Cell Surface Hsp90- and αMβ2 Integrin-Mediated Uptake of Bacterial Flagellins to Activate Inflammasomes by Human Macrophages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
2.3. RNA Preparation and Real-Time Quantitative Polymerase Chain Reaction (qPCR)
2.4. Flow Cytometry
2.5. Cell Surface Biotinylation
2.6. Western Blotting Analysis
2.7. Cross-Linking Reaction
2.8. Co-Immunoprecipitation
2.9. In-Gel Digestion with Trypsin and Extraction of Peptides
2.10. Identification of Proteins by Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS)
2.11. Immunofluorescence
2.12. Nuclear Factor-Kappa B (NF-κB)/Activator Protein 1 (AP-1) Activation Reporter Assay
2.13. ELISA
2.14. Statistical Analysis
3. Results
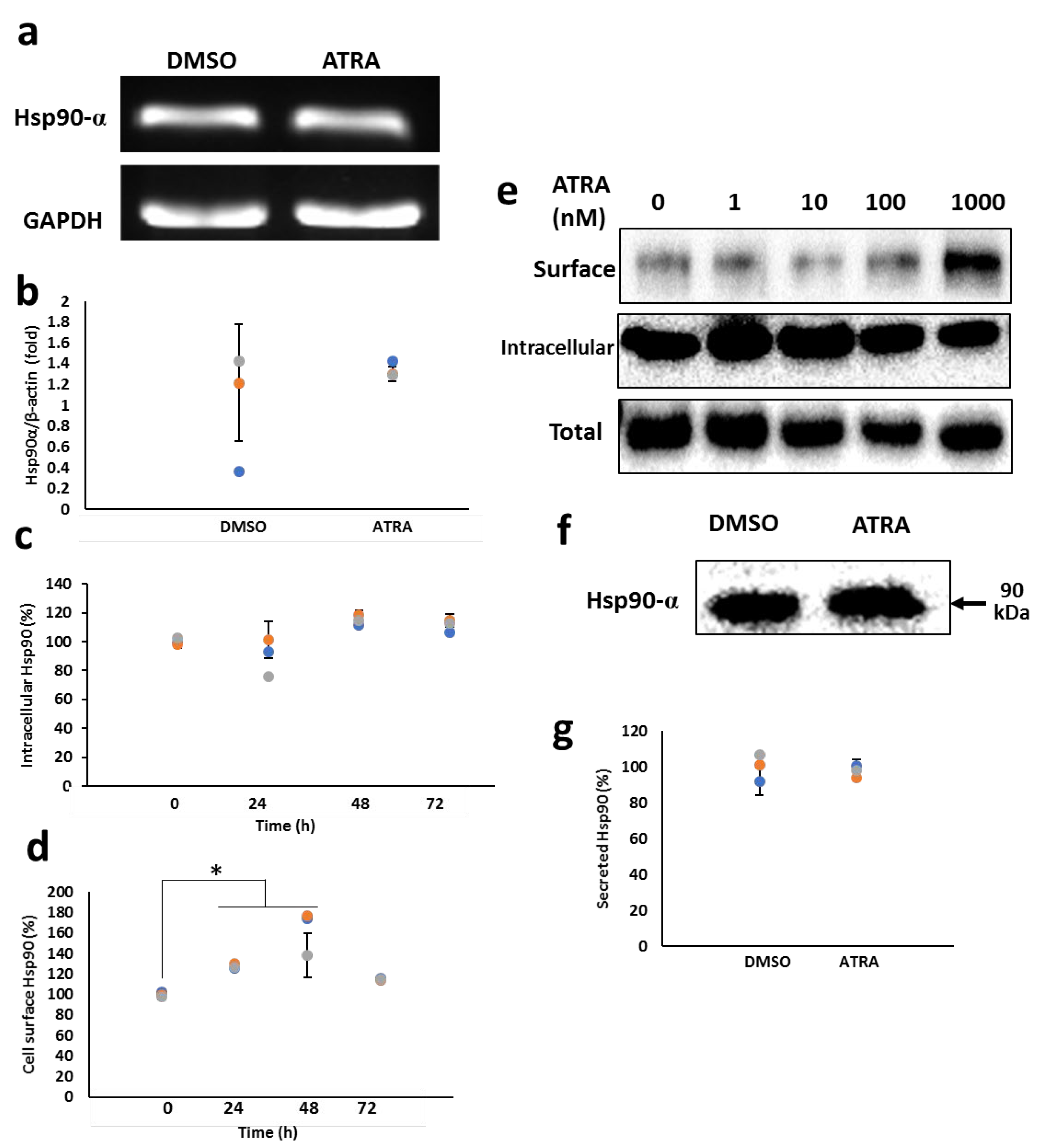
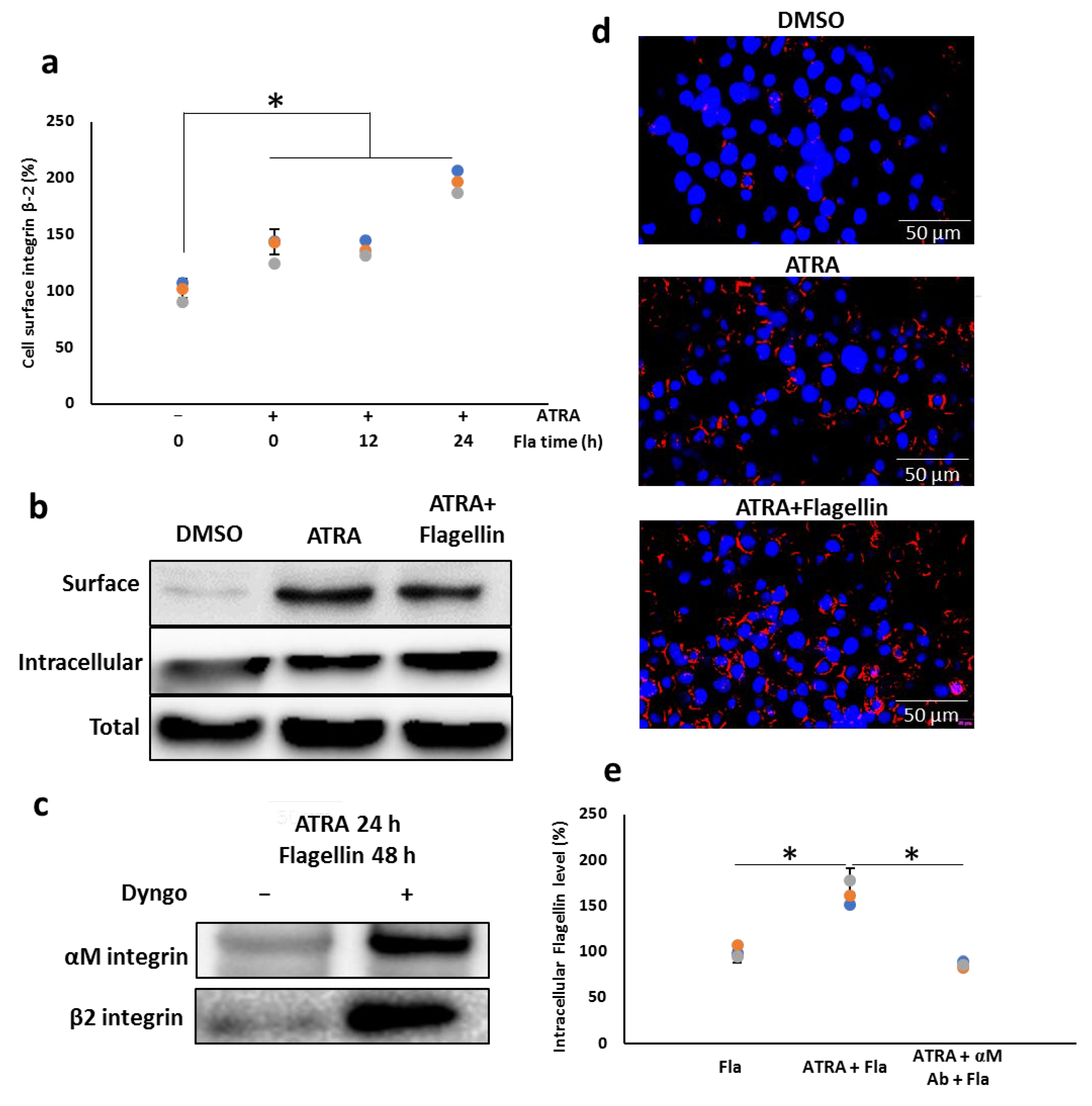
3.1. ATRA Enhances the Cell Surface Hsp90 Protein Level
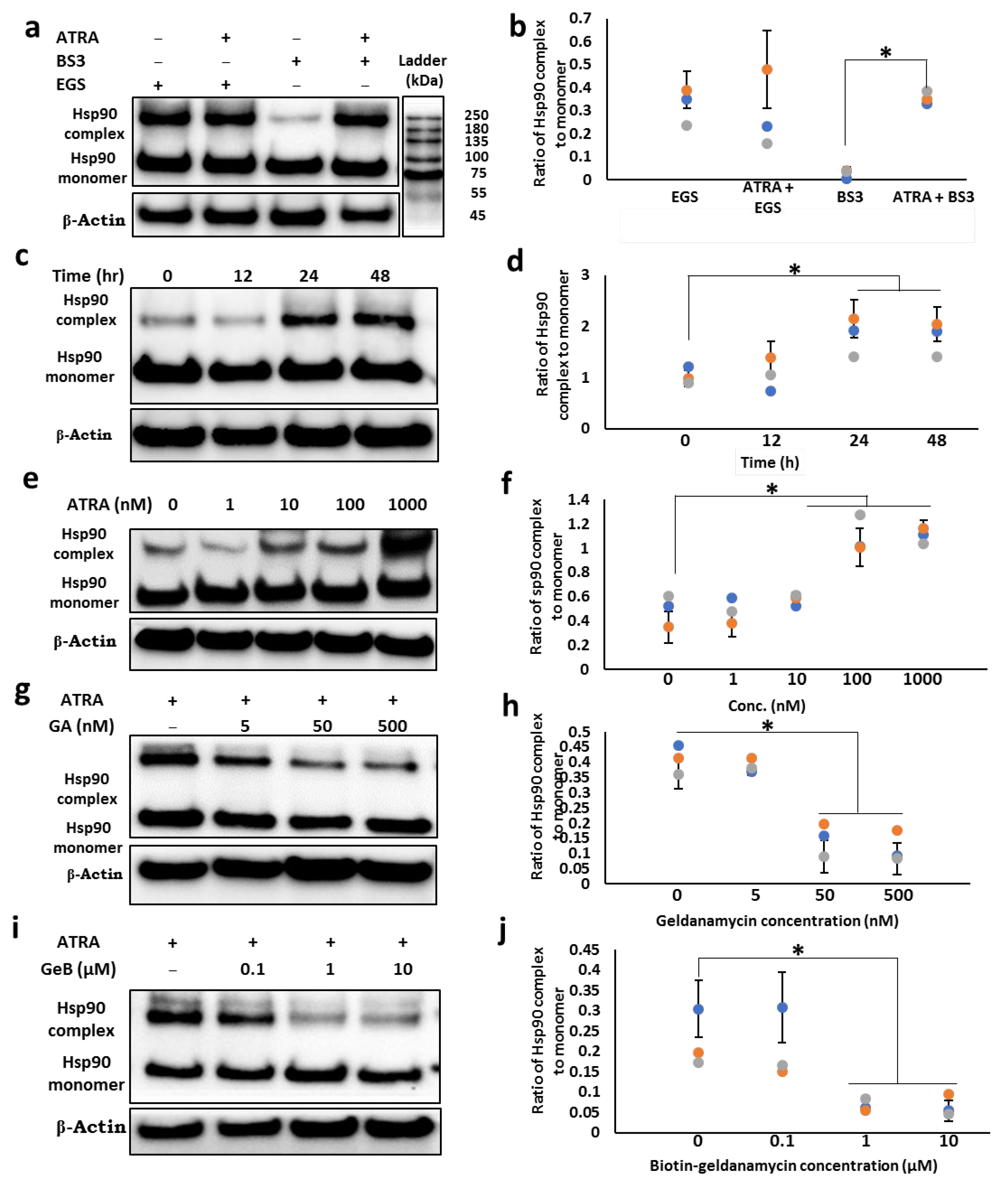
3.2. ATRA Induces the Cell Surface Hsp90 Complex Formation in a Time- and Concentration-Dependent Manner
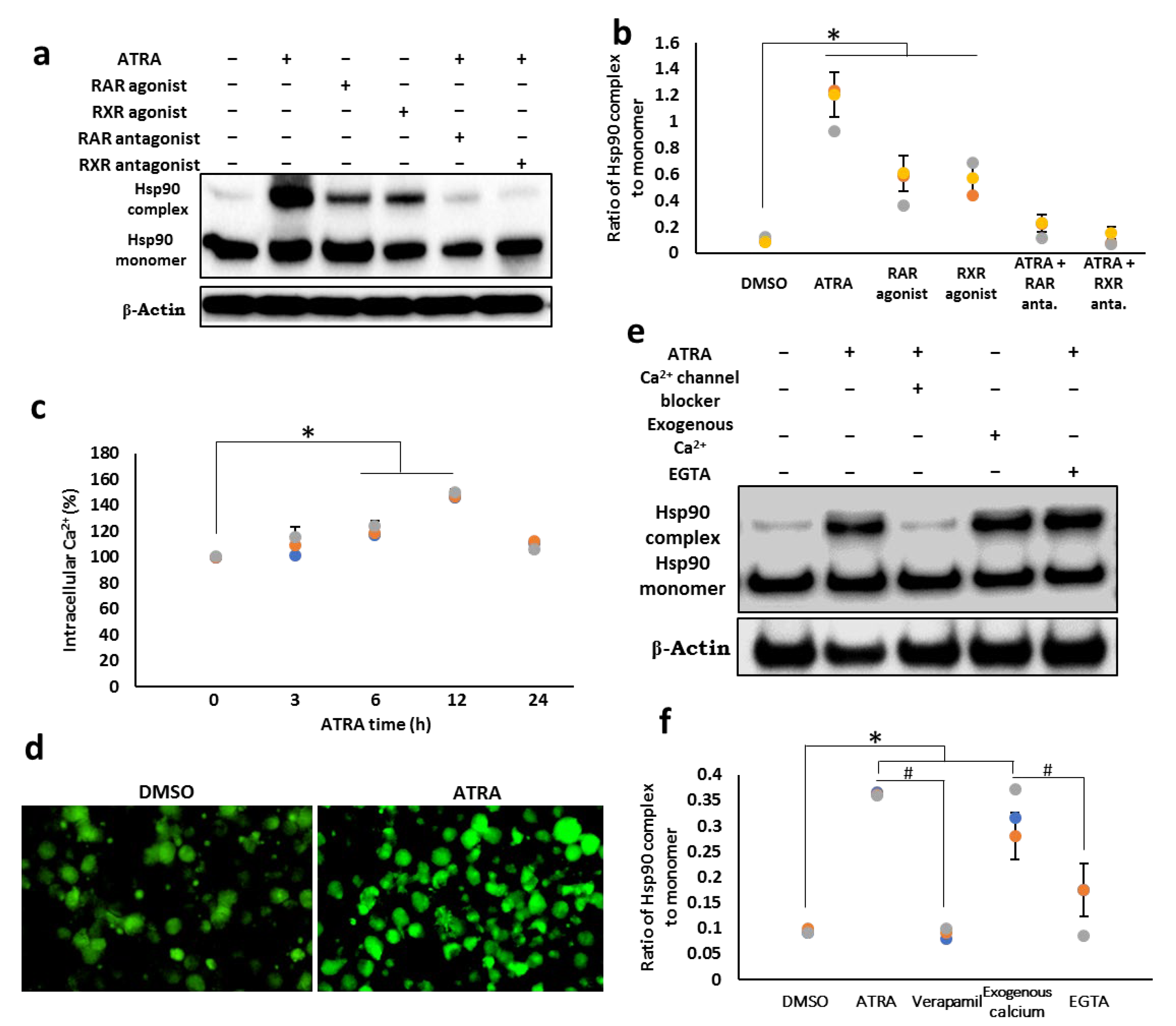
3.3. Cell Surface Hsp90 Complex Formation Depends on RAR/RXR Pathway and Intracellular Calcium Level
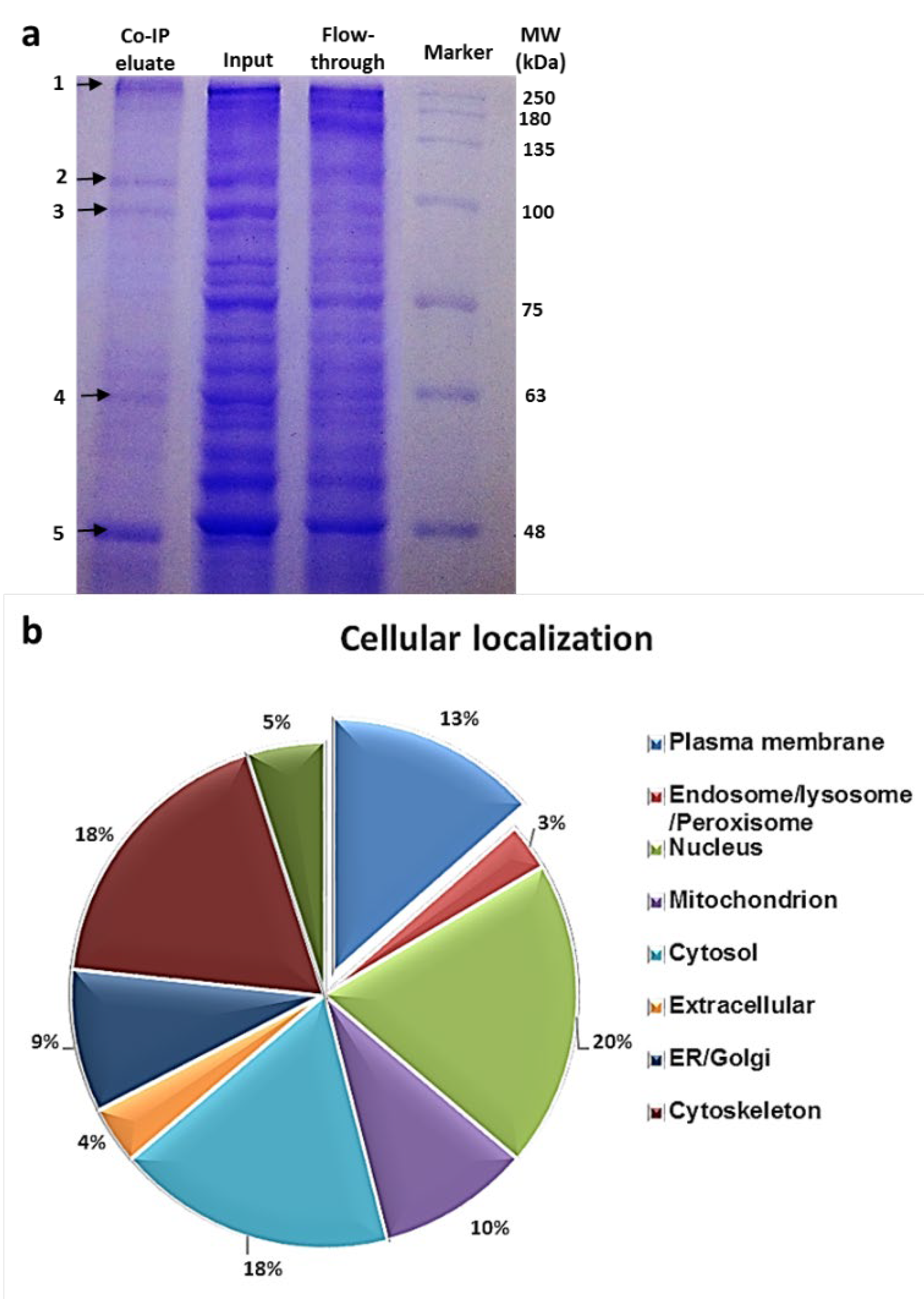
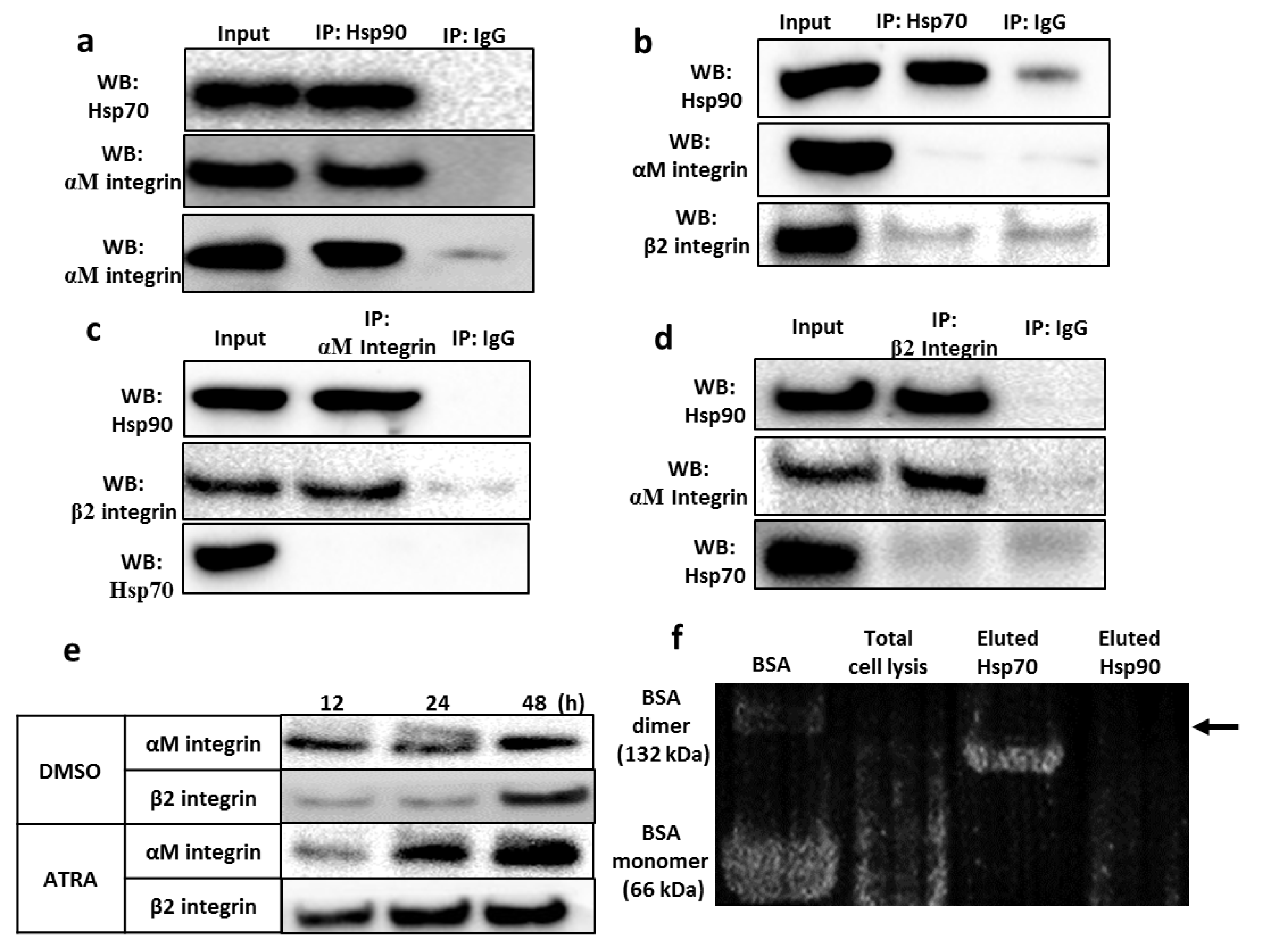
3.4. Identification of the Hsp90 Complex Components
3.5. Validation of the Interaction between the Hsp90 Chaperone and Integrin αMβ2
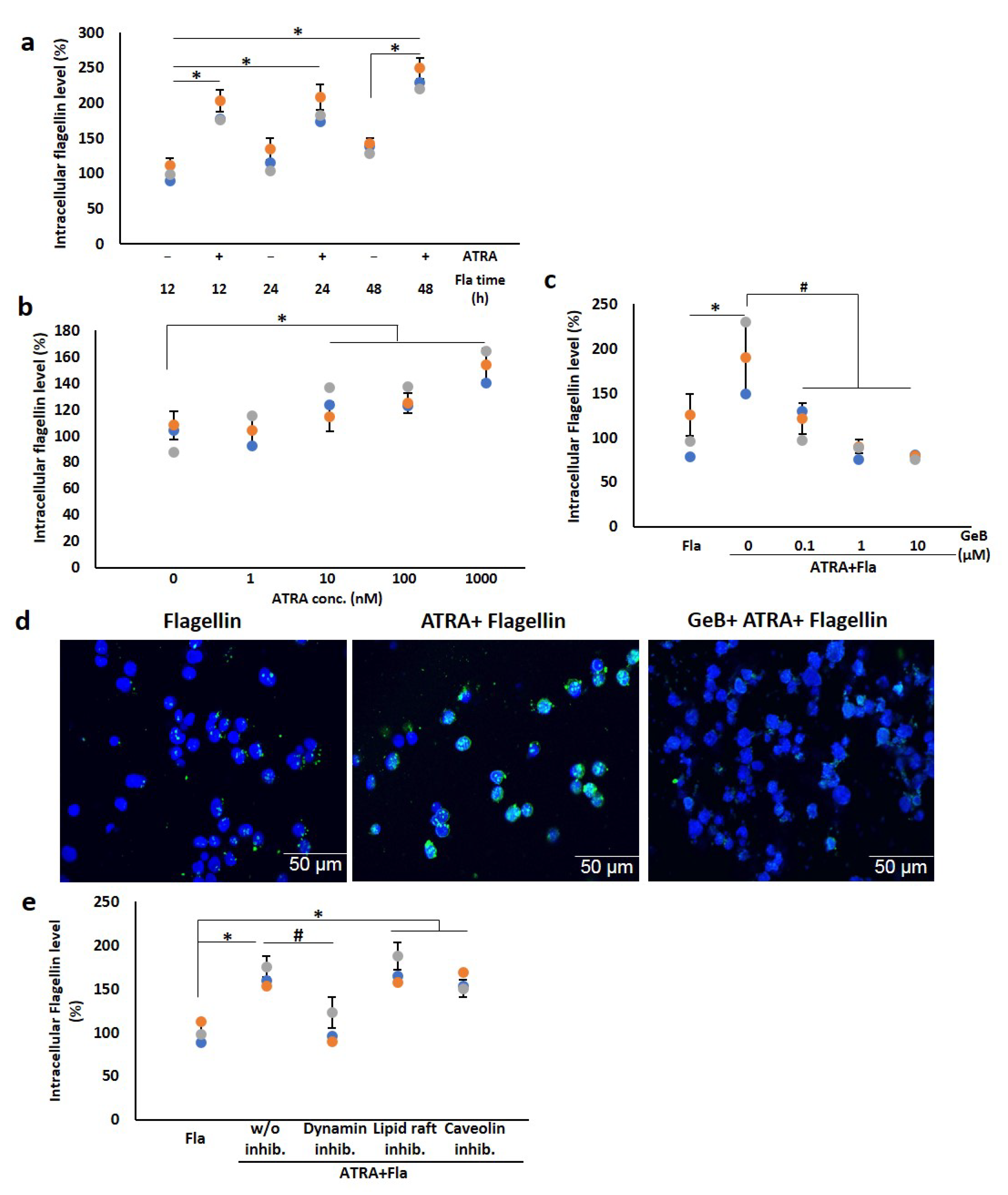
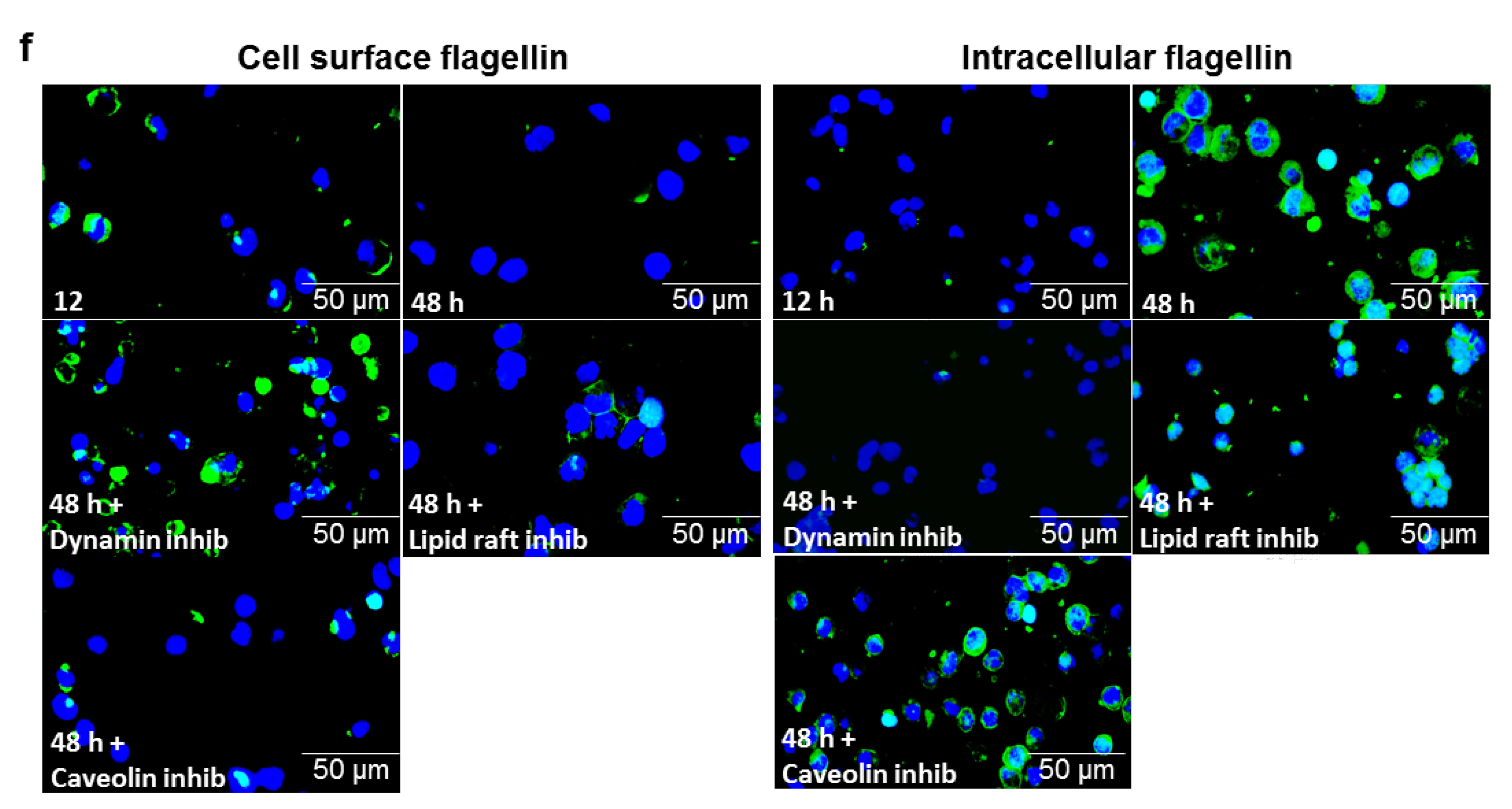
3.6. ATRA Triggers Bacterial Flagellin Internalization in an Hsp90-Dependent Manner
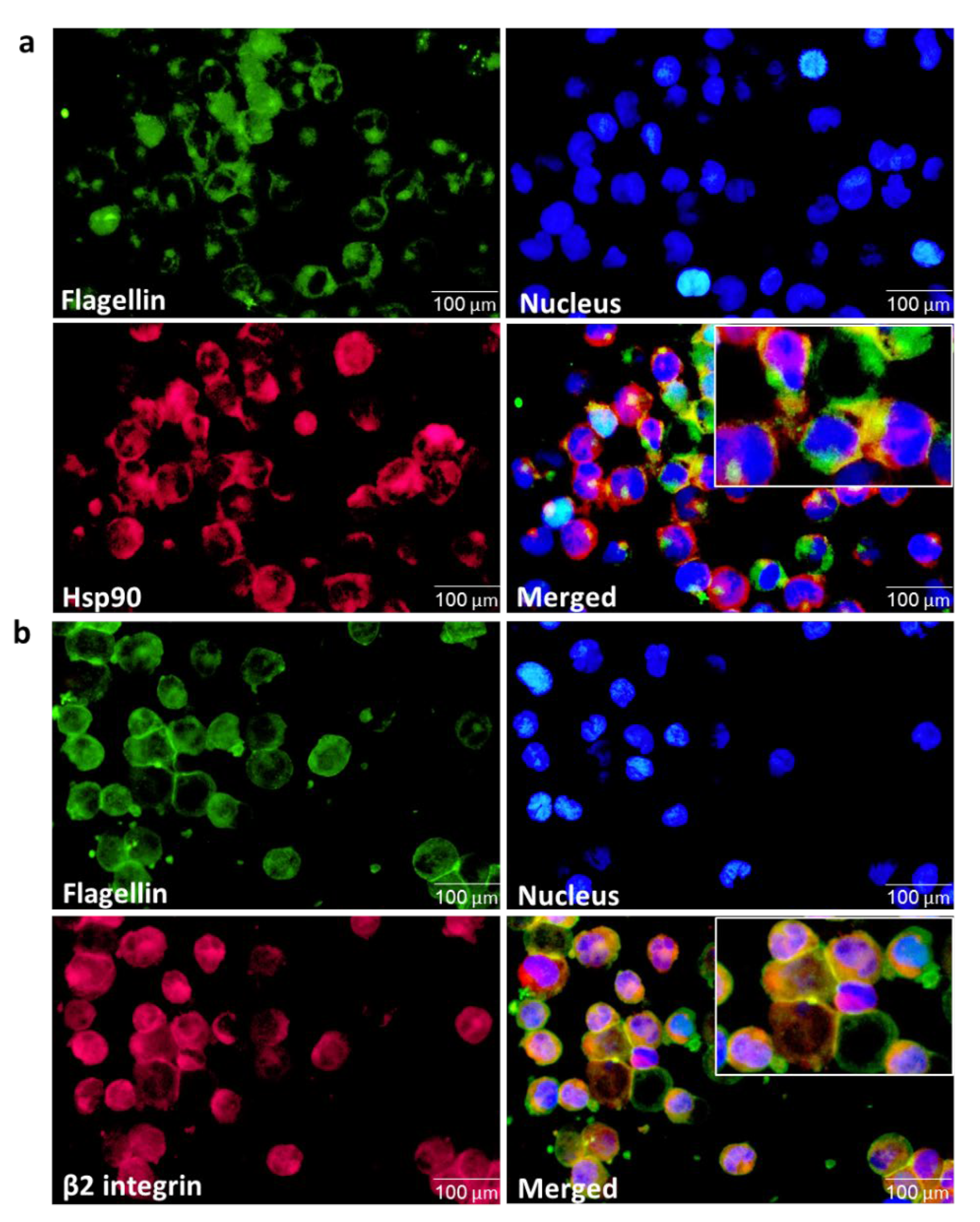
3.7. αMβ2 Integrin Acts as a Carrier for Bacterial Flagellin Internalization
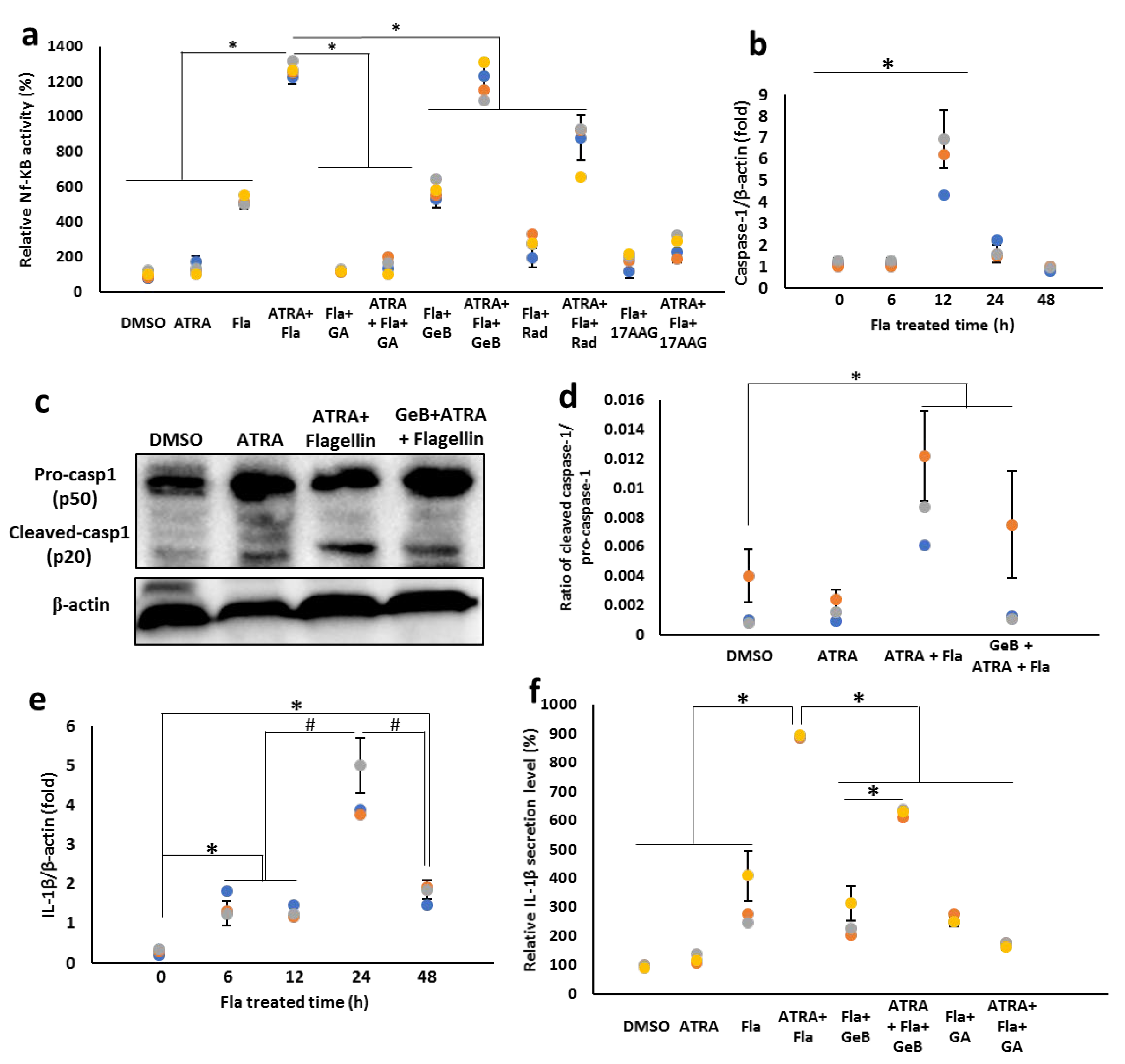
