Regulating the Expression of HIF-1α or lncRNA: Potential Directions for Cancer Therapy
Abstract
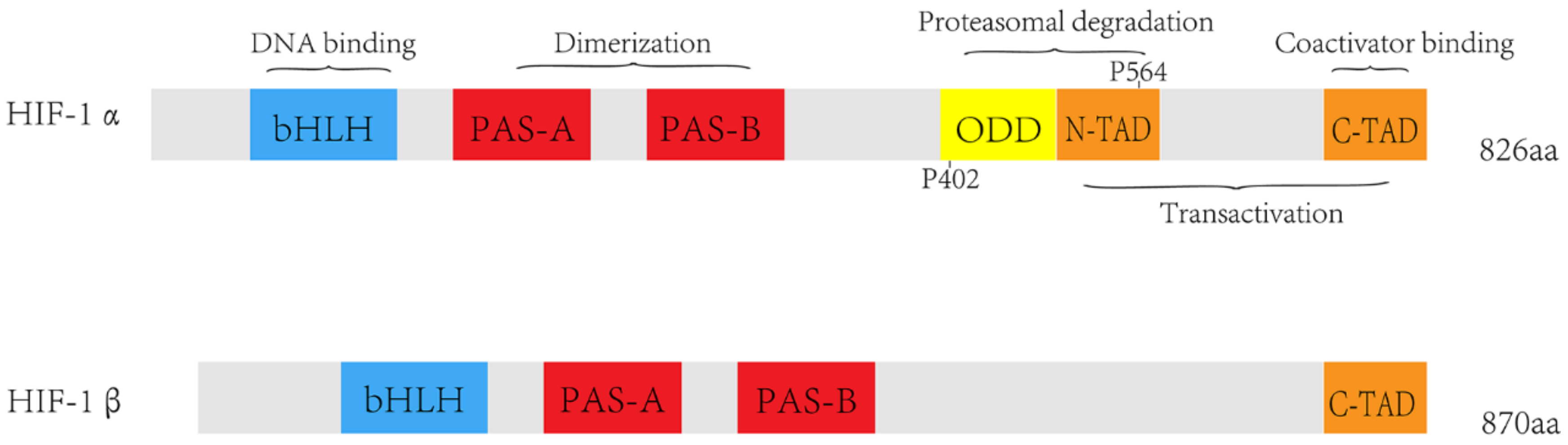
:1. Introduction
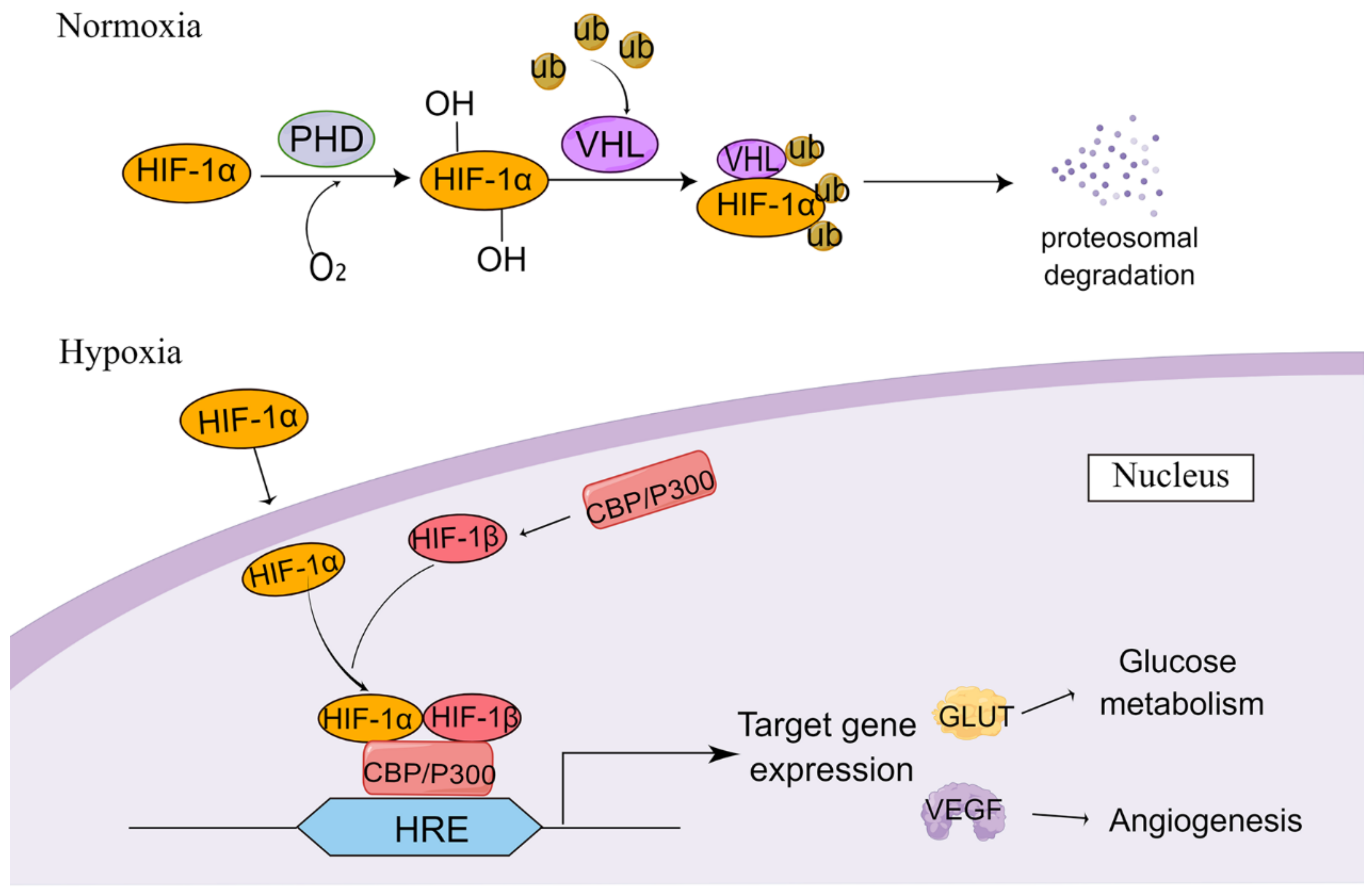
2. Induction of HIF-1α Accumulation in Tumor Cells under Hypoxia
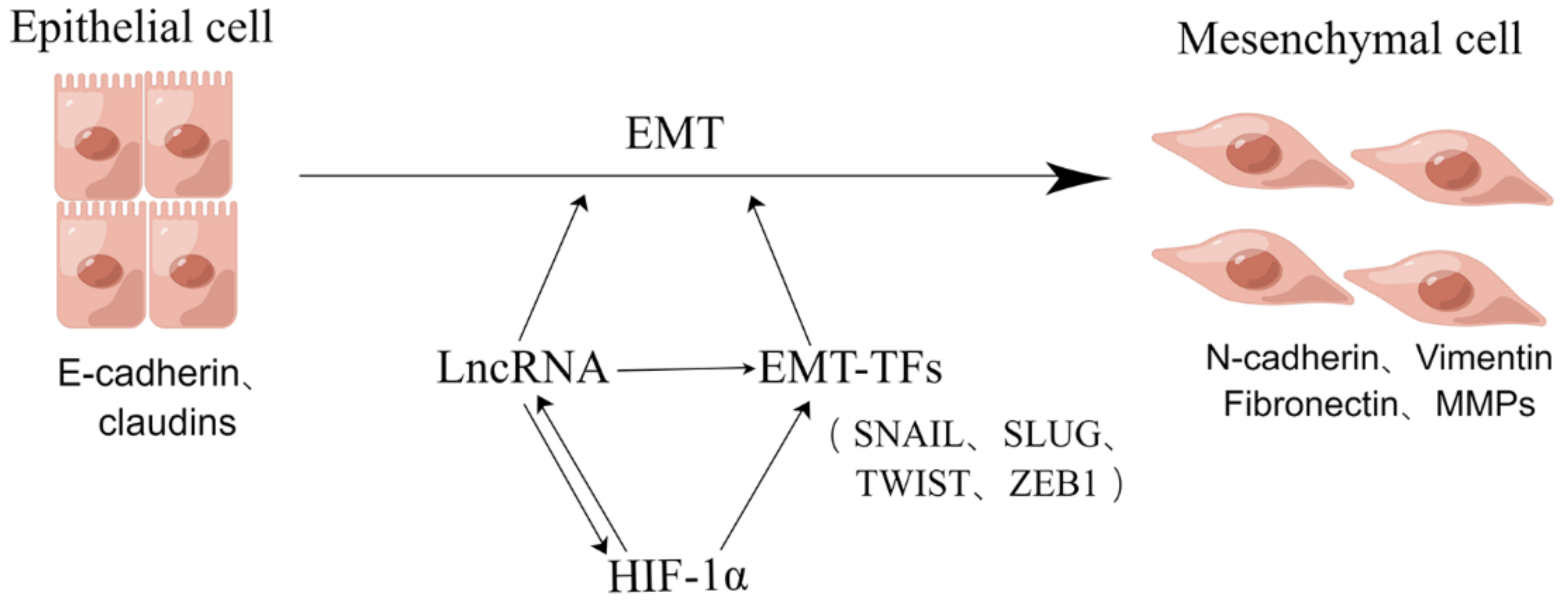
3. The HIF-1α Induced EMT in Hypoxia
4. HIF-1α Can Promote Tumor EMT Progression by Regulating LncRNA Expression under Hypoxia
4.1. LncRNA HOTAIR
4.2. LncRNA RP11-390F4.3
4.3. LncRNA FALEC
4.4. LncRNA GAPLINC
4.5. LncRNA HAS2-AS1
4.6. LncRNA MALAT1
4.7. LncRNA UCA1
4.8. LncRNA H19
4.9. LncRNA BACE1-AS
4.10. LncRNA BX111
4.11. LncRNA CASC9
5. Targeted Drugs That Inhibit the Biological Function of HIF-1α
5.1. HIF-1α Inhibitors That Inhibit HIF-1α mRNA Levels
5.2. HIF-1α Inhibitors That Inhibit HIF-1α Transcriptional Activity
5.3. HIF-1α Inhibitors That Inhibit HIF-1α Protein Synthesis and Accumulation
5.4. HIF-1α Inhibitors That Promote Proteasomal Degradation
5.5. HIF-1α Inhibitor That Inhibits the Formation of HIF-1-a/p300 Complex
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
| LncRNA | Regulated by HIF-1α | Cancer Types | Mechanism and Related Molecules | Biological Function | Reference |
|---|---|---|---|---|---|
| HOTAIR | √ | NSCLC | N.D. | Promoting cell proliferation, migration, and invasion | [61] |
| Cervical cancer | miR-29b/PTEN/PI3K | Promoting cell EMT, invasion, and chemoresistance | [62] | ||
| RP11-390F4.3 | √ | FADU and MCF7 cell lines | Induced EMT-TFs (Snail, Twist1, ZEB1, and ZEB2) | Promoting cell EMT, metastasis, and tumorigenesis | [63,64] |
| FALEC | √ | Prostate cancer | N.D. | Promoting cell proliferation, migration, and invasion | [65] |
| Gastric cancer | ECM1/β-catenin | Promoting cell EMT, metastasis, and invasion | [67] | ||
| GAPLINC | √ | Gastric cancer | N.D. | Promoting cell migration, invasion | [68] |
| HCC | N.D. | Promoting cell EMT, invasion, proliferation, and migration | [69] | ||
| Colorectal cancer | PSF/NONO/SNAI2 | Promoting cell EMT, invasion | [70] | ||
| HAS2-AS1 | √ | OSCC | Stabilize HAS2 expression | Promoting cell EMT, invasion | [71] |
| MALAT1 | √ | Breast cancer | competitively binding to miR-3064-5p | Promoting cell proliferation and migration | [72] |
| OSCC | Induced EMT-TFs (β-catenin, NF-κB) | Promoting cell EMT, invasion, and migration | [73] | ||
| Bladder cancer | miR-124/foxq1/suz12 | Promoting cell EMT | [74,75] | ||
| UCA1 | √ | Breast cancer | N.D. | Promoting cell proliferation | [76] |
| Bladder cancer | miR-143/HMGB1/UCA1 | Promoting cell EMT and invasion | [77] | ||
| H19 | √ | Colorectal cancer | Competitively binding to miR-138 and miR-200a | Promoting cell EMT | [81] |
| BACE1 | √ | HCC | miR-377-3p/CELF1 | Promoting cell EMT, invasion, and metastasis | [82,83] |
| BX111 | √ | Pancreatic cancer | Recruits YB1 to ZEB1 and activates ZEB1 expression | Promoting cell EMT, invasion, and metastasis | [84] |
| CASC9 | √ | Lung Cancer | N.D. | Promoting cell EMT, invasion, and migration | [85] |
References
- Mudassar, F.; Shen, H.; O’Neill, G.; Hau, E. Targeting tumor hypoxia and mitochondrial metabolism with anti-parasitic drugs to improve radiation response in high-grade gliomas. J. Exp. Clin. Cancer Res. 2020, 39, 208. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Li, J.; Zhang, C.; Hu, T.; Li, S.; He, S.; Yan, H.; Tan, Y.; Lei, M.; Wen, M.; et al. The role of hypoxia-inducible factors in tumor angiogenesis and cell metabolism. Genes Dis. 2017, 4, 19–24. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Pastushenko, I.; Blanpain, C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019, 29, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Christofori, G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009, 28, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.Y.; Wu, V.W.C.; Law, H.K.W. Hypoxia-Induced Epithelial-Mesenchymal Transition in Cancers: HIF-1α and beyond. Front. Oncol. 2020, 10, 486. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Xu, Y.; He, T.; Qin, C.; Xu, J. Normal and disease-related biological functions of Twist1 and underlying molecular mechanisms. Cell Res. 2012, 22, 90–106. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Z.; Chen, X.; Zhang, S. Long non-coding RNAs: From disease code to drug role. Acta Pharm. Sin. B 2021, 11, 340–354. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, D.; Xie, H.; Hu, Y. Interplay of long non-coding RNAs and HIF-1α: A new dimension to understanding hypoxia-regulated tumor growth and metastasis. Cancer Lett. 2021, 499, 49–59. [Google Scholar] [CrossRef]
- Epstein, A.C.; Gleadle, J.M.; McNeill, L.A.; Hewitson, K.S.; O’Rourke, J.; Mole, D.R.; Mukherji, M.; Metzen, E.; Wilson, M.I.; Dhanda, A.; et al. C. elegans EGL-9 and Mammalian Homologs Define a Family of Dioxygenases that Regulate HIF by Prolyl Hydroxylation. Cell 2001, 107, 43–54. [Google Scholar] [CrossRef] [Green Version]
