Dectin-1 as a Potential Inflammatory Biomarker for Metabolic Inflammation in Adipose Tissue of Individuals with Obesity
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population and Anthropometric Measurements
2.2. Collection of Subcutaneous AT
2.3. Measurement of Metabolic Inflammatory Markers
2.4. RNA Extraction, cDNA Synthesis, and RT-qPCR Reactions
2.5. Immunohistochemistry (IHC)
2.6. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of the Study Population
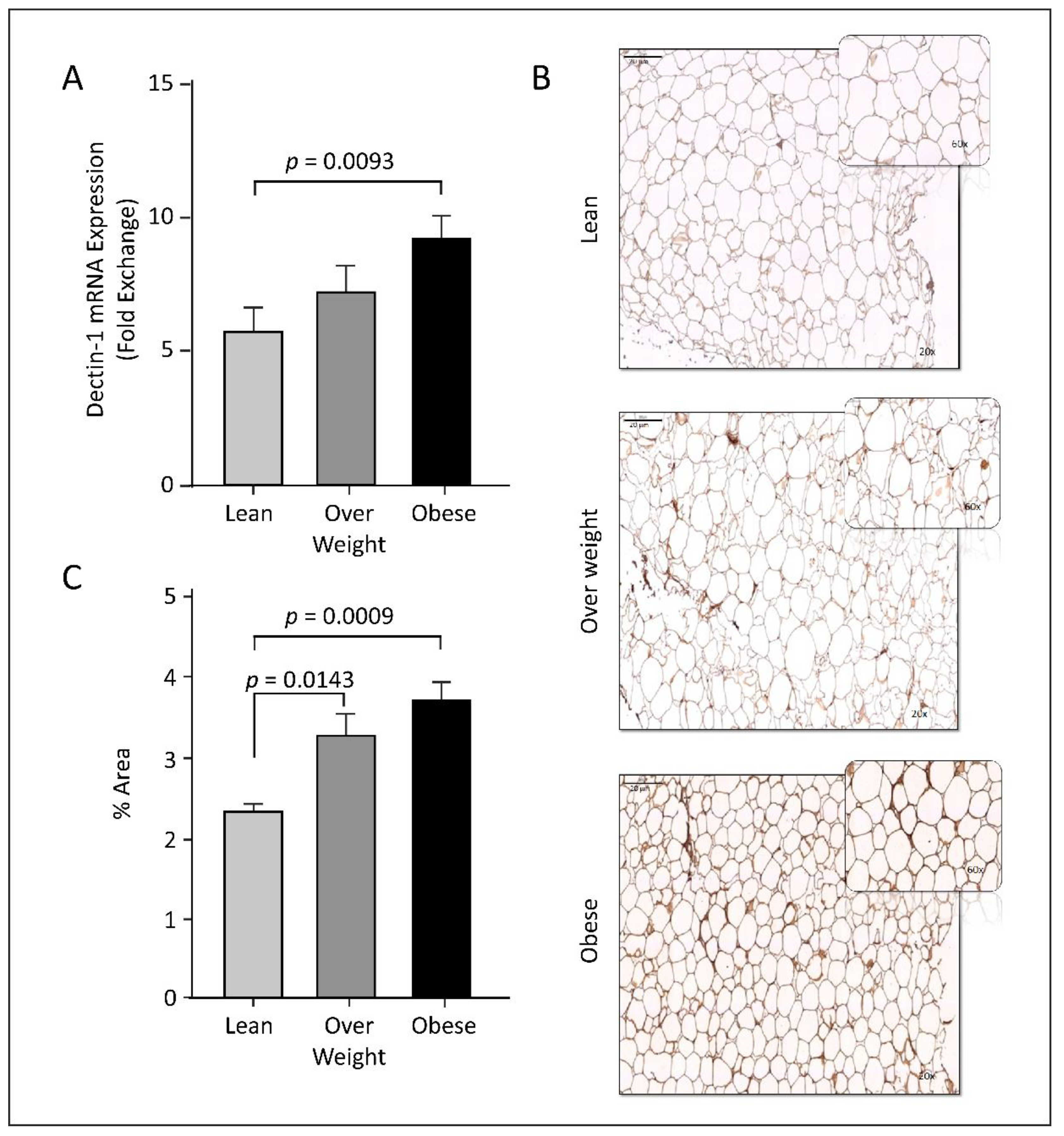
3.2. Dectin-1 Gene Expression Is Associated with Obesity
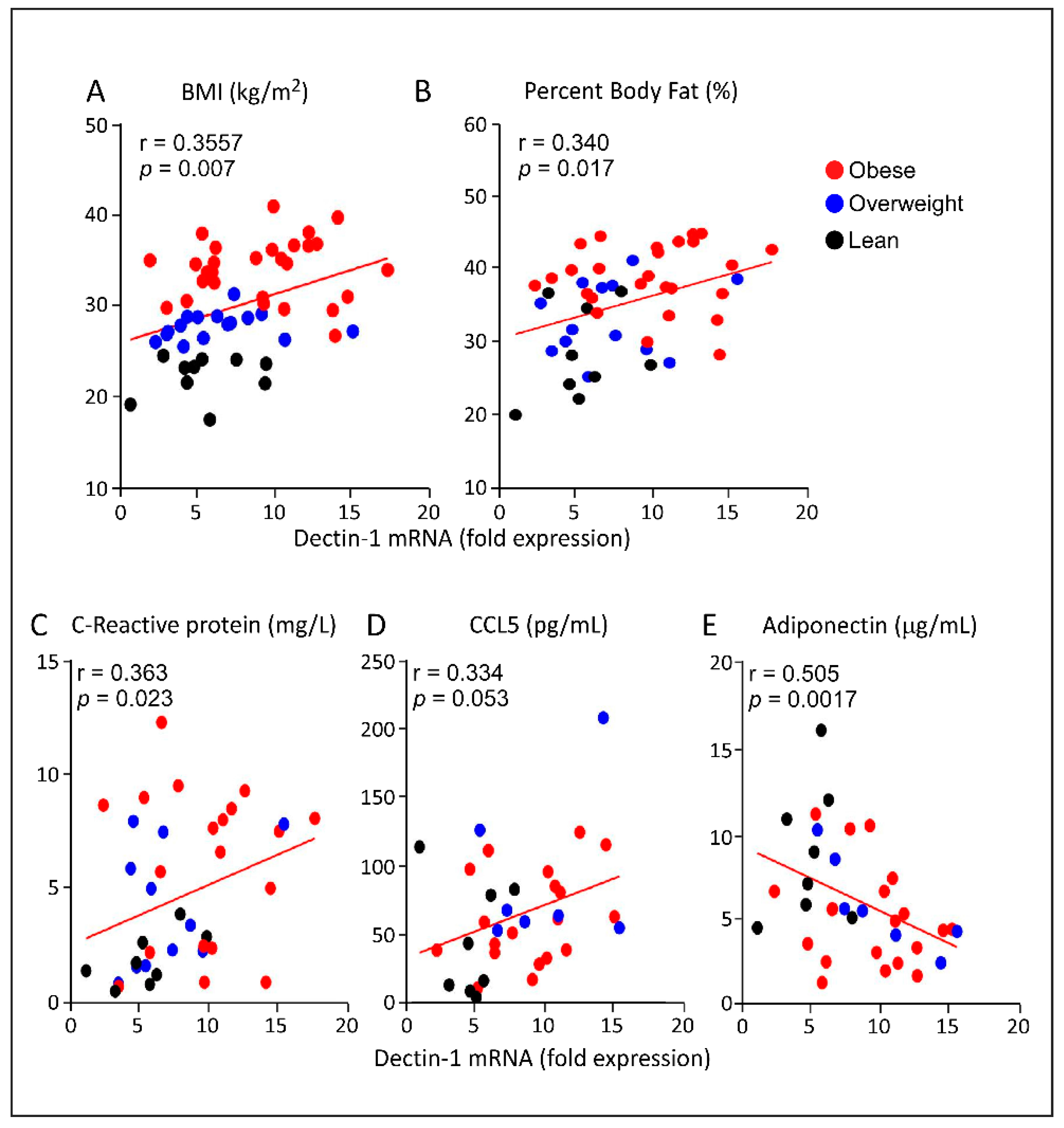
3.3. Increased AT Dectin-1 Gene Expression in Obesity Correlates with the Metabolic and Immune Markers
3.4. Increased Adipose Dectin-1 Gene Expression Is Associated with Inflammatory Signatures
3.5. Increased AT Dectin-1 Gene Expression in Obesity Is Associated with Toll-Like Receptors (TLRs), Downstream Signaling Molecules, and Inflammatory Leukocyte Subpopulations
3.6. Dectin-1 as an Independent Predictor of AT Inflammatory Markers
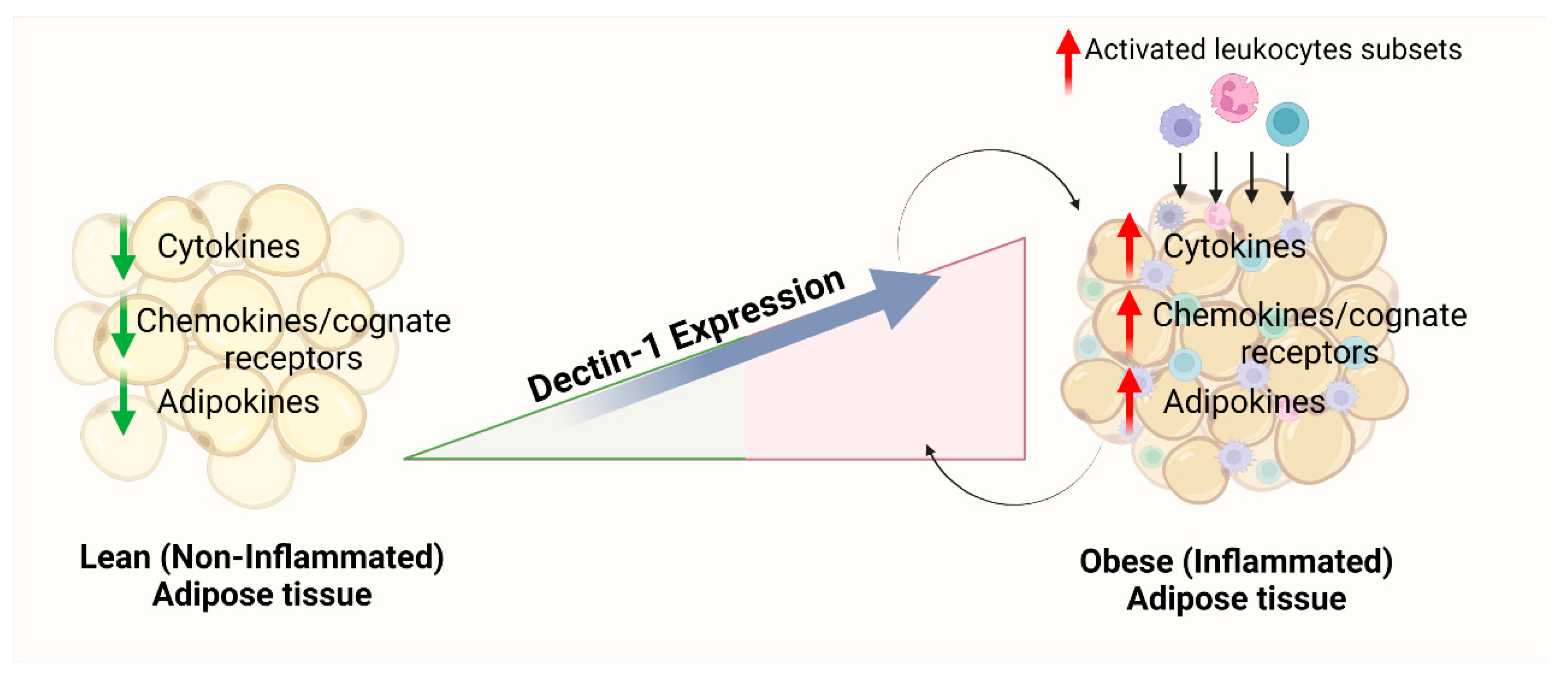
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2015 Obesity Collaborators; Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [PubMed]
- Gregg, E.W.; Shaw, J.E. Global Health Effects of Overweight and Obesity. N. Engl. J. Med. 2017, 377, 80–81. [Google Scholar] [CrossRef] [PubMed]
- Ogden, J.; Flanagan, Z. Beliefs about the causes and solutions to obesity: A comparison of GPs and lay people. Patient Educ. Couns. 2008, 71, 72–78. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fitipaldi, H.; McCarthy, M.I.; Florez, J.C.; Franks, P.W. A Global Overview of Precision Medicine in Type 2 Diabetes. Diabetes 2018, 67, 1911–1922. [Google Scholar] [CrossRef]
- Day, M.J. One Health Approach to Preventing Obesity in People and Their Pets. J. Comp. Pathol. 2017, 156, 293–295. [Google Scholar] [CrossRef]
- Bomberg, E.M.; Ryder, J.R.; Brundage, R.C.; Straka, R.J.; Fox, C.K.; Gross, A.C.; Oberle, M.M.; Bramante, C.T.; Sibley, S.D.; Kelly, A.S. Precision medicine in adult and pediatric obesity: A clinical perspective. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018819863022. [Google Scholar] [CrossRef]
- Lee, L.; Sanders, R.A. Metabolic syndrome. Pediatr. Rev. 2012, 33, 459–466. [Google Scholar] [CrossRef]
- Echahidi, N.; Mohty, D.; Pibarot, P.; Despres, J.P.; O’Hara, G.; Champagne, J.; Philippon, F.; Daleau, P.; Voisine, P.; Mathieu, P. Obesity and metabolic syndrome are independent risk factors for atrial fibrillation after coronary artery bypass graft surgery. Circulation 2007, 116 (Suppl. 11), I213–I219. [Google Scholar] [CrossRef]
- Herrera, B.M.; Lindgren, C.M. The genetics of obesity. Curr. Diabetes Rep. 2010, 10, 498–505. [Google Scholar] [CrossRef]