3.8. Integrin-Carried Flagellin Is Capable of Activating the Inflammasomes
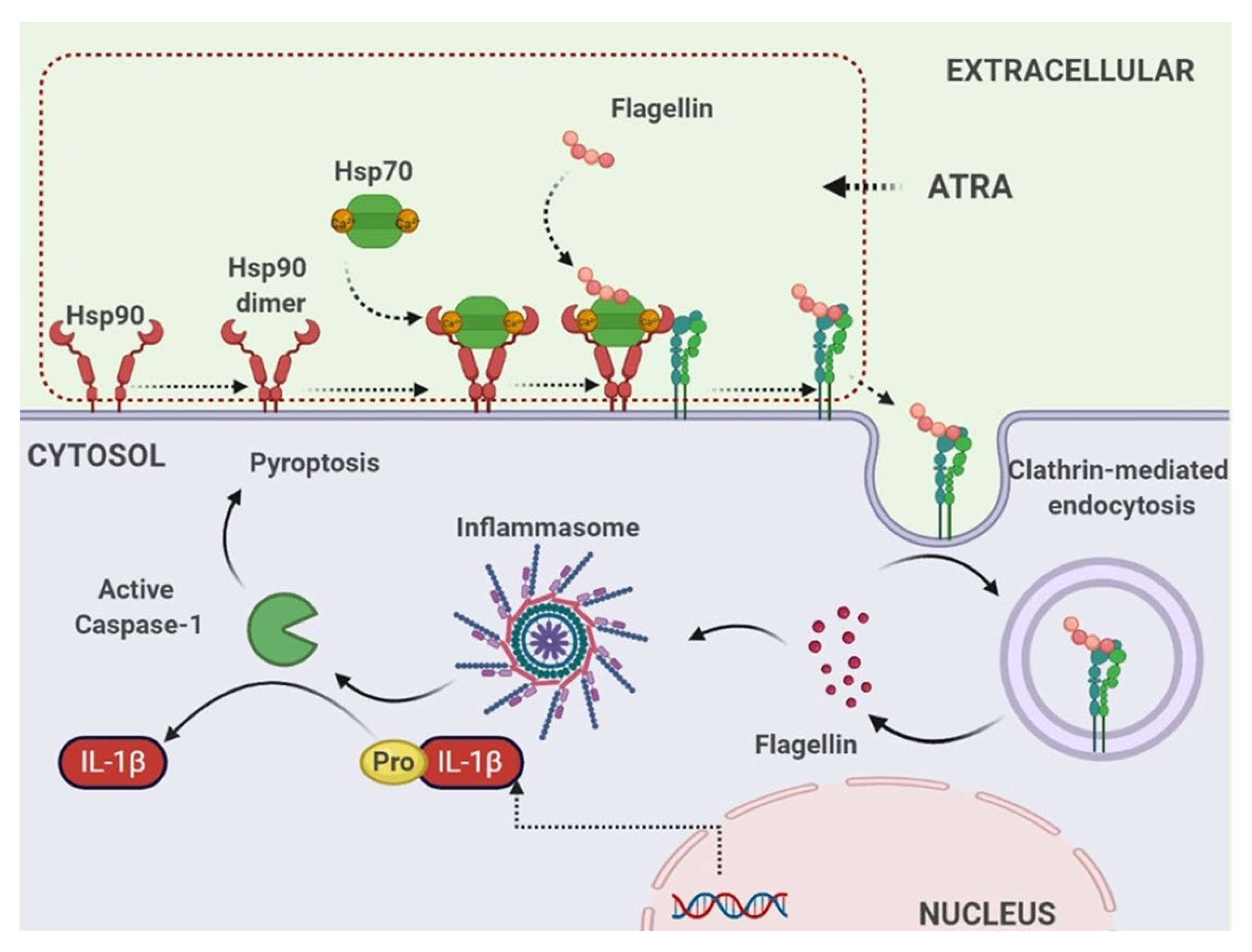
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taipale, M.; Jarosz, D.F.; Lindquist, S. HSP90 at the hub of protein homeostasis: Emerging mechanistic insights. Nat. Rev. Mol. Cell Biol. 2010, 11, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Murshid, A.; Gong, J.; Calderwood, S.K. Hsp90 mediates Efficient Antigen Cross Presentation Through the Scavenger Receptor SREC-I. J. Immunol. 2010, 185, 2903–2917. [Google Scholar] [CrossRef]
- Imai, T.; Kato, Y.; Kajiwara, C.; Mizukami, S.; Ishige, I.; Ichiyanagi, T.; Hikida, M.; Wang, J.Y.; Udono, H. Heat shock protein 90 (HSP90) contributes to cytosolic translocation of extracellular antigen for cross-presentation by dendritic cells. Proc. Natl. Acad. Sci. USA 2011, 108, 16363. [Google Scholar] [CrossRef]
- Murshid, A.; Gong, J.; Calderwood, S.K. Hsp90-peptide complexes stimulate antigen presentation through the class II pathway after binding scavenger receptor SREC-I. Immunobiology 2014, 219, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Barth, H.; Ernst, K. Chaperones and ADP-Ribosylating bacterial toxins. Microb. Toxins 2016, 1–22. [Google Scholar] [CrossRef]
- Ratts, R.; Zeng, H.; Berg, E.A.; Blue, C.; McComb, M.E.; Costello, C.E.; vanderSpek, J.C.; Murphy, J.R. The cytosolic entry of diphtheria toxin catalytic domain requires a host cell cytosolic translocation factor complex. J. Cell Biol. 2003, 160, 139–1150. [Google Scholar] [CrossRef]
- Dmochewitz, L.; Lillich, M.; Kaiser, E.; Jennings, L.D.; Lang, A.E.; Buchner, J.; Fischer, G.; Aktories, K.; Collier, R.J.; Barth, H. Role of CypA and Hsp90 in membrane translocation mediated by anthrax protective antigen. Cell. Microbiol. 2011, 13, 359–373. [Google Scholar] [CrossRef]
