Systematic Annotation Reveals CEP Function in Tomato Root Development and Abiotic Stress Response
Abstract
1. Introduction
2. Results
2.1. Genome-Wide Annotation of Putative Tomato CEP Gene Family
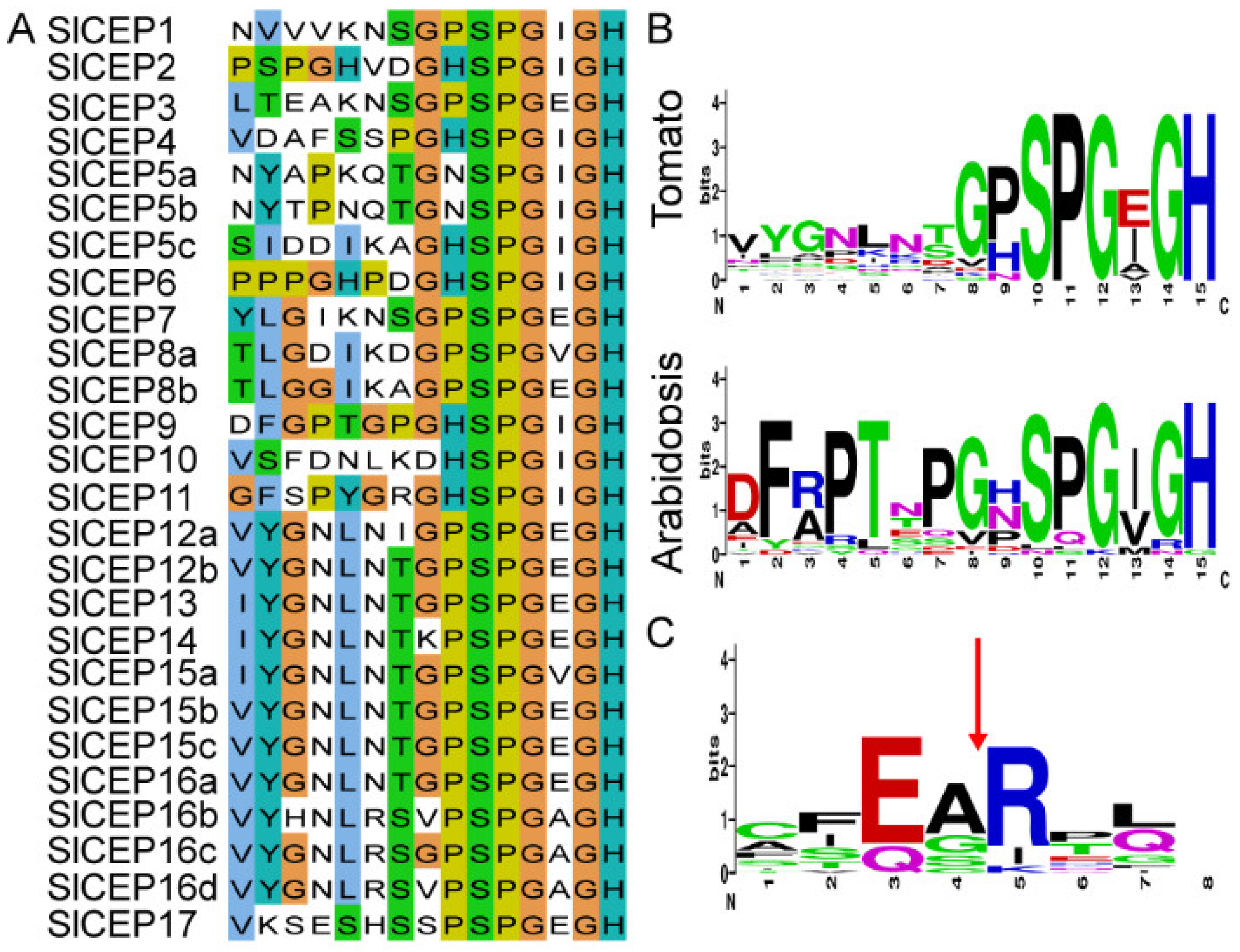
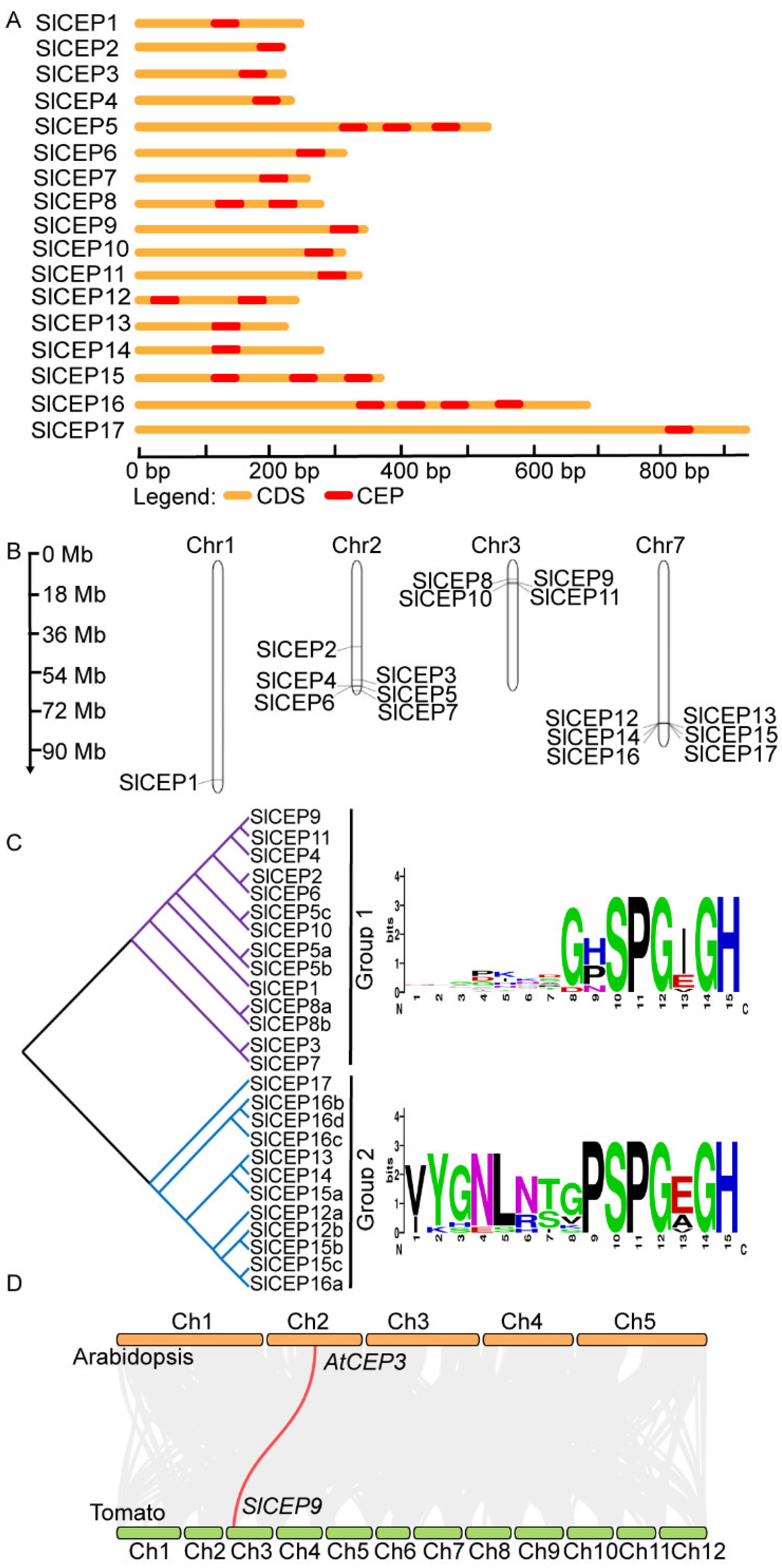
2.2. Motif Analysis, Gene Structure and Chromosome Localization of Slcep Proteins
2.3. Phylogenetic Analysis of SlCEP Proteins
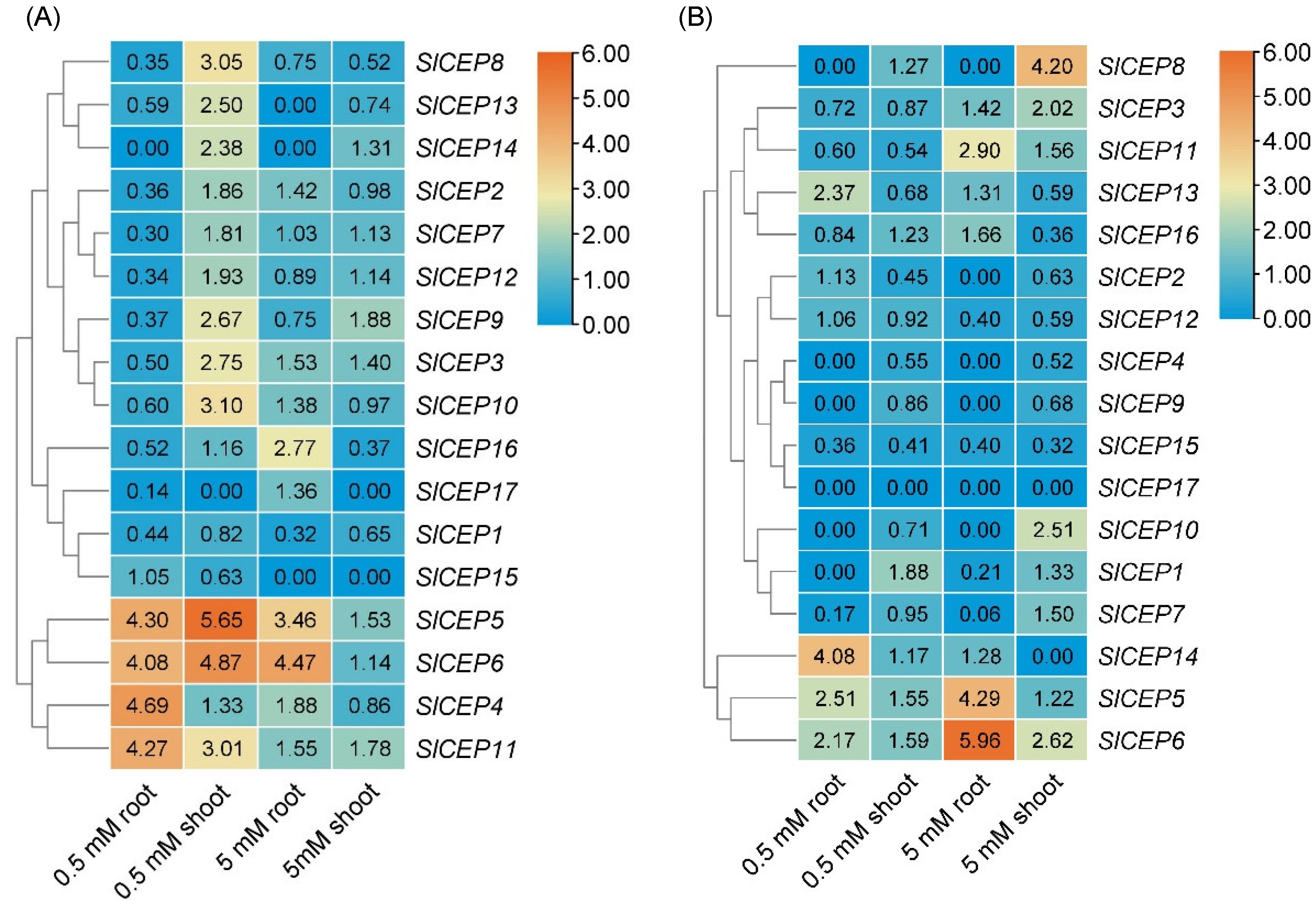
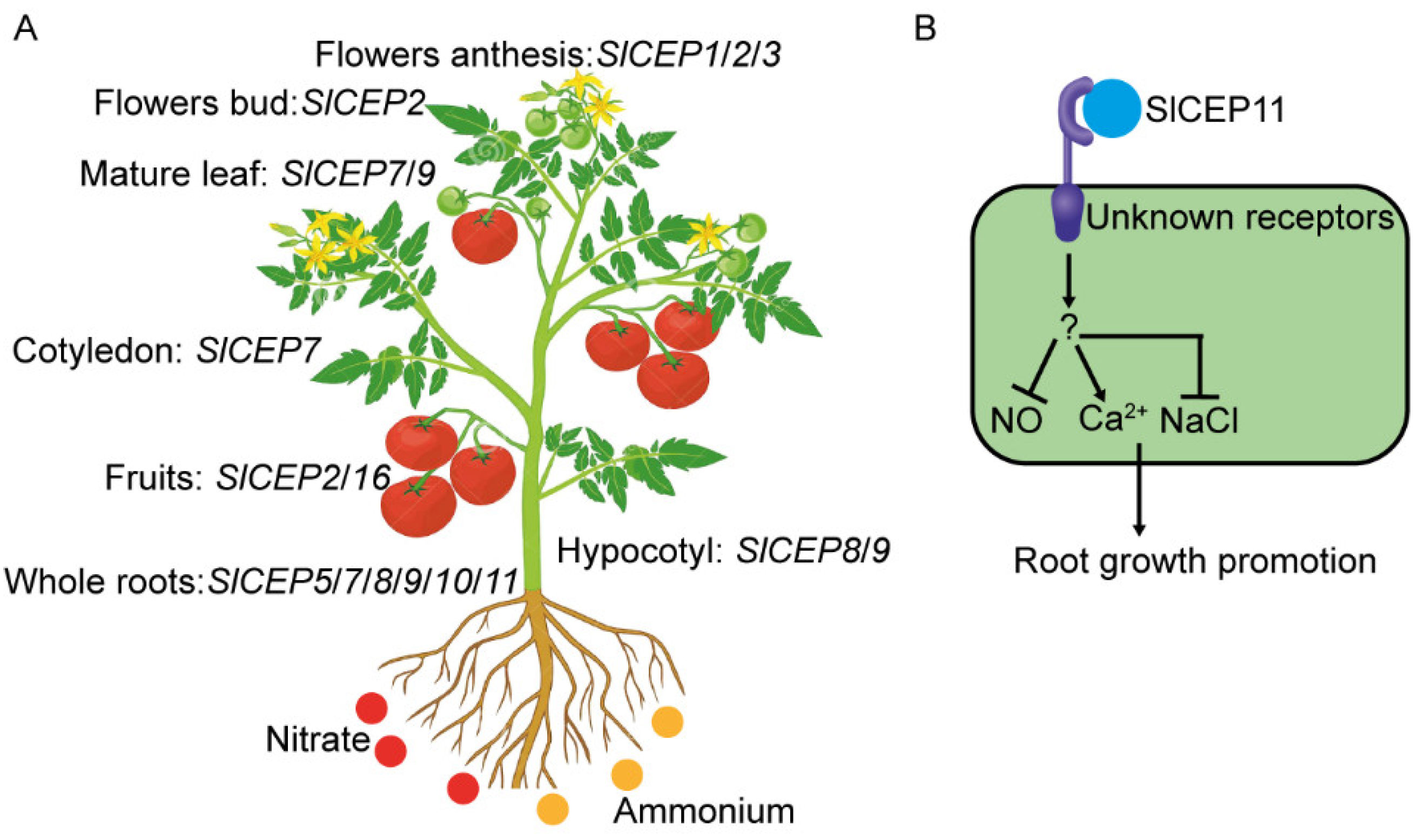
2.4. Distinct Expression Pattern of SlCEP Genes in Response to Developmental and Nitrogen Signal
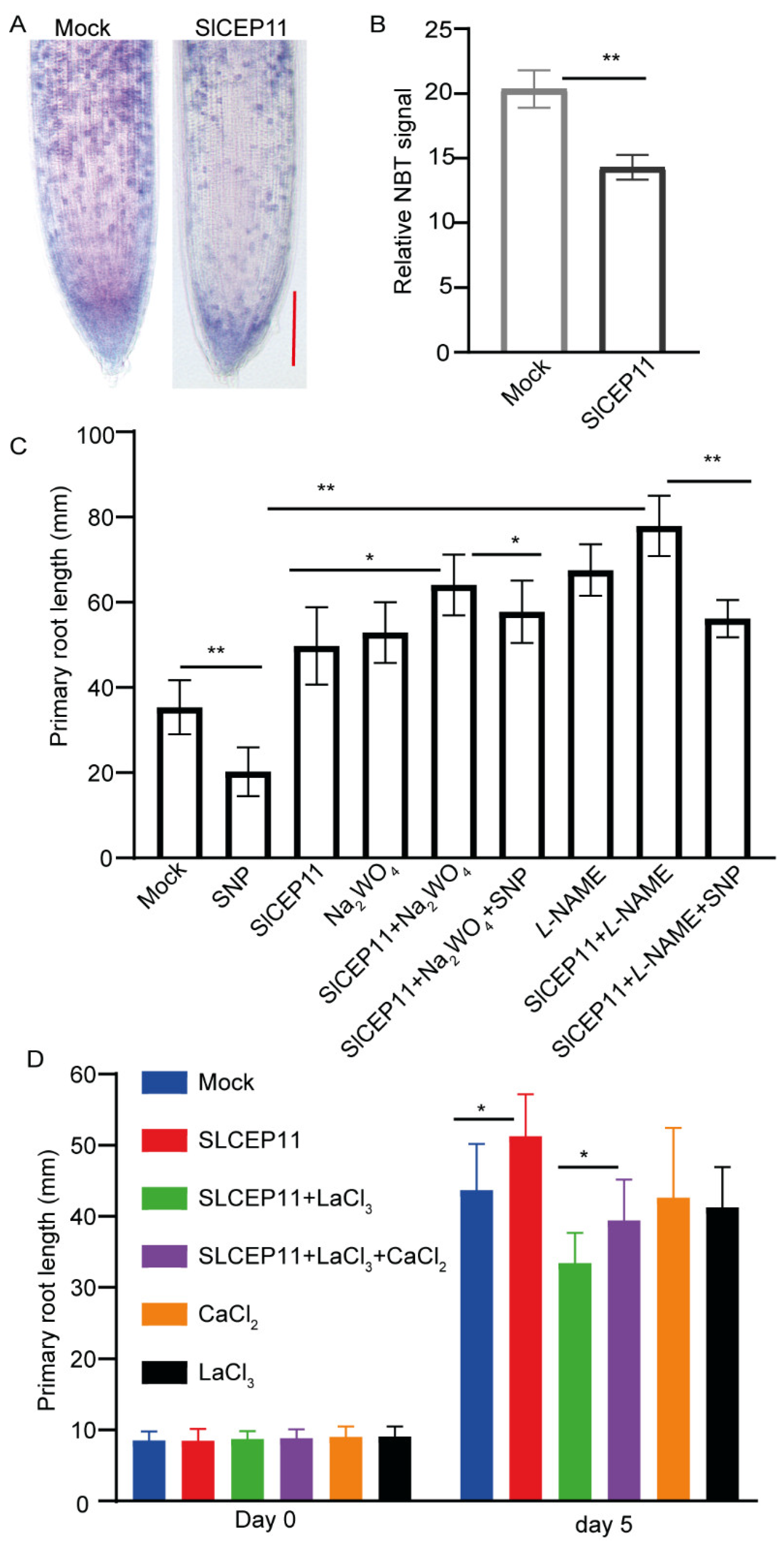
2.5. NO and Ca2+ Mediate CEP Peptide to Promote Tomato Root Growth
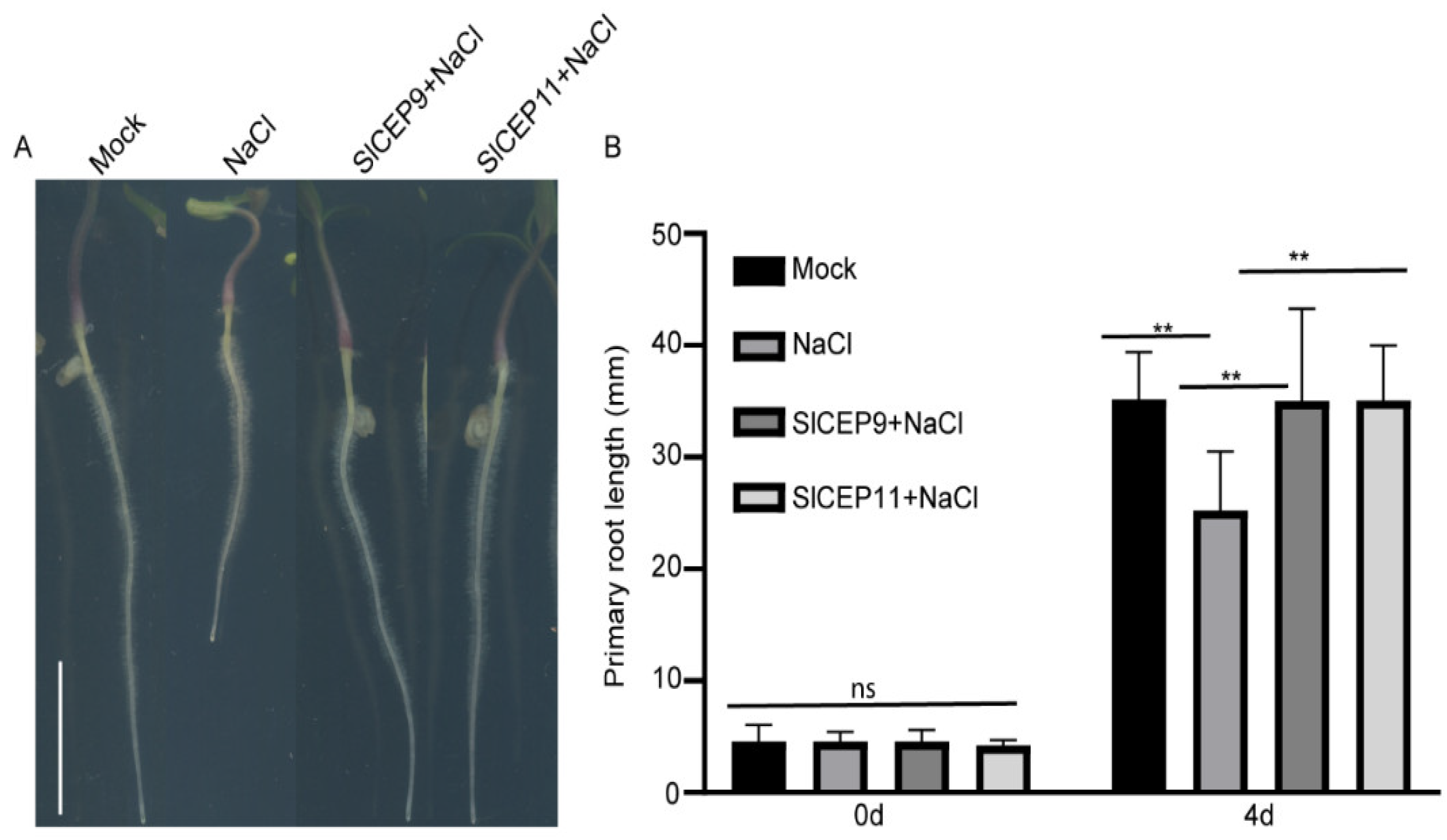
2.6. SlCEP Peptide Promotes Tomato Root Resistance to Salinity
3. Discussion
4. Materials and Methods
4.1. Genome-Wide Annotation of Tomato CEP Peptide Family
4.2. CEP Motif Analysis
4.3. SlCEP Protein Features Analysis
4.4. Genomic Organization and Chromosome Localization
4.5. Alignment and Phylogenetic Analysis
4.6. Gene Duplication Analysis
4.7. SlCEP Gene Expression in Tomato Tissues
4.8. Plant Material and Growth Conditions
4.9. Total RNA Extraction and Gene Expression Analysis
4.10. SlCEP Peptides Treatment
4.11. NBT (Nitroblue Tetrazolium) Staining
4.12. NO and Ca2+ Inhibitor Treatment
4.13. Salinity Treatment and Root Growth Quantification
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CEP | C-TERMINALLY ENCODED PEPTIDE |
| CEPR1 | XYLEM INTERMIXED WITH PHLOEM 1 (XIP1)/ CEP RECEPTOR 1 |
| ROS | Reactive Oxygen Species |
| H2O2 | Hydrogen peroxide |
| NO | Nitric oxide |
| NBT | Nitroblue tetrazolium |
| SNP | Sodium nitroprusside |
| L-NAME | L-NG-Nitro arginine methyl ester |
| RLK | Receptor like kinase |
| CEPD1 | CEPD1 CEP DOWNSTREAM 1 |
| CEPD2 | CEP DOWNSTREAM 1 CEPD 2 |
| CEPDL2 | CEPD-LIKE2 |
| CRA2 | COMPACT ROOT ARCHITECTURE 2 |