- Kissebah, A.H.; Vydelingum, N.; Murray, R.; Evans, D.J.; Hartz, A.J.; Kalkhoff, R.K.; Adams, P.W. Relation of body fat distribution to metabolic complications of obesity. J. Clin. Endocrinol. Metab. 1982, 54, 254–260. [Google Scholar] [CrossRef]
- Walker, K.A.; Gottesman, R.F.; Wu, A.; Knopman, D.S.; Gross, A.L.; Mosley, T.H.; Selvin, E., Jr.; Windham, B.G. Systemic inflammation during midlife and cognitive change over 20 years: The ARIC Study. Neurology 2019, 92, e1256–e1267. [Google Scholar] [PubMed]
- Walker, K.A.; Walston, J.; Gottesman, R.F.; Kucharska-Newton, A.; Palta, P.; Windham, B.G. Midlife Systemic Inflammation Is Associated With Frailty in Later Life: The ARIC Study. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Choi, M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef]
- Johnson, A.M.; Olefsky, J.M. The origins and drivers of insulin resistance. Cell 2013, 152, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Alaniz, M.H.; Takada, J.; Alonso-Vale, M.I.; Lima, F.B. Adipose tissue as an endocrine organ: From theory to practice. J. Pediatr. 2007, 83 (Suppl. 5), S192–S203. [Google Scholar] [CrossRef]
- Ahima, R.S.; Flier, J.S. Adipose tissue as an endocrine organ. Trends Endocrinol. Metab. 2000, 11, 327–332. [Google Scholar] [CrossRef]
- Cinti, S. Pink Adipocytes. Trends Endocrinol. Metab. 2018, 29, 651–666. [Google Scholar] [CrossRef]
- Waki, H.; Tontonoz, P. Endocrine functions of adipose tissue. Annu. Rev. Pathol. 2007, 2, 31–56. [Google Scholar] [CrossRef]
- Pasarica, M.; Sereda, O.R.; Redman, L.M.; Albarado, D.C.; Hymel, D.T.; Roan, L.E.; Rood, J.C.; Burk, D.H.; Smith, S.R. Reduced adipose tissue oxygenation in human obesity: Evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 2009, 58, 718–725. [Google Scholar] [CrossRef]
- Garcia-Martin, R.; Alexaki, V.I.; Qin, N.; Rubin de Celis, M.F.; Economopoulou, M.; Ziogas, A.; Gercken, B.; Kotlabova, K.; Phieler, J.; Ehrhart-Bornstein, M.; et al. Adipocyte-Specific Hypoxia-Inducible Factor 2alpha Deficiency Exacerbates Obesity-Induced Brown Adipose Tissue Dysfunction and Metabolic Dysregulation. Mol. Cell Biol. 2016, 36, 376–393. [Google Scholar] [CrossRef]
- Esteve Rafols, M. Adipose tissue: Cell heterogeneity and functional diversity. Endocrinol. Nutr. 2014, 61, 100–112. [Google Scholar] [CrossRef] [PubMed]
- De Boer, M.D. Obesity, systemic inflammation, and increased risk for cardiovascular disease and diabetes among adolescents: A need for screening tools to target interventions. Nutrition 2013, 29, 379–386. [Google Scholar] [CrossRef]
- Scherer, P.E.; Hill, J.A. Obesity, Diabetes, and Cardiovascular Diseases: A Compendium. Circ. Res. 2016, 118, 1703–1705. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.R.; Brown, G.D.; Reid, D.M.; Willment, J.A.; Martinez-Pomares, L.; Gordon, S.; Wong, S.Y. The beta-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J. Immunol. 2002, 169, 3876–3882. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Taylor, P.R.; Reid, D.M.; Willment, J.A.; Williams, D.L.; Martinez-Pomares, L.; Wong, S.Y.; Gordon, S. Dectin-1 is a major beta-glucan receptor on macrophages. J. Exp. Med. 2002, 196, 407–412. [Google Scholar] [CrossRef]
