Pathophysiology and Therapy of Associated Features of Migraine
Abstract
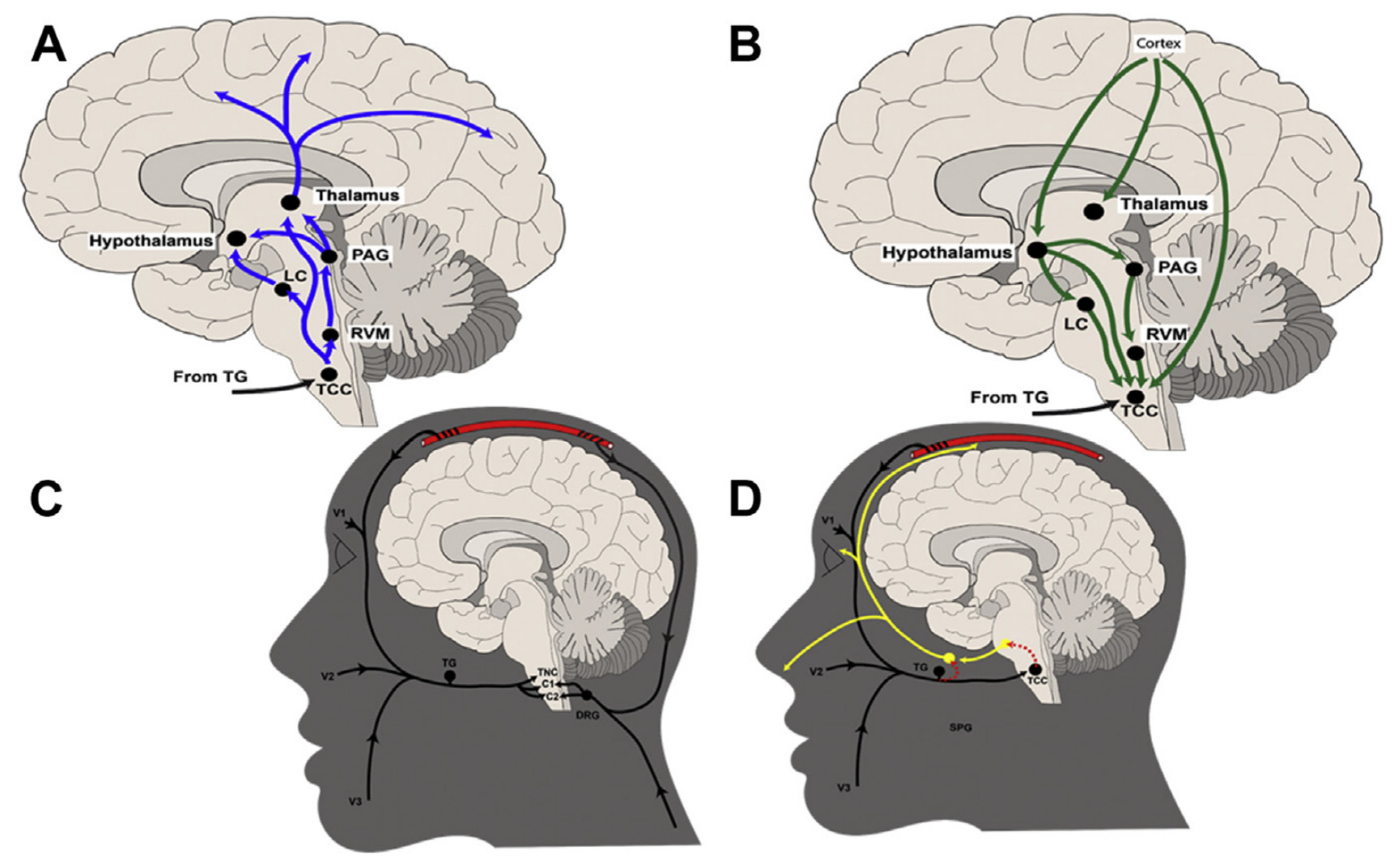
1. Introduction
2. Nausea and Vomiting
2.1. Nausea in Migraine and Conditions Related to Migraine
2.1.1. Cyclic Vomiting Syndrome
2.1.2. Motion Sickness
2.1.3. Pregnancy
2.2. Neuroanatomy and Neuropharmacology
2.3. Treatment of Nausea
2.3.1. Serotonin
2.3.2. Dopamine
2.3.3. Substance P
3. Osmophobia
4. Neuro-Otological Manifestations
4.1. Phonophobia
4.2. Vertigo
4.3. Allodynia
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Willis, T.; Pordage, S. Two Discourses Concerning the Soul of Brutes, Which Is That of the Vital and Sensitive of Man: The First Is Physiological, Shewing the Nature, Parts, Powers, and Affections of the Same; and the Other Is Pathological, Which Unfolds the Diseases which Affect It and Its Primary Seat, to Wit, the Brain and Nervous Stock, and Treats of Their Cures: With Copper Cuts; Thomas Dring, Ch. Harper and John Leigh: London, UK, 1683. [Google Scholar]
- Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [CrossRef] [PubMed]
- Gowers, W.R. A Manual of Diseases of the Nervous System, 3rd ed.; P. Blakiston, Son & Co.: Philadelphia, PA, USA, 1899; p. 1357. [Google Scholar]
- Goadsby, P.; Holland, P.; Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol. Rev. 2017, 97, 553–622. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Suzuki, S.; Shiina, T.; Okamura, M.; Haruyama, Y.; Tatsumoto, M.; Hirata, K. Investigating the relationships between the burden of multiple sensory hypersensitivity symptoms and headache-related disability in patents with migraine. J. Headache Pain 2021, 22, 77. [Google Scholar] [CrossRef] [PubMed]
- Lévêque, Y.; Masson, R.; Fornoni, L.; Moulin, A.; Bidet-Caulet, A.; Caclin, A.; Demarquay, G. Self-perceived attention difficulties are associated with sensory hypersensitivity in migraine. Rev. Neurol. 2020, 176, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Danno, D.; Wolf, J.; Ishizaki, K.; Kikui, S.; Hirata, K.; Takeshima, T. Cranial autonomic symptoms in migraine are related to central sensitization: A prospective study of 164 migraine patients at a tertiary headache center. BMC Neurol. 2022, 22, 89. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.-L.H.; Wang, Y.-F.; Ling, Y.-H.; Lai, K.-L.; Chen, S.-P.; Chen, W.-T.; Treede, R.-D.; Wang, S.-J. Pain sensitivities predict prophylactic treatment outcomes of flunarizine in chronic migraine patients: A prospective study. Cephalalgia 2022, 11, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Stubberud, A.; Buse, D.C.; Kristoffersen, E.S.; Linde, M.; Tronvik, E. Is there a causal relationship between stress and migraine? Current evidence and implications for management. J. Headache Pain 2021, 22, 155. [Google Scholar] [CrossRef]
- Westgate, C.S.J.; Israelsen, I.M.E.; Jensen, R.H.; Eftekhari, S. Understanding the link between obesity and headache- with focus on migraine and idiopathic intracranial hypertension. J. Headache Pain 2021, 22, 123. [Google Scholar] [CrossRef]
- Kang, L.; Tang, W.; Zhang, Y.; Zhang, M.; Liu, J.; Li, Y.; Kong, S.; Zhao, D.; Yu, S. The gut microbiome modulates nitroglycerin-induced migraine-related hyperalgesia in mice. Cephalalgia 2022, 42, 490–499. [Google Scholar] [CrossRef]
- Raibin, K.; Markus, T.E. Cutaneous allodynia in pediatric and adolescent patients and their mothers: A comparative study. Cephalalgia 2022, 42, 579–589. [Google Scholar] [CrossRef]
- Villar-Martinez, M.D.; Goadsby, P.J. Dim the Lights: A Narrative Review of Photophobia in Migraine. TouchREVIEWS Neurol. 2022, 18, 14–21. [Google Scholar] [CrossRef]
- Lipton, R.B.; Buse, D.C.; Saiers, J.; Serrano, D.; Reed, M.L. Healthcare resource utilization and direct costs associated with frequent nausea in episodic migraine: Results from the American Migraine Prevalence and Prevention (AMPP) Study. J. Med. Econ. 2013, 16, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Gajria, K.; Lee, L.K.; Flores, N.M.; Aycardi, E.; Gandhi, S.K. Humanistic and economic burden of nausea and vomiting among migraine sufferers. J. Pain Res. 2017, 10, 689–698. [Google Scholar] [CrossRef]
- Munjal, S.; Singh, P.; Reed, M.L.; Fanning, K.; Schwedt, T.J.; Dodick, D.W.; Buse, D.C.; Lipton, R.B. Most Bothersome Symptom in Persons with Migraine: Results from the Migraine in America Symptoms and Treatment (MAST) Study. Headache 2020, 60, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Lipton, R.B.; Buse, D.C.; Saiers, J.; Fanning, K.M.; Serrano, D.; Reed, M.L. Frequency and burden of headache-related nausea: Results from the American Migraine Prevalence and Prevention (AMPP) study. Headache 2013, 53, 93–103. [Google Scholar] [CrossRef]
- Reed, M.L.; Fanning, K.M.; Serrano, D.; Buse, D.C.; Lipton, R.B. Persistent frequent nausea is associated with progression to chronic migraine: AMPP study results. Headache 2015, 55, 76–87. [Google Scholar] [CrossRef]
- Kovacic, K.; Li, B.U.K. Cyclic vomiting syndrome: A narrative review and guide to management. Headache 2021, 61, 231–243. [Google Scholar] [CrossRef]
- Li, B.U.; Murray, R.D.; Heitlinger, L.A.; Robbins, J.L.; Hayes, J.R. Is cyclic vomiting syndrome related to migraine? J. Pediatr. 1999, 134, 567–572. [Google Scholar] [CrossRef]
- Abell, T.L.; Adams, K.A.; Boles, R.G.; Bousvaros, A.; Chong, S.K.F.; Fleisher, D.R.; Hasler, W.L.; Hyman, P.E.; Issenman, R.M.; Li, B.U.K.; et al. Cyclic vomiting syndrome in adults. Neurogastroenterol. Motil. 2008, 20, 269–284. [Google Scholar] [CrossRef]
- Ellingsen, D.-M.; Garcia, R.G.; Lee, J.; Lin, R.L.; Kim, J.; Thurler, A.H.; Castel, S.; Dimisko, L.; Rosen, B.R.; Hadjikhani, N.; et al. Cyclic Vomiting Syndrome is characterized by altered functional brain connectivity of the insular cortex: A cross-comparison with migraine and healthy adults. Neurogastroenterol. Motil. 2017, 29, e13004. [Google Scholar] [CrossRef] [PubMed]
- Sticht, M.A.; Limebeer, C.L.; Rafla, B.R.; Abdullah, R.A.; Poklis, J.L.; Ho, W.; Niphakis, M.J.; Cravatt, B.F.; Sharkey, K.A.; Lichtman, A.H.; et al. Endocannabinoid regulation of nausea is mediated by 2-arachidonoylglycerol (2-AG) in the rat visceral insular cortex. Neuropharmacology 2016, 102, 92–102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Perisetti, A.; Goyal, H. Endocannabinoid system and cannabis hyperemesis syndrome: A narrative update. Eur. J. Gastroenterol. Hepatol. 2022, 34, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, C.J.; DeSanto, K.; Borgelt, L.; Phillips, K.T.; Monte, A.A. Cannabinoid Hyperemesis Syndrome: Diagnosis, Pathophysiology, and Treatment-a Systematic Review. J. Med. Toxicol. 2017, 13, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, T.; Levinthal, D.; Tarbell, S.E.; Jaradeh, S.S.; Hasler, W.L.; Issenman, R.M.; Adams, K.A.; Sarosiek, I.; Stave, C.; Sharaf, R.N.; et al. Guidelines on management of cyclic vomiting syndrome in adults by the American Neurogastroenterology and Motility Society and the Cyclic Vomiting Syndrome Association. Neurogastroenterol. Motil. 2019, 2 (Suppl. S2), e13604. [Google Scholar] [CrossRef]
- Abouzari, M.; Cheung, D.; Pham, T.; Goshtasbi, K.; Sarna, B.; Tajran, S.; Sahyouni, S.; Lin, H.W.; Djalilian, H.R. The Relationship between Vestibular Migraine and Motion Sickness Susceptibility. Otol. Neurotol. 2020, 41, 1116–1121. [Google Scholar] [CrossRef]
- Kayan, A.; Hood, J.D. Neuro-otological manifestations of migraine. Brain 1984, 107 Pt 4, 1123–1142. [Google Scholar] [CrossRef]
- Marcus, D.A.; Furman, J.M. Prevention of motion sickness with rizatriptan: A double-blind, placebo-controlled pilot study. Med. Sci. Monit. 2006, 12, PI1-7. [Google Scholar]
- Drummond, P.D.; Granston, A. Facial pain increases nausea and headache during motion sickness in migraine sufferers. Brain 2004, 127 Pt 3, 526–534. [Google Scholar] [CrossRef]
- Drummond, P.D.; Granston, A. Painful stimulation of the temple induces nausea, headache and extracranial vasodilation in migraine sufferers. Cephalalgia 2005, 25, 16–22. [Google Scholar] [CrossRef]
- Stadler, M.; Bardiau, F.; Seidel, L.; Albert, A.; Boogaerts, J.G. Difference in risk factors for postoperative nausea and vomiting. Anesthesiology 2003, 98, 46–52. [Google Scholar] [CrossRef]
