TMEM43 Protects against Sepsis-Induced Cardiac Injury via Inhibiting Ferroptosis in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Animal Model
2.2. AAV9 Construction and Viral Delivery
2.3. Echocardiography Measurements
2.4. Detection of Cardiac Injury Markers
2.5. Fe Assays and Malondialdehyde (MDA) Level
2.6. Prussian Blue Staining
2.7. Cell Culture
2.8. Detection of Reactive Oxygen Species (ROS)
2.9. RT-PCR and Western Blot
- Primer used for TMEM43:
- Mouse TMEM43
- Sense: 5′-TGTACCAGTGGGTGGAGACA-3′
- Antisense: 5′-AATGAGGCCTGCTGAGAGAA-3′
- Rat TMEM43
- Sense: 5′- TGTACCAGTGGGTGGAGACA-3′
- Antisense: 5′- ACCTGCCAATCTGGACAAAG-3′
2.10. Data Analysis
3. Results
3.1. The Expression Level of TMEM43 in Injured Hearts
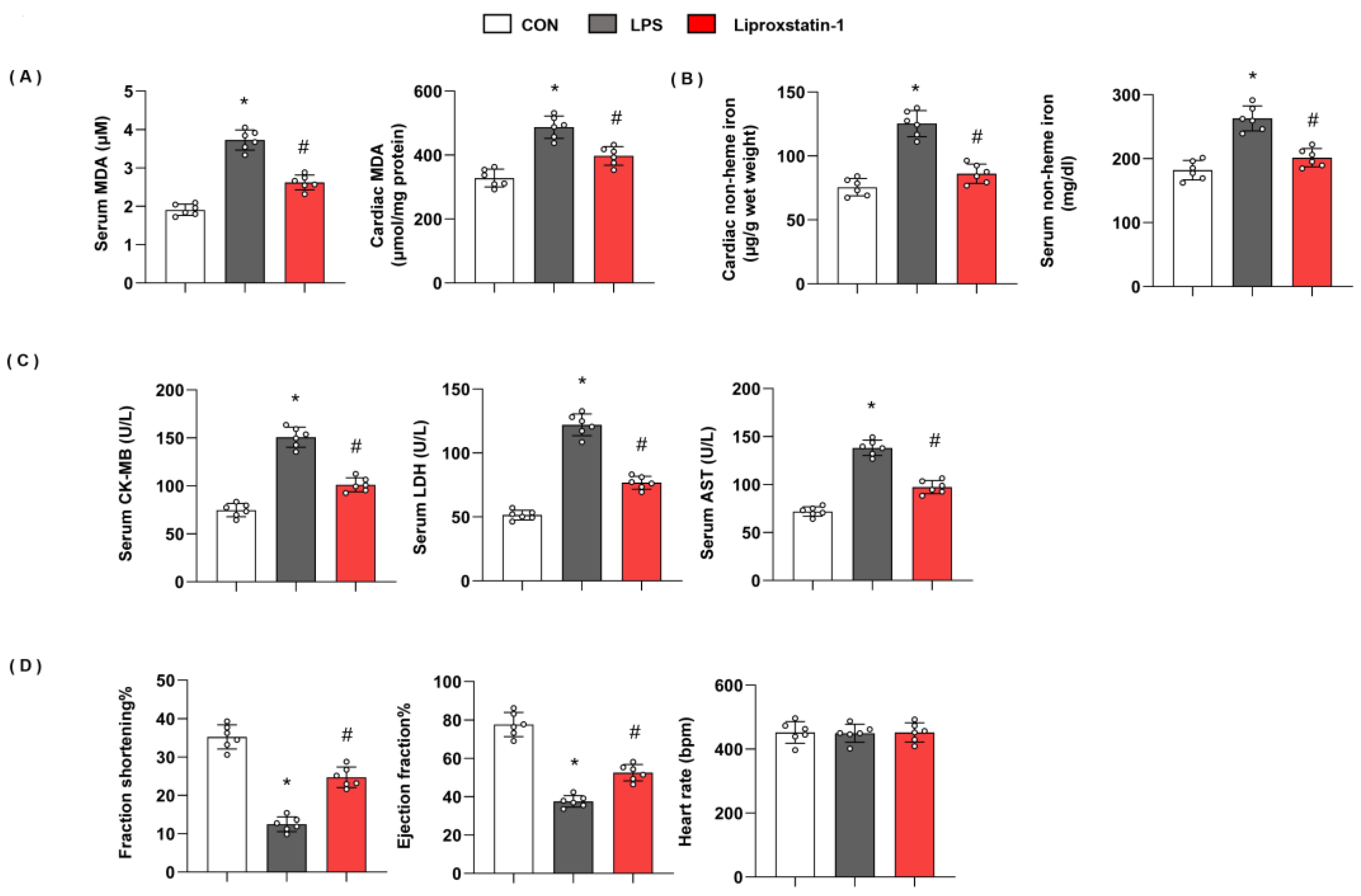
3.2. Ferroptosis Plays an Essential Role in LPS-Induced Cardiac Injury
3.3. TMEM43 Knockdown Aggravates LPS-Induced Cardiac Injury
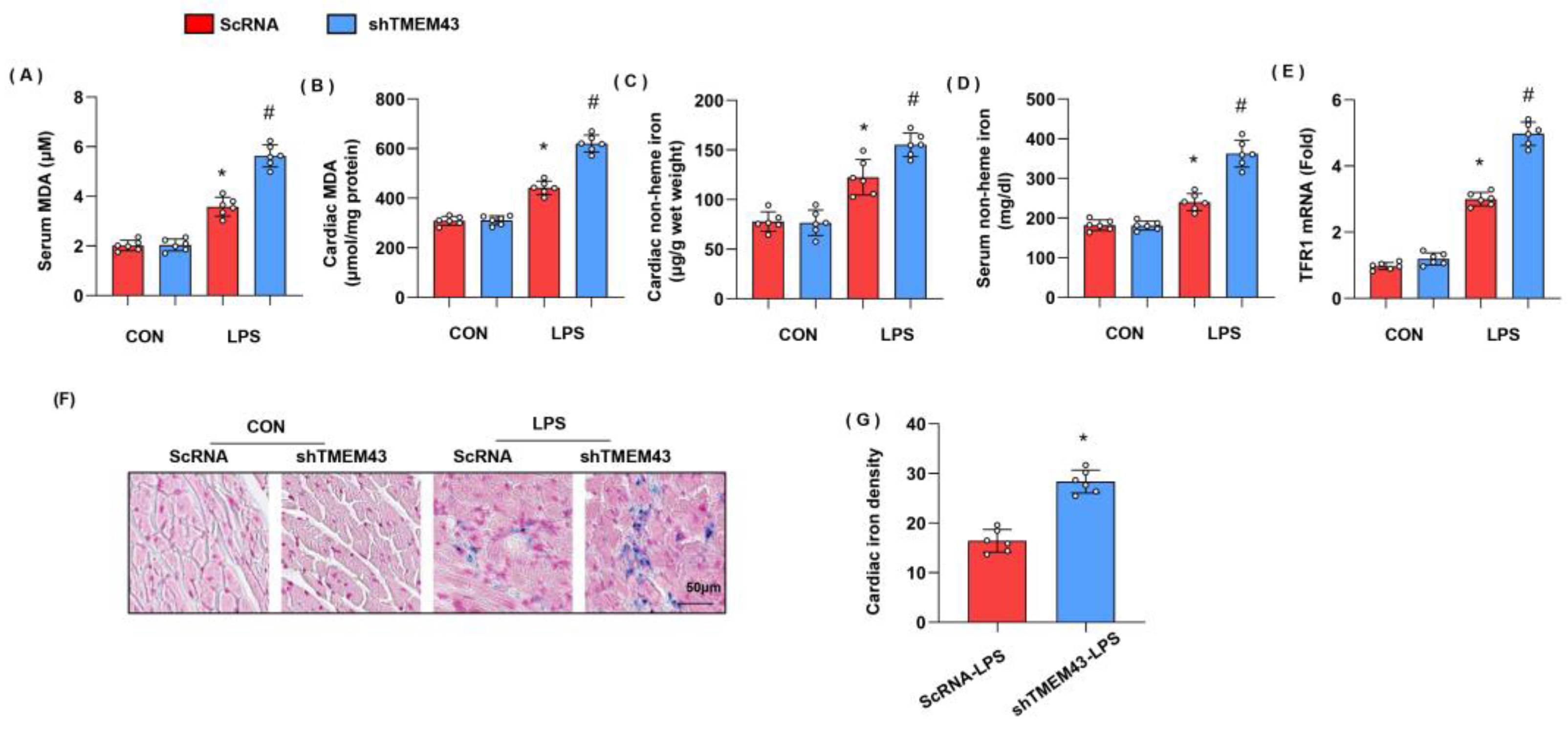
3.4. TMEM43 Knockdown Aggravates LPS-Induced Ferroptosis
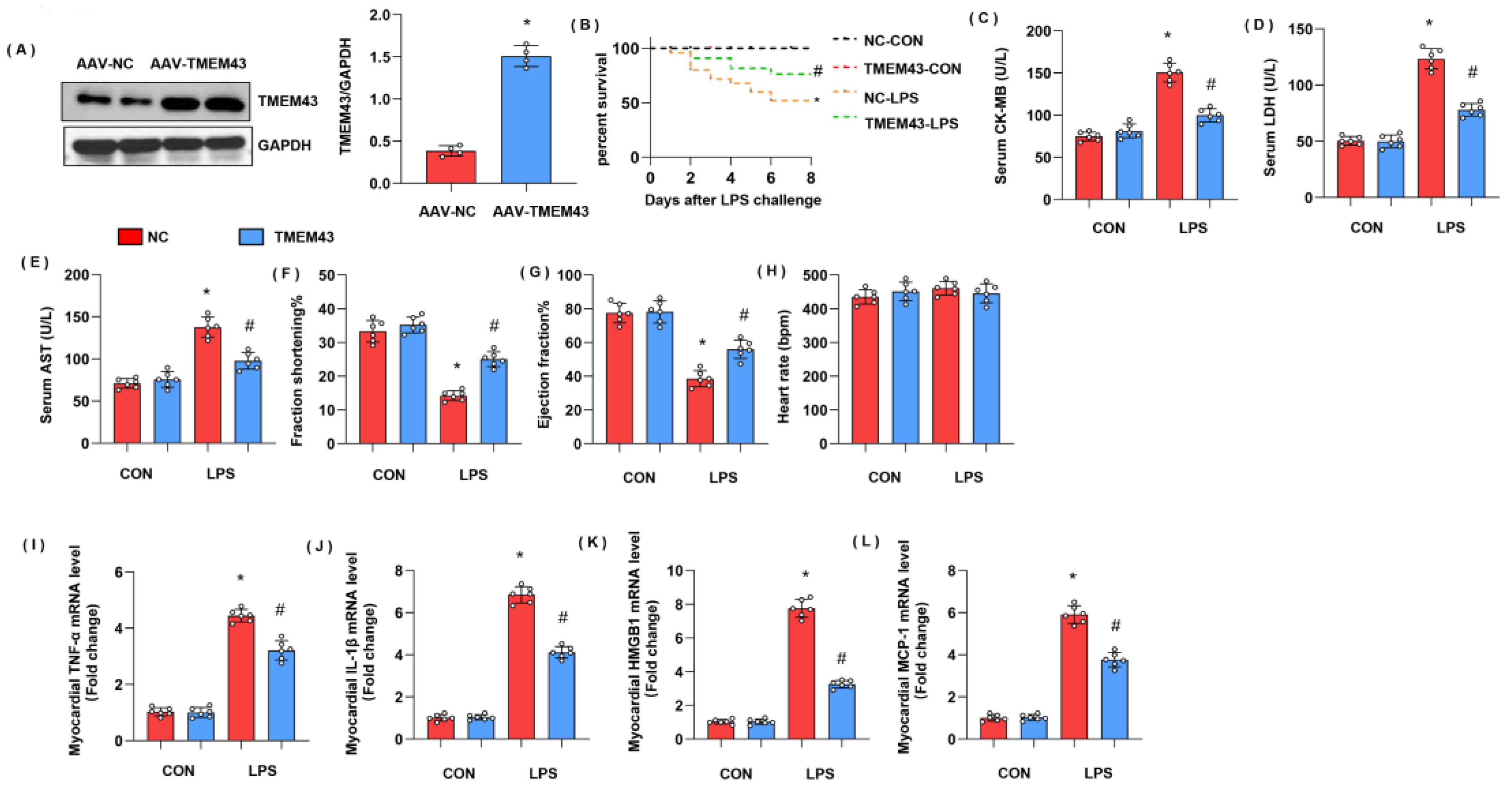
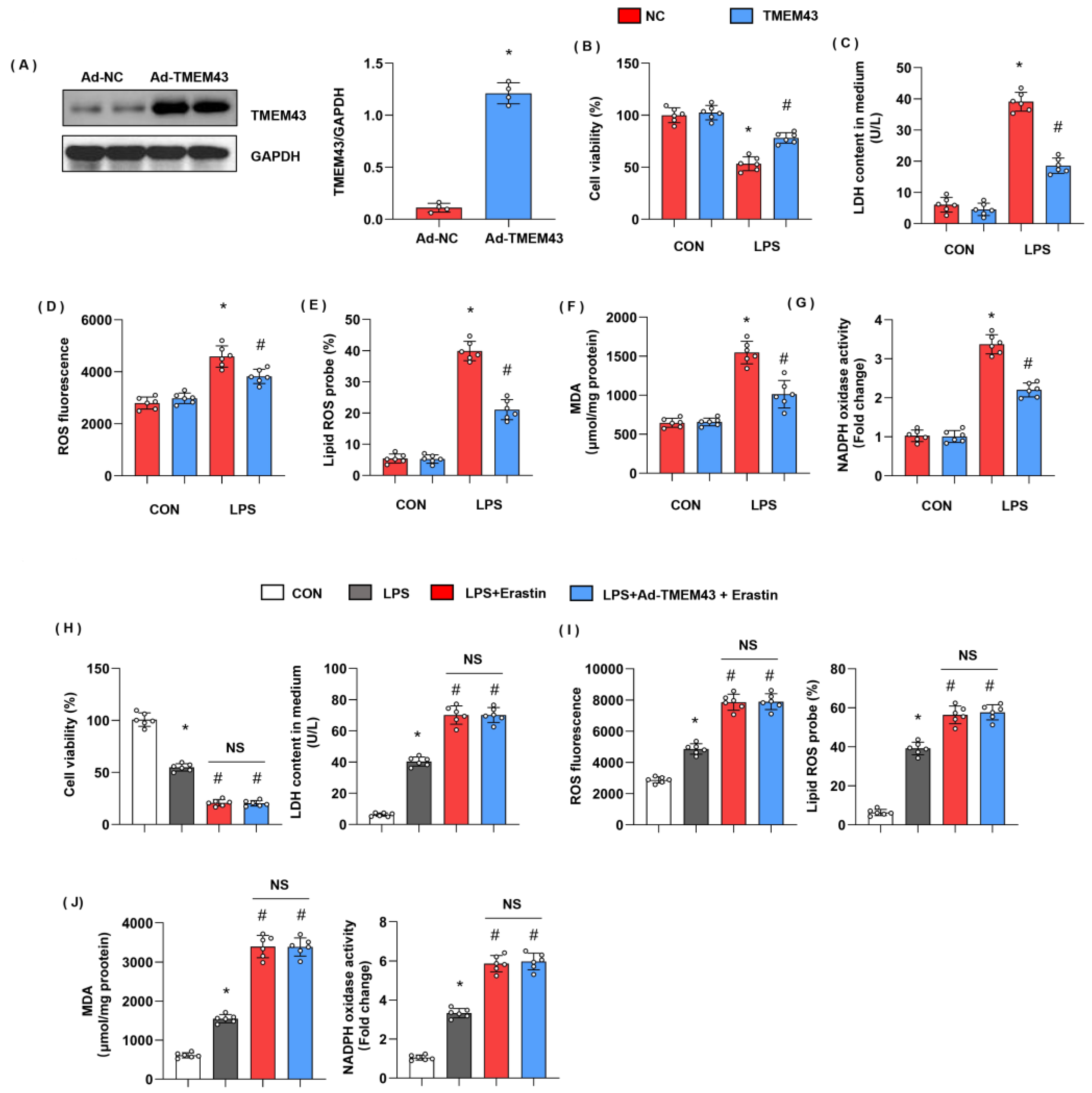
3.5. TMEM43 Overexpression Alleviates LPS-Induced Cardiac Injury
