Overexpression of acdS in Petunia hybrida Improved Flower Longevity and Cadmium-Stress Tolerance by Reducing Ethylene Production in Floral and Vegetative Tissues
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Measurement of Flower Longevity and Ethylene Production
2.3. Expression Analysis of Ethylene Biosynthesis and Signaling Genes
2.4. Cd-Stress Treatment
2.5. Measurement of SPAD Values and Relative Water Content (RWC)
2.6. Analysis of Stomatal Density
2.7. Measurement of Ethylene Production
2.8. Detection of Cd Concentration
2.9. Detection of Hydrogen Peroxide (H2O2) Accumulation
2.10. Detection of the Genes at Transcript Level Involved in Ethylene Biosynthesis and Signaling Pathways, Antioxidant and Proline Activities, and Metal Chelation
2.11. Statistical Analysis
3. Results
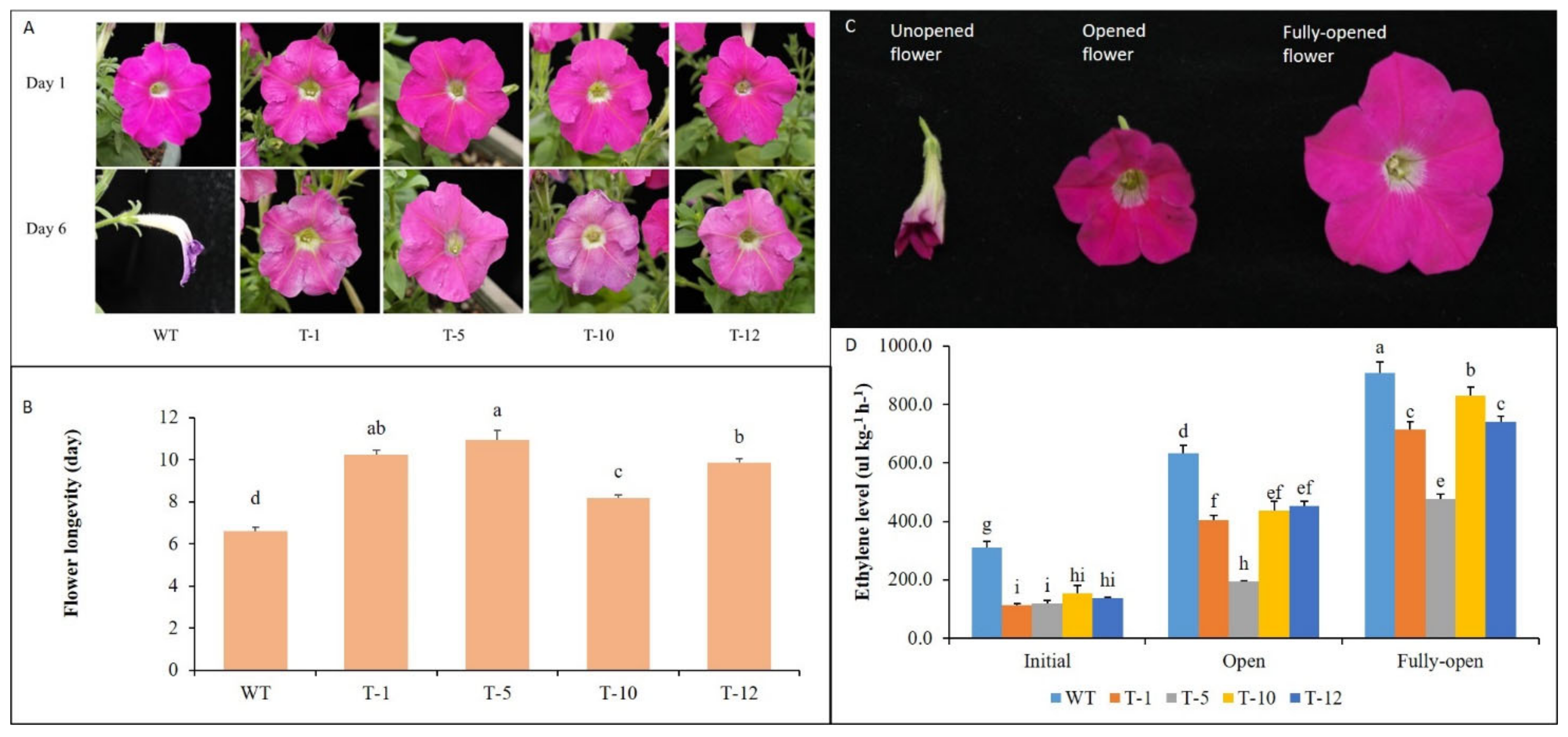
3.1. Flower Longevity of WT and Transgenic Plants
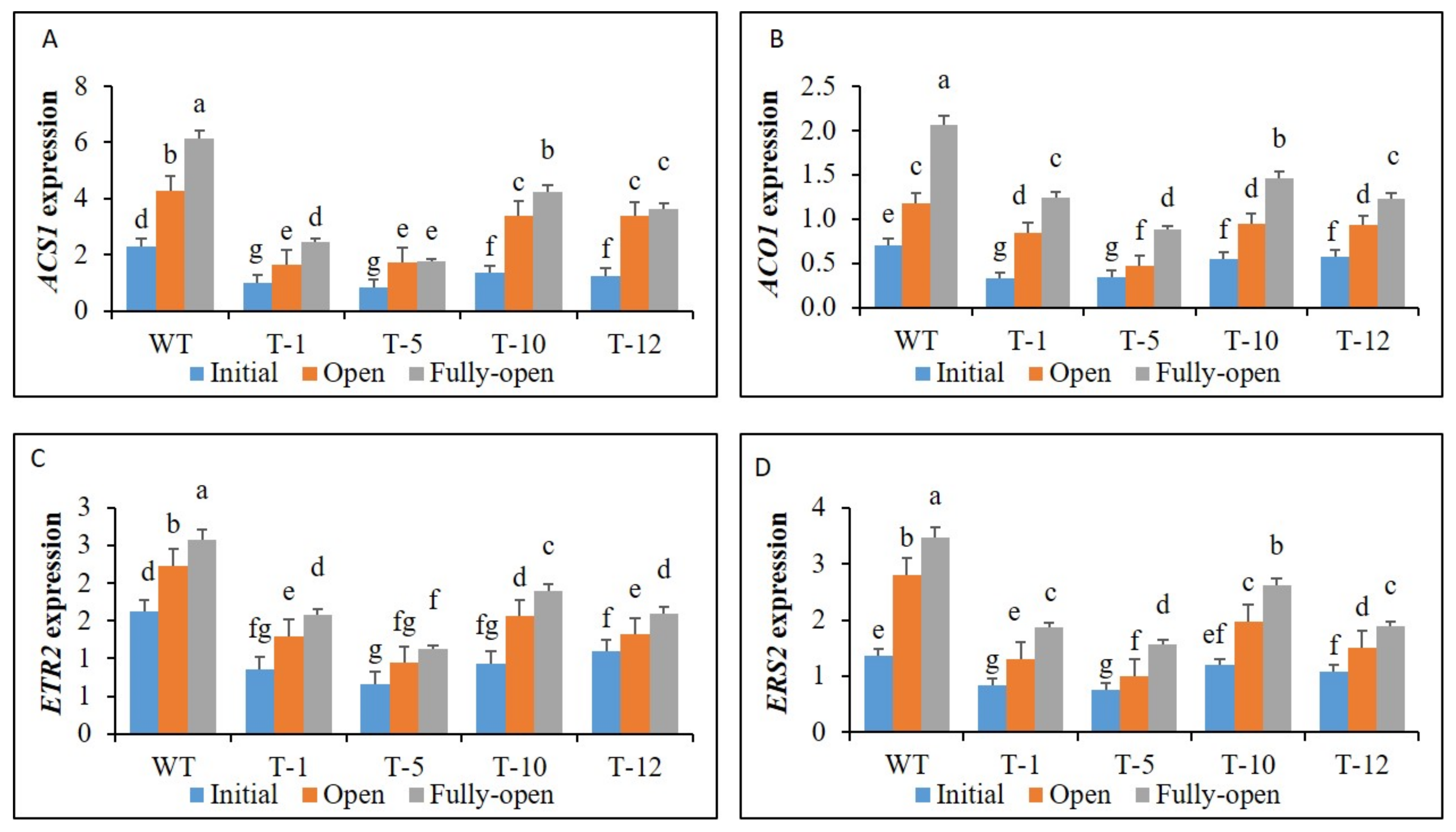
3.2. Expression of Ethylene Biosynthesis and Receptor Genes in Floral Tissues
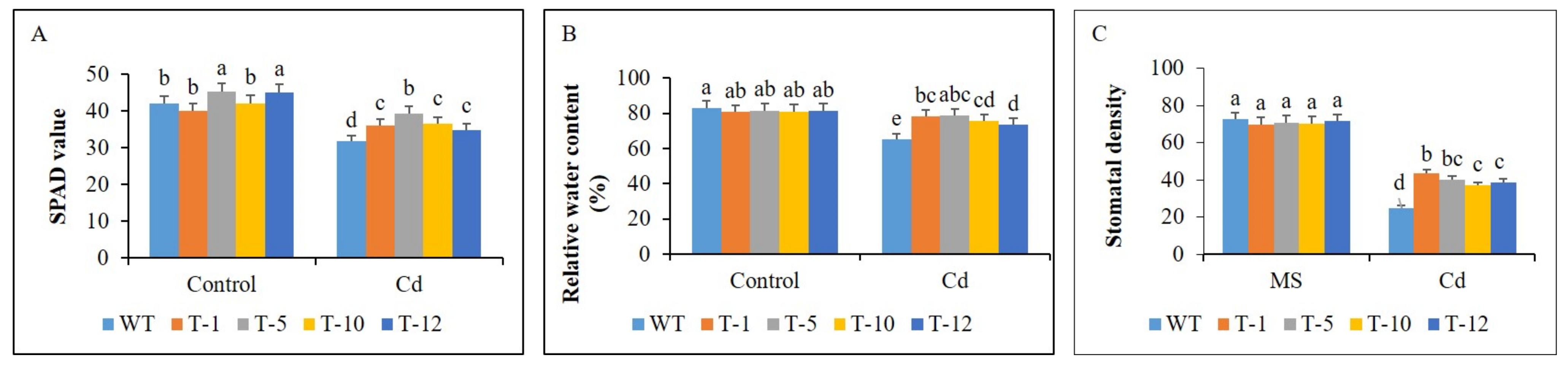
3.3. Plant Growth and Physiological Performance under Control and Cd Stress Conditions
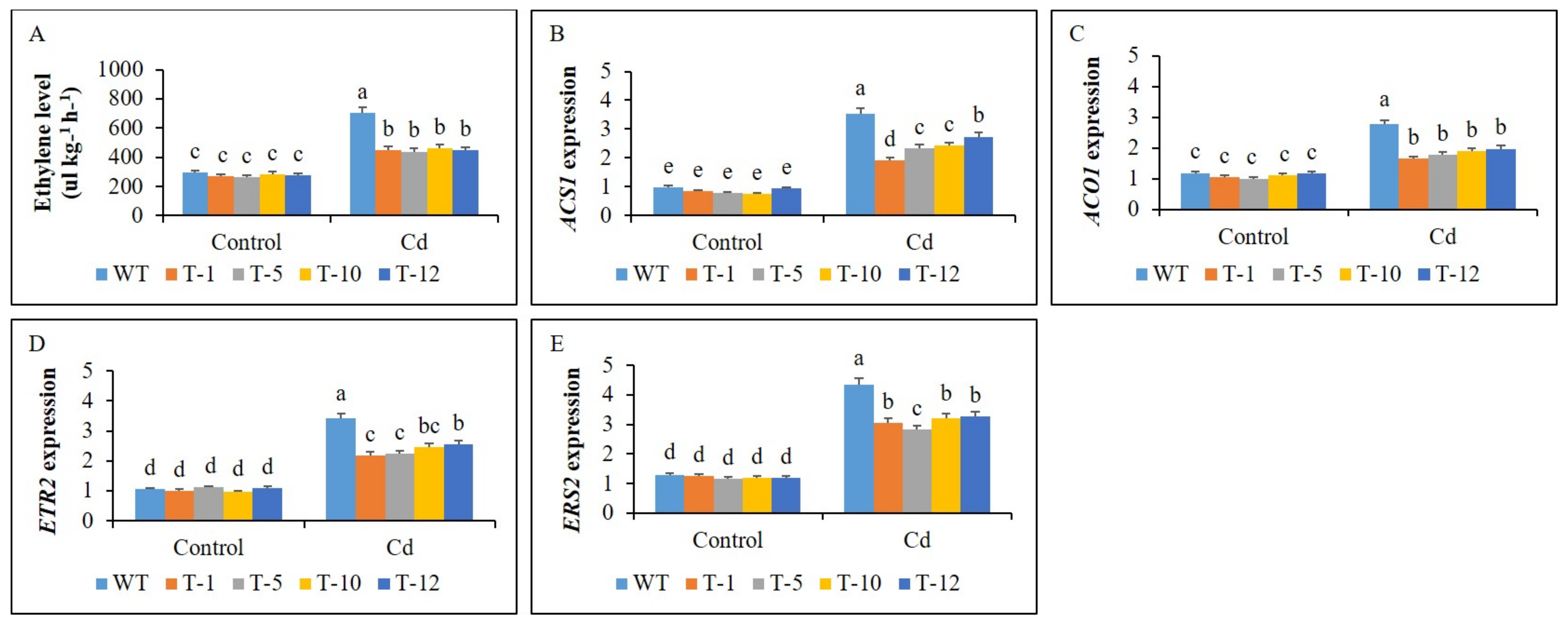
3.4. Ethylene Production and Expression Profiles of Its Biosynthesis and Receptor Genes
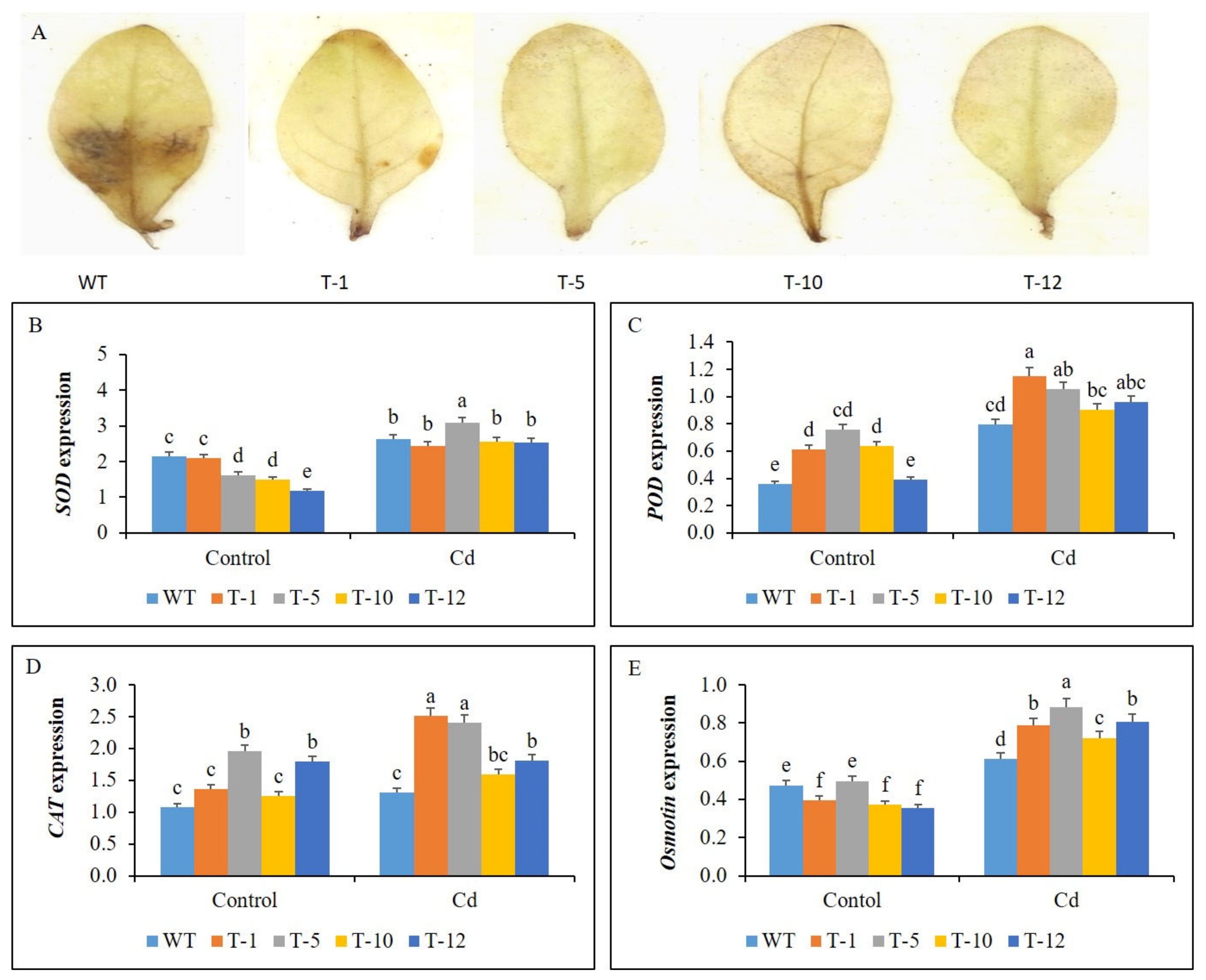
3.5. Hydrogen Peroxide (H2O2) Accumulation in WT and Transgenic Plants
3.6. Expression Profile of Antioxidant- and Proline-Related Genes
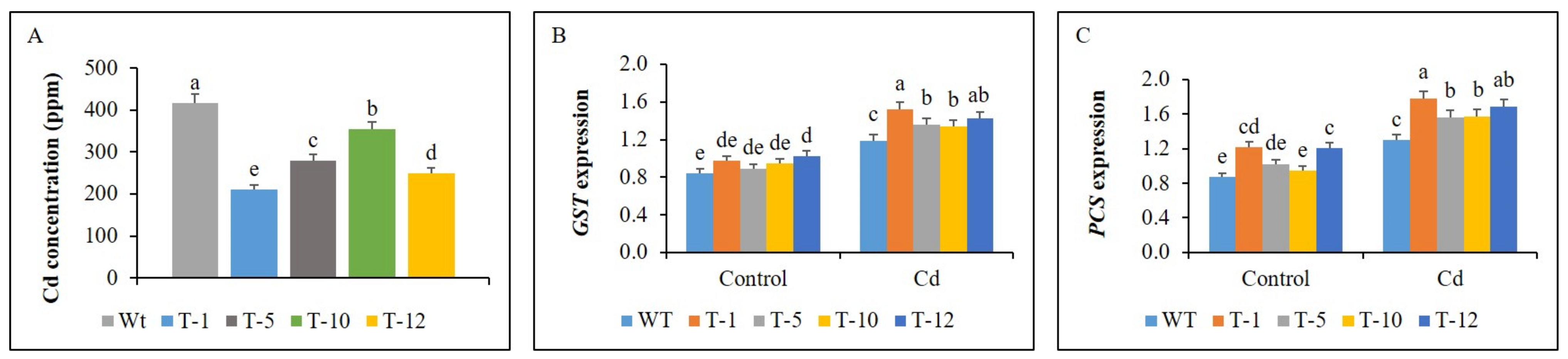