- Talks, K.L.; Turley, H.; Gatter, K.C.; Maxwell, P.H.; Pugh, C.W.; Ratcliffe, P.J.; Harris, A.L. The Expression and Distribution of the Hypoxia-Inducible Factors HIF-1α and HIF-2α in Normal Human Tissues, Cancers, and Tumor-Associated Macrophages. Am. J. Pathol. 2000, 157, 411–421. [Google Scholar] [CrossRef]
- Tao, J.; Yang, G.; Zhou, W.; Qiu, J.; Chen, G.; Luo, W.; Zhao, F.; You, L.; Zheng, L.; Zhang, T.; et al. Targeting hypoxic tumor microenvironment in pancreatic cancer. J. Hematol. Oncol. 2021, 14, 14. [Google Scholar] [CrossRef]
- Balamurugan, K. HIF-1 at the crossroads of hypoxia, inflammation, and cancer. Int. J. Cancer 2016, 138, 1058–1066. [Google Scholar] [CrossRef]
- Infantino, V.; Santarsiero, A.; Convertini, P.; Todisco, S.; Iacobazzi, V. Cancer Cell Metabolism in Hypoxia: Role of HIF-1 as Key Regulator and Therapeutic Target. Int. J. Mol. Sci. 2021, 22, 5703. [Google Scholar] [CrossRef]
- Bhattarai, D.; Xu, X.; Lee, K. Hypoxia-inducible factor-1 (HIF-1) inhibitors from the last decade (2007 to 2016): A “structure-activity relationship” perspective. Med. Res. Rev. 2018, 38, 1404–1442. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Conotte, S.; Belayew, A.; Declèves, A.E.; Legrand, A.; Tassin, A. Hypoxia and Hypoxia-Inducible Factor Signaling in Muscular Dystrophies: Cause and Consequences. Int. J. Mol. Sci. 2021, 22, 7220. [Google Scholar] [CrossRef]
- He, Q.; Ma, Y.; Liu, J.; Zhang, D.; Ren, J.; Zhao, R.; Chang, J.; Guo, Z.N.; Yang, Y. Biological Functions and Regulatory Mechanisms of Hypoxia-Inducible Factor-1α in Ischemic Stroke. Front. Immunol. 2021, 12, 801985. [Google Scholar] [CrossRef]
- Jokilehto, T.; Jaakkola, P.M. The role of HIF prolyl hydroxylases in tumour growth. J. Cell. Mol. Med. 2010, 14, 758–770. [Google Scholar] [CrossRef]
- Baba, Y.; Nosho, K.; Shima, K.; Irahara, N.; Chan, A.T.; Meyerhardt, J.A.; Chung, D.C.; Giovannucci, E.L.; Fuchs, C.S.; Ogino, S. HIF1A overexpression is associated with poor prognosis in a cohort of 731 colorectal cancers. Am. J. Pathol. 2010, 176, 2292–2301. [Google Scholar] [CrossRef]
- Chen, L.; Shi, Y.; Yuan, J.; Han, Y.; Qin, R.; Wu, Q.; Jia, B.; Wei, B.; Wei, L.; Dai, G.; et al. HIF-1 alpha overexpression correlates with poor overall survival and disease-free survival in gastric cancer patients post-gastrectomy. PLoS ONE 2014, 9, e90678. [Google Scholar] [CrossRef]
- Zhang, Y.; Weinberg, R.A. Epithelial-to-mesenchymal transition in cancer: Complexity and opportunities. Front. Med. 2018, 12, 361–373. [Google Scholar] [CrossRef]
- Brabletz, S.; Schuhwerk, H.; Brabletz, T.; Stemmler, M.P. Dynamic EMT: A multi-tool for tumor progression. EMBO J. 2021, 40, e108647. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Mittal, V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. 2018, 13, 395–412. [Google Scholar] [CrossRef]
- Hapke, R.Y.; Haake, S.M. Hypoxia-induced epithelial to mesenchymal transition in cancer. Cancer Lett. 2020, 487, 10–20. [Google Scholar] [CrossRef]
- De Craene, B.; Berx, G. Regulatory networks defining EMT during cancer initiation and progression. Nat. Rev. Cancer 2013, 13, 97–110. [Google Scholar] [CrossRef]
- Gonzalez-Gonzalez, R.; Ortiz-Sarabia, G.; Molina-Frechero, N.; Salas-Pacheco, J.M.; Salas-Pacheco, S.M.; Lavalle-Carrasco, J.; Lopez-Verdin, S.; Tremillo-Maldonado, O.; Bologna-Molina, R. Epithelial-Mesenchymal Transition Associated with Head and Neck Squamous Cell Carcinomas: A Review. Cancers 2021, 13, 3027. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, B.P. Snail—More than EMT. Cell Adhes. Migr. 2010, 4, 199–203. [Google Scholar] [CrossRef]
- Zhu, Y.; Tan, J.; Xie, H.; Wang, J.; Meng, X.; Wang, R. HIF-1α regulates EMT via the Snail and beta-catenin pathways in paraquat poisoning-induced early pulmonary fibrosis. J. Cell. Mol. Med. 2016, 20, 688–697. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jeong, E.K.; Ju, M.K.; Jeon, H.M.; Kim, M.Y.; Kim, C.H.; Park, H.G.; Han, S.I.; Kang, H.S. Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation. Mol. Cancer 2017, 16, 10. [Google Scholar] [CrossRef] [Green Version]
- Loh, C.Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef]
- Djediai, S.; Gonzalez Suarez, N.; El Cheikh-Hussein, L.; Rodriguez Torres, S.; Gresseau, L.; Dhayne, S.; Joly-Lopez, Z.; Annabi, B. MT1-MMP Cooperates with TGF-beta Receptor-Mediated Signaling to Trigger SNAIL and Induce Epithelial-to-Mesenchymal-like Transition in U87 Glioblastoma Cells. Int. J. Mol. Sci. 2021, 22, 13006. [Google Scholar] [CrossRef] [PubMed]
- Borretzen, A.; Gravdal, K.; Haukaas, S.A.; Mannelqvist, M.; Beisland, C.; Akslen, L.A.; Halvorsen, O.J. The epithelial-mesenchymal transition regulators Twist, Slug, and Snail are associated with aggressive tumour features and poor outcome in prostate cancer patients. J. Pathol. Clin. Res. 2021, 7, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, J.; Guedj, N.; Clapéron, A.; Nguyen Ho-Bouldoires, T.H.; Paradis, V.; Fouassier, L. Epithelial-mesenchymal transition in cholangiocarcinoma: From clinical evidence to regulatory networks. J. Hepatol. 2017, 66, 424–441. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, J.; O’Brien, W.T.; Johnson, R.S.; LaManna, J.C.; Chavez, J.C.; Klein, P.S.; Simon, M.C. O2 regulates stem cells through Wnt/β-catenin signalling. Nat. Cell Biol. 2010, 12, 1007–1013. [Google Scholar] [CrossRef]