- Ernst, K.; Schmid, J.; Beck, M.; Hägele, M.; Hohwieler, M.; Hauff, P.; Ückert, A.K.; Anastasia, A.; Fauler, M.; Jank, T.; et al. Hsp70 facilitates trans-membrane transport of bacterial ADP-ribosylating toxins into the cytosol of mammalian cells. Sci. Rep. 2017, 7, 2724. [Google Scholar] [CrossRef]
- Goodman, D.S. Overview of current knowledge of metabolism of vitamin A and carotenoids. J. Natl. Cancer Inst. 1984, 73, 1375–1379. [Google Scholar]
- Pino-Lagos, K.; Guo, Y.; Noelle, R.J. Retinoic acid: A key player in immunity. BioFactors 2010, 36, 430–436. [Google Scholar] [CrossRef]
- Hoang, T.X.; Jung, J.H.; Kim, J.Y. All-Trans retinoic acid enhances bacterial flagellin-stimulated proinflammatory responses in human monocyte THP-1 cells by upregulating CD14. BioMed Res. Int. 2019, 2019, 8059312. [Google Scholar] [CrossRef] [PubMed]
- Trinh, T.A.; Hoang, T.X.; Kim, J.Y. All-trans retinoic acid increases NF-κB activity in PMA-stimulated THP-1 cells upon unmethylated CpG challenge by enhancing cell surface TLR9 expression. Mol. Cell Biochem. 2020, 473, 167–177. [Google Scholar] [CrossRef]
- Wiedermann, U.; Tarkowski, A.; Bremell, T.; Hanson, L.A.; Kahu, H.; Dahlgren, U.I. Vitamin A deficiency predisposes to Staphylococcus aureus infection. Infect. Immun. 1996, 64, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Gewirtz, A.T.; Navas, T.A.; Lyons, S.; Godowski, P.J.; Madara, J.L. Cutting edge: Bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 2001, 167, 1882–1885. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. TLR signaling. Semin. Immunol. 2007, 19, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Kofoed, E.M.; Vance, R.E. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 2011, 477, 592–595. [Google Scholar] [CrossRef]