3.7. Cd Concentration and Expression of GST and PCS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Xu, J.; Kang, B.C.; Naing, A.H.; Bae, S.J.; Kim, J.S.; Kim, H.; Kim, C.K. CRISPR/Cas9-mediated editing of 1-aminocyclopropane-1-carboxylate oxidase1 enhances Petunia flower longevity. Plant Biotechnol. J. 2020, 18, 287–297. [Google Scholar] [CrossRef]
- Xu, J.; Naing, A.H.; Bunch, H.; Jeong, J.; Kim, H.; Kim, C.K. Enhancement of the flower longevity of petunia by CRISPR/Cas9-mediated targeted editing of ethylene biosynthesis genes. Postharvest Biol. Technol. 2021, 174, 111460. [Google Scholar] [CrossRef]
- Huang, L.-C.; Lai, U.-L.; Yang, S.-F.; Chu, M.-J.; Kuo, C.-I.; Tsai, M.-F.; Sun, C.-W. Delayed flower senescence of Petunia hybrida plants transformed with antisense broccoli ACC synthase and ACC oxidase genes. Postharvest Biol. Technol. 2007, 46, 47–53. [Google Scholar] [CrossRef]
- Naing, A.H.; Jeong, H.Y.; Jung, S.K.; Kim, C.K. Overexpression of 1-Aminocyclopropane-1-Carboxylic Acid Deaminase (acdS) Gene in Petunia hybrida Improves Tolerance to Abiotic Stresses. Front. Plant Sci. 2021, 12, 737490. [Google Scholar] [CrossRef] [PubMed]
- Naing, A.H.; Kim, C.K. Application of nano-silver particles to control the postharvest biology of cut fowers: A review. Sci. Hortic. 2020, 270, 109463. [Google Scholar] [CrossRef]
- Honma, M.; Shimomura, T. Metabolism of 1-aminocyclopropane-1-carboxylic acid. Agric. Biol. Chem. 1978, 42, 1825–1831. [Google Scholar]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Grichko, V.P.; Filby, B.; Glick, B.R. Increased ability of transgenic plants expressing the bacterial enzyme ACC deaminase to accumulate Cd, Co, Cu, Ni, Pb, and Zn. J. Biotechnol. 2000, 81, 45–53. [Google Scholar] [CrossRef]