- Georgakopoulos-Soares, I.; Chartoumpekis, D.V.; Kyriazopoulou, V.; Zaravinos, A. EMT Factors and Metabolic Pathways in Cancer. Front. Oncol. 2020, 10, 499. [Google Scholar] [CrossRef]
- Yang, M.H.; Wu, M.Z.; Chiou, S.H.; Chen, P.M.; Chang, S.Y.; Liu, C.J.; Teng, S.C.; Wu, K.J. Direct regulation of TWIST by HIF-1α promotes metastasis. Nat. Cell Biol. 2008, 10, 295–305. [Google Scholar] [CrossRef]
- Hung, J.J.; Yang, M.H.; Hsu, H.S.; Hsu, W.H.; Liu, J.S.; Wu, K.J. Prognostic significance of hypoxia-inducible factor-1alpha, TWIST1 and Snail expression in resectable non-small cell lung cancer. Thorax 2009, 64, 1082–1089. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, X.; Peng, Y.; Wu, M.; Zhang, P.; Xie, R.; Wu, Y.; Yan, Q.; Liu, S.; Wang, J. HIF-1α Promotes Epithelial-Mesenchymal Transition and Metastasis through Direct Regulation of ZEB1 in Colorectal Cancer. PLoS ONE 2015, 10, e0129603. [Google Scholar] [CrossRef]
- Yoo, Y.G.; Christensen, J.; Gu, J.; Huang, L.E. HIF-1α mediates tumor hypoxia to confer a perpetual mesenchymal phenotype for malignant progression. Sci. Signal. 2011, 4(pt4). [Google Scholar] [CrossRef]
- Yalim-Camci, I.; Balcik-Ercin, P.; Cetin, M.; Odabas, G.; Tokay, N.; Sayan, A.E.; Yagci, T. ETS1 is coexpressed with ZEB2 and mediates ZEB2-induced epithelial-mesenchymal transition in human tumors. Mol. Carcinog. 2019, 58, 1068–1081. [Google Scholar] [CrossRef]
- Huber, M.A.; Azoitei, N.; Baumann, B.; Grünert, S.; Sommer, A.; Pehamberger, H.; Kraut, N.; Beug, H.; Wirth, T. NF-κB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J. Clin. Investig. 2004, 114, 569–581. [Google Scholar] [CrossRef]
- Nomura, A.; Majumder, K.; Giri, B.; Dauer, P.; Dudeja, V.; Roy, S.; Banerjee, S.; Saluja, A.K. Inhibition of NF-κB pathway leads to deregulation of epithelial-mesenchymal transition and neural invasion in pancreatic cancer. Lab. Investig. J. Tech. Methods Pathol. 2016, 96, 1268–1278. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, X.; Fan, D. Ginsenoside CK Inhibits Hypoxia-Induced Epithelial-Mesenchymal Transformation through the HIF-1α/NF-κB Feedback Pathway in Hepatocellular Carcinoma. Foods 2021, 10, 1195. [Google Scholar] [CrossRef]
- Umezaki, N.; Nakagawa, S.; Yamashita, Y.I.; Kitano, Y.; Arima, K.; Miyata, T.; Hiyoshi, Y.; Okabe, H.; Nitta, H.; Hayashi, H.; et al. Lysyl oxidase induces epithelial-mesenchymal transition and predicts intrahepatic metastasis of hepatocellular carcinoma. Cancer Sci. 2019, 110, 2033–2043. [Google Scholar] [CrossRef]
- Schietke, R.; Warnecke, C.; Wacker, I.; Schödel, J.; Mole, D.R.; Campean, V.; Amann, K.; Goppelt-Struebe, M.; Behrens, J.; Eckardt, K.U.; et al. The lysyl oxidases LOX and LOXL2 are necessary and sufficient to repress E-cadherin in hypoxia: Insights into cellular transformation processes mediated by HIF-1. J. Biol. Chem. 2010, 285, 6658–6669. [Google Scholar] [CrossRef]
- North, R.A. Molecular physiology of P2X receptors. Physiol. Rev. 2002, 82, 1013–1067. [Google Scholar] [CrossRef]
- Roger, S.; Jelassi, B.; Couillin, I.; Pelegrin, P.; Besson, P.; Jiang, L.H. Understanding the roles of the P2X7 receptor in solid tumour progression and therapeutic perspectives. Biochim. Biophys. Acta 2015, 1848, 2584–2602. [Google Scholar] [CrossRef]
- Zuo, J.; Wen, M.; Li, S.; Lv, X.; Wang, L.; Ai, X.; Lei, M. Overexpression of CXCR4 promotes invasion and migration of non-small cell lung cancer via EGFR and MMP-9. Oncol. Lett. 2017, 14, 7513–7521. [Google Scholar] [CrossRef]
- Cayetano-Salazar, L.; Olea-Flores, M.; Zuñiga-Eulogio, M.D.; Weinstein-Oppenheimer, C.; Fernández-Tilapa, G.; Mendoza-Catalán, M.A.; Zacapala-Gómez, A.E.; Ortiz-Ortiz, J.; Ortuño-Pineda, C.; Navarro-Tito, N. Natural isoflavonoids in invasive cancer therapy: From bench to bedside. Phytother. Res. 2021, 35, 4092–4110. [Google Scholar] [CrossRef]
- Tafani, M.; Schito, L.; Pellegrini, L.; Villanova, L.; Marfe, G.; Anwar, T.; Rosa, R.; Indelicato, M.; Fini, M.; Pucci, B.; et al. Hypoxia-increased RAGE and P2X7R expression regulates tumor cell invasion through phosphorylation of Erk1/2 and Akt and nuclear translocation of NF-κB. Carcinogenesis 2011, 32, 1167–1175. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Q.; Song, W.; Peng, X.; Zhao, R. P2X7 receptor: A critical regulator and potential target for breast cancer. J. Mol. Med. 2021, 99, 349–358. [Google Scholar] [CrossRef]
- Wang, M.Q.; Zhu, W.J.; Gao, P. New insights into long non-coding RNAs in breast cancer: Biological functions and therapeutic prospects. Exp. Mol. Pathol. 2021, 120, 104640. [Google Scholar] [CrossRef] [PubMed]
