Nitric Oxide Trickle Drives Heme into Hemoglobin and Muscle Myoglobin
Abstract
:1. Introduction
2. Material and Methods
2.1. Reagents
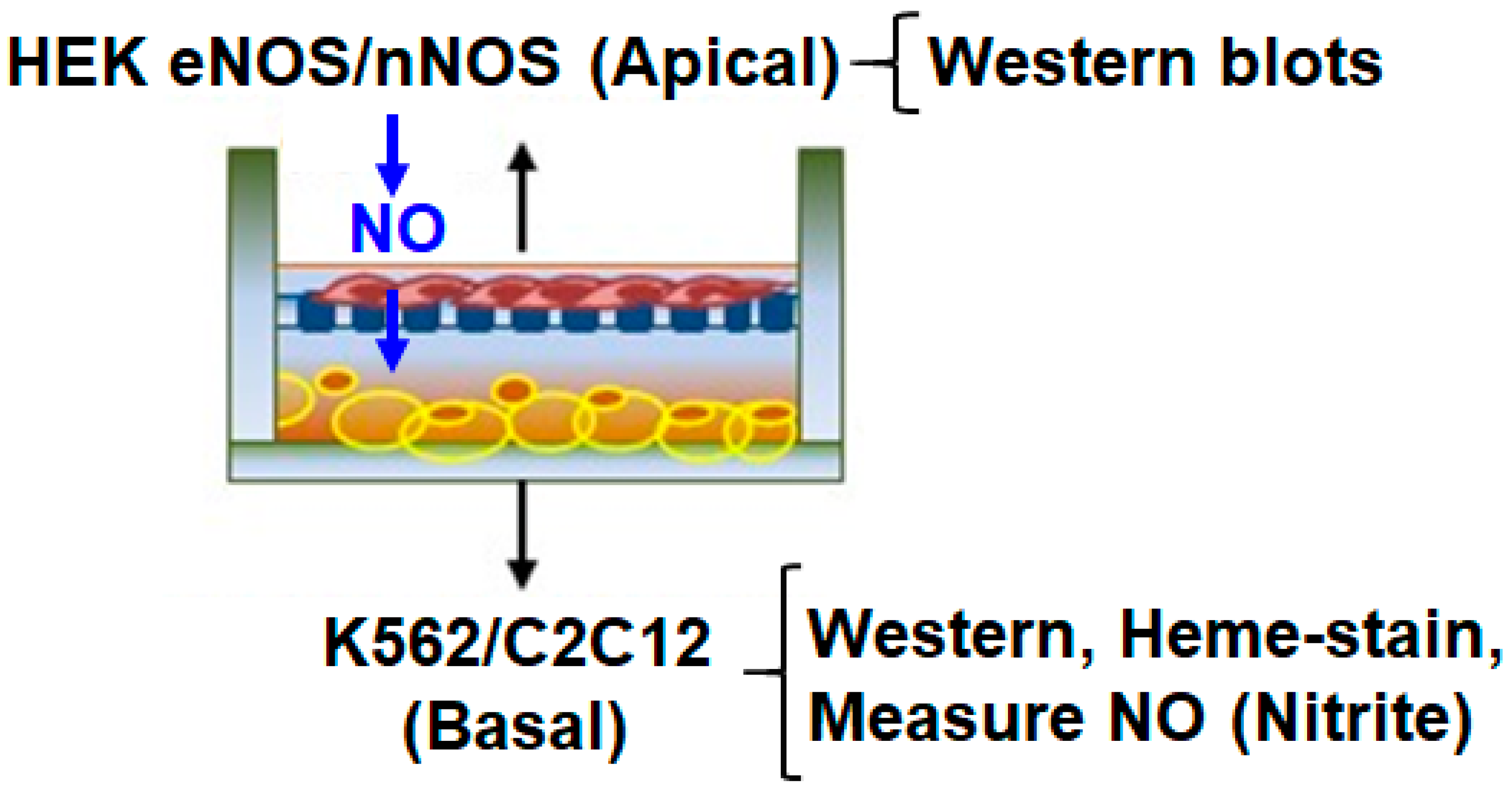
2.2. Transwell Co-Culture and Growth/Induction of Cells
2.3. Statistics
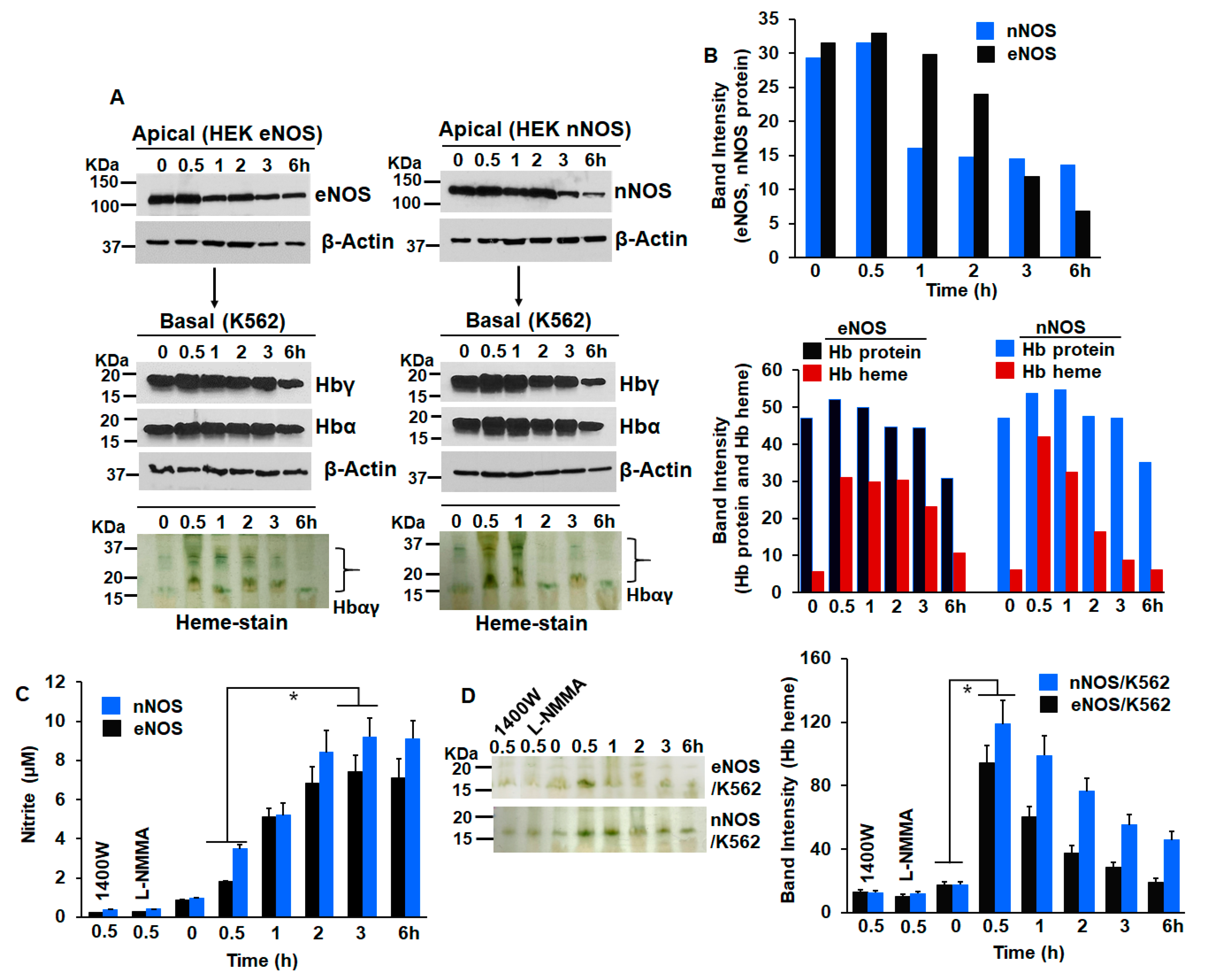
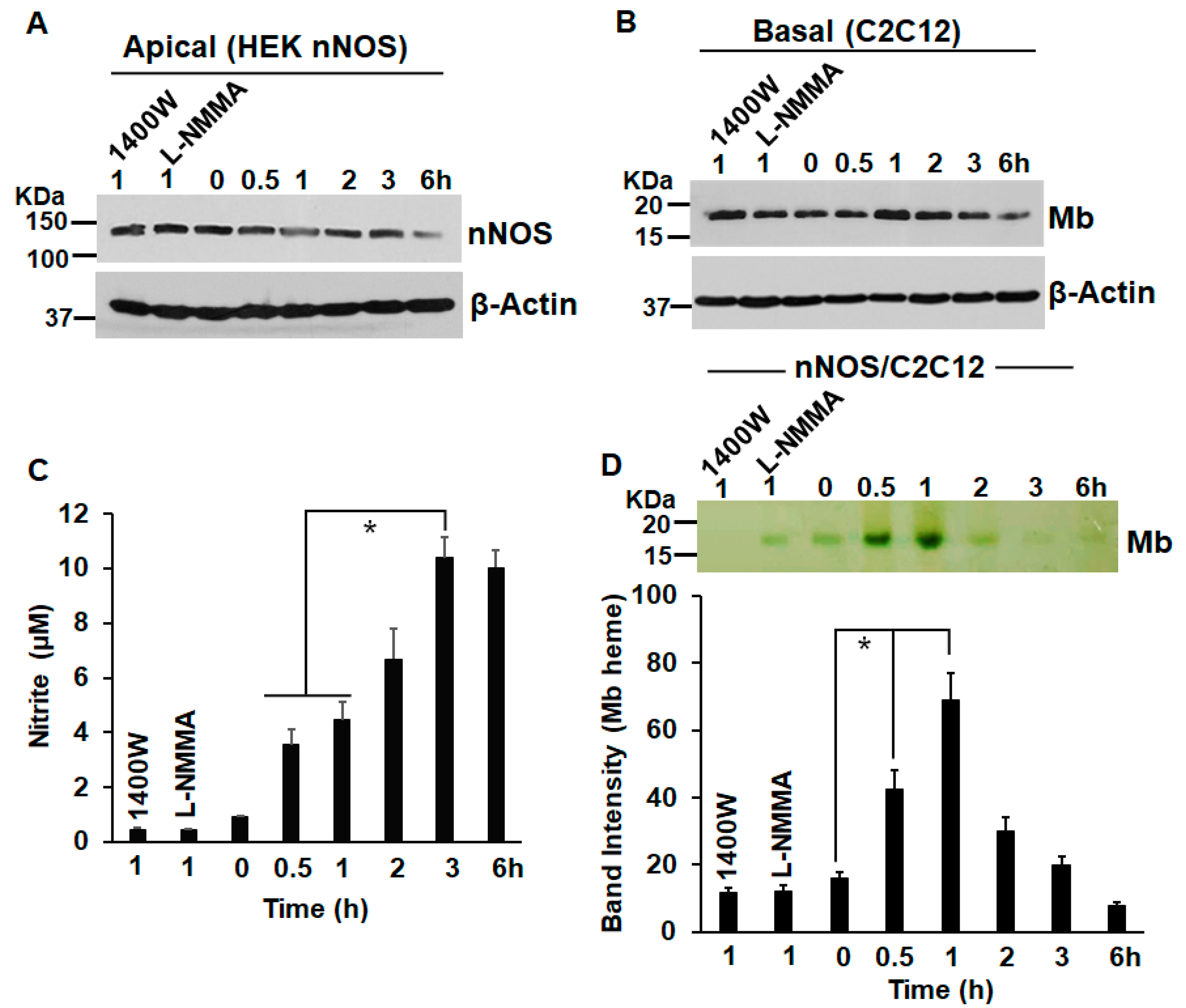
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Chen, K.; Pittman, R.N.; Popel, A.S. Nitric oxide in the vasculature: Where does it come from and where does it go? A quantitative perspective. Antioxid. Redox Signal. 2008, 10, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.M.; Ferrige, A.G.; Moncada, S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987, 327, 524–526. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.C.; Loscalzo, J. Vascular Nitric Oxide: Formation and Function. J. Blood Med. 2010, 2010, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Murad, F. Shattuck Lecture. Nitric oxide and cyclic GMP in cell signaling and drug development. N. Engl. J. Med. 2006, 355, 2003–2011. [Google Scholar] [CrossRef]
- Stuehr, D.J. Mammalian nitric oxide synthases. Biochim. Biophys. Acta 1999, 1411, 217–230. [Google Scholar] [CrossRef]
- Stuehr, D.J.; Haque, M.M. Nitric oxide synthase enzymology in the 20 years after the Nobel Prize. Br. J. Pharmacol. 2019, 176, 177–188. [Google Scholar] [CrossRef]
- Marletta, M.A. Nitric oxide synthase: Aspects concerning structure and catalysis. Cell 1994, 78, 927–930. [Google Scholar] [CrossRef]