- Nie, L.; Shah, S.; Rashid, A.; Burd, G.I.; Dixon, D.G.; Glick, B.R. Phytoremediation of arsenate contaminated soil by transgenic canola and the plant growth-promoting bacterium Entero-bacter cloacae CAL2. Plant Physiol. Biochem. 2002, 40, 355–361. [Google Scholar] [CrossRef]
- Reed, A.J.; Magin, K.M.; Anderson, J.S.; Austin, G.D.; Rangwala, T.; Linde, D.C.; Love, J.N.; Rogers, S.G.; Fuchs, R.L. Delayed ripening tomato plants expressing the enzyme 1-aminocyclopropane-1-carboxylic acid deaminase. 1. Molecular characterization, enzyme expression, and fruit ripening traits. J. Agric. Food Chem. 1995, 43, 1954–1962. [Google Scholar]
- Stearns, J.C.; Saleh, S.; Greenberg, B.M.; Dixon, D.G.; Glick, B.R. Tolerance of transgenic canola expressing 1-aminocyclopropane1-carboxylic acid deaminase to growth inhibition by nickel. Plant Physiol. Biochem. 2005, 43, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Ai, T.N.; Naing, A.H.; Yun, B.-W.; Lim, S.H.; Kim, C.K. Overexpression of RsMYB1 Enhances Anthocyanin Accumulation and Heavy Metal Stress Tolerance in Transgenic Petunia. Front. Plant Sci. 2018, 9, 1388. [Google Scholar] [CrossRef] [PubMed]
- Nriagu, J.O.; Pacyna, J.M. Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature 1988, 333, 134–139. [Google Scholar] [CrossRef]
- Tran, T.A.; Popova, L.P. Functions and toxicity of cadmium in plants: Recent advances and future prospects. Turk. J. Bot. 2013, 37, 1–13. [Google Scholar] [CrossRef]
- Cao, F.; Chen, F.; Sun, H.; Zhang, G.; Chen, Z.-H.; Wu, F. Genomewide transcriptome and functional analysis of two contrasting genotypes reveals key genes for cadmium tolerance in barley. BMC Genomics 2014, 15, 611. [Google Scholar] [CrossRef]