- Rogers, N.C.; Slack, E.C.; Edwards, A.D.; Nolte, M.A.; Schulz, O.; Schweighoffer, E.; Williams, D.L.; Gordon, S.; Tybulewicz, V.L.; Brown, G.D.; et al. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity 2005, 22, 507–517. [Google Scholar] [CrossRef]
- Dennehy, K.M.; Brown, G.D. The role of the beta-glucan receptor Dectin-1 in control of fungal infection. J. Leukoc. Biol. 2007, 82, 253–258. [Google Scholar] [CrossRef]
- Gross, O.; Gewies, A.; Finger, K.; Schafer, M.; Sparwasser, T.; Peschel, C.; Forster, I.; Ruland, J. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature 2006, 442, 651–656. [Google Scholar] [CrossRef]
- Saijo, S.; Fujikado, N.; Furuta, T.; Chung, S.H.; Kotaki, H.; Seki, K.; Sudo, K.; Akira, S.; Adachi, Y.; Ohno, N.; et al. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat. Immunol. 2007, 8, 39–46. [Google Scholar] [CrossRef]
- Taylor, P.R.; Tsoni, S.V.; Willment, J.A.; Dennehy, K.M.; Rosas, M.; Findon, H.; Haynes, K.; Steele, C.; Botto, M.; Gordon, S.; et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat. Immunol. 2007, 8, 31–38. [Google Scholar] [CrossRef]
- Ferwerda, B.; Ferwerda, G.; Plantinga, T.S.; Willment, J.A.; van Spriel, A.B.; Venselaar, H.; Elbers, C.C.; Johnson, M.D.; Cambi, A.; Huysamen, C.; et al. Human dectin-1 deficiency and mucocutaneous fungal infections. N. Engl. J. Med. 2009, 361, 1760–1767. [Google Scholar] [CrossRef] [PubMed]
- Mata-Martinez, P.; Bergon-Gutierrez, M.; Del Fresno, C. Dectin-1 Signaling Update: New Perspectives for Trained Immunity. Front. Immunol. 2022, 13, 812148. [Google Scholar] [CrossRef] [PubMed]
- Castoldi, A.; Andrade-Oliveira, V.; Aguiar, C.F.; Amano, M.T.; Lee, J.; Miyagi, M.T.; Latancia, M.T.; Braga, T.T.; da Silva, M.B.; Ignacio, A.; et al. Dectin-1 Activation Exacerbates Obesity and Insulin Resistance in the Absence of MyD88. Cell Rep. 2017, 19, 2272–2288. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, S.; Akhter, N.; Wilson, A.; Thomas, R.; Arefanian, H.; Al Madhoun, A.; Al-Mulla, F.; Ahmad, R. MIP-1alpha Expression Induced by Co-Stimulation of Human Monocytic Cells with Palmitate and TNF-alpha Involves the TLR4-IRF3 Pathway and Is Amplified by Oxidative Stress. Cells 2020, 9, 1799. [Google Scholar] [CrossRef]
- Kochumon, S.; Madhoun, A.A.; Al-Rashed, F.; Azim, R.; Al-Ozairi, E.; Al-Mulla, F.; Ahmad, R. Adipose tissue gene expression of CXCL10 and CXCL11 modulates inflammatory markers in obesity: Implications for metabolic inflammation and insulin resistance. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820930902. [Google Scholar] [CrossRef]