- Kopp, F.; Mendell, J.T. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell 2018, 172, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, J.D.; Wei, Y.; Khavari, P.A. The functions and unique features of long intergenic non-coding RNA. Nat. Rev. Mol. Cell Biol. 2018, 19, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Barreca, M.M.; Zichittella, C.; Alessandro, R.; Conigliaro, A. Hypoxia-Induced Non-Coding RNAs Controlling Cell Viability in Cancer. Int. J. Mol. Sci. 2021, 22, 1857. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.-C.; Kung, H.-J.; Shih, J.-W. Signaling in and out: Long-noncoding RNAs in tumor hypoxia. J. Biomed. Sci. 2020, 27, 59. [Google Scholar] [CrossRef]
- Huang, X.; Zuo, J. Emerging roles of miR-210 and other non-coding RNAs in the hypoxic response. Acta Biochim. Biophys. Sin. 2014, 46, 220–232. [Google Scholar] [CrossRef]
- Tay, Y.; Rinn, J.; Pandolfi, P.P. The multilayered complexity of ceRNA crosstalk and competition. Nature 2014, 505, 344–352. [Google Scholar] [CrossRef]
- Zheng, F.; Chen, J.; Zhang, X.; Wang, Z.; Chen, J.; Lin, X.; Huang, H.; Fu, W.; Liang, J.; Wu, W.; et al. The HIF-1α antisense long non-coding RNA drives a positive feedback loop of HIF-1α mediated transactivation and glycolysis. Nat. Commun. 2021, 12, 1341. [Google Scholar] [CrossRef]
- Zhou, C.; Ye, L.; Jiang, C.; Bai, J.; Chi, Y.; Zhang, H. Long noncoding RNA HOTAIR, a hypoxia-inducible factor-1alpha activated driver of malignancy, enhances hypoxic cancer cell proliferation, migration, and invasion in non-small cell lung cancer. Tumour Biol. 2015, 36, 9179–9188. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, Q.; Liu, Y.; Wang, X.; Ma, C.; Zhu, W. LncRNA HOTAIR Promotes Chemoresistance by Facilitating Epithelial to Mesenchymal Transition through miR-29b/PTEN/PI3K Signaling in Cervical Cancer. Cells Tissues Organs 2022, 211, 16–29. [Google Scholar] [CrossRef]
- Peng, P.H.; Lai, J.C.-Y.; Hsu, K.W.; Wu, K.J. Hypoxia-induced lncRNA RP11-390F4.3 promotes epithelial-mesenchymal transition (EMT) and metastasis through upregulating EMT regulators. Cancer Lett. 2020, 483, 35–45. [Google Scholar] [CrossRef]
- Peng, P.H.; Hsu, K.W.; Lai, J.C.-Y.; Wu, K.J. The role of hypoxia-induced long noncoding RNAs (lncRNAs) in tumorigenesis and metastasis. Biomed. J. 2021, 44, 521–533. [Google Scholar] [CrossRef]
- Zhao, R.; Sun, F.; Bei, X.; Wang, X.; Zhu, Y.; Jiang, C.; Zhao, F.; Han, B.; Xia, S. Upregulation of the long non-coding RNA FALEC promotes proliferation and migration of prostate cancer cell lines and predicts prognosis of PCa patients. Prostate 2017, 77, 1107–1117. [Google Scholar] [CrossRef]
- Wu, H.; Qiao, F.; Zhao, Y.; Wu, S.; Hu, M.; Wu, T.; Huang, F.; Chen, W.; Sun, D.; Liu, M.; et al. Downregulation of Long Non-coding RNA FALEC Inhibits Gastric Cancer Cell Migration and Invasion Through Impairing ECM1 Expression by Exerting Its Enhancer-Like Function. Front. Genet. 2019, 10, 255. [Google Scholar] [CrossRef]
- Lee, K.M.; Nam, K.; Oh, S.; Lim, J.; Kim, R.K.; Shim, D.; Choi, J.H.; Lee, S.J.; Yu, J.H.; Lee, J.W.; et al. ECM1 regulates tumor metastasis and CSC-like property through stabilization of β-catenin. Oncogene 2015, 34, 6055–6065. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, X.; Zou, H.; Bai, R.; Yang, K.; Tian, Z. Hypoxia Promotes Gastric Cancer Malignancy Partly through the HIF-1α Dependent Transcriptional Activation of the Long Non-coding RNA GAPLINC. Front. Physiol. 2016, 7, 420. [Google Scholar] [CrossRef]
- Zhang, W.; Yao, H.; Wu, Y. Poor expression of long-chain noncoding RNA GAPLINC inhibits epithelial-mesenchymal transition, and invasion and migration of hepatocellular carcinoma cells. Anti-Cancer Drugs 2019, 30, 784–794. [Google Scholar] [CrossRef]
- Yang, P.; Chen, T.; Xu, Z.; Zhu, H.; Wang, J.; He, Z. Long noncoding RNA GAPLINC promotes invasion in colorectal cancer by targeting SNAI2 through binding with PSF and NONO. Oncotarget 2016, 7, 42183–42194. [Google Scholar] [CrossRef] [Green Version]
- Zhu, G.; Wang, S.; Chen, J.; Wang, Z.; Liang, X.; Wang, X.; Jiang, J.; Lang, J.; Li, L. Long noncoding RNA HAS2-AS1 mediates hypoxia-induced invasiveness of oral squamous cell carcinoma. Mol. Carcinog. 2017, 56, 2210–2222. [Google Scholar] [CrossRef]
- Shih, C.H.; Chuang, L.L.; Tsai, M.H.; Chen, L.H.; Chuang, E.Y.; Lu, T.P.; Lai, L.C. Hypoxia-Induced MALAT1 Promotes the Proliferation and Migration of Breast Cancer Cells by Sponging MiR-3064-5p. Front. Oncol. 2021, 11, 658151. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, S.; Cai, G.; Kong, L.; Zhang, T.; Ren, Y.; Wu, Y.; Mei, M.; Zhang, L.; Wang, X. Long Non Coding RNA MALAT1 Promotes Tumor Growth and Metastasis by inducing Epithelial-Mesenchymal Transition in Oral Squamous Cell Carcinoma. Sci. Rep. 2015, 5, 15972. [Google Scholar] [CrossRef]
- Jiao, D.; Li, Z.; Zhu, M.; Wang, Y.; Wu, G.; Han, X. LncRNA MALAT1 promotes tumor growth and metastasis by targeting miR-124/foxq1 in bladder transitional cell carcinoma (BTCC). Am. J. Cancer Res. 2018, 8, 748–760. [Google Scholar]
- Fan, Y.; Shen, B.; Tan, M.; Mu, X.; Qin, Y.; Zhang, F.; Liu, Y. TGF-β-induced upregulation of malat1 promotes bladder cancer metastasis by associating with suz12. Clin. Cancer Res. 2014, 20, 1531–1541. [Google Scholar] [CrossRef]