- Khoronenko, V.E.; Baskakov, D.S. Role of migraine history in the development of postoperative nausea and vomiting in patients undergoing general and combined general-epidural anesthesia. Anesteziol. Reanimatol. 2014, 2, 41–44. [Google Scholar]
- Apfel, C.C.; Kranke, P.; Eberhart, L.H. Comparison of surgical site and patient’s history with a simplified risk score for the prediction of postoperative nausea and vomiting. Anaesthesia 2004, 59, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Heinrichs, L. Linking olfaction with nausea and vomiting of pregnancy, recurrent abortion, hyperemesis gravidarum, and migraine headache. Am. J. Obstet. Gynecol. 2002, 186 (Suppl. S5), 123053. [Google Scholar] [CrossRef] [PubMed]
- Hirst, B.; Noble, B. Migraine as a cause of persistent nausea or vomiting in palliative care: A case series. J. Pain Symptom Manag. 2009, 37, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Sicuteri, F. Dopamine, the second putative protagonist in headache. Headache 1977, 17, 129–131. [Google Scholar] [CrossRef]
- Bes, A.; Dupui, P.; Guell, A.; Bessoles, G.; Geraud, G. Pharmacological exploration of dopamine hypersensitivity in migraine patients. Int. J. Clin. Pharmacol. Res. 1986, 6, 189–192. [Google Scholar]
- Calabresi, P.; Silvestrini, M.; Stratta, F.; Cupini, L.M.; Argiro, G.; Atzei, G.P.; Bernardi, G. l-deprenyl test in migraine: Neuroendocrinological aspects. Cephalalgia 1993, 13, 406–409. [Google Scholar] [CrossRef]
- Cerbo, R.; Barbanti, P.; Buzzi, M.G.; Fabbrini, G.; Brusa, L.; Roberti, C.; Zanette, E.; Lenzi, G.L. Dopamine hypersensitivity in migraine: Role of the apomorphine test. Clin. Neuropharmacol. 1997, 20, 36–41. [Google Scholar] [CrossRef]
- Del Zompo, M.; Cherchi, A.; Palmas, M.A.; Ponti, M.; Bocchetta, A.; Gessa, G.L.; Piccardi, M.P. Association between dopamine receptor genes and migraine without aura in a Sardinian sample. Neurology 1998, 51, 781–786. [Google Scholar] [CrossRef]
- Charbit, A.R.; Akerman, S.; Goadsby, P.J. Dopamine: What’s new in migraine? Curr. Opin. Neurol. 2010, 23, 275–281. [Google Scholar] [CrossRef]
- Walstab, J.; Krüger, D.; Stark, T.; Hofmann, T.; Demir, I.E.; Ceyhan, G.O.; Feistel, B.; Schemann, M.; Niesler, B. Ginger and its pungent constituents non-competitively inhibit activation of human recombinant and native 5-HT3 receptors of enteric neurons. Neurogastroenterol. Motil. 2013, 25, 439–447, e302. [Google Scholar] [CrossRef] [PubMed]
- Malick, A.; Jakubowski, M.; Elmquist, J.K.; Saper, C.B.; Burstein, R. A neurohistochemical blueprint for pain-induced loss of appetite. Proc. Natl. Acad. Sci. USA 2001, 98, 9930–9935. [Google Scholar] [CrossRef] [PubMed]
- Giffin, N.; Ruggiero, L.; Lipton, R.; Silberstein, S.; Tvedskov, J.F.; Olesen, J.; Altman, J.; Goadsby, P.; Macrae, A. Premonitory symptoms in migraine: An electronic diary study. Neurology 2003, 60, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Karsan, N.; Bose, P.R.; Thompson, C.; Newman, J.; Goadsby, P.J. Headache and non-headache symptoms provoked by nitroglycerin in migraineurs: A human pharmacological triggering study. Cephalalgia 2020, 40, 828–841. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.; Gonsalves, S.; Fossa, A.; McLean, S.; Seeger, T.; Obach, S.; Andrews, P. The anti-emetic effects of CP-99,994 in the ferret and the dog: Role of the NK1 receptor. Br. J. Pharmacol. 1995, 115, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Saria, A. The tachykinin NK1 receptor in the brain: Pharmacology and putative functions. Eur. J. Pharmacol. 1999, 375, 51–60. [Google Scholar] [CrossRef]
- McLean, S.; Ganong, A.H.; Seeger, T.F.; Bryce, D.K.; Pratt, K.G.; Reynolds, L.S.; Siok, C.J.; Lowe, J.A.; Heym, J. Activity and distribution of binding sites in brain of a nonpeptide substance P (NK1) receptor antagonist. Science 1991, 251, 437–439. [Google Scholar] [CrossRef]
- Robinson, B.W.; Mishkin, M. Alimentary responses to forebrain stimulation in monkeys. Exp. Brain Res. 1968, 4, 330–366. [Google Scholar] [CrossRef]
- Grélot, L.; Milano, S.; Portillo, F.; Miller, A.D.; Bianchi, A.L. Membrane potential changes of phrenic motoneurons during fictive vomiting, coughing, and swallowing in the decerebrate cat. J. Neurophysiol. 1992, 68, 2110–2119. [Google Scholar] [CrossRef]
- Miller, A.D.; Nonaka, S.; Jakus, J. Brain areas essential or non-essential for emesis. Brain Res. 1994, 647, 255–264. [Google Scholar] [CrossRef]
- Maniyar, F.H.; Sprenger, T.; Schankin, C.; Goadsby, P.J. The origin of nausea in migraine-a PET study. J. Headache Pain 2014, 15, 84. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Holland, P.R. An Update: Pathophysiology of Migraine. Neurol. Clin. 2019, 37, 651–671. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.A.; Lu, G.; Wang, S.; Schweder, P.M.; Hyam, J.A.; Stein, J.F.; Paterson, D.J.; Aziz, T.Z.; Green, A.L. Ventral periaqueductal grey stimulation alters heart rate variability in humans with chronic pain. Exp. Neurol. 2010, 223, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Green, A.L.; Hyam, J.A.; Williams, C.; Wang, S.; Shlugman, D.; Stein, J.F.; Paterson, D.J.; Aziz, T.Z. Intra-operative deep brain stimulation of the periaqueductal grey matter modulates blood pressure and heart rate variability in humans. Neuromodulation 2010, 13, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Huang, Y.; Green, A.L.; Aziz, T.Z.; Xiao, X.; Wang, S. Neurophysiological characteristics in the periventricular/periaqueductal gray correlate with pain perception, sensation, and affect in neuropathic pain patients. Neuroimage Clin. 2021, 32, 102876. [Google Scholar] [CrossRef]
- Aguiar, D.C.; Almeida-Santos, A.F.; Moreira, F.A.; Guimaraes, F.S. Involvement of TRPV1 channels in the periaqueductal grey on the modulation of innate fear responses. Acta Neuropsychiatr. 2015, 27, 97–105. [Google Scholar] [CrossRef]
- McGovern, A.E.; Ajayi, I.E.; Farrell, M.J.; Mazzone, S.B. A neuroanatomical framework for the central modulation of respiratory sensory processing and cough by the periaqueductal grey. J. Thorac. Dis. 2017, 9, 4098–4107. [Google Scholar] [CrossRef]
- Subramanian, H.H.; Balnave, R.J.; Holstege, G. The midbrain periaqueductal gray control of respiration. J. Neurosci. 2008, 28, 12274–12283. [Google Scholar] [CrossRef]
- Oliveras, J.L.; Besson, J.M.; Guilbaud, G.; Liebeskind, J.C. Behavioral and electrophysiological evidence of pain inhibition from midbrain stimulation in the cat. Exp. Brain Res. 1974, 20, 32–44. [Google Scholar] [CrossRef]
- Makovac, E.; Venezia, A.; Hohenschurz-Schmidt, D.; Dipasquale, O.; Jackson, J.B.; Medina, S.; O’Daly, O.; Williams, S.C.R.; McMahon, S.B.; Howard, M.A. The association between pain-induced autonomic reactivity and descending pain control is mediated by the periaqueductal grey. J. Physiol. 2021, 599, 5243–5260. [Google Scholar] [CrossRef]
- Wu, D.; Wang, S.; Stein, J.F.; Aziz, T.Z.; Green, A.L. Reciprocal interactions between the human thalamus and periaqueductal gray may be important for pain perception. Exp. Brain Res. 2014, 232, 527–534. [Google Scholar] [CrossRef]
- Lau, B.K.; Winters, B.L.; Vaughan, C.W. Opioid presynaptic disinhibition of the midbrain periaqueductal grey descending analgesic pathway. Br. J. Pharmacol. 2020, 177, 2320–2332. [Google Scholar] [CrossRef] [PubMed]
- Hyland, N.P.; Abrahams, T.P.; Fuchs, K.; Burmeister, M.A.; Hornby, P.J. Organization and neurochemistry of vagal preganglionic neurons innervating the lower esophageal sphincter in ferrets. J. Comp. Neurol. 2001, 430, 222–234. [Google Scholar] [CrossRef]
- Kannan, H.; Yamashita, H. Connections of neurons in the region of the nucleus tractus solitarius with the hypothalamic paraventricular nucleus: Their possible involvement in neural control of the cardiovascular system in rats. Brain Res. 1985, 329, 205–212. [Google Scholar] [CrossRef]
- Miller, A.D.; Leslie, R.A. The area postrema and vomiting. Front. Neuroendocrinol. 1994, 15, 301–320. [Google Scholar] [CrossRef]
- Hyde, T.M.; Knable, M.B.; Murray, A.M. Distribution of dopamine D1–D4 receptor subtypes in human dorsal vagal complex. Synapse 1996, 24, 224–232. [Google Scholar] [CrossRef]
