alberti, S. Klebsiella pneumoniae AcrAB efflux pump contributes to antimicrobial resistance and virulence. Antimicrob. Agents Chemother. 2010, 54, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.B.; Rajamohan, G. KpnEF, a new member of the Klebsiella pneumoniae cell envelope stress response regulon, is an SMR-type efflux pump involved in broad-spectrum antimicrobial resistance. Antimicrob. Agents Chemother. 2013, 57, 4449–4462. [Google Scholar] [CrossRef]
- Hussein, N.H.; Al-Kadmy, I.M.S.; Taha, B.M.; Hussein, J.D. Mobilized colistin resistance (mcr) genes from 1 to 10: A comprehensive review. Mol. Biol. Rep. 2021, 48, 2897–2907. [Google Scholar] [CrossRef]
- Phetburom, N.; Boueroy, P.; Chopjitt, P.; Hatrongjit, R.; Akeda, Y.; Hamada, S.; Nuanualsuwan, S.; Kerdsin, A. Klebsiella pneumoniae Complex Harboring mcr-1, mcr-7, and mcr-8 Isolates from Slaughtered Pigs in Thailand. Microorganisms 2021, 9, 2436. [Google Scholar] [CrossRef]
- Chen, F.J.; Lauderdale, T.L.; Huang, W.C.; Shiau, Y.R.; Wang, H.Y.; Kuo, S.C. Emergence of mcr-1, mcr-3 and mcr-8 in clinical Klebsiella pneumoniae isolates in Taiwan. Clin. Microbiol. Infect. 2021, 27, 305–307. [Google Scholar] [CrossRef]
- Wang, C.; Feng, Y.; Liu, L.; Wei, L.; Kang, M.; Zong, Z. Identification of novel mobile colistin resistance gene mcr-10. Emerg. Microbes Infect. 2020, 9, 508–516. [Google Scholar] [CrossRef]
- Li, B.; Yin, F.; Zhao, X.; Guo, Y.; Wang, W.; Wang, P.; Zhu, H.; Yin, Y.; Wang, X. Colistin Resistance Gene mcr-1 Mediates Cell Permeability and Resistance to Hydrophobic Antibiotics. Front. Microbiol. 2019, 10, 3015. [Google Scholar] [CrossRef]
- Yang, Q.; Li, M.; Spiller, O.B.; Andrey, D.O.; Hinchliffe, P.; Li, H.; MacLean, C.; Niumsup, P.; Powell, L.; Pritchard, M.; et al. Balancing mcr-1 expression and bacterial survival is a delicate equilibrium between essential cellular defence mechanisms. Nat. Commun. 2017, 8, 2054. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Q.; Liu, Y.Y.; Wu, R.; Xun, H.; Sun, J.; Li, J.; Feng, Y.; Liu, J.H. Impact of mcr-1 on the Development of High Level Colistin Resistance in Klebsiella pneumoniae and Escherichia coli. Front. Microbiol. 2021, 12, 666782. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, D.; Shi, Q.; Quan, J.; Li, X.; Yu, Y. mcr-1 Gene Has No Effect on Colistin Resistance When It Coexists with Inactivated mgrB Gene in Klebsiella pneumoniae. Microb. Drug Resist. 2018, 24, 1117–1120. [Google Scholar] [CrossRef] [PubMed]
- Lippa, A.M.; Goulian, M. Feedback inhibition in the PhoQ/PhoP signaling system by a membrane peptide. PLoS Genet. 2009, 5, e1000788. [Google Scholar] [CrossRef] [PubMed]
- Kidd, T.J.; Mills, G.; Sa-Pessoa, J.; Dumigan, A.; Frank, C.G.; Insua, J.L.; Ingram, R.; Hobley, L.; Bengoechea, J.A. A Klebsiella pneumoniae antibiotic resistance mechanism that subdues host defences and promotes virulence. EMBO Mol. Med. 2017, 9, 430–447. [Google Scholar] [CrossRef]
- Salazar, M.E.; Podgornaia, A.I.; Laub, M.T. The small membrane protein MgrB regulates PhoQ bifunctionality to control PhoP target gene expression dynamics. Mol. Microbiol. 2016, 102, 430–445. [Google Scholar] [CrossRef]
- Bray, A.S.; Smith, R.D.; Hudson, A.W.; Hernandez, G.E.; Young, T.M.; George, H.E.; Ernst, R.K.; Zafar, M.A. MgrB-Dependent Colistin Resistance in Klebsiella pneumoniae Is Associated with an Increase in Host-to-Host Transmission. mBio 2022, 13, e0359521. [Google Scholar] [CrossRef]
