Postnatal and Adult Neurogenesis in Mammals, Including Marsupials
Abstract
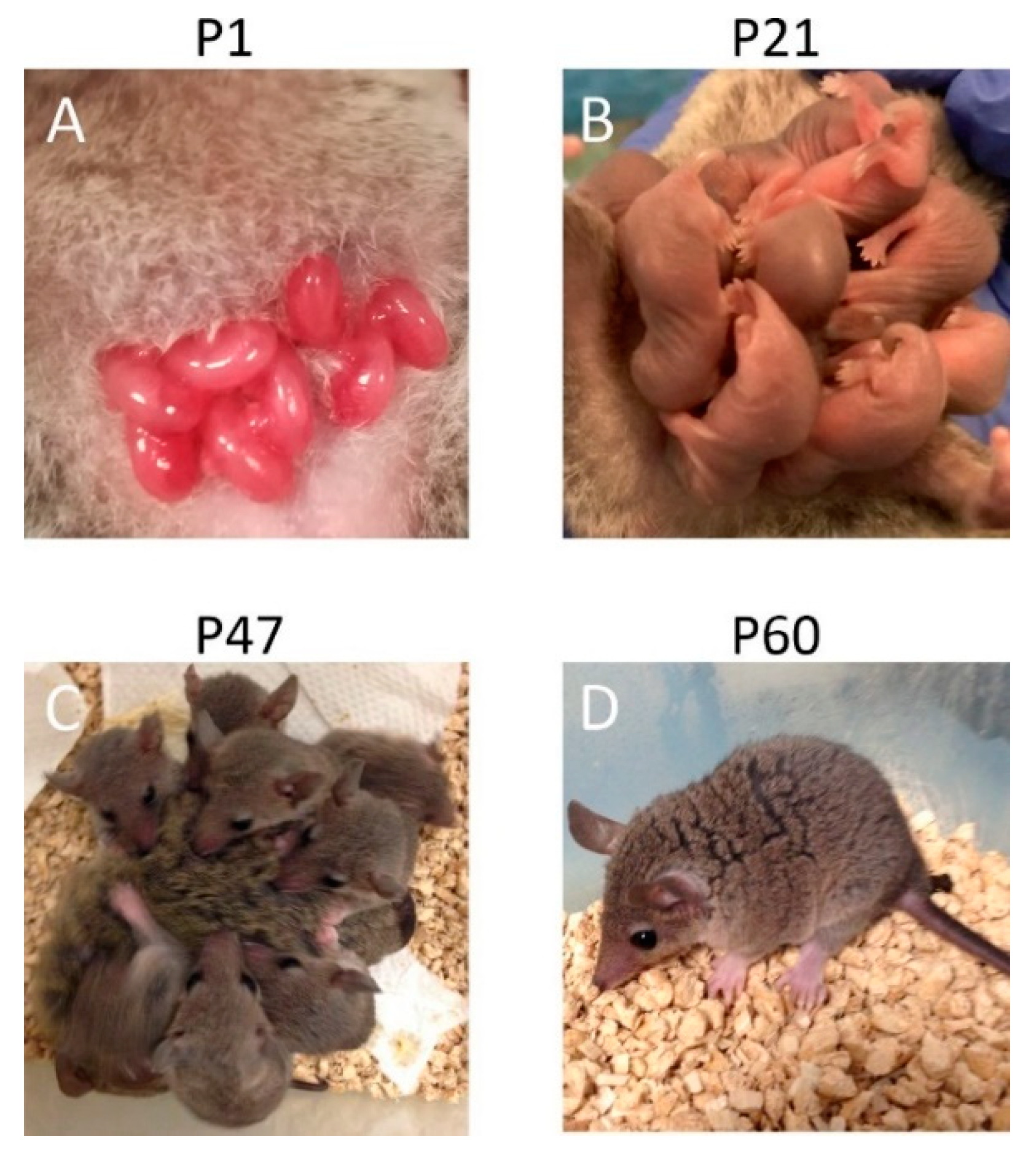
1. Introduction
2. Postnatal Developmental Neurogenesis in Mammals
Postnatal Neurogenesis in the Cerebellum
3. Adult Neurogenesis in Mammals
3.1. Adult Neurogenesis in the SVZ/OB
3.2. Does Adult Neurogenesis Occur in the Piriform Cortex?
3.3. Adult-Born Neurons of the Dentate Gyrus (DG)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ashwell, K.W.S. The Neurobiology of Australian Marsupials. Brain Evolution in the Other Mammalian Radiation; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2010; pp. 18–119. [Google Scholar]
- Chuah, M.I.; Tennent, R.; Teague, R. Developmental anatomy of the primary olfactory pathway in the opossum Monodelphis domestica. Histol. Histopathol. 1997, 12, 799–806. [Google Scholar] [PubMed]
- Sanderson, K.J.; Wilson, P.M. Neurogenesis in septum, amygdala and hippocampus in the marsupial brush-tailed possum (Trichosurus vulpecula). Rev. Bras. Biol. 1997, 57, 323–335. [Google Scholar]
- Sanderson, K.J.; Weller, W.L. Neurogenesis in a marsupial: The brush-tailed possum (Trichosurus vulpecula). II. Sensorimotor pathways. Brain Behav. Evol. 1990, 35, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Butts, T.; Green, M.J.; Wingate, R.J. Development of the cerebellum: Simple steps to make a ‘little brain’. Development 2014, 141, 4031–4041. [Google Scholar] [CrossRef] [PubMed]
- Altman, J.; Das, G.D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965, 124, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Bayer, S.A. Joseph Altman (1925–2016): A life in neurodevelopment. J. Comp. Neurol. 2016, 524, 2933–2943. [Google Scholar] [CrossRef]
- Ramon y Cajal, S. Histologie du Systeme Nerveux de L’homme et des Vertebres. Paris Maloine 1909, 2, 80–106. [Google Scholar]
- Kuhn, H.G.; Dickinson-Anson, H.; Gage, F.H. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996, 16, 2027–2033. [Google Scholar] [CrossRef]
- Lois, C.; Alvarez-Buylla, A. Long-distance neuronal migration in the adult mammalian brain. Science 1994, 264, 1145–1148. [Google Scholar] [CrossRef]
- Rao, M.S.; Hattiangady, B.; Shetty, A.K. The window and mechanisms of major age-related decline in the production of new neurons within the dentate gyrus of the hippocampus. Aging Cell 2006, 5, 545–558. [Google Scholar] [CrossRef]
- Conover, J.C.; Shook, B.A. Aging of the subventricular zone neural stem cell niche. Aging Dis. 2011, 2, 49–63. [Google Scholar] [PubMed]
- Duque, A.; Arellano, J.I.; Rakic, P. An assessment of the existence of adult neurogenesis in humans and value of its rodent models for neuropsychiatric diseases. Mol. Psychiatry 2022, 27, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, D.M.; Bordey, A.; Bonfanti, L. Noncanonical Sites of Adult Neurogenesis in the Mammalian Brain. Cold Spring Harb. Perspect. Biol. 2015, 7, a018846. [Google Scholar] [CrossRef] [PubMed]