- Al-Roub, A.; Al Madhoun, A.; Akhter, N.; Thomas, R.; Miranda, L.; Jacob, T.; Al-Ozairi, E.; Al-Mulla, F.; Sindhu, S.; Ahmad, R. IL-1β and TNFα Cooperativity in Regulating IL-6 Expression in Adipocytes Depends on CREB Binding and H3K14 Acetylation. Cells 2021, 10, 3228. [Google Scholar] [CrossRef]
- Kochumon, S.; Al-Rashed, F.; Abu-Farha, M.; Devarajan, S.; Tuomilehto, J.; Ahmad, R. Adipose tissue expression of CCL19 chemokine is positively associated with insulin resistance. Diabetes Metab. Res. Rev. 2019, 35, e3087. [Google Scholar] [CrossRef]
- Ahmad, R.; Shihab, P.K.; Thomas, R.; Alghanim, M.; Hasan, A.; Sindhu, S.; Behbehani, K. Increased expression of the interleukin-1 receptor-associated kinase (IRAK)-1 is associated with adipose tissue inflammatory state in obesity. Diabetol. Metab. Syndr. 2015, 7, 71. [Google Scholar] [CrossRef]
- Ahmad, R.; Kochumon, S.; Chandy, B.; Shenouda, S.; Koshy, M.; Hasan, A.; Arefanian, H.; Al-Mulla, F.; Sindhu, S. TNF-alpha Drives the CCL4 Expression in Human Monocytic Cells: Involvement of the SAPK/JNK and NF-kappaB Signaling Pathways. Cell Physiol. Biochem. 2019, 52, 908–921. [Google Scholar]
- Sindhu, S.; Akhter, N.; Kochumon, S.; Thomas, R.; Wilson, A.; Shenouda, S.; Tuomilehto, J.; Ahmad, R. Increased Expression of the Innate Immune Receptor TLR10 in Obesity and Type-2 Diabetes: Association with ROS-Mediated Oxidative Stress. Cell Physiol. Biochem. 2018, 45, 572–590. [Google Scholar] [CrossRef]
- Kochumon, S.; Wilson, A.; Chandy, B.; Shenouda, S.; Tuomilehto, J.; Sindhu, S.; Ahmad, R. Palmitate Activates CCL4 Expression in Human Monocytic Cells via TLR4/MyD88 Dependent Activation of NF-kappaB/MAPK/ PI3K Signaling Systems. Cell Physiol. Biochem. 2018, 46, 953–964. [Google Scholar] [CrossRef]
- Abdel-Halim, S.M.; Al Madhoun, A.; Nizam, R.; Melhem, M.; Cherian, P.; Al-Khairi, I.; Haddad, D.; Abu-Farha, M.; Abubaker, J.; Bitar, M.S.; et al. Increased Plasma Levels of Adenylate Cyclase 8 and cAMP Are Associated with Obesity and Type 2 Diabetes: Results from a Cross-Sectional Study. Biology 2020, 9, 244. [Google Scholar] [CrossRef]
- Kochumon, S.; Al Madhoun, A.; Al-Rashed, F.; Thomas, R.; Sindhu, S.; Al-Ozairi, E.; Al-Mulla, F.; Ahmad, R. Elevated adipose tissue associated IL-2 expression in obesity correlates with metabolic inflammation and insulin resistance. Sci. Rep. 2020, 10, 16364. [Google Scholar] [CrossRef]
- Akhter, N.; Wilson, A.; Thomas, R.; Al-Rashed, F.; Kochumon, S.; Al-Roub, A.; Arefanian, H.; Al-Madhoun, A.; Al-Mulla, F.; Ahmad, R.; et al. ROS/TNF-α Crosstalk Triggers the Expression of IL-8 and MCP-1 in Human Monocytic THP-1 Cells via the NF-κB and ERK1/2 Mediated Signaling. Int. J. Mol. Sci. 2021, 22, 10519. [Google Scholar] [CrossRef]