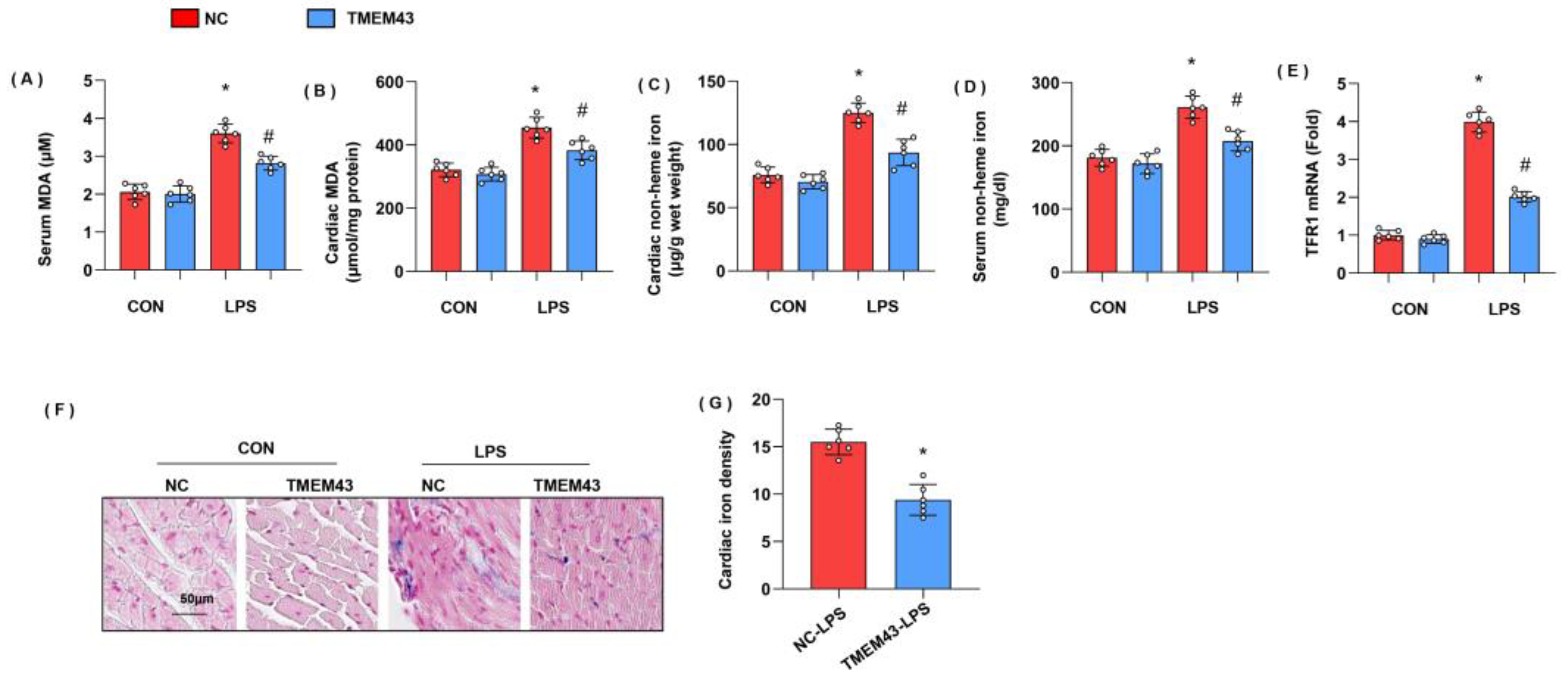
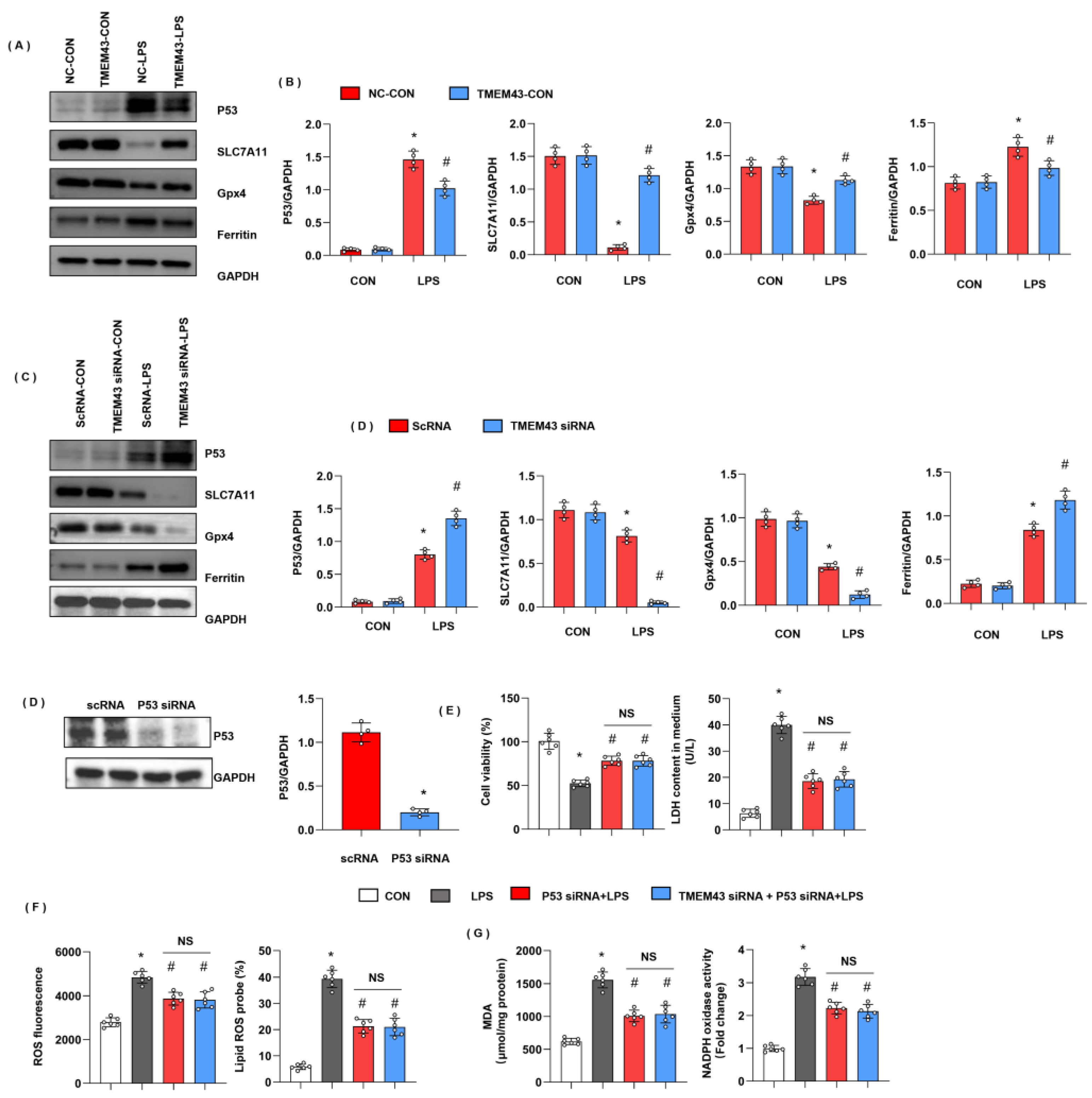
3.6. TMEM43 Overexpression Alleviates LPS-Induced Ferroptosis
3.7. TMEM43 Overexpression In Vitro Ameliorates LPS-Induced Cell Injury
3.8. TMEM43 Knockdown In Vitro Deteriorates LPS-Induced Cell Injury
3.9. TMEM43 Suppresses P53-Mediated Ferroptosis
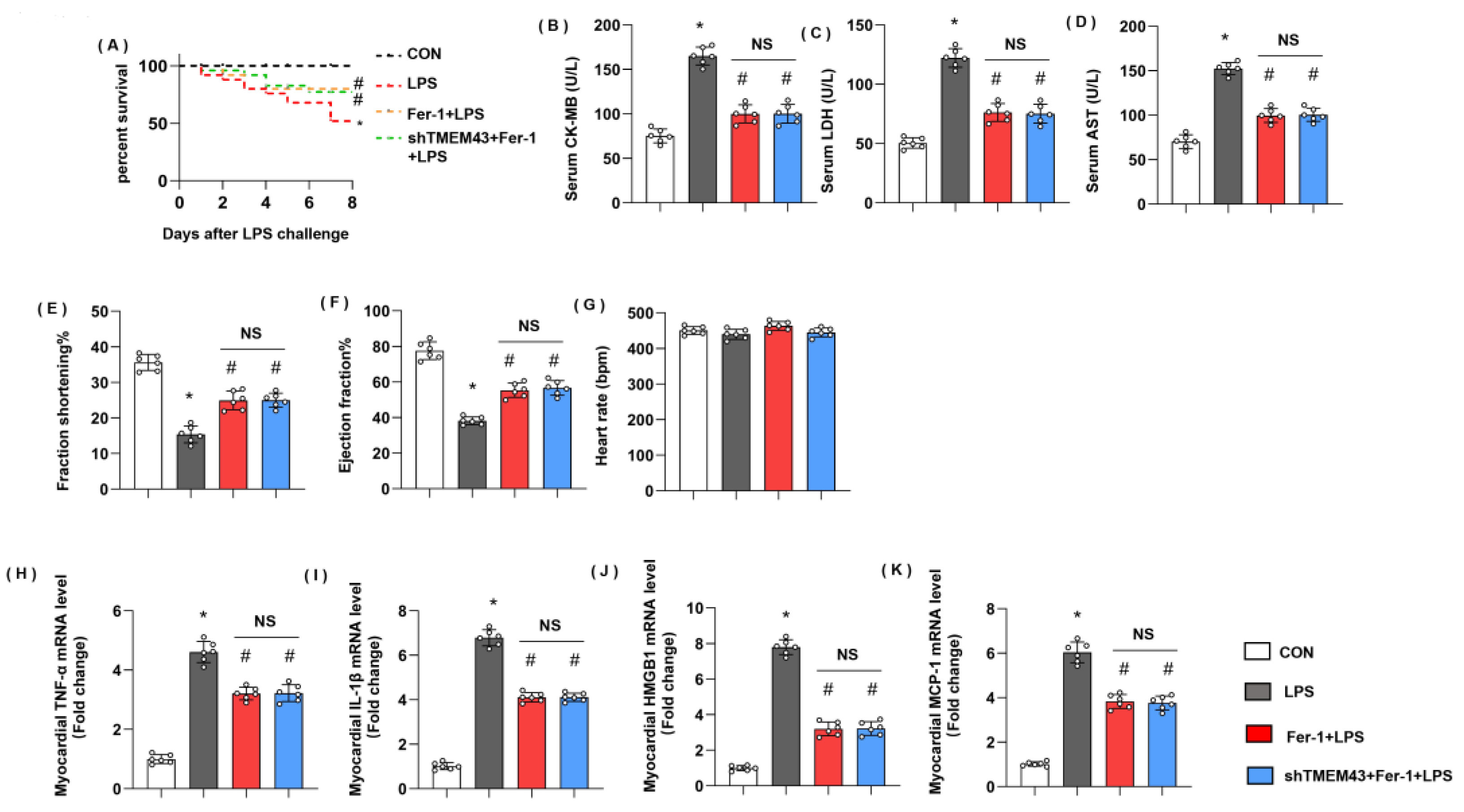
3.10. Ferrostatin-1 Blurs TMEM43 Knockdown-Induced Aggravating Effects In Vivo
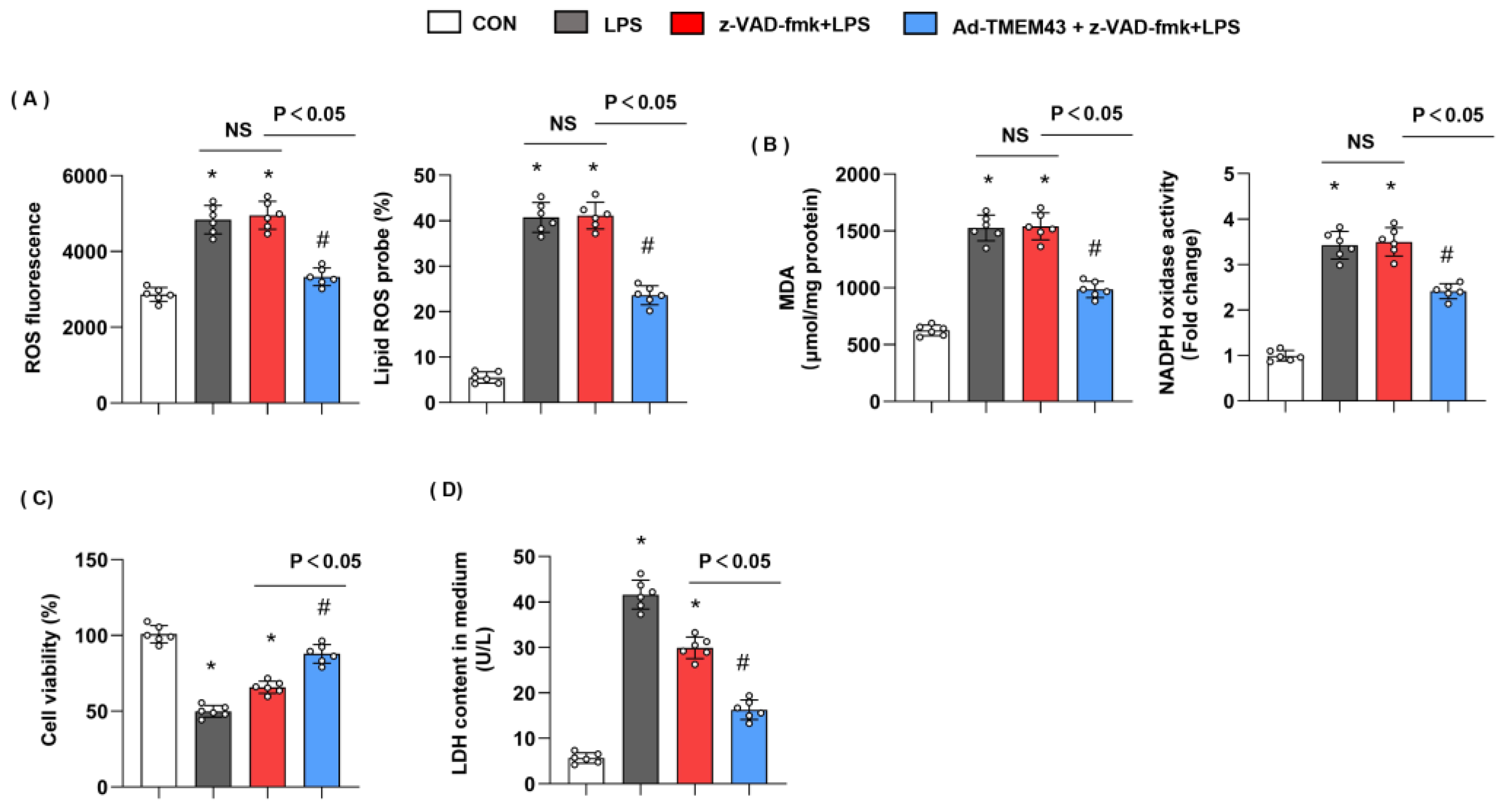
3.11. Apoptosis Inhibitor Could Not Blur the Protective Effects of TMEM43
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hollenberg, S.M.; Singer, M. Pathophysiology of sepsis-induced cardiomyopathy. Nat. Rev. Cardiol. 2021, 18, 424–434. [Google Scholar] [CrossRef] [PubMed]
- L’Heureux, M.; Sternberg, M.; Brath, L.; Turlington, J.; Kashiouris, M.G. Sepsis-Induced Cardiomyopathy: A Comprehensive Review. Curr. Cardiol. Rep. 2020, 22, 35. [Google Scholar] [CrossRef]