| NIN | NODULE INCEPTION |
References
- Van Norman, J.M.; Breakfield, N.W.; Benfey, P.N. Intercellular communication during plant development. Plant Cell 2011, 23, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.; Smith, S.; De Smet, I. Small signaling peptides in Arabidopsis development: How cells communicate over a short distance. Plant Cell 2012, 24, 3198–3217. [Google Scholar] [CrossRef] [PubMed]
- Matsubayashi, Y. Posttranslationally modified small-peptide signals in plants. Annu. Rev. Plant Biol. 2014, 65, 385–413. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, K.; Ogawa, M.; Matsubayashi, Y. Identification of a biologically active, small, secreted peptide in Arabidopsis by in silico gene screening, followed by LC-MS-based structure analysis. Plant J. 2008, 55, 152–160. [Google Scholar] [CrossRef]
- Delay, C.; Imin, N.; Djordjevic, M.A. CEP genes regulate root and shoot development in response to environmental cues and are specific to seed plants. J. Exp. Bot. 2013, 64, 5383–5394. [Google Scholar] [CrossRef] [PubMed]
- Roberts, I.; Smith, S.; De Rybel, B.; Van Den Broeke, J.; Smet, W.; De Cokere, S.; Mispelaere, M.; De Smet, I.; Beeckman, T. The CEP family in land plants: Evolutionary analyses, expression studies, and role in Arabidopsis shoot development. J. Exp. Bot. 2013, 64, 5371–5381. [Google Scholar] [CrossRef]
- Ogilvie, H.A.; Imin, N.; Djordjevic, M.A. Diversification of the C-TERMINALLY ENCODED PEPTIDE (CEP) gene family in angiosperms, and evolution of plant-family specific CEP genes. BMC Genom. 2014, 15, 870. [Google Scholar] [CrossRef]
- Sui, Z.; Wang, T.; Li, H.; Zhang, M.; Li, Y.; Xu, R.; Xing, G.; Ni, Z.; Xin, M. Overexpression of Peptide-Encoding OsCEP6.1 Results in Pleiotropic Effects on Growth in Rice (O. sativa). Front. Plant Sci. 2016, 7, 228. [Google Scholar] [CrossRef]