- Jurkowski, M.P.; Bettio, L.K.; Woo, E.; Patten, A.; Yau, S.Y.; Gil-Mohapel, J. Beyond the Hippocampus and the SVZ: Adult Neurogenesis Throughout the Brain. Front. Cell Neurosci. 2020, 14, 576444. [Google Scholar] [CrossRef] [PubMed]
- Ponti, G.; Peretto, P.; Bonfanti, L. Genesis of neuronal and glial progenitors in the cerebellar cortex of peripuberal and adult rabbits. PLoS ONE 2008, 3, e2366. [Google Scholar] [CrossRef]
- Luo, Z.X.; Yuan, C.X.; Meng, Q.J.; Ji, Q. A Jurassic eutherian mammal and divergence of marsupials and placentals. Nature 2011, 476, 442–445. [Google Scholar] [CrossRef]
- Rose, R.W. Embryonic growth rates of marsupials with a note on monotremes. J. Zool. Lond. 1989, 218, 11–16. [Google Scholar] [CrossRef]
- Hickford, D.; Frankenberg, S.; Renfree, M.B. The tammar wallaby, Macropus eugenii: A model kangaroo for the study of developmental and reproductive biology. Cold Spring Harb. Protoc. 2009, 2009, pdb.emo137. [Google Scholar] [CrossRef]
- Smith, K.K. Early development of the neural plate, neural crest and facial region of marsupials. J. Anat. 2001, 199, 121–131. [Google Scholar] [CrossRef]
- Warner, F.J. The development of the diencephalon in Trichosurus vulpecula. Okajimas Folia Anat. Jpn. 1969, 46, 265–295. [Google Scholar] [CrossRef][Green Version]
- Harman, A.M.; Eastough, N.J.; Beazley, L.D. Development of the visual cortex in a wallaby—Phylogenetic implications. Brain Behav. Evol. 1995, 45, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Renfree, M.B.; Holt, A.B.; Green, S.W.; Carr, J.P.; Cheek, D.B. Ontogeny of the brain in a marsupial (Macropus eugenii) throughout pouch life. I. Brain growth. Brain Behav. Evol. 1982, 20, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Sauerland, C.; Menzies, B.R.; Glatzle, M.; Seeger, J.; Renfree, M.B.; Fietz, S.A. The Basal Radial Glia Occurs in Marsupials and Underlies the Evolution of an Expanded Neocortex in Therian Mammals. Cereb. Cortex 2018, 28, 145–157. [Google Scholar] [CrossRef]
- Harder, J.D.; Stonerook, M.J.; Pondy, J. Gestation and placentation in two New World opossums: Didelphis virginiana and Monodelphis domestica. J. Exp. Zool. 1993, 266, 463–479. [Google Scholar] [CrossRef] [PubMed]
- Saunders, N.R.; Adam, E.; Reader, M.; Møllgård, K. Monodelphis domestica (grey short-tailed opossum): An accessible model for studies of early neocortical development. Anat. Embryol. 1989, 180, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Moreira, M.; Halbert, J.; Valloton, D.; Velten, B.; Chen, C.; Shao, Y.; Liechti, A.; Ascenção, K.; Rummel, C.; Ovchinnikova, S.; et al. Gene expression across mammalian organ development. Nature 2019, 571, 505–509. [Google Scholar] [CrossRef]