- Choudhry, H. UCA1 Overexpression Promotes Hypoxic Breast Cancer Cell Proliferation and Inhibits Apoptosis via HIF-1α Activation. J. Oncol. 2021, 2021, 5512156. [Google Scholar] [CrossRef]
- Luo, J.; Chen, J.; Li, H.; Yang, Y.; Yun, H.; Yang, S.; Mao, X. LncRNA UCA1 promotes the invasion and EMT of bladder cancer cells by regulating the miR-143/HMGB1 pathway. Oncol. Lett. 2017, 14, 5556–5562. [Google Scholar] [CrossRef]
- Wu, W.; Hu, Q.; Nie, E.; Yu, T.; Wu, Y.; Zhi, T.; Jiang, K.; Shen, F.; Wang, Y.; Zhang, J.; et al. Hypoxia induces H19 expression through direct and indirect Hif-1α activity, promoting oncogenic effects in glioblastoma. Sci. Rep. 2017, 7, 45029. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, X.; Kai, J.; Wang, F.; Wang, Z.; Shao, J.; Tan, S.; Chen, A.; Zhang, F.; Wang, S.; et al. HIF-1α-upregulated lncRNA-H19 regulates lipid droplet metabolism through the AMPKα pathway in hepatic stellate cells. Life Sci. 2020, 255, 117818. [Google Scholar] [CrossRef]
- Corrado, C.; Costa, V.; Giavaresi, G.; Calabrese, A.; Conigliaro, A.; Alessandro, R. Long Non Coding RNA H19: A New Player in Hypoxia-Induced Multiple Myeloma Cell Dissemination. Int. J. Mol. Sci. 2019, 20, 801. [Google Scholar] [CrossRef]
- Liang, W.C.; Fu, W.M.; Wong, C.W.; Wang, Y.; Wang, W.M.; Hu, G.X.; Zhang, L.; Xiao, L.J.; Wan, D.C.; Zhang, J.F.; et al. The lncRNA H19 promotes epithelial to mesenchymal transition by functioning as miRNA sponges in colorectal cancer. Oncotarget 2015, 6, 22513–22525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sil, S.; Hu, G.; Liao, K.; Niu, F.; Callen, S.; Periyasamy, P.; Fox, H.S.; Buch, S. HIV-1 Tat-mediated astrocytic amyloidosis involves the HIF-1α/lncRNA BACE1-AS axis. PLoS Biol. 2020, 18, e3000660. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, H.; Tang, L.; Huang, H.; Xu, M.; Lin, Y.; Zhou, L.; Ho, L.; Lu, J.; Ai, X. LncRNA BACE1-AS enhances the invasive and metastatic capacity of hepatocellular carcinoma cells through mediating miR-377-3p/CELF1 axis. Life Sci. 2021, 275, 119288. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.J.; Chen, H.Y.; Ye, Z.; Deng, S.C.; Zhu, S.; Zeng, Z.; He, C.; Liu, M.L.; Huang, K.; Zhong, J.X.; et al. Hypoxia-induced LncRNA-BX111 promotes metastasis and progression of pancreatic cancer through regulating ZEB1 transcription. Oncogene 2018, 37, 5811–5828. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Xie, H.; Duan, L.; Zhao, D.; Ding, J.; Jiang, G. Long Non-Coding RNA CASC9 And HIF-1α Form A Positive Feedback Loop To Facilitate Cell Proliferation And Metastasis In Lung Cancer. OncoTargets Ther. 2019, 12, 9017–9027. [Google Scholar] [CrossRef] [PubMed]
- Sakai, M.; Takahashi, N.; Ikeda, H.; Furutani, Y.; Higuchi, S.; Suzuki, T.; Dohmae, N.; Kobayashi, S.; Harada, H.; Kojima, S.; et al. Design, synthesis, and target identification of new hypoxia-inducible factor 1 (HIF-1) inhibitors containing 1-alkyl-1H-pyrazole-3-carboxamide moiety. Bioorg. Med. Chem. 2021, 46, 116375. [Google Scholar] [CrossRef] [PubMed]
- Newcomb, E.W.; Ali, M.A.; Schnee, T.; Lan, L.; Lukyanov, Y.; Fowkes, M.; Miller, D.C.; Zagzag, D. Flavopiridol downregulates hypoxia-mediated hypoxia-inducible factor-1alpha expression in human glioma cells by a proteasome-independent pathway: Implications for in vivo therapy. Neuro-Oncology 2005, 7, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Greenberger, L.M.; Horak, I.D.; Filpula, D.; Sapra, P.; Westergaard, M.; Frydenlund, H.F.; Albaek, C.; Schrøder, H.; Ørum, H. A RNA antagonist of hypoxia-inducible factor-1alpha, EZN-2968, inhibits tumor cell growth. Mol. Cancer Ther. 2008, 7, 3598–3608. [Google Scholar] [CrossRef]
- Lee, K.; Kim, H.M. A novel approach to cancer therapy using PX-478 as a HIF-1α inhibitor. Arch. Pharmacal Res. 2011, 34, 1583–1585. [Google Scholar] [CrossRef]
- Naik, R.; Han, S.; Lee, K. Chemical biology approach for the development of hypoxia inducible factor (HIF) inhibitor LW6 as a potential anticancer agent. Arch. Pharmacal Res. 2015, 38, 1563–1574. [Google Scholar] [CrossRef]
- Koh, M.Y.; Spivak-Kroizman, T.; Venturini, S.; Welsh, S.; Williams, R.R.; Kirkpatrick, D.L.; Powis, G. Molecular mechanisms for the activity of PX-478, an antitumor inhibitor of the hypoxia-inducible factor-1alpha. Mol. Cancer Ther. 2008, 7, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Jacoby, J.J.; Erez, B.; Korshunova, M.V.; Williams, R.R.; Furutani, K.; Takahashi, O.; Kirkpatrick, L.; Lippman, S.M.; Powis, G.; O’Reilly, M.S.; et al. Treatment with HIF-1α antagonist PX-478 inhibits progression and spread of orthotopic human small cell lung cancer and lung adenocarcinoma in mice. J. Thorac. Oncol. 2010, 5, 940–949. [Google Scholar] [CrossRef]
- Zhu, Y.; Zang, Y.; Zhao, F.; Li, Z.; Zhang, J.; Fang, L.; Li, M.; Xing, L.; Xu, Z.; Yu, J. Inhibition of HIF-1α by PX-478 suppresses tumor growth of esophageal squamous cell cancer in vitro and in vivo. Am. J. Cancer Res. 2017, 7, 1198–1212. [Google Scholar]