- Xu, J.; Li, T.; Gao, Y.; Deng, J.; Gu, J. MgrB affects the acid stress response of Escherichia coli by modulating the expression of iraM. FEMS Microbiol. Lett. 2019, 366, fnz123. [Google Scholar] [CrossRef]
- Yadavalli, S.S.; Goh, T.; Carey, J.N.; Malengo, G.; Vellappan, S.; Nickels, B.E.; Sourjik, V.; Goulian, M.; Yuan, J. Functional determinants of a small protein controlling a broadly conserved bacterial sensor kinase. J. Bacteriol. 2020, 202, e00305-20. [Google Scholar] [CrossRef]
- Leung, L.M.; Cooper, V.S.; Rasko, D.A.; Guo, Q.; Pacey, M.P.; McElheny, C.L.; Mettus, R.T.; Yoon, S.H.; Goodlett, D.R.; Ernst, R.K.; et al. Structural modification of LPS in colistin-resistant, KPC-producing Klebsiella pneumoniae. J. Antimicrob. Chemother. 2017, 72, 3035–3042. [Google Scholar] [CrossRef]
- Ayala-Torres, C.; Hernández, N.; Galeano, A.; Novoa-Aponte, L.; Soto, C.-Y. Zeta potential as a measure of the surface charge of mycobacterial cells. Ann. Microbiol. 2014, 64, 1189–1195. [Google Scholar] [CrossRef]
- Velkov, T.; Deris, Z.Z.; Huang, J.X.; Azad, M.A.; Butler, M.; Sivanesan, S.; Kaminskas, L.M.; Dong, Y.D.; Boyd, B.; Baker, M.A.; et al. Surface changes and polymyxin interactions with a resistant strain of Klebsiella pneumoniae. Innate Immun. 2014, 20, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Gogry, F.A.; Siddiqui, M.T.; Sultan, I.; Husain, F.M.; Al-Kheraif, A.A.; Ali, A.; Haq, Q.M.R. Colistin Interaction and Surface Changes Associated with mcr-1 Conferred Plasmid Mediated Resistance in E. coli and A. veronii Strains. Pharmaceutics 2022, 14, 295. [Google Scholar] [CrossRef]
- Al-Farsi, H.M.; Al-Adwani, S.; Ahmed, S.; Vogt, C.; Ambikan, A.T.; Leber, A.; Al-Jardani, A.; Al-Azri, S.; Al-Muharmi, Z.; Toprak, M.S.; et al. Effects of the Antimicrobial Peptide LL-37 and Innate Effector Mechanisms in Colistin-Resistant Klebsiella pneumoniae With mgrB Insertions. Front. Microbiol. 2019, 10, 2632. [Google Scholar] [CrossRef] [PubMed]
- Formosa, C.; Herold, M.; Vidaillac, C.; Duval, R.E.; Dague, E. Unravelling of a mechanism of resistance to colistin in Klebsiella pneumoniae using atomic force microscopy. J. Antimicrob. Chemother. 2015, 70, 2261–2270. [Google Scholar] [CrossRef]
- Ayerbe-Algaba, R.; Gil-Marqués, M.L.; Miró-Canturri, A.; Parra-Millán, R.; Pachón-Ibáñez, M.E.; Jiménez-Mejías, M.E.; Pachón, J.; Smani, Y. The anthelmintic oxyclozanide restores the activity of colistin against colistin-resistant Gram-negative bacilli. Int. J. Antimicrob. Agents 2019, 54, 507–512. [Google Scholar] [CrossRef]
- Cain, A.K.; Boinett, C.J.; Barquist, L.; Dordel, J.; Fookes, M.; Mayho, M.; Ellington, M.J.; Goulding, D.; Pickard, D.; Wick, R.R.; et al. Morphological, genomic and transcriptomic responses of Klebsiella pneumoniae to the last-line antibiotic colistin. Sci. Rep. 2018, 8, 9868. [Google Scholar] [CrossRef]
- Avalos Vizcarra, I.; Hosseini, V.; Kollmannsberger, P.; Meier, S.; Weber, S.S.; Arnoldini, M.; Ackermann, M.; Vogel, V. How type 1 fimbriae help Escherichia coli to evade extracellular antibiotics. Sci. Rep. 2016, 6, 18109. [Google Scholar] [CrossRef]
- Ierardi, V.; Domenichini, P.; Reali, S.; Chiappara, G.M.; Devoto, G.; Valbusa, U. Klebsiella pneumoniae antibiotic resistance identified by atomic force microscopy. J. Biosci. 2017, 42, 623–636. [Google Scholar] [CrossRef]