- Zhou, Y.; Sarker, U.; Neumann, G.; Ludewig, U. The LaCEP1 peptide modulates cluster root morphology in Lupinus albus. Physiol. Plant. 2019, 166, 525–537. [Google Scholar] [CrossRef]
- Yu, Z.; Xu, Y.; Liu, L.; Guo, Y.; Yuan, X.; Man, X.; Liu, C.; Yang, G.; Huang, J.; Yan, K.; et al. The Importance of Conserved Serine for C-Terminally Encoded Peptides Function Exertion in Apple. Int. J. Mol. Sci. 2019, 20, 775. [Google Scholar] [CrossRef]
- Aggarwal, S.; Kumar, A.; Jain, M.; Sudan, J.; Singh, K.; Kumari, S.; Mustafiz, A. C-terminally encoded peptides (CEPs) are potential mediators of abiotic stress response in plants. Physiol. Mol. Biol. Plants 2020, 26, 2019–2033. [Google Scholar]
- Qiu, Z.; Zhuang, K.; Liu, Y.; Ge, X.; Chen, C.; Hu, S.; Han, H. Functional characterization of C-TERMINALLY ENCODED PEPTIDE (CEP) family in Brassica rapa L. Plant Signal. Behav. 2022, 17, 2021365. [Google Scholar] [CrossRef]
- Liu, Y.; Zuo, T.; Qiu, Z.; Zhuang, K.; Hu, S.; Han, H. Genome-wide identification reveals the function of CEP peptide in cucumber root development. Plant Physiol. Biochem. 2021, 169, 119–126. [Google Scholar] [CrossRef]
- Xu, R.; Li, Y.; Sui, Z.; Lan, T.; Song, W.; Zhang, M.; Zhang, Y.; Xing, J. A C-terminal encoded peptide, ZmCEP1, is essential for kernel development in maize. J. Exp. Bot. 2021, 72, 5390–5406. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, Y.; Xu, Q.; Wan, Y.; Zhang, S.; Yang, G.; Huang, J.; Yan, K.; Zheng, C.; Wu, C. SiCEP3, a C-terminally encoded peptide from Setaria italica, promotes ABA import and signaling. J. Exp. Bot. 2021, 72, 6260–6273. [Google Scholar] [CrossRef]
- Roberts, I.; Smith, S.; Stes, E.; De Rybel, B.; Staes, A.; Van De Cotte, B.; Njo, M.F.; Dedeyne, L.; Demol, H.; Lavenus, J.; et al. CEP5 and XIP1/CEPR1 regulate lateral root initiation in Arabidopsis. J. Exp. Bot. 2016, 67, 4889–4899. [Google Scholar] [CrossRef]