- Hueck, C.J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 1998, 62, 379–433. [Google Scholar] [CrossRef]
- Franchi, L.; Amer, A.; Body-Malapel, M.; Kanneganti, T.D.; Ozoren, N.; Jagirdar, R.; Inohara, N.; Vandenabeele, P.; Bertin, J.; Coyle, A.; et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in salmonella-infected macrophages. Nat. Immunol. 2006, 7, 576–582. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, E.; Liu, F.; Zhang, Y.; Zhong, M.; Li, Y.; Zhou, D.; Chen, Y.; Cao, Y.; Xiao, Y.; et al. Flagellins of Salmonella typhi and nonpathogenic Escherichia coli are differentially recognized through the NLRC4 pathway in macrophages. J. Innate Immun. 2014, 6, 47–57. [Google Scholar] [CrossRef]
- Cho, H.Y.; Choi, E.K.; Lee, S.W.; Kim, K.H.; Park, S.J.; Lee, C.K.; Lee, S.W. All-trans retinoic acid induces TLR-5 expression and cell differentiation and promotes flagellin-mediated cell functions in human THP-1 cells. Immunol. Lett. 2011, 136, 97–107. [Google Scholar] [CrossRef]
- Calderwood, S.K.; Gong, J.; Murshid, A. Extracellular HSPs: The complicated roles of extracellular HSPs in immunity. Front. Immunol. 2016, 7, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oura, J.; Tamura, Y.; Kamiguchi, K.; Kutomi, G.; Sahara, H.; Torigoe, T.; Himi, T.; Sato, N. Extracellular heat shock protein 90 plays a role in translocating chaperoned antigen from endosome to proteasome for generating antigenic peptide to be cross-presented by dendritic cells. Int. Immunol. 2011, 23, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.C.3; Kazmer, S.; Levin, A.A.; Yen, A. Myeloid differentiation and retinoblastoma phosphorylation changes in HL-60 cells induced by retinoic acid receptor-and retinoid X receptor-selective retinoic acid analogs. Blood 1996, 87, 227–237. [Google Scholar]