- MacNair, C.R.; Stokes, J.M.; Carfrae, L.A.; Fiebig-Comyn, A.A.; Coombes, B.K.; Mulvey, M.R.; Brown, E.D. Overcoming mcr-1 mediated colistin resistance with colistin in combination with other antibiotics. Nat. Commun. 2018, 9, 458. [Google Scholar] [CrossRef]
- Vaara, M. Agents that increase the permeability of the outer membrane. Microbiol. Rev. 1992, 56, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Koirala, B.; Hernandez, Y.; Zimmerman, M.; Park, S.; Perlin, D.S.; Brady, S.F. A naturally inspired antibiotic to target multidrug-resistant pathogens. Nature 2022, 601, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Vaara, M. Polymyxin Derivatives that Sensitize Gram-Negative Bacteria to Other Antibiotics. Molecules 2019, 24, 249. [Google Scholar] [CrossRef]
- Moghal, M.R.; Hossain, F.; Yamazaki, M. Action of antimicrobial peptides and cell-penetrating peptides on membrane potential revealed by the single GUV method. Biophys. Rev. 2020, 12, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Sekar, P.C.; Chandrasekhar, G.; Rajasekaran, R. Hydrophobic Residues Confer the Helicity and Membrane Permeability of Ocellatin-1 Antimicrobial Peptide Scaffold Towards Therapeutics. Int. J. Pept. Res. Ther. 2021, 27, 2459–2470. [Google Scholar] [CrossRef]
- Velkov, T.; Roberts, K.D.; Nation, R.L.; Wang, J.; Thompson, P.E.; Li, J. Teaching ‘old’ polymyxins new tricks: New-generation lipopeptides targeting gram-negative ‘superbugs’. ACS Chem. Biol. 2014, 9, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Di Pasqua, R.; Hoskins, N.; Betts, G.; Mauriello, G. Changes in membrane fatty acids composition of microbial cells induced by addiction of thymol, carvacrol, limonene, cinnamaldehyde, and eugenol in the growing media. J. Agric. Food. Chem. 2006, 54, 2745–2749. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.S.X.; Yusoff, K.; Lim, S.-H.E.; Chong, C.-M.; Lai, K.-S. Membrane Disruption Properties of Essential Oils—A Double-Edged Sword? Processes 2021, 9, 595. [Google Scholar] [CrossRef]
- Meng, F.; Liu, Y.; Nie, T.; Tang, C.; Lyu, F.; Bie, X.; Lu, Y.; Zhao, M.; Lu, Z. Plantaricin A, Derived from Lactiplantibacillus plantarum, Reduces the Intrinsic Resistance of Gram-Negative Bacteria to Hydrophobic Antibiotics. Appl. Environ. Microbiol. 2022, 88, e0037122. [Google Scholar] [CrossRef] [PubMed]
- Dhanda, G.; Mukherjee, R.; Basak, D.; Haldar, J. Small-Molecular Adjuvants with Weak Membrane Perturbation Potentiate Antibiotics against Gram-Negative Superbugs. ACS Infect. Dis. 2022, 8, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yap, P.S.-X.; Cheng, W.-H.; Chang, S.-K.; Lim, S.-H.E.; Lai, K.-S. MgrB Mutations and Altered Cell Permeability in Colistin Resistance in Klebsiella pneumoniae. Cells 2022, 11, 2995. https://doi.org/10.3390/cells11192995
Yap PS-X, Cheng W-H, Chang S-K, Lim S-HE, Lai K-S. MgrB Mutations and Altered Cell Permeability in Colistin Resistance in Klebsiella pneumoniae. Cells. 2022; 11(19):2995. https://doi.org/10.3390/cells11192995
Chicago/Turabian StyleYap, Polly Soo-Xi, Wan-Hee Cheng, Sook-Keng Chang, Swee-Hua Erin Lim, and Kok-Song Lai. 2022. "MgrB Mutations and Altered Cell Permeability in Colistin Resistance in Klebsiella pneumoniae" Cells 11, no. 19: 2995. https://doi.org/10.3390/cells11192995
APA StyleYap, P. S.-X., Cheng, W.-H., Chang, S.-K., Lim, S.-H. E., & Lai, K.-S. (2022). MgrB Mutations and Altered Cell Permeability in Colistin Resistance in Klebsiella pneumoniae. Cells, 11(19), 2995. https://doi.org/10.3390/cells11192995