- Tabata, R.; Sumida, K.; Yoshii, T.; Ohyama, K.; Shinohara, H.; Matsubayashi, Y. Perception of root-derived peptides by shoot LRR-RKs mediates systemic N-demand signaling. Science 2014, 346, 343–346. [Google Scholar] [CrossRef]
- Ohkubo, Y.; Tanaka, M.; Tabata, R.; Ogawa-Ohnishi, M.; Matsubayashi, Y. Shoot-to-root mobile polypeptides involved in systemic regulation of nitrogen acquisition. Nat. Plants 2017, 3, 17029. [Google Scholar] [CrossRef]
- Ota, R.; Ohkubo, Y.; Yamashita, Y.; Ogawa-Ohnishi, M.; Matsubayashi, Y. Shoot-to-root mobile CEPD-like 2 integrates shoot nitrogen status to systemically regulate nitrate uptake in Arabidopsis. Nat. Commun. 2020, 11, 641. [Google Scholar] [CrossRef]
- Xie, H.; Zhao, W.; Li, W.; Zhang, Y.; Hajný, J.; Han, H. Small signaling peptides mediate plant adaptions to abiotic environmental stress. Planta 2022, 255, 72. [Google Scholar] [CrossRef]
- Laffont, C.; Ivanovici, A.; Gautrat, P.; Brault, M.; Djordjevic, M.A.; Frugier, F. The NIN transcription factor coordinates CEP and CLE signaling peptides that regulate nodulation antagonistically. Nat. Commun. 2020, 11, 3167. [Google Scholar] [CrossRef] [PubMed]
- Gautrat, P.; Laffont, C.; Frugier, F. Compact Root Architecture 2 Promotes Root Competence for Nodulation through the miR2111 Systemic Effector. Curr. Biol. 2020, 30, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Deng, J.; Chen, H.; Liu, P.; Zheng, L.; Ye, Q.; Li, R.; Brault, M.; Wen, J.; Frugier, F.; et al. A CEP Peptide Receptor-Like Kinase Regulates Auxin Biosynthesis and Ethylene Signaling to Coordinate Root Growth and Symbiotic Nodulation in Medicago truncatula. Plant Cell 2020, 32, 2855–2877. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.; Taleski, M.; Ogilvie, H.A.; Imin, N.; Djordjevic, M.A. CEP–CEPR1 signalling inhibits the sucrose-dependent enhancement of lateral root growth. J. Exp. Bot. 2019, 70, 3955–3967. [Google Scholar] [CrossRef]