- Jeong, J.K.; Dow, S.A.; Young, C.N. Sensory Circumventricular Organs, Neuroendocrine Control, and Metabolic Regulation. Metabolites 2021, 11, 494. [Google Scholar] [CrossRef]
- Stafanini, E.; Clement-Cormier, Y. Detection of dopamine receptors in the area postrema. Eur. J. Pharmacol. 1981, 74, 257–260. [Google Scholar] [CrossRef]
- Amin, A.H.; Crawford, T.B.; Gaddum, J.H. The distribution of substance P and 5-hydroxytryptamine in the central nervous system of the dog. J. Physiol. 1954, 126, 596–618. [Google Scholar] [CrossRef]
- Lipton, R.B.; Schmidt, P.; Diener, H.C. Post Hoc Subanalysis of Two Randomized, Controlled Phase 3 Trials Evaluating Diclofenac Potassium for Oral Solution: Impact of Migraine-Associated Nausea and Prior Triptan Use on Efficacy. Headache 2017, 57, 756–765. [Google Scholar] [CrossRef]
- Chan, T.L.H.; Cowan, R.P.; Woldeamanuel, Y.W. Calcitonin Gene-Related Peptide Receptor Antagonists (Gepants) for the Acute Treatment of Nausea in Episodic Migraine: A Systematic Review and Meta-Analysis. Headache 2020, 60, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Duthie, D.J.; Nimmo, W.S. Adverse effects of opioid analgesic drugs. Br. J. Anaesth. 1987, 59, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Bigal, M.E.; Serrano, D.; Buse, D.; Scher, A.; Stewart, W.F.; Lipton, R.B. Acute migraine medications and evolution from episodic to chronic migraine: A longitudinal population-based study. Headache 2008, 48, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.W.; Rodgers, A.; Bigal, M.E. Impact of recent prior opioid use on rizatriptan efficacy. A post hoc pooled analysis. Headache 2009, 49, 395–403. [Google Scholar] [CrossRef]
- Bonafede, M.; Wilson, K.; Xue, F. Long-term treatment patterns of prophylactic and acute migraine medications and incidence of opioid-related adverse events in patients with migraine. Cephalalgia 2019, 39, 1086–1098. [Google Scholar] [CrossRef]
- Lipton, R.B.; Pascual, J.; Goadsby, P.J.; Massiou, H.; McCarroll, K.A.; Vandormael, K.; Jiang, K.; Lines, C.R. Effect of rizatriptan and other triptans on the nausea symptom of migraine: A post hoc analysis. Headache 2001, 41, 754–763. [Google Scholar] [CrossRef]
- Freitag, F.; Taylor, F.R.; Hamid, M.A.; Ms, A.R.; Hustad, C.M.; Ramsey, K.E.; Skobieranda, F. Elimination of migraine-associated nausea in patients treated with rizatriptan orally disintegrating tablet (ODT): A randomized, double-blind, placebo-controlled study. Headache 2008, 48, 368–377. [Google Scholar] [CrossRef]
- Pierce, M. Oral triptans and nausea: Treatment considerations in migraine. Headache 2013, 1, 17–20. [Google Scholar] [CrossRef]
- Rowat, B.M.; Merrill, C.F.; Davis, A.; South, V. A double-blind comparison of granisetron and placebo for the treatment of acute migraine in the emergency department. Cephalalgia 1991, 11, 207–213. [Google Scholar] [CrossRef]
- Abiri, S.; Chegin, M.; Soleimani, R.; Hatami, N.; Kalani, N.; Rayatdoost, E. Propofol + Granisetron vs. Propofol + Metoclopramide in Symptom Management of Acute Migraine Headache; a Double-Blind Randomized Clinical Trial. Arch. Acad. Emerg. Med. 2022, 10, e19. [Google Scholar]
- Chen, L.; Cai, Z. The efficacy of ginger for the treatment of migraine: A meta-analysis of randomized controlled studies. Am. J. Emerg. Med. 2021, 46, 567–571. [Google Scholar] [CrossRef]
- Maghbooli, M.; Golipour, F.; Moghimi Esfandabadi, A.; Yousefi, M. Comparison between the efficacy of ginger and sumatriptan in the ablative treatment of the common migraine. Phytother. Res. 2014, 28, 412–415. [Google Scholar] [CrossRef]
- Sweetman, S.C. Martindale: The Complete Drug Reference, 36th ed.; Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- Jolliet, P.; Nion, S.; Allain-Veyrac, G.; Tilloy-Fenart, L.; Vanuxeem, D.; Berezowski, V.; Cecchelli, R. Evidence of lowest brain penetration of an antiemetic drug, metopimazine, compared to domperidone, metoclopramide and chlorpromazine, using an in vitro model of the blood-brain barrier. Pharmacol. Res. 2007, 56, 11–17. [Google Scholar] [CrossRef]
- Volans, G.N. The effect of metoclopramide on the absorption of effervescent aspirin in migraine. Br. J. Clin. Pharmacol. 1975, 2, 57–63. [Google Scholar] [CrossRef]
- Eken, C. Critical reappraisal of intravenous metoclopramide in migraine attack: A systematic review and meta-analysis. Am. J. Emerg. Med. 2015, 33, 331–337. [Google Scholar] [CrossRef]
- Aydin, H.D.; Vuralli, D.; Akçali, D.T.; Bolay, H. Metoclopramide inhibits trigeminovascular activation:evidence for effective acute attack treatment in migraine. Turk. J. Med. Sci. 2017, 47, 343–347. [Google Scholar] [CrossRef]
- Soltani, K.M.; Motamed, H.; Eslami, K.; Majdinasab, N.; Kouti, L. Randomised trial of IV metoclopramide vs. IV ketorolac in treatment of acute primary headaches. Am. J. Emerg. Med. 2021, 50, 376–380. [Google Scholar] [CrossRef]
- Doğan, N.; Pekdemir, M.; Yılmaz, S.; Yaka, E.; Karadaş, A.; Durmuş, U.; Avcu, N.; Koçkan, E. Intravenous metoclopramide in the treatment of acute migraines: A randomized, placebo-controlled trial. Acta Neurol. Scand. 2019, 139, 334–339. [Google Scholar] [CrossRef]
- Lapierre, J.; Amin, M.; Hattangadi, S. Prochlorperazine—A review of the literature since 1956. Can. Psychiatr. Assoc. J. 1969, 14, 267–274. [Google Scholar] [CrossRef]
- Golikhatir, I.; Cheraghmakani, H.; Bozorgi, F.; Jahanian, F.; Sazgar, M.; Montazer, S.H. The Efficacy and Safety of Prochlorperazine in Patients with Acute Migraine: A Systematic Review and Meta-Analysis. Headache 2019, 59, 682–700. [Google Scholar] [CrossRef]
- Bigal, M.E.; Bordini, C.A.; Speciali, J.G. Intravenous chlorpromazine in the emergency department treatment of migraines: A randomized controlled trial. J. Emerg. Med. 2002, 23, 141–148. [Google Scholar] [CrossRef]
- Chou, D.E.; Tso, A.R.; Goadsby, P.J. Aprepitant for the management of nausea with inpatient IV dihydroergotamine. Neurology 2016, 87, 1613–1616. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, L.V.; Souza, F.H.; Brunetto, A.T.; Sasse, A.D.; da Silveira Nogueira Lima, J.P. Neurokinin-1 receptor antagonists for chemotherapy-induced nausea and vomiting: A systematic review. J. Natl. Cancer Inst. 2012, 104, 1280–1292. [Google Scholar] [CrossRef]
- Douek, E.E. Smell: Recent theories and their clinical application. J. Laryngol. Otol. 1967, 81, 431–439. [Google Scholar] [CrossRef]
- Courteau, J.P.; Cushman, R.; Bouchard, F.; Quevillon, M.; Chartrand, A.; Bherer, L. Survey of construction workers repeatedly exposed to chlorine over a three to six month period in a pulpmill: I. Exposure and symptomatology. Occup. Environ. Med. 1994, 51, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Peatfield, R.C. Relationships between food, wine, and beer-precipitated migrainous headaches. Headache 1995, 35, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Wantke, F.; Focke, M.; Hemmer, W.; Bracun, R.; Wolf-Abdolvahab, S.; Gotz, M.; Jarisch, R.; Tschabitscher, M.; Gann, M.; Tappler, P.; et al. Exposure to formaldehyde and phenol during an anatomy dissecting course: Sensitizing potency of formaldehyde in medical students. Allergy 2000, 55, 84–87. [Google Scholar] [CrossRef]
- Kelman, L. The triggers or precipitants of the acute migraine attack. Cephalalgia 2007, 27, 394–402. [Google Scholar] [CrossRef]
- Barrett, S.A.; Gifford, E.W. Miwok Material Culture. Bull. Public Mus. City Milwaukee 1933, 2, 117–376. [Google Scholar]
- Benemei, S.; Appendino, G.; Geppetti, P. Pleasant natural scent with unpleasant effects: Cluster headache-like attacks triggered by Umbellularia californica. Cephalalgia 2010, 30, 744–746. [Google Scholar] [CrossRef]
- Nassini, R.; Materazzi, S.; Vriens, J.; Prenen, J.; Benemei, S.; De Siena, G.; La Marca, G.; Andrè, E.; Preti, D.; Avonto, C.; et al. The ‘headache tree’ via umbellulone and TRPA1 activates the trigeminovascular system. Brain 2012, 135 Pt 2, 376–390. [Google Scholar] [CrossRef]
- Zhong, J.; Minassi, A.; Prenen, J.; Taglialatela-Scafati, O.; Appendino, G.; Nilius, B. Umbellulone modulates TRP channels. Pflugers Arch. 2011, 462, 861–870. [Google Scholar] [CrossRef]
