Mineralocorticoid Receptor Antagonists Mitigate Mitral Regurgitation-Induced Myocardial Dysfunction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
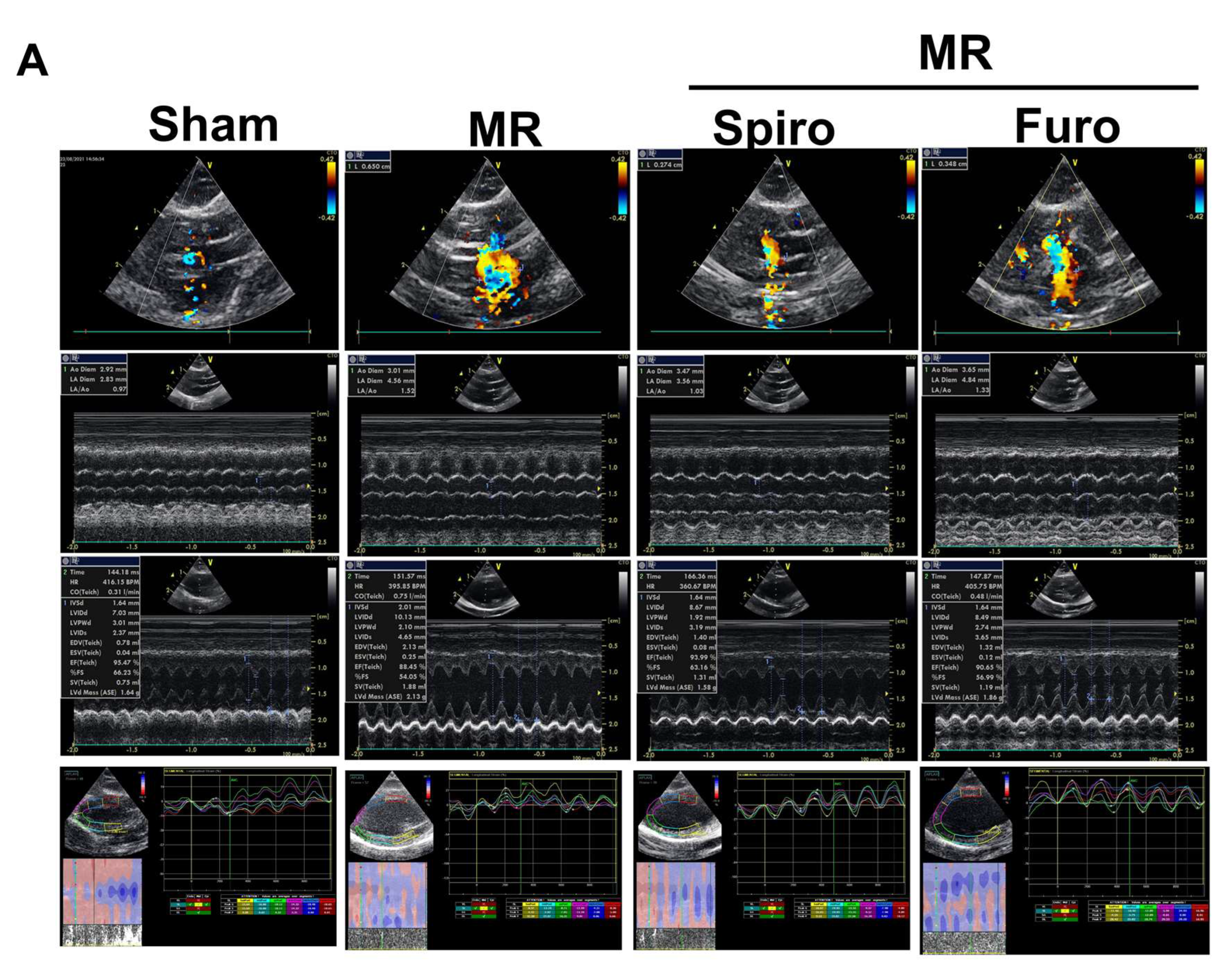
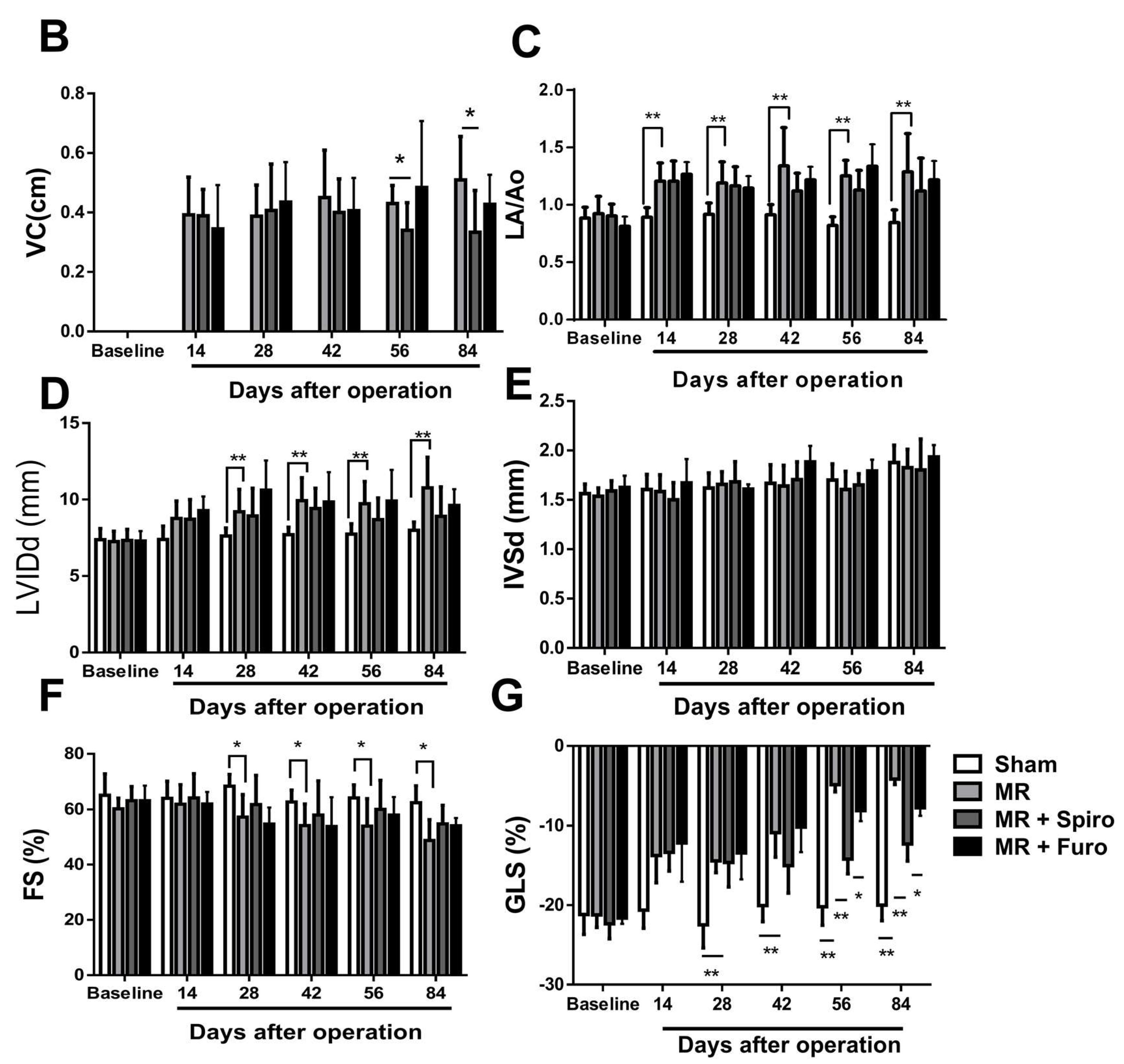
2.2. Echocardiographic and Strain Analyses in Animals
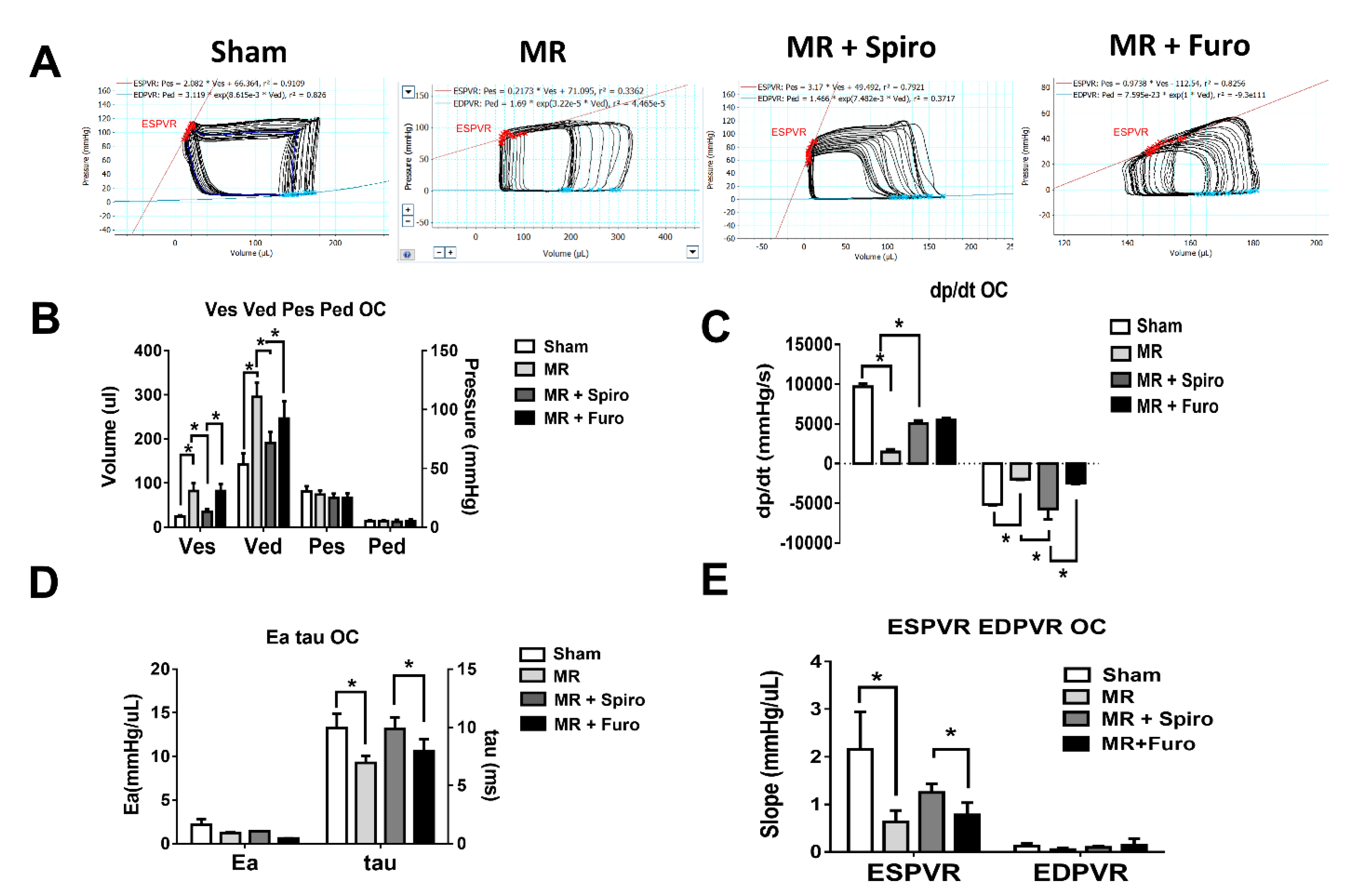
2.3. Hemodynamic Study of Pressure-Volume Loop (PV Loop)
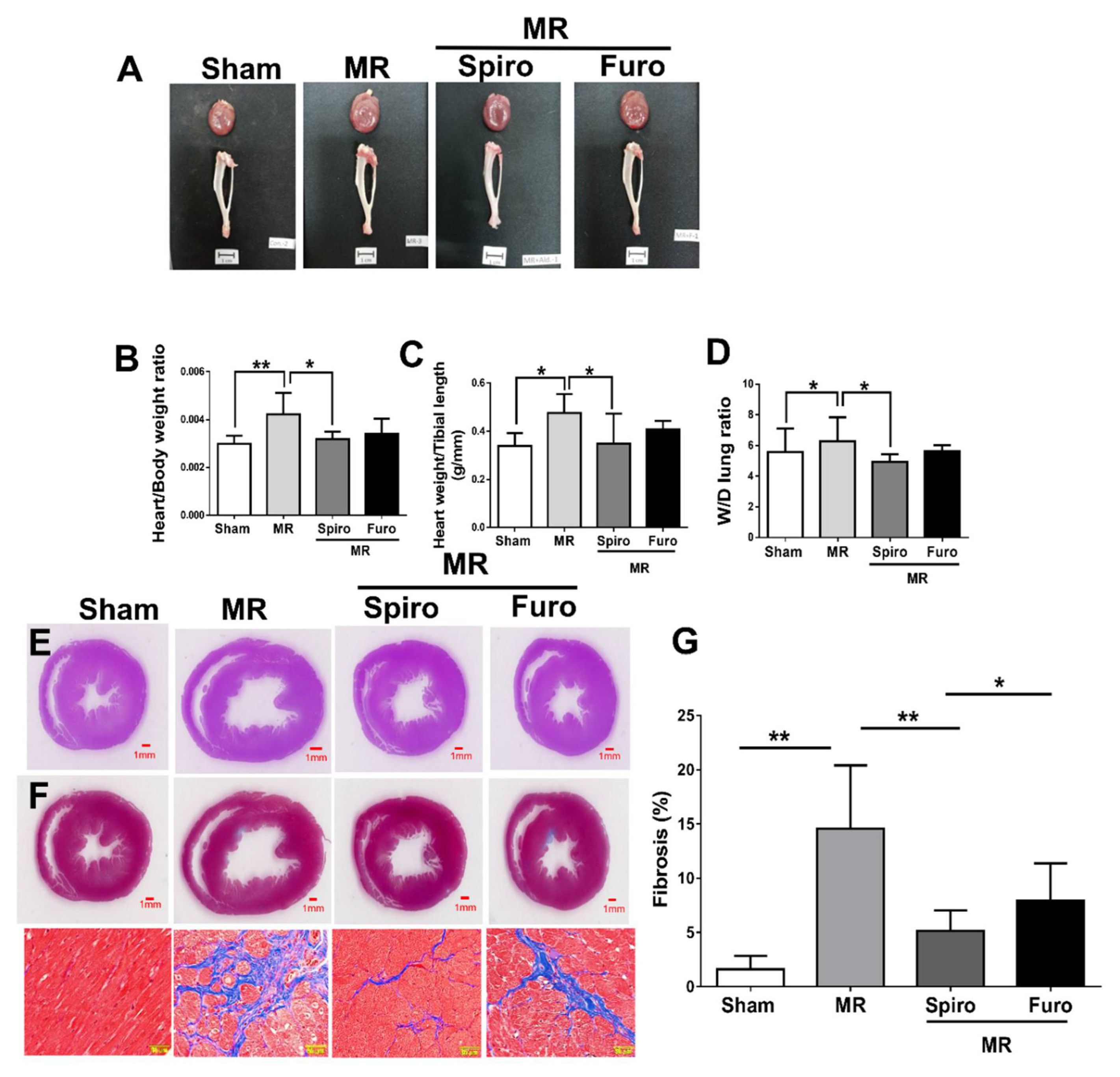
2.4. Histological Analysis
2.5. Western Blot
2.6. Patients and Clinical Study Designs
2.7. Echocardiographic Parameters in Patients
2.8. Statistics
3. Results
3.1. Spiro Improved Myocardial Strain in a Rat Model of MR
3.2. Spiro Improves MR-Induced Volume Overload in Hemodynamic Studies
3.3. Spiro Reduced MR-Induced Myocardial Fibrosis and Apoptosis
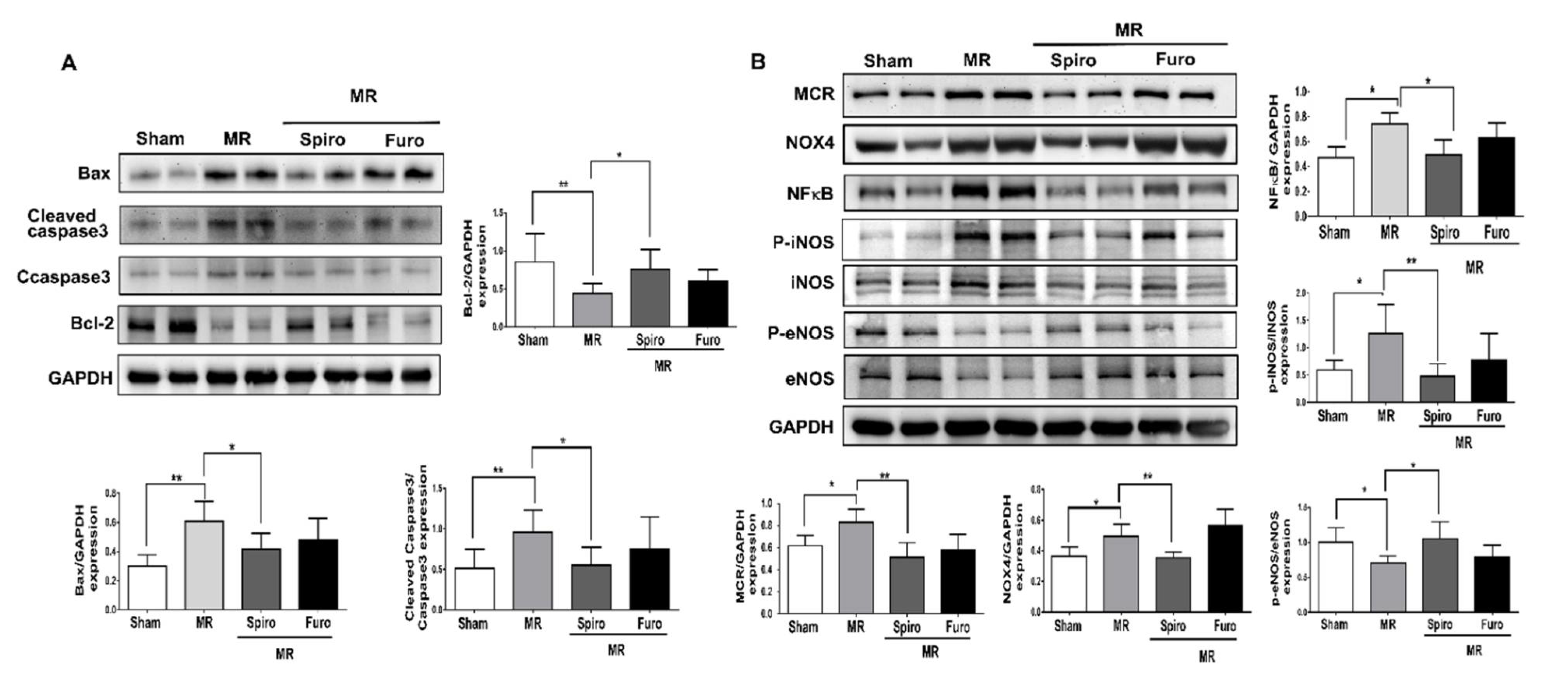
3.4. Spiro Treatment Attenuated Cardiac Apoptosis by Mediating Mineralocorticoid Receptors (MCRs) in MR Rats
3.5. Spiro Improved the Severity of MR in Patients
4. Discussion
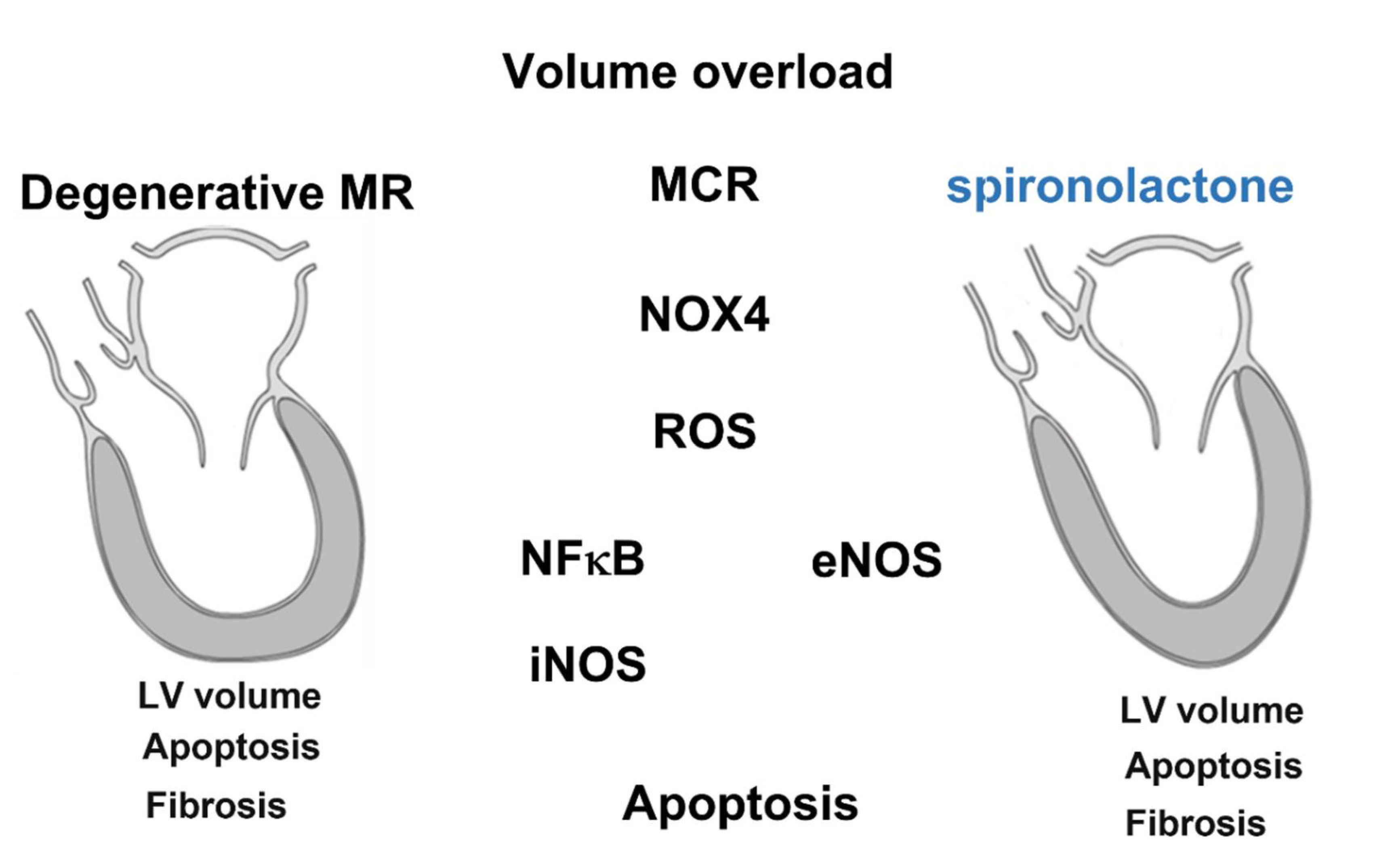
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar] [CrossRef]
- Antoine, C.; Mantovani, F.; Benfari, G.; Mankad, S.V.; Maalouf, J.F.; Michelena, H.I.; Enriquez-Sarano, M. Pathophysiology of Degenerative Mitral Regurgitation: New 3-Dimensional Imaging Insights. Circ Cardiovasc. Imaging 2018, 11, e005971. [Google Scholar] [CrossRef]