- Delay, C.; Chapman, K.; Taleski, M.; Wang, Y.; Tyagi, S.; Xiong, Y.; Imin, N.; Djordjevic, M.A. CEP3 levels affect starvation-related growth responses of the primary root. J. Exp. Bot. 2019, 70, 4763–4774. [Google Scholar] [CrossRef]
- Smith, S.; Zhu, S.; Joos, L.; Roberts, I.; Nikonorova, N.; Vu, L.D.; Stes, E.; Cho, H.; Larrieu, A.; Xuan, W.; et al. The CEP5 Peptide Promotes Abiotic Stress Tolerance, As Revealed by Quantitative Proteomics, and Attenuates the AUX/IAA Equilibrium in Arabidopsis. Mol. Cell Proteom. 2020, 19, 1248–1262. [Google Scholar] [CrossRef]
- Chaudhary, J.; Khatri, P.; Singla, P.; Kumawat, S.; Kumari, A.; Vinaykumar, R.; Vikram, A.; Jindal, S.K.; Kardile, H.; Kumar, R.; et al. Advances in Omics Approaches for Abiotic Stress Tolerance in Tomato. Biology 2019, 8, 90. [Google Scholar] [CrossRef]
- Rothan, C.; Diouf, I.; Causse, M. Trait discovery and editing in tomato. Plant J. 2019, 97, 73–90. [Google Scholar] [CrossRef]
- Taleski, M.; Imin, N.; Djordjevic, M.A. CEP peptide hormones: Key players in orchestrating nitrogen-demand signalling, root nodulation, and lateral root development. J. Exp. Bot. 2018, 69, 1829–1836. [Google Scholar] [CrossRef]
- Stührwohldt, N.; Schaller, A. Regulation of plant peptide hormones and growth factors by post-translational modification. Plant Biol. 2019, 1, 49–63. [Google Scholar] [CrossRef]
- Mei, Y.; Zhao, Y.; Jin, X.; Wang, R.; Xu, N.; Hu, J.; Huang, L.; Guan, R.; Shen, W. L-Cysteine desulfhydrase-dependent hydrogen sulfide is required for methane-induced lateral root formation. Plant Mol. Biol. 2019, 99, 283–298. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Liu, F.; Wang, R.; Huang, L.; Shen, W. Hydrogen peroxide is involved in methane-induced tomato lateral root formation. Plant Cell Rep. 2019, 38, 377–389. [Google Scholar] [CrossRef]
- Jin, X.; Li, Y.; Lu, R.; Cheng, P.; Zhang, Y.; Li, L.; Wang, R.; Cui, J.; Shen, W. Methane-induced lateral root formation requires the participation of nitric oxide signaling. Plant Physiol. Biochem. 2020, 147, 262–271. [Google Scholar] [CrossRef]