- Kapil, V.; Khambata, R.S.; Jones, D.A.; Rathod, K.; Primus, C.; Massimo, G.; Fukuto, J.M.; Ahluwalia, A. The Noncanonical Pathway for In Vivo Nitric Oxide Generation: The Nitrate-Nitrite-Nitric Oxide Pathway. Pharmacol. Rev. 2020, 72, 692–766. [Google Scholar] [CrossRef]
- Bryan, N.S.; Bian, K.; Murad, F. Discovery of the nitric oxide signaling pathway and targets for drug development. Front. Biosci. 2009, 14, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Derbyshire, E.R.; Marletta, M.A. Biochemistry of soluble guanylate cyclase. In Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2009; pp. 17–31. [Google Scholar] [CrossRef]
- Derbyshire, E.R.; Marletta, M.A. Structure and regulation of soluble guanylate cyclase. Annu. Rev. Biochem. 2012, 81, 533–559. [Google Scholar] [CrossRef] [PubMed]
- Cokic, V.P.; Schechter, A.N. Effects of nitric oxide on red blood cell development and phenotype. Curr. Top. Dev. Biol. 2008, 82, 169–215. [Google Scholar] [CrossRef] [PubMed]
- Cokic, V.P.; Smith, R.D.; Beleslin-Cokic, B.B.; Njoroge, J.M.; Miller, J.L.; Gladwin, M.T.; Schechter, A.N. Hydroxyurea induces fetal hemoglobin by the nitric oxide-dependent activation of soluble guanylyl cyclase. J. Clin. Invest. 2003, 111, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Flonta, S.E.; Arena, S.; Pisacane, A.; Michieli, P.; Bardelli, A. Expression and functional regulation of myoglobin in epithelial cancers. Am. J. Pathol. 2009, 175, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Dai, Y.; Biswas, P.; Stuehr, D.J. Myoglobin maturation is driven by the hsp90 chaperone machinery and by soluble guanylyl cyclase. FASEB J. 2019, 33, 9885–9896. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Garee, G.; Sweeny, E.A.; Nakamura, Y.; Stuehr, D.J. Hsp90 chaperones hemoglobin maturation in erythroid and nonerythroid cells. Proc. Natl. Acad. Sci. USA 2018, 115, E1117–E1126. [Google Scholar] [CrossRef]
- Dai, Y.; Faul, E.M.; Ghosh, A.; Stuehr, D.J. NO rapidly mobilizes cellular heme to trigger assembly of its own receptor. Proc. Natl. Acad. Sci. USA 2022, 119, e2115774119. [Google Scholar] [CrossRef]
- Ghosh, A.; Stasch, J.P.; Papapetropoulos, A.; Stuehr, D.J. Nitric oxide and heat shock protein 90 activate soluble guanylate cyclase by driving rapid change in its subunit interactions and heme content. J. Biol. Chem. 2014, 289, 15259–15271. [Google Scholar] [CrossRef]
- Ghosh, A.; Chawla-Sarkar, M.; Stuehr, D.J. Hsp90 interacts with inducible NO synthase client protein in its heme-free state and then drives heme insertion by an ATP-dependent process. FASEB J. 2011, 25, 2049–2060. [Google Scholar] [CrossRef] [Green Version]
- Hausladen, A.; Rafikov, R.; Angelo, M.; Singel, D.J.; Nudler, E.; Stamler, J.S. Assessment of nitric oxide signals by triiodide chemiluminescence. Proc. Natl. Acad. Sci. USA 2007, 104, 2157–2162. [Google Scholar] [CrossRef]
- Chowdhury, A.A.; Chaudhuri, J.; Biswas, N.; Manna, A.; Chatterjee, S.; Mahato, S.K.; Chaudhuri, U.; Jaisankar, P.; Bandyopadhyay, S. Synergistic apoptosis of CML cells by buthionine sulfoximine and hydroxychavicol correlates with activation of AIF and GSH-ROS-JNK-ERK-iNOS pathway. PLoS ONE 2013, 8, e73672. [Google Scholar] [CrossRef] [PubMed]