- Strecker, T.; Dux, M.; Messlinger, K. Nitric oxide releases calcitonin-gene-related peptide from rat dura mater encephali promoting increases in meningeal blood flow. J. Vasc. Res. 2002, 39, 489–496. [Google Scholar] [CrossRef]
- Nicoletti, P.; Trevisani, M.; Manconi, M.; Gatti, R.; De Siena, G.; Zagli, G.; Benemei, S.; Capone, J.A.; Geppetti, P.; Pini, L.A. Ethanol causes neurogenic vasodilation by TRPV1 activation and CGRP release in the trigeminovascular system of the guinea pig. Cephalalgia 2008, 28, 9–17. [Google Scholar] [CrossRef]
- Trevisani, M.; Smart, D.; Gunthorpe, M.J.; Tognetto, M.; Barbieri, M.; Campi, B.; Amadesi, S.; Gray, J.; Jerman, J.C.; Brough, S.J.; et al. Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1. Nat. Neurosci. 2002, 5, 546–551. [Google Scholar] [CrossRef]
- Andre, E.; Campi, B.; Materazzi, S.; Trevisani, M.; Amadesi, S.; Massi, D.; Creminon, C.; Vaksman, N.; Nassini, R.; Civelli, M.; et al. Cigarette smoke-induced neurogenic inflammation is mediated by alpha, beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J. Clin. Investig. 2008, 118, 2574–2582. [Google Scholar]
- Fujita, F.; Uchida, K.; Moriyama, T.; Shima, A.; Shibasaki, K.; Inada, H.; Sokabe, T.; Tominaga, M. Intracellular alkalization causes pain sensation through activation of TRPA1 in mice. J. Clin. Investig. 2008, 118, 4049–4057. [Google Scholar] [CrossRef]
- Bessac, B.F.; Sivula, M.; von Hehn, C.A.; Escalera, J.; Cohn, L.; Jordt, S.E. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J. Clin. Investig. 2008, 118, 1899–1910. [Google Scholar] [CrossRef]
- McNamara, C.R.; Mandel-Brehm, J.; Bautista, D.M.; Siemens, J.; Deranian, K.L.; Zhao, M.; Hayward, N.J.; Chong, J.A.; Julius, D.; Moran, M.M.; et al. TRPA1 mediates formalin-induced pain. Proc. Natl. Acad. Sci. USA 2007, 104, 13525–13530. [Google Scholar] [CrossRef]
- Silva-Néto, R.P.; Peres, M.F.; Valença, M.M. Odorant substances that trigger headaches in migraine patients. Cephalalgia 2014, 34, 14–21. [Google Scholar] [CrossRef]
- Martins, B.; Costa, A. Migraine Improvement during COVID-19 Pandemic—A Case Report on the Wonders of a Mask. Headache 2020, 60, 2608–2609. [Google Scholar] [CrossRef]
- Schulte, L.H.; Jürgens, T.P.; May, A. Photo-, osmo- and phonophobia in the premonitory phase of migraine: Mistaking symptoms for triggers? J. Headache Pain 2015, 16, 015–0495. [Google Scholar] [CrossRef]
- Hoffmann, J.; Recober, A. Migraine and triggers: Post hoc ergo propter hoc? Curr. Pain Headache Rep. 2013, 17, 370. [Google Scholar] [CrossRef]
- Tanik, N.; Bektas, M. Development of quality of life assessment questionnaire associated with osmophobia in people with migraine. Pain Med. 2021, 21, 1006–1014. [Google Scholar] [CrossRef]
- Baldacci, F.; Lucchesi, C.; Ulivi, M.; Cafalli, M.; Vedovello, M.; Vergallo, A.; Prete, E.D.; Nuti, A.; Bonuccelli, U.; Gori, S. Clinical features associated with ictal osmophobia in migraine. Neurol. Sci. 2015, 36, 43–46. [Google Scholar] [CrossRef]
- Albanês Oliveira Bernardo, A.; Lys Medeiros, F.; Sampaio Rocha-Filho, P.A. Osmophobia and Odor-Triggered Headaches in Children and Adolescents: Prevalence, Associated Factors, and Importance in the Diagnosis of Migraine. Headache 2020, 60, 954–966. [Google Scholar] [CrossRef]
- Lovati, C.; Giani, L.; Capiluppi, E.; Preziosa, G.; D’Amico, D.; Mariani, C. O067. Osmophobia in allodynic migraine: Role of frequency of attacks and headache duration. J. Headache Pain 2015, 16 (Suppl. S1), 1129–2377. [Google Scholar] [CrossRef][Green Version]
- Lovati, C.; Lombardo, D.; Peruzzo, S.; Bellotti, A.; Capogrosso, C.A.; Pantoni, L. Osmophobia in migraine: Multifactorial investigation and population-based survey. Neurol. Sci. 2020, 41 (Suppl. S2), 453–454. [Google Scholar] [CrossRef]
- Delussi, M.; Laporta, A.; Fraccalvieri, I.; de Tommaso, M. Osmophobia in primary headache patients: Associated symptoms and response to preventive treatments. J. Headache Pain 2021, 22, 109. [Google Scholar] [CrossRef]
- Lovati, C.; Giani, L.; Castoldi, D.; Mariotti D’Alessandro, C.; DeAngeli, F.; Capiluppi, E.; D’Amico, D.; Mariani, C. Osmophobia in allodynic migraineurs: Cause or consequence of central sensitization? Neurol. Sci. 2015, 1, 145–147. [Google Scholar] [CrossRef]
- Wang, Y.F.; Fuh, J.L.; Chen, S.P.; Wu, J.C.; Wang, S.J. Clinical correlates and diagnostic utility of osmophobia in migraine. Cephalalgia 2012, 32, 1180–1188. [Google Scholar] [CrossRef]
- Park, S.P.; Seo, J.G.; Lee, W.K. Osmophobia and allodynia are critical factors for suicidality in patients with migraine. J. Headache Pain 2015, 16, 44. [Google Scholar] [CrossRef] [PubMed]
- Saçmacı, H.; Cengiz, G.F.; Aktürk, T. Impact of dissociative experiences in migraine and its close relationship with osmophobia. Neurol. Res. 2020, 42, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Zanchin, G.; Dainese, F.; Mainardi, F.; Mampreso, E.; Perin, C.; Maggioni, F. Osmophobia in primary headaches. J. Headache Pain 2005, 6, 213–215. [Google Scholar] [CrossRef] [PubMed]
- De Carlo, D.; Toldo, I.; Dal Zotto, L.; Perissinotto, E.; Sartori, S.; Gatta, M.; Balottin, U.; Mazzotta, G.; Moscato, D.; Raieli, V.; et al. Osmophobia as an early marker of migraine: A follow-up study in juvenile patients. Cephalalgia 2012, 32, 401–406. [Google Scholar] [CrossRef]
- Rocha-Filho, P.A.; Marques, K.S.; Torres, R.C.; Leal, K.N. Osmophobia and Headaches in Primary Care: Prevalence, Associated Factors, and Importance in Diagnosing Migraine. Headache 2015, 55, 840–845. [Google Scholar] [CrossRef]
- Silva-Néto, R.P.; Rodrigues, Â.B.; Cavalcante, D.C.; Ferreira, P.H.; Nasi, E.P.; Sousa, K.M.; Peres, M.F.; Valença, M.M. May headache triggered by odors be regarded as a differentiating factor between migraine and other primary headaches? Cephalalgia 2017, 37, 20–28. [Google Scholar] [CrossRef]
- Chalmer, M.A.; Hansen, T.F.; Olesen, J. Nosographic analysis of osmophobia and field testing of diagnostic criteria including osmophobia. Cephalalgia 2019, 39, 38–43. [Google Scholar] [CrossRef]
- Terrin, A.; Mainardi, F.; Lisotto, C.; Mampreso, E.; Fuccaro, M.; Maggioni, F.; Zanchin, G. A prospective study on osmophobia in migraine versus tension-type headache in a large series of attacks. Cephalalgia 2020, 40, 337–346. [Google Scholar] [CrossRef]
- Blau, J.N.; Solomon, F. Smell and other sensory disturbances in migraine. J. Neurol. 1985, 232, 275–276. [Google Scholar] [CrossRef]
- Porta-Etessam, J.; Casanova, I.; García-Cobos, R.; Lapeña, T.; Fernández, M.J.; García-Ramos, R.; Serna, C. Osmophobia analysis in primary headache. Neurologia 2009, 24, 315–317. [Google Scholar] [PubMed]
- Kayabaşoglu, G.; Altundag, A.; Kotan, D.; Dizdar, D.; Kaymaz, R. Osmophobia and olfactory functions in patients with migraine. Eur. Arch. Otorhinolaryngol. 2017, 274, 817–821. [Google Scholar] [CrossRef]
- Kandemir, S.; Pamuk, A.E.; Habipoğlu, Y.; Özel, G.; Bayar Muluk, N.; Kılıç, R. Olfactory acuity based on Brief Smell Identification Test (BSIT®) in migraine patients with and without aura: A cross-sectional, controlled study. Auris Nasus Larynx 2021, 49, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Marmura, M.J.; Monteith, T.S.; Anjum, W.; Doty, R.L.; Hegarty, S.E.; Keith, S.W. Olfactory function in migraine both during and between attacks. Cephalalgia 2014, 34, 977–985. [Google Scholar] [CrossRef]
- Hirsch, A.R. Osmophobia and taste abnormality in migraineurs: A tertiary care study. Headache 2005, 45, 763–764. [Google Scholar] [CrossRef]
- Doğan, A.; Bayar Muluk, N.; Şahan, M.H.; Asal, N.; Inal, M.; Ergün, U. Olfactory bulbus volume and olfactory sulcus depth in migraine patients: An MRI evaluation. Eur. Arch. Otorhinolaryngol. 2018, 275, 2005–2011. [Google Scholar] [CrossRef] [PubMed]
- Aktürk, T.; Tanık, N.; Serin, H.; Saçmacı, H.; İnan, L.E. Olfactory bulb atrophy in migraine patients. Neurol. Sci. 2019, 40, 127–132. [Google Scholar] [CrossRef]
- Demarquay, G.; Royet, J.P.; Mick, G.; Ryvlin, P. Olfactory hypersensitivity in migraineurs: A H(2)(15)O-PET study. Cephalalgia 2008, 28, 1069–1080. [Google Scholar] [CrossRef]