- Tian, W.; Wang, C.; Gao, Q.; Li, L.; Luan, S. Calcium spikes, waves and oscillations in plant development and biotic interactions. Nat. Plants 2020, 6, 750–759. [Google Scholar] [CrossRef]
- Song, X.F.; Guo, P.; Ren, S.C.; Xu, T.T.; Liu, C.M. Antagonistic peptide technology for functional dissection of CLV3/ESR genes in Arabidopsis. Plant Physiol. 2013, 161, 1076–1085. [Google Scholar] [CrossRef]
- Czyzewicz, N.; Wildhagen, M.; Cattaneo, P.; Stahl, Y.; Pinto, K.G.; Aalen, R.B.; Butenko, M.A.; Simon, R.; Hardtke, C.S.; De Smet, I. Antagonistic peptide technology for functional dissection of CLE peptides revisited. J. Exp. Bot. 2015, 66, 5367–5374. [Google Scholar] [CrossRef]
- Hsieh, Y.H.; Wei, Y.H.; Lo, J.C.; Pan, H.Y.; Yang, S.Y. Arbuscular mycorrhizal symbiosis enhances tomato lateral root formation by modulating CEP2 peptide expression. New Phytol. 2022, 235, 292–305. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, J.; Yang, S.; Song, Y. Identification and expression analysis of the LRR-RLK gene family in tomato (Solanum lycopersicum) Heinz 1706. Genome 2015, 58, 121–134. [Google Scholar] [CrossRef]
- Gaillochet, C.; Develtere, W.; Jacobs, T.B. CRISPR screens in plants: Approaches, guidelines, and future prospects. Plant Cell 2021, 33, 794–813. [Google Scholar] [CrossRef]
- Shinohara, H.; Matsubayashi, Y. Photoaffinity Labeling of Plant Receptor Kinases. Methods Mol Biol. 2017, 1621, 59–68. [Google Scholar]
- Eljebbawi, A.; Guerrero, Y.D.C.R.; Dunand, C.; Estevez, J.M. Highlighting reactive oxygen species as multitaskers in root development. iScience 2020, 24, 101978. [Google Scholar] [CrossRef] [PubMed]
- Mase, K.; Tsukagoshi, H. Reactive Oxygen Species Link Gene Regulatory Networks During Arabidopsis Root Development. Front. Plant Sci. 2021, 12, 660274. [Google Scholar] [CrossRef] [PubMed]