- Helms, C.C.; Gladwin, M.T.; Kim-Shapiro, D.B. Erythrocytes and Vascular Function: Oxygen and Nitric Oxide. Front. Physiol. 2018, 9, 125. [Google Scholar] [CrossRef]
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015, 129, 83–94. [Google Scholar] [CrossRef]
- Waheed, S.M.; Ghosh, A.; Chakravarti, R.; Biswas, A.; Haque, M.M.; Panda, K.; Stuehr, D.J. Nitric oxide blocks cellular heme insertion into a broad range of heme proteins. Free Radic. Biol. Med. 2010, 48, 1548–1558. [Google Scholar] [CrossRef] [PubMed]
- Albakri, Q.A.; Stuehr, D.J. Intracellular assembly of inducible NO synthase is limited by nitric oxide-mediated changes in heme insertion and availability. J. Biol. Chem. 1996, 271, 5414–5421. [Google Scholar] [CrossRef] [PubMed]
- Tupta, B.; Stuehr, E.; Sumi, M.P.; Sweeny, E.A.; Smith, B.; Stuehr, D.J.; Ghosh, A. GAPDH is involved in the heme-maturation of myoglobin and hemoglobin. FASEB J. 2022, 36, e22099. [Google Scholar] [CrossRef]
- Thomas, D.D.; Wink, D.A. NOS2 as an Emergent Player in Progression of Cancer. Antioxid. Redox Signal. 2017, 26, 963–965. [Google Scholar] [CrossRef]
- Cobbs, C.S.; Brenman, J.E.; Aldape, K.D.; Bredt, D.S.; Israel, M.A. Expression of nitric oxide synthase in human central nervous system tumors. Cancer Res. 1995, 55, 727–730. [Google Scholar]
- Maman, S.; Sagi-Assif, O.; Yuan, W.; Ginat, R.; Meshel, T.; Zubrilov, I.; Keisari, Y.; Lu, W.; Lu, W.; Witz, I.P. The Beta Subunit of Hemoglobin (HBB2/HBB) Suppresses Neuroblastoma Growth and Metastasis. Cancer Res. 2017, 77, 14–26. [Google Scholar] [CrossRef]