- Asgar, A.W.; Mack, M.J.; Stone, G.W. Secondary mitral regurgitation in heart failure: Pathophysiology, prognosis, and therapeutic considerations. J. Am. Coll. Cardiol. 2015, 65, 1231. [Google Scholar] [CrossRef]
- Gorini, S.; Kim, S.K.; Infante, M.; Mammi, C.; La Vignera, S.; Fabbri, A.; Jaffe, I.Z.; Caprio, M. Role of Aldosterone and Mineralocorticoid Receptor in Cardiovascular Aging. Front. Endocrinol. 2019, 10, 584. [Google Scholar] [CrossRef]
- Ibarrola, J.; Garcia-Pena, A.; Matilla, L.; Bonnard, B.; Sadaba, R.; Arrieta, V.; Alvarez, V.; Fernandez-Celis, A.; Gainza, A.; Navarro, A.; et al. A New Role for the Aldosterone/Mineralocorticoid Receptor Pathway in the Development of Mitral Valve Prolapse. Circ. Res. 2020, 127, e80–e93. [Google Scholar] [CrossRef]
- Nagase, M.; Ayuzawa, N.; Kawarazaki, W.; Ishizawa, K.; Ueda, K.; Yoshida, S.; Fujita, T. Oxidative stress causes mineralocorticoid receptor activation in rat cardiomyocytes: Role of small GTPase Rac1. Hypertension 2012, 59, 500–506. [Google Scholar] [CrossRef]
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N. Engl. J. Med. 1999, 341, 709–717. [Google Scholar] [CrossRef]
- Lin, Y.W.; Chen, C.Y.; Shih, J.Y.; Cheng, B.C.; Chang, C.P.; Lin, M.T.; Ho, C.H.; Chen, Z.C.; Fisch, S.; Chang, W.T. Dapagliflozin Improves Cardiac Hemodynamics and Mitigates Arrhythmogenesis in Mitral Regurgitation-Induced Myocardial Dysfunction. J. Am. Heart Assoc. 2021, 10, e019274. [Google Scholar] [CrossRef]