- Diaz, M.; Sanchez-Barrena, M.J.; Gonzalez-Rubio, J.M.; Rodriguez, L.; Fernandez, D.; Antoni, R.; Yunta, C.; Belda-Palazon, B.; Gonzalez-Guzman, M.; Peirats-Llobet, M.; et al. Calcium-dependent oligomerization of CAR proteins at cell membrane modulates ABA signaling. Proc. Natl. Acad. Sci. USA 2016, 113, E396. [Google Scholar] [CrossRef] [PubMed]
- Schittenhelm, L.; Hilkens, C.M.; Morrison, V.L. β(2) Integrins as regulators of dendritic cell, monocyte, and macrophage function. Front. Immunol. 2017, 8, 1866. [Google Scholar] [CrossRef]
- Sriram, M.; Osipiuk, J.; Freeman, B.; Morimoto, R.; Joachimiak, A. Human Hsp70 molecular chaperone binds two calcium ions within the ATPase domain. Structure 1997, 5, 403–414. [Google Scholar] [CrossRef]
- Na, B.H.; Hoang, T.X.; Kim, J.Y. Hsp90 inhibition reduces TLR5 surface expression and NF-κB activation in human myeloid leukemia THP-1 cells. Biomed. Res. Int. 2018, 2018, 4319369. [Google Scholar] [CrossRef]
- Sidera, K.; Patsavoudi, E. Extracellular HSP90: Conquering the cell surface. Cell Cycle 2008, 7, 1564–1568. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Guan, S.; Fan, J.; Cheng, C.F.; Brigh, A.M.; Chinn, C.; Chen, M.; Woodley, D.T. Extracellular heat shock protein-90α: Linking hypoxia to skin cell motility and wound healing. EMBO J. 2007, 26, 1221–1233. [Google Scholar] [CrossRef]