- Mangraviti, A.; Raghavan, T.; Volpin, F.; Skuli, N.; Gullotti, D.; Zhou, J.; Asnaghi, L.; Sankey, E.; Liu, A.; Wang, Y.; et al. HIF-1α- Targeting Acriflavine Provides Long Term Survival and Radiological Tumor Response in Brain Cancer Therapy. Sci. Rep. 2017, 7, 14978. [Google Scholar] [CrossRef]
- Deng, X.; Song, Q.; Zhang, Y.; Liu, W.; Hu, H.; Zhang, Y. Tumour microenvironment-responsive nanoplatform based on biodegradable liposome-coated hollow MnO(2) for synergistically enhanced chemotherapy and photodynamic therapy. J. Drug Target. 2022, 30, 334–347. [Google Scholar] [CrossRef]
- McLean, L.; Soto, U.; Agama, K.; Francis, J.; Jimenez, R.; Pommier, Y.; Sowers, L.; Brantley, E. Aminoflavone induces oxidative DNA damage and reactive oxidative species-mediated apoptosis in breast cancer cells. Int. J. Cancer 2008, 122, 1665–1674. [Google Scholar] [CrossRef]
- Terzuoli, E.; Puppo, M.; Rapisarda, A.; Uranchimeg, B.; Cao, L.; Burger, A.M.; Ziche, M.; Melillo, G. Aminoflavone, a ligand of the aryl hydrocarbon receptor, inhibits HIF-1α expression in an AhR-independent fashion. Cancer Res. 2010, 70, 6837–6848. [Google Scholar] [CrossRef]
- Plastino, F.; Santana-Garrido, Á.; Pesce, N.A.; Aronsson, M.; Lardner, E.; Mate, A.; Kvanta, A.; Vázquez, C.M.; André, H. Echinomycin mitigates ocular angiogenesis by transcriptional inhibition of the hypoxia-inducible factor-1. Exp. Eye Res. 2021, 206, 108518. [Google Scholar] [CrossRef]
- Bailey, C.M.; Liu, Y.; Peng, G.; Zhang, H.; He, M.; Sun, D.; Zheng, P.; Liu, Y.; Wang, Y. Liposomal formulation of HIF-1α inhibitor echinomycin eliminates established metastases of triple-negative breast cancer. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102278. [Google Scholar] [CrossRef]
- Vlaminck, B.; Toffoli, S.; Ghislain, B.; Demazy, C.; Raes, M.; Michiels, C. Dual effect of echinomycin on hypoxia-inducible factor-1 activity under normoxic and hypoxic conditions. FEBS J. 2007, 274, 5533–5542. [Google Scholar] [CrossRef]
- Lee, K.; Qian, D.Z.; Rey, S.; Wei, H.; Liu, J.O.; Semenza, G.L. Anthracycline chemotherapy inhibits HIF-1 transcriptional activity and tumor-induced mobilization of circulating angiogenic cells. Proc. Natl. Acad. Sci. USA 2009, 106, 2353–2358. [Google Scholar] [CrossRef] [PubMed]
- Befani, C.D.; Vlachostergios, P.J.; Hatzidaki, E.; Patrikidou, A.; Bonanou, S.; Simos, G.; Papandreou, C.N.; Liakos, P. Bortezomib represses HIF-1α protein expression and nuclear accumulation by inhibiting both PI3K/Akt/TOR and MAPK pathways in prostate cancer cells. J. Mol. Med. 2012, 90, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Shao, Y.; Pan, Y.; Li, Y.; Wang, Y.; Wang, L.; Wang, X.; Shao, K.; Wang, S.; Liu, N.; et al. Total synthesis and biological evaluation of 7-hydroxyneolamellarin A as hypoxia-inducible factor-1α inhibitor for cancer therapy. Bioorg. Med. Chem. Lett. 2021, 50, 128338. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Cui, P.; Yu, M.; Han, J.; Li, H.; Xiu, R. Melatonin modulates the expression of VEGF and HIF-1 alpha induced by CoCl2 in cultured cancer cells. J. Pineal Res. 2008, 44, 121–126. [Google Scholar] [CrossRef]
- Owczarek, A.; Gieczewska, K.B.; Polanska, M.; Paterczyk, B.; Gruza, A.; Winiarska, K. Melatonin Lowers HIF-1α Content in Human Proximal Tubular Cells (HK-2) Due to Preventing Its Deacetylation by Sirtuin 1. Front. Physiol. 2020, 11, 572911. [Google Scholar] [CrossRef]
- Hwang, S.J.; Jung, Y.; Song, Y.S.; Park, S.; Park, Y.; Lee, H.J. Enhanced anti-angiogenic activity of novel melatonin-like agents. J. Pineal Res. 2021, 71, e12739. [Google Scholar] [CrossRef]
- Flannigan, K.L.; Agbor, T.A.; Motta, J.P.; Ferraz, J.G.; Wang, R.; Buret, A.G.; Wallace, J.L. Proresolution effects of hydrogen sulfide during colitis are mediated through hypoxia-inducible factor-1α. FASEB J. 2015, 29, 1591–1602. [Google Scholar] [CrossRef]
- Kai, S.; Tanaka, T.; Daijo, H.; Harada, H.; Kishimoto, S.; Suzuki, K.; Takabuchi, S.; Takenaga, K.; Fukuda, K.; Hirota, K. Hydrogen sulfide inhibits hypoxia- but not anoxia-induced hypoxia-inducible factor 1 activation in a von hippel-lindau- and mitochondria-dependent manner. Antioxid. Redox Signal. 2012, 16, 203–216. [Google Scholar] [CrossRef]
- Lu, S.; Gao, Y.; Huang, X.; Wang, X. GYY4137, a hydrogen sulfide (H2S) donor, shows potent anti-hepatocellular carcinoma activity through blocking the STAT3 pathway. Int. J. Oncol. 2014, 44, 1259–1267. [Google Scholar] [CrossRef]
- Sheng, H.; Huang, Y.; Xiao, Y.; Zhu, Z.; Shen, M.; Zhou, P.; Guo, Z.; Wang, J.; Wang, H.; Dai, W.; et al. ATR inhibitor AZD6738 enhances the antitumor activity of radiotherapy and immune checkpoint inhibitors by potentiating the tumor immune microenvironment in hepatocellular carcinoma. J. Immunother. Cancer 2020, 8, e000340. [Google Scholar] [CrossRef]
- Fallone, F.; Britton, S.; Nieto, L.; Salles, B.; Muller, C. ATR controls cellular adaptation to hypoxia through positive regulation of hypoxia-inducible factor 1 (HIF-1) expression. Oncogene 2013, 32, 4387–4396. [Google Scholar] [CrossRef]