- Nagata, K.; Obata, K.; Xu, J.; Ichihara, S.; Noda, A.; Kimata, H.; Kato, T.; Izawa, H.; Murohara, T.; Yokota, M. Mineralocorticoid receptor antagonism attenuates cardiac hypertrophy and failure in low-aldosterone hypertensive rats. Hypertension 2006, 47, 656–664. [Google Scholar] [CrossRef]
- Pacher, P.; Nagayama, T.; Mukhopadhyay, P.; Batkai, S.; Kass, D.A. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat. Protoc. 2008, 3, 1422–1434. [Google Scholar] [CrossRef] [Green Version]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef] [PubMed]
- Dhalla, N.S.; Elimban, V.; Bartekova, M.; Adameova, A. Involvement of Oxidative Stress in the Development of Subcellular Defects and Heart Disease. Biomedicines 2022, 10, 393. [Google Scholar] [CrossRef] [PubMed]
- Gladden, J.D.; Ahmed, M.I.; Litovsky, S.H.; Schiros, C.G.; Lloyd, S.G.; Gupta, H.; Denney, T.S., Jr.; Darley-Usmar, V.; McGiffin, D.C.; Dell’Italia, L.J. Oxidative stress and myocardial remodeling in chronic mitral regurgitation. Am. J. Med. Sci. 2011, 342, 114–119. [Google Scholar] [CrossRef]
- Levine, R.A.; Jerosch-Herold, M.; Hajjar, R.J. Mitral Valve Prolapse: A Disease of Valve and Ventricle. J. Am. Coll. Cardiol. 2018, 72, 835–837. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Li, J.; Zhai, Y.; Li, Q.; Xie, D.; Zhang, J.; Xiao, Y.; Gao, X. Spironolactone suppresses aldosterone-induced Kv1.5 expression by attenuating mineralocorticoid receptor-Nox1/2/4-mediated ROS generation in neonatal rat atrial myocytes. Biochem. Biophys. Res. Commun. 2019, 520, 379–384. [Google Scholar] [CrossRef]
- McManus, D.D.; Freedman, J.E. MicroRNAs in platelet function and cardiovascular disease. Nat. Rev. Cardiol. 2015, 12, 711–717. [Google Scholar] [CrossRef]
- Cezar, M.D.; Damatto, R.L.; Pagan, L.U.; Lima, A.R.; Martinez, P.F.; Bonomo, C.; Rosa, C.M.; Campos, D.H.; Cicogna, A.C.; Gomes, M.J.; et al. Early Spironolactone Treatment Attenuates Heart Failure Development by Improving Myocardial Function and Reducing Fibrosis in Spontaneously Hypertensive Rats. Cell. Physiol. Biochem. 2015, 36, 1453–1466. [Google Scholar] [CrossRef]
- Milliez, P.; Deangelis, N.; Rucker-Martin, C.; Leenhardt, A.; Vicaut, E.; Robidel, E.; Beaufils, P.; Delcayre, C.; Hatem, S.N. Spironolactone reduces fibrosis of dilated atria during heart failure in rats with myocardial infarction. Eur. Heart J. 2005, 26, 2193–2199. [Google Scholar] [CrossRef]
- Rokutan, H.; Suckow, C.; von Haehling, S.; Strassburg, S.; Bockmeyer, B.; Doehner, W.; Waller, C.; Bauersachs, J.; von Websky, K.; Hocher, B.; et al. Furosemide induces mortality in a rat model of chronic heart failure. Int. J. Cardiol. 2012, 160, 20–25. [Google Scholar] [CrossRef]