- Binder, R.J.; Blachere, N.E.; Srivastava, P.K. Heat shock protein chaperoned peptides but not free peptides introduced into the cytosol are presented efficiently by major histocompatibility complex I molecules. J. Biol. Chem. 2001, 276, 17163–17171. [Google Scholar] [CrossRef]
- Pearl, L.H.; Prodromou, C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Ann. Rev. Biochem. 2006, 75, 271–294. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Weidenauer, L.; Luengo, T.M.; Pieters, E.C.; Echeverría, P.C.; Bernasconi, L.; Wider, D.; Sadian, Y.; Koopman, M.B.; Villemin, M.; et al. The Hsp70-Hsp90 co-chaperone Hop/Stip1 shifts the proteostatic balance from folding towards degradation. Nat. Commun. 2020, 11, 5975. [Google Scholar] [CrossRef]
- Sung, N.; Lee, J.; Kim, J.H.; Chang, C.; Joachimiak, A.; Lee, S.; Tsai, F.T. Mitochondrial Hsp90 is a ligand-activated molecular chaperone coupling ATP binding to dimer closure through a coiled-coil intermediate. Proc. Natl. Acad. Sci. USA 2016, 113, 2952–2957. [Google Scholar] [CrossRef] [PubMed]
- Rüdiger, S.; Buchberger, A.; Bukau, B. Interaction of Hsp70 chaperones with substrates. Nat. Struct. Biol. 1997, 4, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Mizel, S.B.; West, A.P.; Hantgan, R.R. Identification of a sequence in human Toll-like receptor 5 required for the binding of Gram-negative flagellin. J. Biol. Chem. 2003, 278, 23624–23629. [Google Scholar] [CrossRef] [PubMed]
- Wayne, N.; Bolon, D.N. Dimerization of Hsp90 is required for in vivo function. Design and analysis of monomers and dimers. J. Biol. Chem. 2007, 282, 35386–35395. [Google Scholar] [CrossRef]
- Tan, S.M. The leucocyte β2 (CD18) integrins: The structure, functional regulation and signalling properties. Biosci. Rep. 2012, 32, 241. [Google Scholar] [CrossRef]