- Ahmad, R.; Al-Mass, A.; Atizado, V.; Al-Hubail, A.; Al-Ghimlas, F.; Al-Arouj, M.; Bennakhi, A.; Dermime, S.; Behbehani, K. Elevated expression of the toll like receptors 2 and 4 in obese individuals: Its significance for obesity-induced inflammation. J. Inflamm. 2012, 9, 48. [Google Scholar] [CrossRef]
- Al-Rashed, F.; Sindhu, S.; Al Madhoun, A.; Ahmad, Z.; AlMekhled, D.; Azim, R.; Al-Kandari, S.; Wahid, M.A.; Al-Mulla, F.; Ahmad, R. Elevated resting heart rate as a predictor of inflammation and cardiovascular risk in healthy obese individuals. Sci. Rep. 2021, 11, 13883. [Google Scholar] [CrossRef]
- del Fresno, C.; Soulat, D.; Roth, S.; Blazek, K.; Udalova, I.; Sancho, D.; Ruland, J.; Ardavin, C. Interferon-beta production via Dectin-1-Syk-IRF5 signaling in dendritic cells is crucial for immunity to C. albicans. Immunity 2013, 38, 1176–1186. [Google Scholar] [CrossRef]
- Lu, J.; Zhao, J.; Meng, H.; Zhang, X. Adipose Tissue-Resident Immune Cells in Obesity and Type 2 Diabetes. Front. Immunol. 2019, 10, 1173. [Google Scholar] [CrossRef]
- Ohashi, K.; Ouchi, N.; Matsuzawa, Y. Anti-inflammatory and anti-atherogenic properties of adiponectin. Biochimie 2012, 94, 2137–2142. [Google Scholar] [CrossRef]
- Forny-Germano, L.; De Felice, F.G.; Vieira, M. The Role of Leptin and Adiponectin in Obesity-Associated Cognitive Decline and Alzheimer’s Disease. Front. Neurosci. 2018, 12, 1027. [Google Scholar] [CrossRef]
- Coelho, M.; Oliveira, T.; Fernandes, R. Biochemistry of adipose tissue: An endocrine organ. Arch. Med. Sci. 2013, 9, 191–200. [Google Scholar] [CrossRef]
- Cortez-Espinosa, N.; Garcia-Hernandez, M.H.; Reynaga-Hernandez, E.; Cortes-Garcia, J.D.; Corral-Fernandez, N.E.; Rodriguez-Rivera, J.G.; Bravo-Ramirez, A.; Gonzalez-Amaro, R.; Portales-Perez, D.P. Abnormal expression and function of Dectin-1 receptor in type 2 diabetes mellitus patients with poor glycemic control (HbA1c>8%). Metabolism 2012, 61, 1538–1546. [Google Scholar] [CrossRef]
- Lauw, F.N.; Pajkrt, D.; Hack, C.E.; Kurimoto, M.; van Deventer, S.J.; van der Poll, T. Proinflammatory effects of IL-10 during human endotoxemia. J. Immunol. 2000, 165, 2783–2789. [Google Scholar] [CrossRef]
- Islam, H.; Chamberlain, T.C.; Mui, A.L.; Little, J.P. Elevated Interleukin-10 Levels in COVID-19: Potentiation of Pro-Inflammatory Responses or Impaired Anti-Inflammatory Action? Front. Immunol. 2021, 12, 677008. [Google Scholar] [CrossRef]
- Tilg, H.; van Montfrans, C.; van den Ende, A.; Kaser, A.; van Deventer, S.J.; Schreiber, S.; Gregor, M.; Ludwiczek, O.; Rutgeerts, P.; Gasche, C.; et al. Treatment of Crohn’s disease with recombinant human interleukin 10 induces the proinflammatory cytokine interferon gamma. Gut 2002, 50, 191–195. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef]
- Moschen, A.R.; Molnar, C.; Enrich, B.; Geiger, S.; Ebenbichler, C.F.; Tilg, H. Adipose and liver expression of interleukin (IL)-1 family members in morbid obesity and effects of weight loss. Mol. Med. 2011, 17, 840–845. [Google Scholar] [CrossRef]