- Stanzani, G.; Duchen, M.R.; Singer, M. The role of mitochondria in sepsis-induced cardiomyopathy. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 759–773. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Yu, M.M.; Shou, S.T.; Chai, Y.F. Sepsis-Induced Cardiomyopathy: Mechanisms and Treatments. Front. Immunol. 2017, 8, 1021. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.T.; Liu, D.W.; Zhang, H.M.; Su, L.X. Induction and deduction in sepsis-induced cardiomyopathy: Five typical categories. Chin. Med. J. 2020, 133, 2205–2211. [Google Scholar] [CrossRef]
- Wei, X.; Yi, X.; Zhu, X.H.; Jiang, D.S. Posttranslational Modifications in Ferroptosis. Oxidative Med. Cell. Longev. 2020, 2020, 8832043. [Google Scholar] [CrossRef]
- Wu, X.; Li, Y.; Zhang, S.; Zhou, X. Ferroptosis as a novel therapeutic target for cardiovascular disease. Theranostics 2021, 11, 3052–3059. [Google Scholar] [CrossRef]
- Qiu, Y.; Cao, Y.; Cao, W.; Jia, Y.; Lu, N. The Application of Ferroptosis in Diseases. Pharmacol. Res. 2020, 159, 104919. [Google Scholar] [CrossRef]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef]
- Li, N.; Jiang, W.; Wang, W.; Xiong, R.; Wu, X.; Geng, Q. Ferroptosis and its emerging roles in cardiovascular diseases. Pharmacol. Res. 2021, 166, 105466. [Google Scholar] [CrossRef]