- Godfrey, E.W.; Schwarte, R.C. The role of nitric oxide signaling in the formation of the neuromuscular junction. J. Neurocytol. 2003, 32, 591–602. [Google Scholar] [CrossRef]
- Ikuta, T.; Sellak, H.; Odo, N.; Adekile, A.D.; Gaensler, K.M. Nitric Oxide-cGMP Signaling Stimulates Erythropoiesis through Multiple Lineage-Specific Transcription Factors: Clinical Implications and a Novel Target for Erythropoiesis. PLoS ONE 2016, 11, e0144561. [Google Scholar] [CrossRef]
- De Palma, C.; Clementi, E. Nitric oxide in myogenesis and therapeutic muscle repair. Mol. Neurobiol. 2012, 46, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Bloch, K.D.; Ichinose, F.; Roberts, J.D., Jr.; Zapol, W.M. Inhaled NO as a therapeutic agent. Cardiovasc. Res. 2007, 75, 339–348. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumi, M.P.; Tupta, B.; Ghosh, A. Nitric Oxide Trickle Drives Heme into Hemoglobin and Muscle Myoglobin. Cells 2022, 11, 2838. https://doi.org/10.3390/cells11182838
Sumi MP, Tupta B, Ghosh A. Nitric Oxide Trickle Drives Heme into Hemoglobin and Muscle Myoglobin. Cells. 2022; 11(18):2838. https://doi.org/10.3390/cells11182838
Chicago/Turabian StyleSumi, Mamta P., Blair Tupta, and Arnab Ghosh. 2022. "Nitric Oxide Trickle Drives Heme into Hemoglobin and Muscle Myoglobin" Cells 11, no. 18: 2838. https://doi.org/10.3390/cells11182838
APA StyleSumi, M. P., Tupta, B., & Ghosh, A. (2022). Nitric Oxide Trickle Drives Heme into Hemoglobin and Muscle Myoglobin. Cells, 11(18), 2838. https://doi.org/10.3390/cells11182838