- Gorecki, L.; Andrs, M.; Rezacova, M.; Korabecny, J. Discovery of ATR kinase inhibitor berzosertib (VX-970, M6620): Clinical candidate for cancer therapy. Pharmacol. Ther. 2020, 210, 107518. [Google Scholar] [CrossRef]
- Pires, I.M.; Olcina, M.M.; Anbalagan, S.; Pollard, J.R.; Reaper, P.M.; Charlton, P.A.; McKenna, W.G.; Hammond, E.M. Targeting radiation-resistant hypoxic tumour cells through ATR inhibition. Br. J. Cancer 2012, 107, 291–299. [Google Scholar] [CrossRef]
- Ni, J.; Ni, A. Histone deacetylase inhibitor induced pVHL-independent degradation of HIF-1α and hierarchical quality control of pVHL via chaperone system. PLoS ONE 2021, 16, e0248019. [Google Scholar] [CrossRef]
- Hutt, D.M.; Roth, D.M.; Vignaud, H.; Cullin, C.; Bouchecareilh, M. The histone deacetylase inhibitor, Vorinostat, represses hypoxia inducible factor 1 alpha expression through translational inhibition. PLoS ONE 2014, 9, e106224. [Google Scholar] [CrossRef]
- Bertozzi, D.; Marinello, J.; Manzo, S.G.; Fornari, F.; Gramantieri, L.; Capranico, G. The natural inhibitor of DNA topoisomerase I, camptothecin, modulates HIF-1α activity by changing miR expression patterns in human cancer cells. Mol. Cancer Ther. 2014, 13, 239–248. [Google Scholar] [CrossRef]
- Tian, X.; Nguyen, M.; Foote, H.P.; Caster, J.M.; Roche, K.C.; Peters, C.G.; Wu, P.; Jayaraman, L.; Garmey, E.G.; Tepper, J.E.; et al. CRLX101, a Nanoparticle-Drug Conjugate Containing Camptothecin, Improves Rectal Cancer Chemoradiotherapy by Inhibiting DNA Repair and HIF1α. Cancer Res. 2017, 77, 112–122. [Google Scholar] [CrossRef]
- Conley, S.J.; Baker, T.L.; Burnett, J.P.; Theisen, R.L.; Lazarus, D.; Peters, C.G.; Clouthier, S.G.; Eliasof, S.; Wicha, M.S. CRLX101, an investigational camptothecin-containing nanoparticle-drug conjugate, targets cancer stem cells and impedes resistance to antiangiogenic therapy in mouse models of breast cancer. Breast Cancer Res. Treat. 2015, 150, 559–567. [Google Scholar] [CrossRef]
- Puppo, M.; Battaglia, F.; Ottaviano, C.; Delfino, S.; Ribatti, D.; Varesio, L.; Bosco, M.C. Topotecan inhibits vascular endothelial growth factor production and angiogenic activity induced by hypoxia in human neuroblastoma by targeting hypoxia-inducible factor-1alpha and -2alpha. Mol. Cancer Ther. 2008, 7, 1974–1984. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, Y.; Peng, G.; Liu, N.; Tian, H.; Pan, D.; Liu, L.; Yang, X.; Li, C.; Li, W.; et al. Topotecan prevents hypoxia-induced pulmonary arterial hypertension and inhibits hypoxia-inducible factor-1α and TRPC channels. Int. J. Biochem. Cell Biol. 2018, 104, 161–170. [Google Scholar] [CrossRef]
- Coltella, N.; Valsecchi, R.; Ponente, M.; Ponzoni, M.; Bernardi, R. Synergistic Leukemia Eradication by Combined Treatment with Retinoic Acid and HIF Inhibition by EZN-2208 (PEG-SN38) in Preclinical Models of PML-RARα and PLZF-RARα-Driven Leukemia. Clin. Cancer Res. 2015, 21, 3685–3694. [Google Scholar] [CrossRef]
- Sapra, P.; Kraft, P.; Pastorino, F.; Ribatti, D.; Dumble, M.; Mehlig, M.; Wang, M.; Ponzoni, M.; Greenberger, L.M.; Horak, I.D. Potent and sustained inhibition of HIF-1α and downstream genes by a polyethyleneglycol-SN38 conjugate, EZN-2208, results in anti-angiogenic effects. Angiogenesis 2011, 14, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Valsecchi, R.; Coltella, N.; Magliulo, D.; Bongiovanni, L.; Scarfò, L.; Ghia, P.; Ponzoni, M.; Bernardi, R. EZN-2208 treatment suppresses chronic lymphocytic leukaemia by interfering with environmental protection and increases response to fludarabine. Open Biol. 2020, 10, 190262. [Google Scholar] [CrossRef]
- Duan, J.; Li, Y.; Gao, H.; Yang, D.; He, X.; Fang, Y.; Zhou, G. Phenolic compound ellagic acid inhibits mitochondrial respiration and tumor growth in lung cancer. Food Funct. 2020, 11, 6332–6339. [Google Scholar] [CrossRef]
- Zhu, J.; Su, C.; Chen, Y.; Hao, X.; Jiang, J. Electroacupuncture on ST36 and GB39 Acupoints Inhibits Synovial Angiogenesis via Downregulating HIF-1α/VEGF Expression in a Rat Model of Adjuvant Arthritis. Evid.-Based Complement. Altern. Med. 2019, 2019, 5741931. [Google Scholar] [CrossRef]
- Su, S.; Dou, H.; Wang, Z.; Zhang, Q. Bufalin inhibits ovarian carcinoma via targeting mTOR/HIF-α pathway. Basic Clin. Pharmacol. Toxicol. 2021, 128, 224–233. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, C.; Xu, L.; Zang, K.; Ning, Z.; Jiang, F.; Chi, H.; Zhu, X.; Meng, Z. Bufalin suppresses hepatocellular carcinoma invasion and metastasis by targeting HIF-1α via the PI3K/AKT/mTOR pathway. Oncotarget 2016, 7, 20193–20208. [Google Scholar] [CrossRef]
- Kocemba-Pilarczyk, K.A.; Ostrowska, B.; Trojan, S.; Aslan, E.; Kusior, D.; Lasota, M.; Lenouvel, C.; Dulińska-Litewka, J. Targeting the hypoxia pathway in malignant plasma cells by using 17-allylamino-17-demethoxygeldanamycin. Acta Biochim. Pol. 2018, 65, 101–109. [Google Scholar] [CrossRef]