- Zannad, F.; McMurray, J.J.; Krum, H.; van Veldhuisen, D.J.; Swedberg, K.; Shi, H.; Vincent, J.; Pocock, S.J.; Pitt, B.; Group, E.-H.S. Eplerenone in patients with systolic heart failure and mild symptoms. N. Engl. J. Med. 2011, 364, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Pitt, B.; Pfeffer, M.A.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Claggett, B.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; et al. Spironolactone for heart failure with preserved ejection fraction. N. Engl. J. Med. 2014, 370, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, S.; Orban, M.; Adamo, M.; Giannini, C.; Melica, B.; Karam, N.; Praz, F.; Kalbacher, D.; Koell, B.; Stolz, L.; et al. Guideline-directed medical therapy in patients undergoing transcatheter edge-to-edge repair for secondary mitral regurgitation. Eur. J. Heart Fail. 2022, in press. [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.; Byun, J.J.; Colvin, M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2021 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, e263–e421. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Ayuzawa, N.; Nagase, M.; Ueda, K.; Nishimoto, M.; Kawarazaki, W.; Marumo, T.; Aiba, A.; Sakurai, T.; Shindo, T.; Fujita, T. Rac1-Mediated Activation of Mineralocorticoid Receptor in Pressure Overload-Induced Cardiac Injury. Hypertension 2016, 67, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Zhang, M.; Benkhoff, S.; Mieth, A.; Pliquett, R.; Kosowski, J.; Kruse, C.; Luedike, P.; Michaelis, U.R.; Weissmann, N.; et al. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ. Res. 2012, 110, 1217–1225. [Google Scholar] [CrossRef] [Green Version]

| Parameters | Spironolactone (N = 213) | Furosemide (N = 252) | p Value |
|---|---|---|---|
| Age (y/o) | 61.8 ± 14.8 | 61.1 ± 16.5 | |
| Male gender, n (%) | 109 (51.1) | 117 (46.4) | 0.35 |
| BMI (kg/m2) | 22.9 ± 5.08 | 23.3 ± 4.6 | 0.24 |
| Heart rate (bpm) | 80 ± 17.2 | 80.1 ± 17.5 | 0.78 |
| SBP (mmHg) | 159.6 ± 30.6 | 160.2 ± 32.5 | 0.36 |
| DBP (mmHg) | 76.5 ± 16.4 | 78.9 ± 17.3 | 0.43 |
| Hypertension, n (%) | 153 (71.8) | 178 (70.6) | 0.42 |
| Diabetes, n (%) | 148 (69.4) | 182 (72.2) | 0.29 |
| CAD, n (%) | 53 (24.8) | 63 (25) | 0.25 |
| HF NYFcII, n (%) | 161 (75.5) | 205 (81.3) | 0.28 |
| HF NYFcIII, n (%) | 52 (24.4) | 47 (18.6) | 0.15 |
| Hyperlipidemia, n (%) | 49 (23) | 58 (23) | 0.74 |
| CKD (including H/D), n (%) | 61 (28.6) | 59 (23.4) | 0.12 |
| eGFR (mL/min/1.73m2) | 51.1 ± 30.5 | 55.1 ± 30.9 | 0.83 |
| ALT(mg/dL) | 24.1 ± 33.8 | 28.1 ± 44.9 | 0.53 |
| Total cholesterol(mg/dL) | 149.3 ± 42.7 | 146.8 ± 41.3 | 0.45 |
| LDL(mg/dL) | 83.7 ± 33.28926 | 81.1 ± 34.4 | 0.25 |
| NT-proBNP (pg/mL) | 331.1 ± 756.1 | 299.3 ± 465.2 | 0.29 |
| Concomitant medications | |||
| ACEIs/ARBs | 102 (47.8) | 118 (46.8) | 0.42 |