- Li, Y.; Cao, Y.; Xiao, J.; Shang, J.; Tan, Q.; Ping, F.; Huang, W.; Wu, F.; Zhang, H.; Zhang, X. Inhibitor of apoptosis-stimulating protein of p53 inhibits ferroptosis and alleviates intestinal ischemia/reperfusion-induced acute lung injury. Cell Death Differ. 2020, 27, 2635–2650. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ma, H.; Li, J.; Meng, C.; Zou, J.; Wang, H.; Liu, K.; Liu, M.; Xiao, X.; Zhang, H.; et al. HSF1 functions as a key defender against palmitic acid-induced ferroptosis in cardiomyocytes. J. Mol. Cell. Cardiol. 2021, 150, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, W.; Zhou, H.; Wu, Q.; Duan, M.; Liu, C.; Wu, H.; Deng, W.; Shen, D.; Tang, Q. Ferritinophagy-mediated ferroptosis is involved in sepsis-induced cardiac injury. Free Radic. Biol. Med. 2020, 160, 303–318. [Google Scholar] [CrossRef]
- Shinomiya, H.; Kato, H.; Kuramoto, Y.; Watanabe, N.; Tsuruda, T.; Arimura, T.; Miyashita, Y.; Miyasaka, Y.; Mashimo, T.; Takuwa, A.; et al. Aberrant accumulation of TMEM43 accompanied by perturbed transmural gene expression in arrhythmogenic cardiomyopathy. FASEB J. 2021, 35, e21994. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Jiang, C.; Li, Y.; Yang, D.; Ma, Y.; Zhang, B.; Li, X.; Zhang, P.; Hu, X.; Zhao, X.; et al. TMEM43-S358L mutation enhances NF-kappaB-TGFbeta signal cascade in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Protein Cell 2019, 10, 104–119. [Google Scholar] [CrossRef]
- Rouhi, L.; Cheedipudi, S.M.; Chen, S.N.; Fan, S.; Lombardi, R.; Chen, X.; Coarfa, C.; Robertson, M.J.; Gurha, P.; Marian, A.J. Haploinsufficiency of Tmem43 in cardiac myocytes activates the DNA damage response pathway leading to a late-onset senescence-associated pro-fibrotic cardiomyopathy. Cardiovasc. Res. 2021, 117, 2377–2394. [Google Scholar] [CrossRef]
- Gu, Q.; Xu, F.; Orgil, B.O.; Khuchua, Z.; Munkhsaikhan, U.; Johnson, J.N.; Alberson, N.R.; Pierre, J.F.; Black, D.D.; Dong, D.; et al. Systems genetics analysis defines importance of TMEM43/LUMA for cardiac- and metabolic-related pathways. Physiol. Genom. 2022, 54, 22–35. [Google Scholar] [CrossRef]