- Nagaraju, G.P.; Park, W.; Wen, J.; Mahaseth, H.; Landry, J.; Farris, A.B.; Willingham, F.; Sullivan, P.S.; Proia, D.A.; El-Hariry, I.; et al. Antiangiogenic effects of ganetespib in colorectal cancer mediated through inhibition of HIF-1α and STAT-3. Angiogenesis 2013, 16, 903–917. [Google Scholar] [CrossRef]
- Zhang, P.C.; Liu, X.; Li, M.M.; Ma, Y.Y.; Sun, H.T.; Tian, X.Y.; Wang, Y.; Liu, M.; Fu, L.S.; Wang, Y.F.; et al. AT-533, a novel Hsp90 inhibitor, inhibits breast cancer growth and HIF-1α/VEGF/VEGFR-2-mediated angiogenesis in vitro and in vivo. Biochem. Pharmacol. 2020, 172, 113771. [Google Scholar] [CrossRef]
- Lee, S.H.; Jee, J.G.; Bae, J.S.; Liu, K.H.; Lee, Y.M. A group of novel HIF-1α inhibitors, glyceollins, blocks HIF-1α synthesis and decreases its stability via inhibition of the PI3K/AKT/mTOR pathway and Hsp90 binding. J. Cell. Physiol. 2015, 230, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, X.; Lu, H.; Fan, Z. 1, 9-Pyrazoloanthrones downregulate HIF-1α and sensitize cancer cells to cetuximab-mediated anti-EGFR therapy. PLoS ONE 2010, 5, e15823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.; Li, X.; Luo, Z.; Liu, J.; Fan, Z. Cetuximab reverses the Warburg effect by inhibiting HIF-1-regulated LDH-A. Mol. Cancer Ther. 2013, 12, 2187–2199. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kang, J.E.; Park, S.K.; Jin, Y.; Chung, K.S.; Kim, H.M.; Lee, K.; Kang, M.R.; Lee, M.K.; Song, K.B.; et al. LW6, a novel HIF-1 inhibitor, promotes proteasomal degradation of HIF-1α via upregulation of VHL in a colon cancer cell line. Biochem. Pharmacol. 2010, 80, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Al-Hashem, F.; Al-Humayed, S.; Amin, S.N.; Kamar, S.S.; Mansy, S.S.; Hassan, S.; Abdel-Salam, L.O.; Ellatif, M.A.; Alfaifi, M.; Haidara, M.A.; et al. Metformin inhibits mTOR-HIF-1α axis and profibrogenic and inflammatory biomarkers in thioacetamide-induced hepatic tissue alterations. J. Cell. Physiol. 2019, 234, 9328–9337. [Google Scholar] [CrossRef]
- Kang, Y.T.; Hsu, W.C.; Ou, C.C.; Tai, H.C.; Hsu, H.T.; Yeh, K.T.; Ko, J.L. Metformin Mitigates Nickel-Elicited Angiopoietin-Like Protein 4 Expression via HIF-1α for Lung Tumorigenesis. Int. J. Mol. Sci. 2020, 21, 619. [Google Scholar] [CrossRef]
- Lei, R.; Zhang, S.; Wang, Y.; Dai, S.; Sun, J.; Zhu, C. Metformin Inhibits Epithelial-to-Mesenchymal Transition of Keloid Fibroblasts via the HIF-1α/PKM2 Signaling Pathway. Int. J. Med. Sci. 2019, 16, 960–966. [Google Scholar] [CrossRef]
- Shao, S.; Zhao, L.; An, G.; Zhang, L.; Jing, X.; Luo, M.; Li, W.; Meng, D.; Ning, Q.; Zhao, X.; et al. Metformin suppresses HIF-1α expression in cancer-associated fibroblasts to prevent tumor-stromal cross talk in breast cancer. FASEB J. 2020, 34, 10860–10870. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, L.; Zhang, Y.; Chen, W.; Liu, D.; Hou, H.; Ding, L.; Li, X. Ginsenoside 20(S)-Rg3 targets HIF-1α to block hypoxia-induced epithelial-mesenchymal transition in ovarian cancer cells. PLoS ONE 2014, 9, e103887. [Google Scholar] [CrossRef]
- Viziteu, E.; Grandmougin, C.; Goldschmidt, H.; Seckinger, A.; Hose, D.; Klein, B.; Moreaux, J. Chetomin, targeting HIF-1α/p300 complex, exhibits antitumour activity in multiple myeloma. Br. J. Cancer 2016, 114, 519–523. [Google Scholar] [CrossRef]
- Reece, K.M.; Richardson, E.D.; Cook, K.M.; Campbell, T.J.; Pisle, S.T.; Holly, A.J.; Venzon, D.J.; Liewehr, D.J.; Chau, C.H.; Price, D.K.; et al. Epidithiodiketopiperazines (ETPs) exhibit in vitro antiangiogenic and in vivo antitumor activity by disrupting the HIF-1α/p300 complex in a preclinical model of prostate cancer. Mol. Cancer 2014, 13, 91. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, R.; Zhao, R.; Chen, G.; Cai, Y.; Jin, J. A novel function of novobiocin: Disrupting the interaction of HIF 1α and p300/CBP through direct binding to the HIF1α C-terminal activation domain. PLoS ONE 2013, 8, e62014. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.M.; Shen, G.; Neckers, L.; Blake, H.; Holzbeierlein, J.; Cronk, B.; Blagg, B.S. Hsp90 inhibitors identified from a library of novobiocin analogues. J. Am. Chem. Soc. 2005, 127, 12778–12779. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Zhang, Y.; Ding, Y.; Huang, J.; Yao, J.; Xie, Z.; Lv, Y.; Zuo, J. Regulating the Expression of HIF-1α or lncRNA: Potential Directions for Cancer Therapy. Cells 2022, 11, 2811. https://doi.org/10.3390/cells11182811
Zhang M, Zhang Y, Ding Y, Huang J, Yao J, Xie Z, Lv Y, Zuo J. Regulating the Expression of HIF-1α or lncRNA: Potential Directions for Cancer Therapy. Cells. 2022; 11(18):2811. https://doi.org/10.3390/cells11182811
Chicago/Turabian StyleZhang, Minghui, Yu Zhang, Yubo Ding, Jialu Huang, Jingwei Yao, Zhuoyi Xie, Yufan Lv, and Jianhong Zuo. 2022. "Regulating the Expression of HIF-1α or lncRNA: Potential Directions for Cancer Therapy" Cells 11, no. 18: 2811. https://doi.org/10.3390/cells11182811
APA StyleZhang, M., Zhang, Y., Ding, Y., Huang, J., Yao, J., Xie, Z., Lv, Y., & Zuo, J. (2022). Regulating the Expression of HIF-1α or lncRNA: Potential Directions for Cancer Therapy. Cells, 11(18), 2811. https://doi.org/10.3390/cells11182811