- Hoene, M.; Weigert, C. The role of interleukin-6 in insulin resistance, body fat distribution and energy balance. Obes. Rev. 2008, 9, 20–29. [Google Scholar] [CrossRef]
- Hueso, L.; Ortega, R.; Selles, F.; Wu-Xiong, N.Y.; Ortega, J.; Civera, M.; Ascaso, J.F.; Sanz, M.J.; Real, J.T.; Piqueras, L. Upregulation of angiostatic chemokines IP-10/CXCL10 and I-TAC/CXCL11 in human obesity and their implication for adipose tissue angiogenesis. Int. J. Obes. 2018, 42, 1406–1417. [Google Scholar] [CrossRef]
- Jialal, I.; Kaur, H.; Devaraj, S. Toll-like receptor status in obesity and metabolic syndrome: A translational perspective. J. Clin. Endocrinol. Metab. 2014, 99, 39–48. [Google Scholar] [CrossRef]
- Ahmad, R.; Kochumon, S.; Thomas, R.; Atizado, V.; Sindhu, S. Increased adipose tissue expression of TLR8 in obese individuals with or without type-2 diabetes: Significance in metabolic inflammation. J. Inflamm. 2016, 13, 38. [Google Scholar] [CrossRef][Green Version]
- Dennehy, K.M.; Ferwerda, G.; Faro-Trindade, I.; Pyz, E.; Willment, J.A.; Taylor, P.R.; Kerrigan, A.; Tsoni, S.V.; Gordon, S.; Meyer-Wentrup, F.; et al. Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. Eur. J. Immunol. 2008, 38, 500–506. [Google Scholar] [CrossRef]
- Ferwerda, G.; Meyer-Wentrup, F.; Kullberg, B.J.; Netea, M.G.; Adema, G.J. Dectin-1 synergizes with TLR2 and TLR4 for cytokine production in human primary monocytes and macrophages. Cell Microbiol. 2008, 10, 2058–2066. [Google Scholar] [CrossRef]
- Underhill, D.M. Collaboration between the innate immune receptors dectin-1, TLRs, and Nods. Immunol. Rev. 2007, 219, 75–87. [Google Scholar] [CrossRef]
- Sindhu, S.; Kochumon, S.; Thomas, R.; Bennakhi, A.; Al-Mulla, F.; Ahmad, R. Enhanced Adipose Expression of Interferon Regulatory Factor (IRF)-5 Associates with the Signatures of Metabolic Inflammation in Diabetic Obese Patients. Cells 2020, 9, 730. [Google Scholar] [CrossRef]
- Sindhu, S.; Thomas, R.; Kochumon, S.; Wilson, A.; Abu-Farha, M.; Bennakhi, A.; Al-Mulla, F.; Ahmad, R. Increased Adipose Tissue Expression of Interferon Regulatory Factor (IRF)-5 in Obesity: Association with Metabolic Inflammation. Cells 2019, 8, 1418. [Google Scholar] [CrossRef]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347–2355. [Google Scholar] [CrossRef]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative activation of macrophages: An immunologic functional perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef]
- Fuentes, L.; Roszer, T.; Ricote, M. Inflammatory mediators and insulin resistance in obesity: Role of nuclear receptor signaling in macrophages. Mediat. Inflamm. 2010, 2010, 219583. [Google Scholar] [CrossRef]
- Cucak, H.; Grunnet, L.G.; Rosendahl, A. Accumulation of M1-like macrophages in type 2 diabetic islets is followed by a systemic shift in macrophage polarization. J. Leukoc. Biol. 2014, 95, 149–160. [Google Scholar] [CrossRef]