- Gao, L.; Liu, Y.; Guo, S.; Xiao, L.; Wu, L.; Wang, Z.; Liang, C.; Yao, R.; Zhang, Y. LAZ3 protects cardiac remodeling in diabetic cardiomyopathy via regulating miR-21/PPARa signaling. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3322–3338. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, P.; Zhai, B.; Zhang, M.; Xiang, Y.; Fang, J.; Xu, S.; Gao, Y.; Chen, X.; Sui, X.; et al. The emerging role of ferroptosis in inflammation. Biomed. Pharmacother. 2020, 127, 110108. [Google Scholar] [CrossRef]
- Pei, Z.; Liu, Y.; Liu, S.; Jin, W.; Luo, Y.; Sun, M.; Duan, Y.; Ajoolabady, A.; Sowers, J.R.; Fang, Y.; et al. FUNDC1 insufficiency sensitizes high fat diet intake-induced cardiac remodeling and contractile anomaly through ACSL4-mediated ferroptosis. Metabolism 2021, 122, 154840. [Google Scholar] [CrossRef]
- Zhao, W.K.; Zhou, Y.; Xu, T.T.; Wu, Q. Ferroptosis: Opportunities and Challenges in Myocardial Ischemia-Reperfusion Injury. Oxidative Med. Cell. Longev. 2021, 2021, 9929687. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Z.; Li, M.; Wang, F.; Jia, Y.; Zhang, F.; Shao, J.; Chen, A.; Zheng, S. P53-dependent induction of ferroptosis is required for artemether to alleviate carbon tetrachloride-induced liver fibrosis and hepatic stellate cell