- Babina, M.; Henz, B.M. All-trans retinoic acid down-regulates expression and function of I 2 integrins by human monocytes: Opposite effects on monocytic cell lines. Eur. J. Immunol. 2003, 33, 616–625. [Google Scholar] [CrossRef]
- Bunting, M.; Harris, E.S.; McIntyre, T.M.; Prescott, S.M.; Zimmerman, G.A. Leukocyte adhesion deficiency syndromes: Adhesion and tethering defects involving beta 2 integrins and selectin ligands. Curr. Opin. Hematol. 2002, 9, 30–35. [Google Scholar] [CrossRef]
- Machacek, C.; Supper, V.; Leksa, V.; Mitulovic, G.; Spittler, A.; Drbal, K.; Suchanek, M.; Ohradanova-Repic, A.; Stockinger, H. Folate receptor β regulates integrin CD11b/CD18 adhesion of a macrophage subset to collagen. J. Immunol. 2016, 197, 2229–2238. [Google Scholar] [CrossRef]
- Ho, W.H.; Lee, D.Y.; Chang, G.D. Proteomic identification of a novel Hsp90-containing protein–mineral complex which can be induced in cells in response to massive calcium influx. J. Proteome Res. 2012, 11, 3160. [Google Scholar] [CrossRef]
- Reyes Ruiz, V.M.; Ramirez, J.; Naseer, N.; Palacio, N.M.; Siddarthan, I.J.; Yan, B.M.; Boyer, M.A.; Pensinger, D.A.; Sauer, J.D.; Shin, S. Broad detection of bacterial type III secretion system and flagellin proteins by the human NAIP/NLRC4 inflammasome. Proc. Natl. Acad. Sci. USA 2017, 114, 13242–13247. [Google Scholar] [CrossRef] [Green Version]
- Lage, S.L.; Buzzo, C.L.; Amaral, E.P.; Matteucci, K.C.; Massis, L.M.; Icimoto, M.Y.; Carmona, A.K.; D’lmperio Lima, M.R.; Rodrigues, M.M.; Ferreira, L.C.; et al. Cytosolic flagellin-induced lysosomal pathway regulates inflammasome-dependent and -independent macrophage responses. Proc. Natl. Acad. Sci. USA 2013, 110, E3321–E3330. [Google Scholar] [CrossRef] [PubMed]