- Goerdt, S.; Politz, O.; Schledzewski, K.; Birk, R.; Gratchev, A.; Guillot, P.; Hakiy, N.; Klemke, C.D.; Dippel, E.; Kodelja, V.; et al. Alternative versus classical activation of macrophages. Pathobiology 1999, 67, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Hao, N.B.; Lu, M.H.; Fan, Y.H.; Cao, Y.L.; Zhang, Z.R.; Yang, S.M. Macrophages in tumor microenvironments and the progression of tumors. Clin. Dev. Immunol. 2012, 2012, 948098. [Google Scholar] [CrossRef]
- Gottfried, E.; Kunz-Schughart, L.A.; Weber, A.; Rehli, M.; Peuker, A.; Muller, A.; Kastenberger, M.; Brockhoff, G.; Andreesen, R.; Kreutz, M. Expression of CD68 in non-myeloid cell types. Scand. J. Immunol. 2008, 67, 453–463. [Google Scholar] [CrossRef]
- Veloso, P.; Fernandez, A.; Terraza-Aguirre, C.; Alvarez, C.; Vernal, R.; Escobar, A.; Hernandez, M. Macrophages skew towards M1 profile through reduced CD163 expression in symptomatic apical periodontitis. Clin. Oral Investig. 2020, 24, 4571–4581. [Google Scholar] [CrossRef]
| Metabolic Markers | Lean | Overweight | Obese | Lean vs. Overweight | Lean vs. Obese |
|---|---|---|---|---|---|
| n = 10 (3M/7F) (Mean ± SD) | n = 19 (12M/7F) (Mean ± SD) | n = 30 (15M/15F) (Mean ± SD) | (p-Value) | (p-Value) | |
| Age (years) | 42.70 ± 8.17 | 43.68 ± 11.12 | 45.20 ± 13.12 | 0.807 | 0.572 |
| Weight (kg) | 62.93 ± 11.90 | 79.56 ± 9.92 | 94.48 ± 14.06 | 0.0004 | <0.0001 |
| Height (cm) | 1.66 ± 0.12 | 1.68 ± 0.11 | 1.64 ± 0.11 | 0.68 | 0.754 |
| BMI (kg/m2) | 22.82 ± 2.35 | 28.27 ± 1.19 | 34.88 ± 3.22 | <0.0001 | <0.0001 |
| Waist (cm) | 81.33 ± 12.44 | 95.19 ± 8.81 | 107.15 ± 12.83 | 0.003 | <0.0001 |
| Body fat (%) | 28.37 ± 6.27 | 32.52 ± 4.87 | 39.47 ± 4.28 | 0.073 | <0.0001 |
| FBG (mmol/L) | 4.97 ± 0.64 | 5.26 ± 0.68 | 5.37 ± 0.76 | 0.282 | 0.14 |
| TGL (mmol/L) | 0.63 ± 0.24 | 1.19 ± 0.64 | 1.34 ± 0.85 | 0.002 | <0.0001 |
| Chol (mmol/L) | 5.30 ± 1.11 | 4.98 ± 0.73 | 5.05 ± 1.14 | 0.348 | 0.544 |
| HDL (mmol/L) | 1.69 ± 0.51 | 1.27 ± 0.30 | 1.17 ± 0.24 | 0.009 | 0.01 |
| LDL (mmol/L) | 3.31 ± 0.93 | 3.18 ± 0.67 | 3.29 ± 1.01 | 0.677 | 0.963 |
| HbA1c (%) | 5.66 ± 0.46 | 5.49 ± 0.45 | 5.70 ± 0.65 | 0.35 | 0.857 |
| HOMA-IR | 1.40 ± 0.64 | 1.71 ± 0.98 | 4.40 ± 3.80 | 0.413 | 0.009 |
| WBC | 5.57 ± 1.60 | 6.11 ± 1.43 | 6.49 ± 1.97 | 0.379 | 0.206 |
| Inflammatory Markers | Spearman Correlation | ||
|---|---|---|---|
| r-Value | p-Value | n | |
| Interleukins | |||
| IL-1β | 0.323 * | 0.033 | 44 |
| IL-2 | 0.247 | 0.067 | 56 |
| IL-5 | −0.048 | 0.733 | 52 |
| IL-6 | 0.183 | 0.195 | 52 |
| IL-8 | 0.569 ** | <0.0001 | 49 |
| IL-10 | 0.564 ** | <0.0001 | 55 |
| IL-12A | 0.188 | 0.227 | 43 |
| IL13 | −0.082 | 0.562 | 52 |
| IL-18 | 0.496 ** | 0.0002 | 53 |
| IL-23A | 0.549 ** | <0.0001 | 57 |
| IL-33 | −0.03 | 0.83 | 55 |