- León, J. Protein Tyrosine Nitration in Plant Nitric Oxide Signaling. Front. Plant Sci. 2022, 13, 859374. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Bhatoee, M.; Singh, P.; Kaladhar, V.C.; Yadav, N.; Paul, D.; Loake, G.J.; Gupta, K.J. Detection of Nitric Oxide from Chickpea Using DAF Fluorescence and Chemiluminescence Methods. Curr. Protoc. 2022, 2, e420. [Google Scholar] [CrossRef]
- Saand, M.A.; Xu, Y.P.; Li, W.; Wang, J.P.; Cai, X.Z. Cyclic nucleotide gated channel gene family in tomato: Genome-wide identification and functional analyses in disease resistance. Front. Plant Sci. 2015, 6, 303. [Google Scholar] [CrossRef]
- Munir, S.; Khan, M.R.; Song, J.; Munir, S.; Zhang, Y.; Ye, Z.; Wang, T. Genome-wide identification, characterization and expression analysis of calmodulin-like (CML) proteins in tomato (Solanum lycopersicum). Plant Physiol. Biochem. 2016, 102, 167–179. [Google Scholar] [CrossRef]
- Wang, J.P.; Xu, Y.P.; Munyampundu, J.P.; Liu, T.Y.; Cai, X.Z. Calcium-dependent protein kinase (CDPK) and CDPK-related kinase (CRK) gene families in tomato: Genome-wide identification and functional analyses in disease resistance. Mol. Genet Genom. 2016, 291, 661–676. [Google Scholar] [CrossRef]
- Hu, Z.; Lv, X.; Xia, X.; Zhou, J.; Shi, K.; Yu, J.; Zhou, Y. Genome-Wide Identification and Expression Analysis of Calcium-dependent Protein Kinase in Tomato. Front. Plant Sci. 2016, 7, 469. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Vidal, E.A.; Alvarez, J.M.; Araus, V.; Riveras, E.; Brooks, M.D.; Krouk, G.; Ruffel, S.; Lejay, L.; Crawford, N.M.; Coruzzi, G.M.; et al. Nitrate in 2020: Thirty Years from Transport to Signaling Networks. Plant Cell 2020, 32, 2094–2119. [Google Scholar] [CrossRef]