- Stankewitz, A.; May, A. Increased limbic and brainstem activity during migraine attacks following olfactory stimulation. Neurology 2011, 77, 476–482. [Google Scholar] [CrossRef]
- Anderson, A.K.; Christoff, K.; Stappen, I.; Panitz, D.; Ghahremani, D.G.; Glover, G.; Gabrieli, J.D.; Sobel, N. Dissociated neural representations of intensity and valence in human olfaction. Nat. Neurosci. 2003, 6, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Hillert, L.; Jovanovic, H.; Ahs, F.; Savic, I. Women with multiple chemical sensitivity have increased harm avoidance and reduced 5-HT(1A) receptor binding potential in the anterior cingulate and amygdala. PLoS ONE 2013, 8, e54781. [Google Scholar] [CrossRef] [PubMed]
- Hillert, L.; Musabasic, V.; Berglund, H.; Ciumas, C.; Savic, I. Odor processing in multiple chemical sensitivity. Hum. Brain Mapp. 2007, 28, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Del Casale, A.; Ferracuti, S.; Mosca, A.; Pomes, L.M.; Fiaschè, F.; Bonanni, L.; Borro, M.; Gentile, G.; Martelletti, P.; Simmaco, M. Multiple Chemical Sensitivity Syndrome: A Principal Component Analysis of Symptoms. Int. J. Environ. Res. Public Health 2020, 17, 6551. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Okamura, M.; Haruyama, Y.; Suzuki, S.; Shiina, T.; Kobashi, G.; Hirata, K. Exploring the contributing factors to multiple chemical sensitivity in patients with migraine. J. Occup. Health 2022, 64, 1348–9585. [Google Scholar] [CrossRef]
- Coleman, E.R.; Grosberg, B.M.; Robbins, M.S. Olfactory hallucinations in primary headache disorders: Case series and literature review. Cephalalgia 2011, 31, 1477–1489. [Google Scholar] [CrossRef]
- Mainardi, F.; Rapoport, A.; Zanchin, G.; Maggioni, F. Scent of aura? Clinical features of olfactory hallucinations during a migraine attack (OHM). Cephalalgia 2017, 37, 154–160. [Google Scholar] [CrossRef]
- Henkin, R.I.; Levy, L.M.; Lin, C.S. Taste and smell phantoms revealed by brain functional MRI (fMRI). J. Comput. Assist. Tomogr. 2000, 24, 106–123. [Google Scholar] [CrossRef]
- Henkin, R.I.; Potolicchio, S.J.; Levy, L.M. Olfactory Hallucinations without Clinical Motor Activity: A Comparison of Unirhinal with Birhinal Phantosmia. Brain Sci. 2013, 3, 1483–1553. [Google Scholar] [CrossRef]
- Saberi, A.; Nemati, S.; Amlashi, T.T.; Tohidi, S.; Bakhshi, F. Phonophobia and migraine features in patients with definite meniere’s disease: Pentad or triad/tetrad? Acta Otolaryngol. 2020, 140, 548–552. [Google Scholar] [CrossRef]
- Mohammadi, M.; Taziki Balajelini, M.H.; Rajabi, A. Migraine and risk of sudden sensorineural hearing loss: A systematic review and meta-analysis. Laryngoscope Investig. Otolaryngol. 2020, 5, 1089–1095. [Google Scholar] [CrossRef]
- Karsan, N.; Bose, P.; Newman, J.; Goadsby, P.J. Are some patient-perceived migraine triggers simply early manifestations of the attack? J. Neurol. 2021, 268, 1885–1893. [Google Scholar] [CrossRef] [PubMed]
- Woodhouse, A.; Drummond, P.D. Mechanisms of increased sensitivity to noise and light in migraine headache. Cephalalgia 1993, 13, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Vingen, J.V.; Pareja, J.A.; Støren, O.; White, L.R.; Stovner, L.J. Phonophobia in migraine. Cephalalgia 1998, 18, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Main, A.; Dowson, A.; Gross, M. Photophobia and phonophobia in migraineurs between attacks. Headache 1997, 37, 492–495. [Google Scholar] [CrossRef]
- Ashkenazi, A.; Mushtaq, A.; Yang, I.; Oshinsky, M.L. Ictal and interictal phonophobia in migraine-a quantitative controlled study. Cephalalgia 2009, 29, 1042–1048. [Google Scholar] [CrossRef]
- Wang, W.; Timsit-Berthier, M.; Schoenen, J. Intensity dependence of auditory evoked potentials is pronounced in migraine: An indication of cortical potentiation and low serotonergic neurotransmission? Neurology 1996, 46, 1404–1409. [Google Scholar] [CrossRef]
- Irimia, P.; Cittadini, E.; Paemeleire, K.; Cohen, A.S.; Goadsby, P.J. Unilateral photophobia or phonophobia in migraine compared with trigeminal autonomic cephalalgias. Cephalalgia 2008, 28, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.W.; Seifert, T.; Kailasam, J.; Mathew, N.T. The use of questions to determine the presence of photophobia and phonophobia during migraine. Headache 2008, 48, 395–397. [Google Scholar] [CrossRef]
- Bolay, H.; Bayazit, Y.A.; Gunduz, B.; Ugur, A.K.; Akcali, D.; Altunyay, S.; Ilica, S.; Babacan, A. Subclinical dysfunction of cochlea and cochlear efferents in migraine: An otoacoustic emission study. Cephalalgia 2008, 28, 309–317. [Google Scholar] [CrossRef]
- Murdin, L.; Premachandra, P.; Davies, R. Sensory dysmodulation in vestibular migraine: An otoacoustic emission suppression study. Laryngoscope 2010, 120, 1632–1636. [Google Scholar] [CrossRef]
- Albanese, M.; Di Girolamo, S.; Silvani, L.; Ciaschi, E.; Chiaramonte, B.; Conti, M.; Passali, F.M.; Di Gioia, B.; Mercuri, N.B.; Di Stadio, A. Distortion Product Otoacoustic Emissions and Their Suppression as Predictors of Peripheral Auditory Damage in Migraine: A Case-Control Study. J. Clin. Med. 2021, 10, 5007. [Google Scholar] [CrossRef]
- Joffily, L.; de Melo Tavares de Lima, M.A.; Vincent, M.B.; Frota, S.M. Assessment of otoacoustic emission suppression in women with migraine and phonophobia. Neurol. Sci. 2016, 37, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, L.A.; Luebke, A.E. CGRP- and cholinergic-containing fibers project to guinea pig outer hair cells. Hear. Res. 2002, 172, 14–17. [Google Scholar] [CrossRef]
- Ambrosini, A.; Rossi, P.; De Pasqua, V.; Pierelli, F.; Schoenen, J. Lack of habituation causes high intensity dependence of auditory evoked cortical potentials in migraine. Brain 2003, 126 Pt 9, 2009–2015. [Google Scholar] [CrossRef]
- Siniatchkin, M.; Kropp, P.; Neumann, M.; Gerber, W.; Stephani, U. Intensity dependence of auditory evoked cortical potentials in migraine families. Pain 2000, 85, 247–254. [Google Scholar] [CrossRef]
- Afra, J.; Proietti Cecchini, A.; Sándor, P.S.; Schoenen, J. Comparison of visual and auditory evoked cortical potentials in migraine patients between attacks. Clin. Neurophysiol. 2000, 111, 1124–1129. [Google Scholar] [CrossRef]
- Hegerl, U.; Juckel, G. Intensity dependence of auditory evoked potentials as an indicator of central serotonergic neurotransmission: A new hypothesis. Biol. Psychiatry 1993, 33, 173–187. [Google Scholar] [CrossRef]
- Sand, T.; Zhitniy, N.; White, L.R.; Stovner, L.J. Brainstem auditory-evoked potential habituation and intensity-dependence related to serotonin metabolism in migraine: A longitudinal study. Clin. Neurophysiol. 2008, 119, 1190–1200. [Google Scholar] [CrossRef]
- Viganò, A.; Torrieri, M.C.; Toscano, M.; Puledda, F.; Petolicchio, B.; Sasso D’Elia, T.; Verzina, A.; Ruggiero, S.; Altieri, M.; Vicenzini, E.; et al. Neurophysiological correlates of clinical improvement after greater occipital nerve (GON) block in chronic migraine: Relevance for chronic migraine pathophysiology. J. Headache Pain 2018, 19, 73. [Google Scholar] [CrossRef]
- Sándor, P.S.; Afra, J.; Ambrosini, A.; Schoenen, J. Prophylactic treatment of migraine with beta-blockers and riboflavin: Differential effects on the intensity dependence of auditory evoked cortical potentials. Headache 2000, 40, 30–35. [Google Scholar] [CrossRef]
- Schlake, H.P.; Grotemeyer, K.H.; Hofferberth, B.; Husstedt, I.W.; Wiesner, S. Brainstem auditory evoked potentials in migraine-evidence of increased side differences during the pain-free interval. Headache 1990, 30, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Podoshin, L.; Ben-David, J.; Pratt, H.; Fradis, M.; Sharf, B.; Weller, B.; Wajsbort, J.; Zellinger, M. Auditory brainstem evoked potentials in patients with migraine. Headache 1987, 27, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Ganji, S. Basilar artery migraine: EEG and evoked potential patterns during acute stage. Headache 1986, 26, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Ganji, S.; Hellman, S.; Stagg, S.; Furlow, J. Episodic coma due to acute basilar artery migraine: Correlation of EEG and brainstem auditory evoked potential patterns. Clin. Electroencephalogr. 1993, 24, 44–48. [Google Scholar] [CrossRef]