- Chmielowska-Bąk, J.; Lefèvre, I.; Lutts, S.; Deckert, J. Short term signaling responses in roots of young soybean seedlings exposed to cadmium stress. J. Plant Physiol. 2013, 170, 1585–1594. [Google Scholar] [CrossRef]
- Naing, A.H.; Maung, T.T.; Kim, C.K. The ACC deaminaseproducing plant growth-promoting bacteria: Influences of bacterial strains and ACC deaminase activities in plant tolerance to abiotic stress. Physiol. Plant. 2021, 173, 1992–2012. [Google Scholar] [CrossRef]
- Arteca, R.N.; Arteca, J.M. Heavy-metal-induced ethylene production in Arabidopsis thaliana. J. Plant Physiol. 2007, 164, 1480–1488. [Google Scholar] [CrossRef]
- Asgher, M.; Khan, N.A.; Khan, M.I.R.; Fatma, M.; Masood, A. Ethylene production is associated with alleviation of cadmium-induced oxidative stress by sulfur in mustard types differing in ethylene sensitivity. Ecotoxicol. Environ. Saf. 2014, 106, 54–61. [Google Scholar] [CrossRef]
- Herbette, S.; Taconnat, L.; Hugouvieux, V.; Piette, L.; Magniette, M.-L.; Cuine, S.; Auroy, P.; Richaud, P.; Forestier, C.; Bourguignon, J.; et al. Genome-wide transcriptome profiling of the early cadmium response of Arabidopsis roots and shoots. Biochimie 2006, 88, 1751–1765. [Google Scholar] [CrossRef]
- Iakimova, E.T.; Woltering, E.J.; Kapchina-Toteva, V.M.; Harren, F.J.M.; Cristescu, S.M. Cadmium toxicity in cultured tomato cells—Role of ethylene, proteases and oxidative stress in cell death signaling. Cell Biol. Int. 2008, 32, 1521–1529. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Nazir, F.; Asgher, M.; Per, T.S.; Khan, N.A. Selenium and sulfur influence ethylene formation and alleviate cadmium-induced oxidative stress by improving proline and glutathione production in wheat. J. Plant Physiol. 2015, 173, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Serrano, M.; Romero-Puertas, M.C.; Zabalza, A.; Corpas, F.J.; Gómez, M.; Del río, L.A.; Sandalio, L.M. Cadmium effect on oxidative metabolism of pea (Pisum sativum L.) roots. Imaging of reactive oxygen species and nitric oxide accumulation in vivo. Plant Cell Environ. 2006, 29, 1532–1534. [Google Scholar] [CrossRef]
- Schellingen, K.; Van Der Straeten, D.; Vandenbussche, F.; Prinsen, E.; Remans, T.; Vangronsveld, J.; Cuypers, A. Cadmium-induced ethylene production and responses in Arabidopsis thaliana rely on ACS2 and ACS6 gene expression. BMC Plant Biol. 2014, 14, 214. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Trampczynska, A.; Clemens, S. Comparative transcriptome analysis of toxic metal responses in Arabidopsis thaliana and the Cd2+- hypertolerant facultative metallophyte Arabidopsis halleri. Plant Cell Environ. 2006, 29, 950–963. [Google Scholar] [CrossRef] [PubMed]
- Belimov, A.A.; Hontzeas, N.; Safronova, V.I.; Demchinskaya, S.V.; Piluzza, G.; Bullitta, S.; Glick, B.R. Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L.). Czern. Soil Biol. Biochem. 2005, 37, 241–250. [Google Scholar] [CrossRef]
- Mitra, S.; Pramanik, K.; Sarkar, A.; Ghosh, P.K.; Soren, T.; Maiti, T.K. Bioaccumulation of cadmium by Enterobacter sp. and enhancement of rice seedling growth under cadmium stress. Ecotoxicol. Environ. Saf. 2018, 156, 183–196. [Google Scholar] [CrossRef]