| TNF-α | 0.491 ** | 0.0002 | 53 |
| Cytokine/chemokines receptors | |||
| IL-2RA | 0.100 | 0.454 | 58 |
| CCR1 | 0.446 ** | 0.001 | 55 |
| CCR2 | 0.470 ** | 0.001 | 51 |
| CCR5 | 0.681 ** | <0.0001 | 57 |
| CC chemokine ligands | |||
| CCL2 | 0.266 * | 0.05 | 55 |
| CCL3 | 0.541 ** | <0.0001 | 54 |
| CCL5 | 0.527 ** | 0.0001 | 47 |
| CCL7 | 0.515 ** | <0.0001 | 54 |
| CCL8 | 0.215 | 0.138 | 49 |
| CCL11 | 0.194 | 0.159 | 54 |
| CCL15 | 0.029 | 0.831 | 57 |
| CCL18 | 0.569 ** | <0.0001 | 56 |
| CCL19 | 0.24 | 0.072 | 57 |
| CCL20 | 0.624 ** | <0.0001 | 56 |
| CXC chemokine ligands | |||
| CXCL9 | 0.260 * | 0.049 | 58 |
| CXCL10 | 0.438 ** | 0.001 | 56 |
| CXCL11 | 0.446 ** | 0.001 | 57 |
| Inflammatory Markers | Spearman Correlation | ||
|---|---|---|---|
| r-Value | p-Value | n | |
| TLRs and downstream signaling markers | |||
| TLR2 | 0.663 ** | 0.0001 | 50 |
| TLR3 | 0.229 | 0.106 | 51 |
| TLR4 | 0.054 | 0.718 | 48 |
| TLR7 | 0.485 ** | 0.0001 | 58 |
| TLR8 | 0.652 ** | 0.0001 | 55 |
| TLR9 | −0.073 | 0.59 | 57 |
| TLR10 | 0.423 ** | 0.001 | 54 |
| MyD88 | 0.408 ** | 0.002 | 57 |
| IRAK1 | 0.360 ** | 0.007 | 55 |
| IRF3 | 0.157 | 0.286 | 48 |
| IRF5 | 0.492 ** | 0.0002 | 54 |
| Monocyte/macrophage surface markers | |||
| CD16 | 0.719 ** | <0.0001 | 56 |
| CD68 | 0.502 ** | <0.0001 | 57 |
| CD86 | 0.679 ** | <0.0001 | 55 |
| CD163 | 0.603 ** | <0.0001 | 57 |
| ANOVA (Sig) R2 = 0.55; p < 0.0001 | ||
|---|---|---|
| Predictor Variable | Scandalized Confinement (β) | p-Value |
| CCR5 | 0.803 | 0.0002 |
| CCL20 | 0.024 | 0.0004 |
| TLR2 | 1.754 | <0.0001 |
| MyD88 | 2.036 | 0.019 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Madhoun, A.; Kochumon, S.; Al-Rashed, F.; Sindhu, S.; Thomas, R.; Miranda, L.; Al-Mulla, F.; Ahmad, R. Dectin-1 as a Potential Inflammatory Biomarker for Metabolic Inflammation in Adipose Tissue of Individuals with Obesity. Cells 2022, 11, 2879. https://doi.org/10.3390/cells11182879
Al Madhoun A, Kochumon S, Al-Rashed F, Sindhu S, Thomas R, Miranda L, Al-Mulla F, Ahmad R. Dectin-1 as a Potential Inflammatory Biomarker for Metabolic Inflammation in Adipose Tissue of Individuals with Obesity. Cells. 2022; 11(18):2879. https://doi.org/10.3390/cells11182879
Chicago/Turabian StyleAl Madhoun, Ashraf, Shihab Kochumon, Fatema Al-Rashed, Sardar Sindhu, Reeby Thomas, Lavina Miranda, Fahd Al-Mulla, and Rasheed Ahmad. 2022. "Dectin-1 as a Potential Inflammatory Biomarker for Metabolic Inflammation in Adipose Tissue of Individuals with Obesity" Cells 11, no. 18: 2879. https://doi.org/10.3390/cells11182879
APA StyleAl Madhoun, A., Kochumon, S., Al-Rashed, F., Sindhu, S., Thomas, R., Miranda, L., Al-Mulla, F., & Ahmad, R. (2022). Dectin-1 as a Potential Inflammatory Biomarker for Metabolic Inflammation in Adipose Tissue of Individuals with Obesity. Cells, 11(18), 2879. https://doi.org/10.3390/cells11182879