| β-Blocker | 45 (21.1) | 63 (25) | 0.34 |
| Statin | 34 (15.9) | 48 (19) | 0.63 |
| Antiplatelet/anticoagulants | 63 (29.6) | 76 (30.1) | 0.28 |
| Parameters | Spironolactone (N = 213) | Furosemide (N = 252) | p Value |
|---|---|---|---|
| Echocardiographic Characteristics | |||
| Left atrial volume index (mL/m2) | |||
| Baseline | 51.7 ± 20.67 | 50.7 ± 19.74 | 0.6 |
| Follow-up | 45.1 ± 15.87 | 47.9 ± 16.63 | 0.06 |
| Changes | −6.62 ± 19.21 | −2.88 ± 17 | 0.02 |
| LVESV, mL | |||
| Baseline | 39.8 ± 12.86 | 40 ± 16.84 | 0.9 |
| Follow-up | 35.7 ± 13.5 | 37.8 ± 13.9 | 0.11 |
| Changes | −4.1 ± 11.4 | −2.2 ± 10.3 | 0.06 |
| LVEDV, mL | |||
| Baseline | 89.2 ± 23.3 | 91.9 ± 24.73 | 0.13 |
| Follow-up | 76.9 ± 22.2 | 83.5 ± 21.5 | 0.01 |
| Changes | −12.3 ± 22.12 | −8.4 ± 21.06 | 0.05 |
| LVEF (%) | |||
| Baseline | 51.4 ± 10.1 | 52.5 ± 10.1 | 0.64 |
| Follow-up | 53.7 ± 9.3 | 51.9 ± 9.9 | 0.21 |
| Changes | 2.3 ± 11.8 | −0.5 ± 9.7 | 0.06 |
| LV mass index (g/m2) | |||
| Baseline | 117.5 ± 34.2 | 115.3 ± 38.5 | 0.95 |
| Follow-up | 107.1 ± 34.8 | 107.2 ± 28.3 | 0.4 |
| Changes | −10.4 ± 35.1 | −8.1 ± 31.5 | 0.12 |
| EROA of MR (cm2) | |||
| Baseline | 0.62 ± 0.09 | 0.57 ± 0.1 | 0.06 |
| Follow-up | 0.39 ± 0.14 | 0.5 ± 0.11 | 0.01 |
| Changes | −0.23 ± 0.15 | −0.07 ± 0.11 | 0.01 |
| Vena contracta (cm) | |||
| Baseline | 1.02 ± 0.47 | 0.94 ± 0.43 | 0.07 |