- Pramanik, K.; Mitra, S.; Sarkar, A.; Maiti, T.K. Alleviation of phytotoxic effects of cadmium on rice seedlings by cadmium resistant PGPR strain Enterobacter aerogenes MCC 3092. J. Hazard. Mater. 2018, 351, 317–329. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, L.; Wang, Y.; Yang, B.; Chen, S. Enhancement of heavy metal accumulation by tissue specific coexpression of iaaM and ACC deaminase genes in plants. Chemosphere 2008, 72, 564–571. [Google Scholar] [CrossRef]
- Sun, P.; Tian, Q.Y.; Chen, J.; Zhang, W.H. Aluminium-induced inhibition of root elongation in Arabidopsis is mediated by ethylene and auxin. J. Exp. Bot. 2010, 61, 347–356. [Google Scholar] [CrossRef]
- Montero-Palmero, M.B.; Martín-Barranco, A.; Escobar, C.; Hernández, L.E. Early transcriptional responses to mercury: A role for ethylene in mercury-induced stress. New Phytol. 2014, 201, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Mostofa, M.G.; Hossain, M.A.; Fujita, M.; Tran, L.S.P. Physiological and biochemical mechanisms associated with trehalose-induced copperstress tolerance in rice. Sci. Rep. 2015, 5, 11433. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.L.; Shen, L.; Wang, J.Q.; Sheng, J.P. Rapid inactivation of chloroplastic ascorbate peroxidase is responsible for oxidative modification to Rubisco in tomato (Lycopersicon esculentum) under cadmium stress. J. Integr. Plant Biol. 2008, 50, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Yusuf, M.A.; Singh, P.; Sardar, M.; Sarin, N.B. Histochemical Detection of superoxide and H2O2 accumulation in Brassica juncea seedlings. Bio-protocol 2014, 4, e1108. [Google Scholar] [CrossRef]