- Brunjes, P.C.; Jazaeri, A.; Sutherland, M.J. Olfactory bulb organization and development in Monodelphis domestica (grey short-tailed opossum). J. Comp. Neurol. 1992, 320, 544–554. [Google Scholar] [CrossRef]
- Tepper, B.; Bartkowska, K.; Okrasa, M.; Ngati, S.; Braszak, M.; Turlejski, K.; Djavadian, R. Downregulation of TrkC Receptors Increases Dendritic Arborization of Purkinje Cells in the Developing Cerebellum of the Opossum, Monodelphis domestica. Front. Neuroanat. 2020, 14, 56. [Google Scholar] [CrossRef]
- Reynolds, M.L.; Cavanagh, M.E.; Dziegielewska, K.M.; Hinds, L.A.; Saunders, N.R.; Tyndale-Biscoe, C.H. Postnatal development of the telencephalon of the tammar wallaby (Macropus eugenii). An accessible model of neocortical differentiation. Anat. Embryol 1985, 173, 81–94. [Google Scholar] [CrossRef]
- Curley, J.P.; Jordan, E.R.; Swaney, W.T.; Izraelit, A.; Kammel, S.; Champagne, F.A. The meaning of weaning: Influence of the weaning period on behavioral development in mice. Dev. Neurosci. 2009, 31, 318–331. [Google Scholar] [CrossRef]
- Shimada, M.; Nakamura, T. Time of neuron origin in mouse hypothalamic nuclei. Exp. Neurol. 1973, 41, 163–173. [Google Scholar] [CrossRef]
- Shimogori, T.; A Lee, D.; Miranda-Angulo, A.; Yang, Y.; Wang, H.; Jiang, L.; Yoshida, A.C.; Kataoka, A.; Mashiko, H.; Avetisyan, M.; et al. A genomic atlas of mouse hypothalamic development. Nat. Neurosci. 2010, 13, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Marotte, L.R.; Mai, J.K.; Ashwell, K.W. Early development of the hypothalamus of a wallaby (Macropus eugenii). J. Comp. Neurol. 2002, 453, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Rivkees, S.A.; Fox, C.A.; Jacobson, C.D.; Reppert, S.M. Anatomic and functional development of the suprachiasmatic nuclei in the gray short-tailed opossum. J. Neurosci. 1988, 8, 4269–4276. [Google Scholar] [CrossRef] [PubMed]
- Noctor, S.C.; Flint, A.C.; Weissman, T.A.; Dammerman, R.S.; Kriegstein, A.R. Neurons derived from radial glial cells establish radial units in neocortex. Nature 2001, 409, 714–720. [Google Scholar] [CrossRef]
- Rakic, P. Evolution of the neocortex: A perspective from developmental biology. Nat. Rev. Neurosci. 2009, 10, 724–735. [Google Scholar] [CrossRef]
- Katz, M.J.; Lasek, R.J.; Silver, J. Ontophyletics of the nervous system: Development of the corpus callosum and evolution of axon tracts. Proc. Natl. Acad. Sci. USA 1983, 80, 5936–5940. [Google Scholar] [CrossRef]
- Cheung, A.F.; Kondo, S.; Abdel-Mannan, O.; Chodroff, R.A.; Sirey, T.M.; Bluy, L.E.; Webber, N.; DeProto, J.; Karlen, S.J.; Krubitzer, L.; et al. The subventricular zone is the developmental milestone of a 6-layered neocortex: Comparisons in metatherian and eutherian mammals. Cereb. Cortex 2010, 20, 1071–1081. [Google Scholar] [CrossRef]