activation. IUBMB Life 2019, 71, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Deng, H.; Hu, S.; Zhang, Y.; Zheng, L.; Liu, M.; Chen, Y.; Wei, J.; Yang, H.; Lv, X. Role of Ferroptosis in Lung Diseases. J. Inflamm. Res. 2021, 14, 2079–2090. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, F.; Yin, H.L.; Huang, Z.J.; Lin, Z.T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Jia, J.; Zheng, J.; Zhou, Y.; Jia, D.; Wang, J. Recent Progress of Ferroptosis in Lung Diseases. Front. Cell Dev. Biol. 2021, 9, 789517. [Google Scholar] [CrossRef]
- Jiang, L.; Kon, N.; Li, T.; Wang, S.J.; Su, T.; Hibshoosh, H.; Baer, R.; Gu, W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 2015, 520, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.; Kon, N.; Chen, D.; Li, T.; Liu, T.; Jiang, L.; Song, S.; Tavana, O.; Gu, W. ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway. Nat. Cell Biol. 2019, 21, 579–591. [Google Scholar] [CrossRef]
- Park, E.; Chung, S.W. ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis. 2019, 10, 822. [Google Scholar] [CrossRef]
- Gao, M.; Monian, P.; Pan, Q.; Zhang, W.; Xiang, J.; Jiang, X. Ferroptosis is an autophagic cell death process. Cell Res. 2016, 26, 1021–1032. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Cao, Z.; Gui, F.; Zhang, M.; Wu, X.; Peng, H.; Yu, B.; Li, W.; Ai, F.; Zhang, J. TMEM43 Protects against Sepsis-Induced Cardiac Injury via Inhibiting Ferroptosis in Mice. Cells 2022, 11, 2992. https://doi.org/10.3390/cells11192992
Chen Z, Cao Z, Gui F, Zhang M, Wu X, Peng H, Yu B, Li W, Ai F, Zhang J. TMEM43 Protects against Sepsis-Induced Cardiac Injury via Inhibiting Ferroptosis in Mice. Cells. 2022; 11(19):2992. https://doi.org/10.3390/cells11192992
Chicago/Turabian StyleChen, Zhen, Zhe Cao, Feng Gui, Mengli Zhang, Xian Wu, Huan Peng, Bo Yu, Wei Li, Fen Ai, and Jun Zhang. 2022. "TMEM43 Protects against Sepsis-Induced Cardiac Injury via Inhibiting Ferroptosis in Mice" Cells 11, no. 19: 2992. https://doi.org/10.3390/cells11192992
APA StyleChen, Z., Cao, Z., Gui, F., Zhang, M., Wu, X., Peng, H., Yu, B., Li, W., Ai, F., & Zhang, J. (2022). TMEM43 Protects against Sepsis-Induced Cardiac Injury via Inhibiting Ferroptosis in Mice. Cells, 11(19), 2992. https://doi.org/10.3390/cells11192992