- Klee, H.J.; Hayford, M.B.; Kretzmer, K.A.; Barry, G.F.; Kishore, G.M. Control of ethylene synthesis by expression of a bacterial enzyme in transgenic tomato plants. Plant Cell 1991, 3, 1187–1193. [Google Scholar]
- Klee, H.J.; Kishore, G.M. Control of fruit ripening and senescence in plants. U.S. Patent 5,702,933, 30 December 1997. [Google Scholar]
- Hall, J.L. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef]
- Gill, S.S.; Khan, N.A.; Anjum, N.A.; Tuteja, N. Amelioration of cadmium stress in crop plants by nutrients management: Morphological, physiological and biochemical aspects. Plant Stress 2011, 5, 1–23. [Google Scholar]
- Keunen, E.; Schellingen, K.; Vangronsveld, J.; Cuypers, A. Ethylene and Metal Stress: Small Molecule, Big Impact. Front. Plant Sci. 2016, 7, 23. [Google Scholar] [CrossRef]
- Yuan, H.M.; Xu, H.H.; Liu, W.C.; Lu, Y.T. Copper regulates primary root elongation through PIN1-mediated auxin redistribution. Plant Cell Physiol. 2013, 54, 766–778. [Google Scholar] [CrossRef]
- Bittsánszky, A.; Kömives, T.; Gullner, G.; Gyulai, G.; Kiss, J.; Heszky, L.; Radimszky, L.; Rennenberg, H. Ability of transgenic poplars with elevated glutathione content to tolerate zinc(2+) stress. Environ. Interact. 2005, 31, 251–254. [Google Scholar] [CrossRef]
- Cairns, N.G.; Pasternak, M.; Wachter, A.; Cobbett, C.S.; Meyer, A.J. Maturation of Arabidopsis seeds is dependent on glutathione biosynthesis within the embryo. Plant Physiol. 