- Puzzolo, E.; Mallamaci, A. Cortico-cerebral histogenesis in the opossum Monodelphis domestica: Generation of a hexalaminar neocortex in the absence of a basal proliferative compartment. Neural. Dev. 2010, 5, 8. [Google Scholar] [CrossRef]
- Bartkowska, K.; Gajerska, M.; Turlejski, K.; Djavadian, R.L. Expression of TrkC receptors in the developing brain of the Monodelphis opossum and its effect on the development of cortical cells. PLoS ONE 2013, 8, e74346. [Google Scholar] [CrossRef]
- Bartkowska, K.; Tepper, B.; Gawda, A.; Jarosik, M.; Sobolewska, P.; Turlejski, K.; Djavadian, R.L. Inhibition of TrkB- and TrkC-Signaling Pathways Affects Neurogenesis in the Opossum Developing Neocortex. Cereb. Cortex 2019, 29, 3666–3675. [Google Scholar] [CrossRef] [PubMed]
- Polleux, F.; Dehay, C.; Kennedy, H. The timetable of laminar neurogenesis contributes to the specification of cortical areas in mouse isocortex. J. Comp. Neurol. 1997, 385, 95–116. [Google Scholar] [CrossRef]
- Sansom, S.N.; Livesey, F.J. Gradients in the brain: The control of the development of form and function in the cerebral cortex. Cold Spring Harb. Perspect. Biol. 2009, 1, a002519. [Google Scholar] [CrossRef]
- Lorente de N´, R. Studies on the structure of the cerebral cortex II. Continuation of the study of the Ammonic system. J. Psychol. Neurol. 1934, 46, 113–177. [Google Scholar]
- Insausti, R. Comparative anatomy of the entorhinal cortex and hippocampus in mammals. Hippocampus 1993, 3, 19–26. [Google Scholar] [CrossRef]
- Amaral, D.G. A Golgi study of cell types in the hilar region of the hippocampus in the rat. J. Comp. Neurol. 1978, 182, 851–914. [Google Scholar] [CrossRef]
- Angevine, J.B., Jr. Time of neuron origin in the hippocampal region. An autoradiographic study in the mouse. Exp. Neurol. Suppl. 1965, 2, 1–70. [Google Scholar]
- Bond, A.M.; Berg, D.A.; Lee, S.; Garcia-Epelboim, A.S.; Adusumilli, V.S.; Ming, G.L.; Song, H. Differential Timing and Coordination of Neurogenesis and Astrogenesis in Developing Mouse Hippocampal Subregions. Brain Sci. 2020, 10, 909. [Google Scholar] [CrossRef]
- Harman, A.M. Development and cell generation in the hippocampus of a marsupial, the quokka wallaby (Setonix brachyurus). Brain Res. Dev. Brain Res. 1997, 104, 41–54. [Google Scholar] [CrossRef]
- Nagayama, S.; Homma, R.; Imamura, F. Neuronal organization of olfactory bulb circuits. Front. Neural Circuits 2014, 8, 98. [Google Scholar] [CrossRef]
- Treloar, H.B.; Miller, A.M.; Ray, A.; Greer, C.A. Development of the Olfactory System. In The Neurobiology of Olfaction; Menini, A., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2010; Chapter 5. Available online: https://www.ncbi.nlm.nih.gov/books/NBK55972/ (accessed on 1 July 2022).