- Benna, P.; Bianco, C.; Costa, P.; Piazza, D.; Bergamasco, B. Visual evoked potentials and brainstem auditory evoked potentials in migraine and transient ischemic attacks. Cephalalgia 1985, 5 (Suppl. 2), 53–58. [Google Scholar] [CrossRef]
- Kalita, J.; Misra, U.K.; Bansal, R. Phonophobia and brainstem excitability in migraine. Eur. J. Neurosci. 2021, 53, 1988–1997. [Google Scholar] [CrossRef]
- Weiller, C.; May, A.; Limmroth, V.; Juptner, M.; Kaube, H.; Schayck, R.V.; Coenen, H.H.; Diener, H.C. Brain stem activation in spontaneous human migraine attacks. Nat. Med. 1995, 1, 658–660. [Google Scholar] [CrossRef]
- Ashkenazi, A.; Yang, I.; Mushtaq, A.; Oshinsky, M.L. Is phonophobia associated with cutaneous allodynia in migraine? J. Neurol. Neurosurg. Psychiatry 2010, 81, 1256–1260. [Google Scholar] [CrossRef]
- Akdal, G.; Ozge, A.; Ergor, G. The prevalence of vestibular symptoms in migraine or tension-type headache. J. Vestib. Res. 2013, 23, 101–106. [Google Scholar] [CrossRef]
- Kuritzky, A.; Ziegler, D.K.; Hassanein, R. Vertigo, motion sickness and migraine. Headache 1981, 21, 227–231. [Google Scholar] [CrossRef]
- Lee, H.; Sohn, S.I.; Jung, D.K.; Cho, Y.W.; Lim, J.G.; Yi, S.D.; Yi, H.A. Migraine and isolated recurrent vertigo of unknown cause. Neurol. Res. 2002, 24, 663–665. [Google Scholar] [CrossRef] [PubMed]
- Moretti, G.; Manzoni, G.C.; Caffarra, P.; Parma, M. “Benign recurrent vertigo” and its connection with migraine. Headache 1980, 20, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Reploeg, M.D.; Goebel, J.A. Migraine-associated dizziness: Patient characteristics and management options. Otol. Neurotol. 2002, 23, 364–371. [Google Scholar] [CrossRef]
- Vukovic, V.; Plavec, D.; Galinovic, I.; Lovrencic-Huzjan, A.; Budisic, M.; Demarin, V. Prevalence of vertigo, dizziness, and migrainous vertigo in patients with migraine. Headache 2007, 47, 1427–1435. [Google Scholar] [CrossRef]
- Dieterich, M.; Obermann, M.; Celebisoy, N. Vestibular migraine: The most frequent entity of episodic vertigo. J. Neurol. 2016, 263 (Suppl. 1), S82–S89. [Google Scholar] [CrossRef] [PubMed]
- Cass, S.P.; Furman, J.M.; Ankerstjerne, K.; Balaban, C.; Yetiser, S.; Aydogan, B. Migraine-related vestibulopathy. Ann. Otol. Rhinol. Laryngol. 1997, 106, 182–189. [Google Scholar] [CrossRef]
- Cutrer, F.M.; Baloh, R.W. Migraine-associated dizziness. Headache 1992, 32, 300–304. [Google Scholar] [CrossRef]
- Van Leeuwen, R.B.; Colijn, C.; van Esch, B.F.; Schermer, T.R. Benign Recurrent Vertigo: The Course of Vertigo Attacks Compared to Patients with Menière’s Disease and Vestibular Migraine. Front. Neurol. 2022, 13, 817812. [Google Scholar] [CrossRef]
- Prell, T.; Finn, S.; Zipprich, H.M.; Axer, H. What Predicts Improvement of Dizziness after Multimodal and Interdisciplinary Day Care Treatment? J. Clin. Med. 2022, 11, 2005. [Google Scholar] [CrossRef] [PubMed]
- Formeister, E.J.; Rizk, H.G.; Kohn, M.A.; Sharon, J.D. The Epidemiology of Vestibular Migraine: A Population-based Survey Study. Otol. Neurotol. 2018, 39, 1037–1044. [Google Scholar] [CrossRef]
- Lempert, T.; Olesen, J.; Furman, J.; Waterston, J.; Seemungal, B.; Carey, J.; Bisdorff, A.; Versino, M.; Evers, S.; Newman-Toker, D. Vestibular migraine: Diagnostic criteria. J. Vestib. Res. 2012, 22, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Chae, R.; Krauter, R.; Pasquesi, L.L.; Sharon, J.D. Broadening the vestibular migraine diagnosis criteria: A prospective cohort study on vestibular migraine subtypes. J. Vestib. Res. 2022, 2, VES-210117. [Google Scholar] [CrossRef] [PubMed]
- Neuhauser, H.K.; Radtke, A.; von Brevern, M.; Feldmann, M.; Lezius, F.; Ziese, T.; Lempert, T. Migrainous vertigo: Prevalence and impact on quality of life. Neurology 2006, 67, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, M.; Brandt, T. Episodic vertigo related to migraine (90 cases): Vestibular migraine? J. Neurol. 1999, 246, 883–892. [Google Scholar] [CrossRef]
- Eggers, S.D.; Staab, J.P.; Neff, B.A.; Goulson, A.M.; Carlson, M.L.; Shepard, N.T. Investigation of the coherence of definite and probable vestibular migraine as distinct clinical entities. Otol. Neurotol. 2011, 32, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Teggi, R.; Colombo, B.; Albera, R.; Asprella Libonati, G.; Balzanelli, C.; Batuecas Caletrio, A.; Casani, A.; Espinoza-Sanchez, J.M.; Gamba, P.; Lopez-Escamez, J.A.; et al. Clinical Features, Familial History, and Migraine Precursors in Patients with Definite Vestibular Migraine: The VM-Phenotypes Projects. Headache 2018, 58, 534–544. [Google Scholar] [CrossRef]
- Li, Z.Y.; Shen, B.; Si, L.H.; Ling, X.; Li, K.Z.; Yang, X. Clinical characteristics of definite vestibular migraine diagnosed according to criteria jointly formulated by the Bárány Society and the International Headache Society. Braz. J. Otorhinolaryngol. 2022, 21, 1–5. [Google Scholar] [CrossRef]
- Von Brevern, M.; Zeise, D.; Neuhauser, H.; Clarke, A.H.; Lempert, T. Acute migrainous vertigo: Clinical and oculographic findings. Brain 2005, 128 Pt 2, 365–374. [Google Scholar] [CrossRef]
- Çelebisoy, N.; Kısabay Ak, A.; Özdemir, H.N.; Gökçay, F.; Durmaz, G.S.; Kartı, D.T.; Toydemir, H.E.; Yayla, V.; Çolpak Işıkay, A.; Erkent, İ.; et al. Vestibular migraine, demographic and clinical features of 415 patients: A multicenter study. Clin. Neurol. Neurosurg. 2022, 215, 107201. [Google Scholar] [CrossRef]
- Kısabay Ak, A.; Çelebisoy, N.; Özdemir, H.N.; Gökçay, F.; Saruhan Durmaz, G.; Top Kartı, D.; Ertaşoğlu Toydemir, H.; Yayla, V.; Çolpak Işıkay, A.; Erkent, İ.; et al. Factors determining the response to treatment in patients with vestibular migraine. Neurol. Res. 2022, 29, 1–8. [Google Scholar] [CrossRef]
- Seo, T.; Okano, Y.; Aomi, M.; Yamada, Z.; Kasugai, S.; Nakamura, M.; Miyamoto, Y.; Koizuka, I. Endolymphatic hydrops presumption tests for patients with vestibular migraine. Acta Otolaryngol. 2022, 142, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Lei, P.; Chen, C.; Liu, Y.; Xia, K.; Liu, B. Non-contrast MRI of Inner Ear Detected Differences of Endolymphatic Drainage System between Vestibular Migraine and Unilateral Ménière’s Disease. Front. Neurol. 2022, 13, 814518. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, L.B.; Yan, F.; Liu, Y.F.; Nguyen, S.A.; Rizk, H.G. Does cognitive dysfunction correlate with dizziness severity in patients with vestibular migraine? Am. J. Otolaryngol. 2021, 42, 103124. [Google Scholar] [CrossRef] [PubMed]
- Karsan, N.; Perez-Rodriguez, A.; Nagaraj, K.; Bose, P.R.; Goadsby, P.J. The migraine postdrome: Spontaneous and triggered phenotypes. Cephalalgia 2021, 41, 721–730. [Google Scholar] [CrossRef]
- Carvalho, G.F.; Luedtke, K.; Pinheiro, C.F.; Moraes, R.; Lemos, T.W.; Carneiro, C.G.; Bigal, M.E.; Dach, F.; Bevilaqua-Grossi, D. Migraine and balance impairment: Influence of subdiagnosis, otoneurological function, falls, and psychosocial factors. Headache 2022, 62, 548–557. [Google Scholar] [CrossRef]
- Nocini, R.; Baraldi, C.; Apa, E.; Ciorba, A.; Monzani, D.; Palma, S. Visually Evoked Postural Responses (VEPRs) in Children with Vestibular Migraine. Children 2021, 9, 14. [Google Scholar] [CrossRef]
- Lim, Y.H.; Kim, J.S.; Lee, H.W.; Kim, S.H. Postural Instability Induced by Visual Motion Stimuli in Patients With Vestibular Migraine. Front. Neurol. 2018, 9, 433. [Google Scholar] [CrossRef]
- So, C.W.; Bent, L.R. Increased vestibular contribution to posture control in individuals with chronic headache. J. Vestib. Res. 2009, 19, 49–58. [Google Scholar]
- Ishizaki, K.; Mori, N.; Takeshima, T.; Fukuhara, Y.; Ijiri, T.; Kusumi, M.; Yasui, K.; Kowa, H.; Nakashima, K. Static stabilometry in patients with migraine and tension-type headache during a headache-free period. Psychiatry Clin. Neurosci. 2002, 56, 85–90. [Google Scholar] [CrossRef]
- Teggi, R.; Colombo, B.; Bernasconi, L.; Bellini, C.; Comi, G.; Bussi, M. Migrainous vertigo: Results of caloric testing and stabilometric findings. Headache 2009, 49, 435–444. [Google Scholar] [CrossRef]