2006, 141, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Gasic, K.; Korban, S.S. Expression of Arabidopsis phytochelatin synthase in Indian mustard (Brassica juncea) plants enhances tolerance for Cd and Zn. Planta 2007, 225, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Gasic, K.; Korban, S.S. Transgenic Indian mustard (Brassica juncea) plants expressing an Arabidopsis phytochelatin synthase (AtPCS1) exhibit enhanced As and Cd tolerance. Plant Mol. Biol. 2007, 64, 361–369. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naing, A.H.; Campol, J.R.; Chung, M.Y.; Kim, C.K. Overexpression of acdS in Petunia hybrida Improved Flower Longevity and Cadmium-Stress Tolerance by Reducing Ethylene Production in Floral and Vegetative Tissues. Cells 2022, 11, 3197. https://doi.org/10.3390/cells11203197
Naing AH, Campol JR, Chung MY, Kim CK. Overexpression of acdS in Petunia hybrida Improved Flower Longevity and Cadmium-Stress Tolerance by Reducing Ethylene Production in Floral and Vegetative Tissues. Cells. 2022; 11(20):3197. https://doi.org/10.3390/cells11203197
Chicago/Turabian StyleNaing, Aung Htay, Jova Riza Campol, Mi Young Chung, and Chang Kil Kim. 2022. "Overexpression of acdS in Petunia hybrida Improved Flower Longevity and Cadmium-Stress Tolerance by Reducing Ethylene Production in Floral and Vegetative Tissues" Cells 11, no. 20: 3197. https://doi.org/10.3390/cells11203197
APA StyleNaing, A. H., Campol, J. R., Chung, M. Y., & Kim, C. K. (2022). Overexpression of acdS in Petunia hybrida Improved Flower Longevity and Cadmium-Stress Tolerance by Reducing Ethylene Production in Floral and Vegetative Tissues. Cells, 11(20), 3197. https://doi.org/10.3390/cells11203197