- Brunjes, P.C.; Frazier, L.L. Maturation and plasticity in the olfactory system of vertebrates. Brain Res. 1986, 396, 1–45. [Google Scholar] [CrossRef]
- Ashwell, K.W.; Marotte, L.R.; Cheng, G. Development of the olfactory system in a wallaby (Macropus eugenii). Brain Behav. Evol. 2008, 71, 216–230. [Google Scholar] [CrossRef]
- Sotelo, C. Viewing the cerebellum through the eyes of Ramón Y Cajal. Cerebellum 2008, 7, 517–522. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sultan, F.; Glickstein, M. The cerebellum: Comparative and animal studies. Cerebellum 2007, 6, 168–176. [Google Scholar] [CrossRef]
- Sánchez-Villagra, M.R.; Sultan, F. The cerebellum at birth in therian mammals, with special reference to rodents. Brain Behav. Evol. 2002, 59, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Altman, J.; Bayer, S.A. Prenatal development of the cerebellar system in the rat. I. Cytogenesis and histogenesis of the deep nuclei and the cortex of the cerebellum. J. Comp. Neurol. 1978, 179, 23–48. [Google Scholar] [CrossRef] [PubMed]
- Sotelo, C. Cellular and genetic regulation of the development of the cerebellar system. Prog. Neurobiol. 2004, 72, 295–339. [Google Scholar] [CrossRef] [PubMed]
- Altman, J.; Bayer, S.A. Embryonic development of the rat cerebellum. III. Regional differences in the time of origin, migration, and settling of Purkinje cells. J. Comp. Neurol. 1985, 231, 42–65. [Google Scholar] [CrossRef]
- Rakic, P.; Sidman, R.L. Histogenesis of cortical layers in human cerebellum, particularly the lamina dissecans. J. Comp. Neurol. 1970, 139, 473–500. [Google Scholar] [CrossRef]
- Hatten, M.E.; Heintz, N. Mechanisms of neural patterning and specification in the developing cerebellum. Annu. Rev. Neurosci. 1995, 18, 85–408. [Google Scholar] [CrossRef]
- Consalez, G.G.; Goldowitz, D.; Casoni, F.; Hawkes, R. Origins, Development, and Compartmentation of the Granule Cells of the Cerebellum. Front. Neural Circuits 2021, 14, 611841. [Google Scholar] [CrossRef] [PubMed]
- Abrahám, H.; Tornóczky, T.; Kosztolányi, G.; Seress, L. Cell formation in the cortical layers of the developing human cerebellum. Int. J. Dev. Neurosci. 2001, 19, 53–62. [Google Scholar] [CrossRef]
- Grabiec, M.; Turlejski, K.; Djavadian, R.L. The partial 5-HT1A receptor agonist buspirone enhances neurogenesis in the opossum (Monodelphis domestica). Eur. Neuropsychopharmacol. 2009, 19, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Harman, A.; Meyer, P.; Ahmat, A. Neurogenesis in the hippocampus of an adult marsupial. Brain Behav. Evol. 2003, 62, 1–12. [Google Scholar] [CrossRef]
- Kaplan, M.S.; Bell, D.H. Mitotic neuroblasts in the 9-day-old and 11-month-old rodent hippocampus. J. Neurosci. 1984, 4, 1429–1441. [Google Scholar] [CrossRef]
- Guidi, S.; Ciani, E.; Severi, S.; Contestabile, A.; Bartesaghi, R. Postnatal neurogenesis in the dentate gyrus of the guinea pig. Hippocampus 2005, 15, 285–301. [Google Scholar] [CrossRef]
- Amrein, I. Adult hippocampal neurogenesis in natural populations of mammals. Cold Spring Harb. Perspect. Biol. 2015, 7, a021295. [Google Scholar] [CrossRef]
- Bekiari, C.; Grivas, I.; Tsingotjidou, A.; Papadopoulos, G.C. Adult neurogenesis and gliogenesis in the dorsal and ventral canine hippocampus. J. Comp. Neurol. 2020, 528, 1216–1230. [Google Scholar] [CrossRef]
- Altman, J. Autoradiographic investigation of cell proliferation in the brains of rats and cats. Anat. Rec. 1963, 145, 573–591. [Google Scholar] [CrossRef]