- Janiak-Kiszka, J.; Nowaczewska, M.; Wierzbiński, R.; Kaźmierczak, W.; Kaźmierczak, H. The visual-ocular and vestibulo-ocular reflexes in vestibular migraine. Otolaryngol. Pol. 2021, 76, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Marano, E.; Marcelli, V.; Di Stasio, E.; Bonuso, S.; Vacca, G.; Manganelli, F.; Marciano, E.; Perretti, A. Trigeminal stimulation elicits a peripheral vestibular imbalance in migraine patients. Headache 2005, 45, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Kim, Y.K.; Kim, H.J.; Kim, J.S. Altered brain metabolism in vestibular migraine: Comparison of interictal and ictal findings. Cephalalgia 2014, 34, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Obermann, M.; Wurthmann, S.; Steinberg, B.S.; Theysohn, N.; Diener, H.C.; Naegel, S. Central vestibular system modulation in vestibular migraine. Cephalalgia 2014, 34, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Marcelli, V.; Esposito, F.; Corvino, V.; Marcuccio, L.; Giannone, A.; Conforti, R.; Marciano, E.; Tedeschi, G.; Tessitore, A. Abnormal thalamic function in patients with vestibular migraine. Neurology 2014, 82, 2120–2126. [Google Scholar] [CrossRef] [PubMed]
- Fasold, O.; von Brevern, M.; Kuhberg, M.; Ploner, C.J.; Villringer, A.; Lempert, T.; Wenzel, R. Human vestibular cortex as identified with caloric stimulation in functional magnetic resonance imaging. Neuroimage 2002, 17, 1384–1393. [Google Scholar] [CrossRef]
- Koo, J.W.; Balaban, C.D. Serotonin-induced plasma extravasation in the murine inner ear: Possible mechanism of migraine-associated inner ear dysfunction. Cephalalgia 2006, 26, 1310–1319. [Google Scholar] [CrossRef]
- Ahn, S.K.; Balaban, C.D. Distribution of 5-HT1B and 5-HT1D receptors in the inner ear. Brain Res. 2010, 1346, 92–101. [Google Scholar] [CrossRef]
- Vass, Z.; Steyger, P.S.; Hordichok, A.J.; Trune, D.R.; Jancso, G.; Nuttall, A.L. Capsaicin stimulation of the cochlea and electric stimulation of the trigeminal ganglion mediate vascular permeability in cochlear and vertebro-basilar arteries: A potential cause of inner ear dysfunction in headache. Neuroscience 2001, 103, 189–201. [Google Scholar] [CrossRef]
- Halberstadt, A.L.; Balaban, C.D. Organization of projections from the raphe nuclei to the vestibular nuclei in rats. Neuroscience 2003, 120, 573–594. [Google Scholar] [CrossRef]
- Byun, Y.J.; Levy, D.A.; Nguyen, S.A.; Brennan, E.; Rizk, H.G. Treatment of Vestibular Migraine: A Systematic Review and Meta-analysis. Laryngoscope 2021, 131, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Bayer, O.; Adrion, C.; Al Tawil, A.; Mansmann, U.; Strupp, M. Results and lessons learnt from a randomized controlled trial: Prophylactic treatment of vestibular migraine with metoprolol (PROVEMIG). Trials 2019, 20, 813. [Google Scholar] [CrossRef]
- Neuhauser, H.; Radtke, A.; von Brevern, M.; Lempert, T. Zolmitriptan for treatment of migrainous vertigo: A pilot randomized placebo-controlled trial. Neurology 2003, 60, 882–883. [Google Scholar] [CrossRef]
- Stancel-Lewis, J.; Lau, J.W.L.; Male, A.; Korres, G.; Rogel-Salazar, J.; Pavlou, M.; Bamiou, D.E. Vestibular Rehabilitation Therapy for the Treatment of Vestibular Migraine, and the Impact of Traumatic Brain Injury on Outcome: A Retrospective Study. Otol. Neurotol. 2022, 43, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Zhang, Y.; Pan, Q.; Wang, Y.; Wen, Q.; Fan, X.; Qin, G.; Zhang, D.; Chen, L.; Zhang, Y.; et al. Calcitonin gene-related peptide receptor antagonist BIBN4096BS regulates synaptic transmission in the vestibular nucleus and improves vestibular function via PKC/ERK/CREB pathway in an experimental chronic migraine rat model. J. Headache Pain 2022, 23, 35. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, J.L.; Fife, T.D. New Anti-CGRP Medications in the Treatment of Vestibular Migraine. Front. Neurol. 2022, 12, 799002. [Google Scholar] [CrossRef]
- Selby, G.; Lance, J.W. Observations on 500 cases of migraine and allied vascular headache. J. Neurol. Neurosurg. Psychiatry 1960, 23, 23–32. [Google Scholar] [CrossRef]
- Ashkenazi, A.; Sholtzow, M.; Shaw, J.W.; Burstein, R.; Young, W.B. Identifying cutaneous allodynia in chronic migraine using a practical clinical method. Cephalalgia 2007, 27, 111–117. [Google Scholar] [CrossRef]
- Nahman-Averbuch, H.; Shefi, T.; Schneider, V.J.; Li, D., 2nd; Ding, L.; King, C.D.; Coghill, R.C. Quantitative sensory testing in patients with migraine: A systematic review and meta-analysis. Pain 2018, 159, 1202–1223. [Google Scholar] [CrossRef]
- Ashkenazi, A.; Silberstein, S.; Jakubowski, M.; Burstein, R. Improved identification of allodynic migraine patients using a questionnaire. Cephalalgia 2007, 27, 325–329. [Google Scholar] [CrossRef]
- Lipton, R.B.; Bigal, M.E.; Ashina, S.; Burstein, R.; Silberstein, S.; Reed, M.L.; Serrano, D.; Stewart, W.F. Cutaneous allodynia in the migraine population. Ann. Neurol. 2008, 63, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Bigal, M.E.; Ashina, S.; Burstein, R.; Reed, M.L.; Buse, D.; Serrano, D.; Lipton, R.B.; Group, A. Prevalence and characteristics of allodynia in headache sufferers: A population study. Neurology 2008, 70, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Burstein, R.; Yarnitsky, D.; Goor-Aryeh, I.; Ransil, B.J.; Bajwa, Z.H. An association between migraine and cutaneous allodynia. Ann. Neurol. 2000, 47, 614–624. [Google Scholar] [CrossRef]
- Bevilaqua-Grossi, D.; Lipton, R.B.; Napchan, U.; Grosberg, B.; Ashina, S.; Bigal, M.E. Temporomandibular disorders and cutaneous allodynia are associated in individuals with migraine. Cephalalgia 2010, 30, 425–432. [Google Scholar] [CrossRef]
- Lovati, C.; D’Amico, D.; Rosa, S.; Suardelli, M.; Mailland, E.; Bertora, P.; Pomati, S.; Mariani, C.; Bussone, G. Allodynia in different forms of migraine. Neurol. Sci. 2007, 28 (Suppl. S2), S220–S221. [Google Scholar] [CrossRef]
- Akerman, S.; Karsan, N.; Bose, P.; Hoffmann, J.R.; Holland, P.R.; Romero-Reyes, M.; Goadsby, P.J. Nitroglycerine triggers triptan-responsive cranial allodynia and trigeminal neuronal hypersensitivity. Brain 2019, 142, 103–119. [Google Scholar] [CrossRef]
- Ladda, J.; Straube, A.; Forderreuther, S.; Krause, P.; Eggert, T. Quantitative sensory testing in cluster headache: Increased sensory thresholds. Cephalalgia 2006, 26, 1043–1050. [Google Scholar] [CrossRef]
- Burstein, R.; Cutrer, M.F.; Yarnitsky, D. The development of cutaneous allodynia during a migraine attack clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain 2000, 123 Pt 8, 1703–1709. [Google Scholar] [CrossRef]
- Burstein, R.; Collins, B.; Jakubowski, M. Defeating migraine pain with triptans: A race against the development of cutaneous allodynia. Ann. Neurol. 2004, 55, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Cady, R.; Martin, V.; Mauskop, A.; Rodgers, A.; Hustad, C.; Ramsey, K.; Skobieranda, F. Symptoms of cutaneous sensitivity pre-treatment and post-treatment: Results from the rizatriptan TAME studies. Cephalalgia 2007, 27, 1055–1060. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Zanchin, G.; Geraud, G.; de Klippel, N.; Diaz-Insa, S.; Gobel, H.; Cunha, L.; Ivanoff, N.; Falques, M.; Fortea, J. Early vs. non-early intervention in acute migraine—‘Act when Mild (AwM)’. A double-blind, placebo-controlled trial of almotriptan. Cephalalgia 2008, 28, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Cady, R.K.; Freitag, F.G.; Mathew, N.T.; Elkind, A.H.; Mao, L.; Fisher, A.C.; Biondi, D.M.; Finlayson, G.; Greenberg, S.J.; Hulihan, J.F. Allodynia-associated symptoms, pain intensity and time to treatment: Predicting treatment response in acute migraine intervention. Headache 2009, 49, 350–363. [Google Scholar] [CrossRef]
- Ezzati, A.; Fanning, K.M.; Buse, D.C.; Pavlovic, J.M.; Armand, C.E.; Reed, M.L.; Martin, V.T.; Lipton, R.B. Predictive models for determining treatment response to nonprescription acute medications in migraine: Results from the American Migraine Prevalence and Prevention Study. Headache 2022, 11, 14312. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, H.; Kenar, S.G.; Gursoy, G.T.; Bektas, H. The impacts of masks and disinfectants on migraine patients in the COVID-19 pandemic. J. Clin. Neurosci. 2022, 97, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Sivilotti, L.; Woolf, C.J. The contribution of GABAA and glycine receptors to central sensitization: Disinhibition and touch-evoked allodynia in the spinal cord. J. Neurophysiol. 1994, 72, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Strassman, A.M.; Raymond, S.A.; Burstein, R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature 1996, 384, 560–564. [Google Scholar] [CrossRef]