| Follow-up | 1.04 ± 0.37 | 0.95 ± 0.36 | 0.13 |
| Changes | −0.01 ± 0.4 | 0.01 ± 0.45 | 0.5 |
| Regurgitation volume (mL) | |||
| Baseline | 70.6 ± 19.8 | 64.5 ± 16.03 | 0.1 |
| Follow-up | 56.1 ± 20.5 | 55.0 ± 17.8 | 0.5 |
| Changes | −14.5 ± 23.2 | −9.4 ± 19.05 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, W.-T.; Lin, Y.-W.; Chen, C.-Y.; Chen, Z.-C.; Shih, J.-Y.; Wu, C.-C.; Luo, C.-Y.; Liu, P.-Y. Mineralocorticoid Receptor Antagonists Mitigate Mitral Regurgitation-Induced Myocardial Dysfunction. Cells 2022, 11, 2750. https://doi.org/10.3390/cells11172750
Chang W-T, Lin Y-W, Chen C-Y, Chen Z-C, Shih J-Y, Wu C-C, Luo C-Y, Liu P-Y. Mineralocorticoid Receptor Antagonists Mitigate Mitral Regurgitation-Induced Myocardial Dysfunction. Cells. 2022; 11(17):2750. https://doi.org/10.3390/cells11172750
Chicago/Turabian StyleChang, Wei-Ting, Yu-Wen Lin, Chin-Yu Chen, Zhih-Cherng Chen, Jhih-Yuan Shih, Chia-Ching Wu, Chwan-Yau Luo, and Ping-Yen Liu. 2022. "Mineralocorticoid Receptor Antagonists Mitigate Mitral Regurgitation-Induced Myocardial Dysfunction" Cells 11, no. 17: 2750. https://doi.org/10.3390/cells11172750
APA StyleChang, W.-T., Lin, Y.-W., Chen, C.-Y., Chen, Z.-C., Shih, J.-Y., Wu, C.-C., Luo, C.-Y., & Liu, P.-Y. (2022). Mineralocorticoid Receptor Antagonists Mitigate Mitral Regurgitation-Induced Myocardial Dysfunction. Cells, 11(17), 2750. https://doi.org/10.3390/cells11172750