- Lévy, F.; Batailler, M.; Meurisse, M.; Migaud, M. Adult Neurogenesis in Sheep: Characterization and Contribution to Reproduction and Behavior. Front. Neurosci. 2017, 11, 570. [Google Scholar] [CrossRef]
- Low, V.F.; Faull, R.L.; Bennet, L.; Gunn, A.J.; Curtis, M.A. Neurogenesis and progenitor cell distribution in the subgranular zone and subventricular zone of the adult sheep brain. Neuroscience 2013, 244, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Bartkowska, K.; Djavadian, R.L.; Taylor, J.R.; Turlejski, K. Generation recruitment and death of brain cells throughout the life cycle of Sorex shrews (Lipotyphla). Eur. J. Neurosci. 2008, 27, 1710–1721. [Google Scholar] [CrossRef] [PubMed]
- Bartkowska, K.; Turlejski, K.; Tepper, B.; Rychlik, L.; Vogel, P.; Djavadian, R. Effects of Brain Size on Adult Neurogenesis in Shrews. Int. J. Mol. Sci. 2021, 22, 7664. [Google Scholar] [CrossRef]
- Patzke, N.; Kaswera, C.; Gilissen, E.; Ihunwo, A.O.; Manger, P.R. Adult neurogenesis in a giant otter shrew (Potamogale velox). Neuroscience 2013, 238, 270–279. [Google Scholar] [CrossRef]
- Gould, E.; McEwen, B.S.; Tanapat, P.; Galea, L.A.; Fuchs, E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J. Neurosci. 1997, 17, 2492–2498. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.Q.; Luo, R.; Tu, T.; Yang, C.; Jiang, J.; Zhang, B.; Bi, R.; Tu, E.; Yao, Y.G.; Yan, X.X. Doublecortin-Expressing Neurons in Chinese Tree Shrew Forebrain Exhibit Mixed Rodent and Primate-Like Topographic Characteristics. Front. Neuroanat. 2021, 15, 727883. [Google Scholar] [CrossRef] [PubMed]
- Patzke, N.; LeRoy, A.; Ngubane, N.W.; Bennett, N.C.; Medger, K.; Gravett, N.; Kaswera-Kyamakya, C.; Gilissen, E.; Chawana, R.; Manger, P.R. The distribution of doublecortin-immunopositive cells in the brains of four afrotherian mammals: The Hottentot golden mole (Amblysomus hottentotus), the rock hyrax (Procavia capensis), the eastern rock sengi (Elephantulus myurus) and the four-toed sengi (Petrodromus tetradactylus). Brain Behav. Evol. 2014, 84, 227–241. [Google Scholar] [CrossRef]
- Bartkowska, K.; Turlejski, K.; Grabiec, M.; Ghazaryan, A.; Yavruoyan, E.; Djavadian, R.L. Adult neurogenesis in the hedgehog (Erinaceus concolor) and mole (Talpa europaea). Brain Behav. Evol. 2010, 76, 128–143. [Google Scholar] [CrossRef]
- Chawana, R.; Alagaili, A.; Patzke, N.; Spocter, M.A.; Mohammed, O.B.; Kaswera, C.; Gilissen, E.; Bennett, N.C.; Ihunwo, A.O.; Manger, P.R. Microbats appear to have adult hippocampal neurogenesis, but post-capture stress causes a rapid decline in the number of neurons expressing doublecortin. Neuroscience 2014, 277, 724–733. [Google Scholar] [CrossRef]
- Chawana, R.; Patzke, N.; Bhagwandin, A.; Kaswera-Kyamakya, C.; Gilissen, E.; Bertelsen, M.F.; Hemingway, J.; Manger, P.R. Adult hippocampal neurogenesis in Egyptian fruit bats from three different environments: Are interpretational variations due to the environment or methodology? J. Comp. Neurol. 2020, 528, 2994–3007. [Google Scholar] [CrossRef]
- Eckenhoff, M.F.; Rakic, P. Nature and fate of proliferative cells in the hippocampal dentate gyrus during the life span of the rhesus monkey. J. Neurosci. 1988, 8, 729–747. [Google Scholar] [CrossRef]
- Kornack, D.R.; Rakic, P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc. Natl. Acad. Sci. USA 1999, 96, 5768–5773. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, P.S.; Perfilieva, E.; Björk-Eriksson, T.; Alborn, A.M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998, 4, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Gould, E.; Reeves, A.J.; Fallah, M.; Tanapat, P.; Gross, C.G.; Fuchs, E. Hippocampal neurogenesis in adult Old World primates. Proc. Natl. Acad. Sci. USA 1999, 96, 5263–5267. [Google Scholar] [CrossRef]