- Hosmani, P.S.; Flores-Gonzalez, M.; van de Geest, H.; Maumus, F.; Bakker, L.V.; Schijlen, E.; van Haarst, J.; Cordewener, J.; Sanchez-Perez, G.; Peters, S.; et al. An improved de novo assembly and annotation of the tomato reference genome using single-molecule sequencing, Hi-C proximity ligation and optical maps. bioRxiv 2019. bioRxiv:767764. [Google Scholar]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Crooks, G.E.; Hon, G.; Chandonia, J.M.; Brenner, S.E. WebLogo: A sequence logo generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Chao, J.; Li, Z.; Sun, Y.; Aluko, O.O.; Wu, X.; Wang, Q.; Liu, G. MG2C: A user-friendly online tool for drawing genetic maps. Mol. Hortic. 2021, 1, 16. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Procter, J.B.; Carstairs, G.M.; Soares, B.; Mourão, K.; Ofoegbu, T.C.; Barton, D.; Lui., L.; Menard., A.; Sherstnev., N.; Roldan-Martinez., D.; et al. Alignment of Biological Sequences with Jalview. Methods Mol. Biol. 2021, 2231, 203–224. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Shen, Z.; Zhuang, K.; Qiu, Z.; Deng, H.; Ke, Q.; Liu, H.; Han, H. Systematic Annotation Reveals CEP Function in Tomato Root Development and Abiotic Stress Response. Cells 2022, 11, 2935. https://doi.org/10.3390/cells11192935
Liu D, Shen Z, Zhuang K, Qiu Z, Deng H, Ke Q, Liu H, Han H. Systematic Annotation Reveals CEP Function in Tomato Root Development and Abiotic Stress Response. Cells. 2022; 11(19):2935. https://doi.org/10.3390/cells11192935
Chicago/Turabian StyleLiu, Dan, Zeping Shen, Keqing Zhuang, Ziwen Qiu, Huiming Deng, Qinglin Ke, Haoju Liu, and Huibin Han. 2022. "Systematic Annotation Reveals CEP Function in Tomato Root Development and Abiotic Stress Response" Cells 11, no. 19: 2935. https://doi.org/10.3390/cells11192935
APA StyleLiu, D., Shen, Z., Zhuang, K., Qiu, Z., Deng, H., Ke, Q., Liu, H., & Han, H. (2022). Systematic Annotation Reveals CEP Function in Tomato Root Development and Abiotic Stress Response. Cells, 11(19), 2935. https://doi.org/10.3390/cells11192935