- Hoskin, K.L.; Bulmer, D.C.; Lasalandra, M.; Jonkman, A.; Goadsby, P.J. Fos expression in the midbrain periaqueductal grey after trigeminovascular stimulation. J. Anat. 2001, 198 Pt 1, 29–35. [Google Scholar] [CrossRef]
- Knight, Y.E.; Goadsby, P.J. The periaqueductal grey matter modulates trigeminovascular input: A role in migraine? Neuroscience 2001, 106, 793–800. [Google Scholar] [CrossRef]
- Pozo-Rosich, P.; Storer, R.J.; Charbit, A.R.; Goadsby, P.J. Periaqueductal gray calcitonin gene-related peptide modulates trigeminovascular neurons. Cephalalgia 2015, 35, 1298–1307. [Google Scholar] [CrossRef]
- Akerman, S.; Goadsby, P.J. Neuronal PAC1 receptors mediate delayed activation and sensitization of trigeminocervical neurons: Relevance to migraine. Sci. Transl. Med. 2015, 7, 308ra157. [Google Scholar] [CrossRef]
- Sessle, B.J.; Hu, J.W.; Amano, N.; Zhong, G. Convergence of cutaneous, tooth pulp, visceral, neck and muscle afferents onto nociceptive and non-nociceptive neurones in trigeminal subnucleus caudalis (medullary dorsal horn) and its implications for referred pain. Pain 1986, 27, 219–235. [Google Scholar] [CrossRef]
- Burstein, R.; Yamamura, H.; Malick, A.; Strassman, A.M. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J. Neurophysiol. 1998, 79, 964–982. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Kopruszinski, C.M.; Moutal, A.; Ikegami, D.; Khanna, R.; Chen, Y.; Ross, S.; Mackenzie, K.; Stratton, J.; Dodick, D.W.; et al. Dysregulation of serum prolactin links the hypothalamus with female nociceptors to promote migraine. Brain 2022, 145, 2894–2909. [Google Scholar] [CrossRef] [PubMed]
- Burstein, R.; Jakubowski, M.; Garcia-Nicas, E.; Kainz, V.; Bajwa, Z.; Hargreaves, R.; Becerra, L.; Borsook, D. Thalamic sensitization transforms localized pain into widespread allodynia. Ann. Neurol. 2010, 68, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Shields, K.G.; Goadsby, P.J. Propranolol modulates trigeminovascular responses in thalamic ventroposteromedial nucleus: A role in migraine? Brain 2005, 128 Pt 1, 86–97. [Google Scholar] [CrossRef]
- Noseda, R.; Jakubowski, M.; Kainz, V.; Borsook, D.; Burstein, R. Cortical projections of functionally identified thalamic trigeminovascular neurons: Implications for migraine headache and its associated symptoms. J. Neurosci. 2011, 31, 14204–14217. [Google Scholar] [CrossRef]
- Moulton, E.A.; Becerra, L.; Maleki, N.; Pendse, G.; Tully, S.; Hargreaves, R.; Burstein, R.; Borsook, D. Painful heat reveals hyperexcitability of the temporal pole in interictal and ictal migraine States. Cereb. Cortex 2011, 21, 435–448. [Google Scholar] [CrossRef]
- Lai, K.L.; Liao, K.K.; Fuh, J.L.; Wang, S.J. Subcortical hyperexcitability in migraineurs: A high-frequency oscillation study. Can. J. Neurol. Sci. 2011, 38, 309–316. [Google Scholar] [CrossRef]
- Ambrosini, A.; Schoenen, J. Electrophysiological response patterns of primary sensory cortices in migraine. J. Headache Pain 2006, 7, 377–388. [Google Scholar] [CrossRef]
- Schwedt, T.J.; Chong, C.D. Correlations between brain cortical thickness and cutaneous pain thresholds are atypical in adults with migraine. PLoS ONE 2014, 9, e99791. [Google Scholar]
- Schwedt, T.J.; Berisha, V.; Chong, C.D. Temporal lobe cortical thickness correlations differentiate the migraine brain from the healthy brain. PLoS ONE 2015, 10, e0116687. [Google Scholar] [CrossRef] [PubMed]
- Tso, A.R.; Trujillo, A.; Guo, C.C.; Goadsby, P.J.; Seeley, W.W. The anterior insula shows heightened interictal intrinsic connectivity in migraine without aura. Neurology 2015, 84, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Wang, Y.; Pan, Q.; Tian, R.; Zhang, D.; Qin, G.; Zhou, J.; Chen, L. MicroRNA-155-5p promotes neuroinflammation and central sensitization via inhibiting SIRT1 in a nitroglycerin-induced chronic migraine mouse model. J. Neuroinflamm. 2021, 18, 287. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tang, X.; Li, J.; Hu, B.; Yang, W.; Zhan, M.; Ma, T.; Xu, S. IL-17 crosses the blood-brain barrier to trigger neuroinflammation: A novel mechanism in nitroglycerin-induced chronic migraine. J. Headache Pain 2022, 23, 1. [Google Scholar] [CrossRef]
- Irimia, P.; Martinez-Valbuena, I.; Minguez-Olaondo, A.; Dominguez-Vivero, C.; Sanchez-Arias, J.A.; Martinez-Vila, E.; Luquin, M.-R.; Leira, R. Interictal amylin levels in chronic migraine patients: A case-control study. Cephalalgia 2021, 41, 604–612. [Google Scholar] [CrossRef]
- De Luca, C.; Baldacci, F.; Mazzucchi, S.; Lombardo, I.; Curto, L.; Ulivi, M.; Chico, L.; Papa, M.; Siciliano, G.; Gori, S. CGRP Inhibitors and Oxidative Stress Biomarkers in Resistant Migraine: A Real-Life Study with Erenumab, Fremanezumab, and Galcanezumab. J. Clin. Med. 2021, 10, 4586. [Google Scholar] [CrossRef]
- Greco, R.; Demartini, C.; Francavilla, M.; Zanaboni, A.M.; Tassorelli, C. Dual Inhibition of FAAH and MAGL Counteracts Migraine-like Pain and Behavior in an Animal Model of Migraine. Cells 2021, 10, 2543. [Google Scholar] [CrossRef]
- Paige, C.; Plasencia-Fernandez, I.; Kume, M.; Papalampropoulou-Tsiridou, M.; Lorenzo, L.E.; David, E.T.; He, L.; Mejia, G.L.; Driskill, C.; Ferrini, F.; et al. A Female-Specific Role for Calcitonin Gene-Related Peptide (CGRP) in Rodent Pain Models. J. Neurosci. 2022, 42, 1930–1944. [Google Scholar] [CrossRef]
- De Logu, F.; Nassini, R.; Hegron, A.; Landini, L.; Jensen, D.D.; Latorre, R.; Ding, J.; Marini, M.; de Araujo, D.S.M.; Ramírez-Garcia, P.; et al. Schwann cell endosome CGRP signals elicit periorbital mechanical allodynia in mice. Nat. Commun. 2022, 13, 646. [Google Scholar] [CrossRef]
- Iannone, L.F.; De Logu, F.; Geppetti, P.; De Cesaris, F. The role of TRP ion channels in migraine and headache. Neurosci. Lett. 2022, 768, 136380. [Google Scholar] [CrossRef]
- Schwedt, T.J.; Krauss, M.J.; Frey, K.; Gereau, I.R.W. Episodic and chronic migraineurs are hypersensitive to thermal stimuli between migraine attacks. Cephalalgia 2011, 31, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Vega, C.C.; Ahn, D.D.; Bischoff, C.; Wang, W.; Horne, D.; Wang, J.; Gavva, N.; Dussor, G. Meningeal transient receptor potential channel M8 activation causes cutaneous facial and hindpaw allodynia in a preclinical rodent model of headache. Cephalalgia 2016, 36, 185–193. [Google Scholar] [CrossRef]
- Ren, L.; Dhaka, A.; Cao, Y.Q. Function and postnatal changes of dural afferent fibers expressing TRPM8 channels. Mol. Pain 2015, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Askari-Zahabi, K.; Abbasnejad, M.; Kooshki, R.; Raoof, M.; Esmaeili-Mahani, S.; Pourrahimi, A.M.; Zamyad, M. The role of basolateral amygdala orexin 1 receptors on the modulation of pain and psychosocial deficits in nitroglycerin-induced migraine model in adult male rats. Korean J. Pain 2022, 35, 22–32. [Google Scholar] [CrossRef]
- Lance, J.W. Impact commentaries. Observations on 500 cases of migraine and allied vascular headache. J. Neurol. Neurosurg. Psychiatry 2012, 83, 673–674. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villar-Martinez, M.D.; Goadsby, P.J. Pathophysiology and Therapy of Associated Features of Migraine. Cells 2022, 11, 2767. https://doi.org/10.3390/cells11172767
Villar-Martinez MD, Goadsby PJ. Pathophysiology and Therapy of Associated Features of Migraine. Cells. 2022; 11(17):2767. https://doi.org/10.3390/cells11172767
Chicago/Turabian StyleVillar-Martinez, Maria Dolores, and Peter J. Goadsby. 2022. "Pathophysiology and Therapy of Associated Features of Migraine" Cells 11, no. 17: 2767. https://doi.org/10.3390/cells11172767
APA StyleVillar-Martinez, M. D., & Goadsby, P. J. (2022). Pathophysiology and Therapy of Associated Features of Migraine. Cells, 11(17), 2767. https://doi.org/10.3390/cells11172767