- Barth, H. Exploring the role of host cell chaperones/PPIases during cellular up-take of bacterial ADP-ribosylating toxins as basis for novel pharmacological strategies to protect mammalian cells against these virulence factors. Naunyn-Schmied. Arch. Pharmacol. 2011, 383, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ross, A.C. Retinoic acid regulates cell cycle progression and cell differentiation in human monocytic THP-1 cells. Exp. Cell Res. 2004, 297, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Twomey, B.M.; McCallum, S.; Isenberg, D.A.; Latchman, D.S. Elevation of heat shock protein synthesis and hsp gene transcription during monocyte to macrophage differentiation of U937 cells. Clin. Exp. Immunol. 1993, 93, 178–183. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoang, T.X.; Kim, J.Y. Cell Surface Hsp90- and αMβ2 Integrin-Mediated Uptake of Bacterial Flagellins to Activate Inflammasomes by Human Macrophages. Cells 2022, 11, 2878. https://doi.org/10.3390/cells11182878
Hoang TX, Kim JY. Cell Surface Hsp90- and αMβ2 Integrin-Mediated Uptake of Bacterial Flagellins to Activate Inflammasomes by Human Macrophages. Cells. 2022; 11(18):2878. https://doi.org/10.3390/cells11182878
Chicago/Turabian StyleHoang, Thi Xoan, and Jae Young Kim. 2022. "Cell Surface Hsp90- and αMβ2 Integrin-Mediated Uptake of Bacterial Flagellins to Activate Inflammasomes by Human Macrophages" Cells 11, no. 18: 2878. https://doi.org/10.3390/cells11182878
APA StyleHoang, T. X., & Kim, J. Y. (2022). Cell Surface Hsp90- and αMβ2 Integrin-Mediated Uptake of Bacterial Flagellins to Activate Inflammasomes by Human Macrophages. Cells, 11(18), 2878. https://doi.org/10.3390/cells11182878