- Xie, Y.-W.; Li, Z.-Y.; Du, J.; Chen, Y.; Chen, B.-Y.; Wang, T.-T.; Huang, Z.; Hou, S.; Wang, Y. Visualization of Rostral Migratory Stream in the Developing Rat Brain by In Vivo Electroporation. Cell Mol. Neurobiol. 2018, 38, 1067–1079. [Google Scholar] [CrossRef]
- Dayer, A.G.; Ford, A.A.; Cleaver, K.M.; Yassaee, M.; Cameron, H.A. Short-term and long-term survival of new neurons in the rat dentate gyrus. J. Comp. Neurol. 2003, 460, 563–572. [Google Scholar] [CrossRef]
- Schlessinger, A.R.; Cowan, W.M.; Gottlieb, D.I. An autoradiographic study of the time of origin and the pattern of granule cell migration in the dentate gyrus of the rat. J. Comp. Neurol. 1975, 159, 149–175. [Google Scholar] [CrossRef]
- Fukushima, N.; Kato, T.; Li, Z.; Yokouchi, K.; Moriizumi, T. Adult neurogenesis and gliogenesis in the rat olfactory nervous system. Chem. Sens. 2005, 30 (Suppl. S1), i113–i114. [Google Scholar] [CrossRef]
- Coviello, S.; Gramuntell, Y.; Castillo-Gomez, E.; Nacher, J. Effects of Dopamine on the Immature Neurons of the Adult Rat Piriform Cortex. Front. Neurosci. 2020, 14, 574234. [Google Scholar] [CrossRef]
- Xu, Y.; Tamamaki, N.; Noda, T.; Kimura, K.; Itokazu, Y.; Matsumoto, N.; Dezawa, M.; Ide, C. Neurogenesis in the ependymal layer of the adult rat 3rd ventricle. Exp. Neurol. 2005, 192, 251–264. [Google Scholar] [CrossRef]
- Pérez-Martín, M.; Cifuentes, M.; Grondona, J.M.; López-Avalos, M.D.; Gómez-Pinedo, U.; García-Verdugo, J.M.; Fernández-Llebrez, P. IGF-I stimulates neurogenesis in the hypothalamus of adult rats. Eur. J. Neurosci. 2010, 31, 1533–1548. [Google Scholar] [CrossRef] [PubMed]
- Dayer, A.G.; Cleaver, K.M.; Abouantoun, T.; Cameron, H.A. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J. Cell Biol. 2005, 168, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Hay, M.; Amilhon, B.; Jean, A.; Moyse, E. In vivo neurogenesis in the dorsal vagal complex of the adult rat brain-stem. Neuroscience 2005, 130, 75–90. [Google Scholar] [CrossRef]
- Batista-Brito, R.; Close, J.; Machold, R.; Fishell, G. The distinct temporal origins of olfactory bulb interneuron subtypes. J. Neurosci. 2008, 28, 3966–3975. [Google Scholar] [CrossRef]
- Matsue, K.; Minakawa, S.; Kashiwagi, T.; Toda, K.; Sato, T.; Shioda, S.; Seki, T. Dentate granule progenitor cell properties are rapidly altered soon after birth. Brain Struct. Funct. 2018, 223, 357–369. [Google Scholar] [CrossRef]
- Olaleye, O.O.; Ihunwo, A.O. Adult neurogenesis in the four-striped mice (Rhabdomys pumilio). Neural. Regen. Res. 2014, 9, 1907–1911. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, L.A.; Ng, K.; Zhou, Q.Y.; Ribak, C.E. Subventricular zone-derived, newly generated neurons populate several olfactory and limbic forebrain regions. Epilepsy Behav. 2009, 14 (Suppl. S1), 74–80. [Google Scholar] [CrossRef] [PubMed]
- Salvi, R.; Steigleder, T.; Schlachetzki, J.C.; Waldmann, E.; Schwab, S.; Winner, B.; Winkler, J.; Kohl, Z. Distinct Effects of Chronic Dopaminergic Stimulation on Hippocampal Neurogenesis and Striatal Doublecortin Expression in Adult Mice. Front. Neurosci. 2016, 10, 77. [Google Scholar] [CrossRef]
- Klempin, F.; Kronenberg, G.; Cheung, G.; Kettenmann, H.; Kempermann, G. Properties of doublecortin-(DCX)-expressing cells in the piriform cortex compared to the neurogenic dentate gyrus of adult mice. PLoS ONE 2011, 6, e25760. [Google Scholar] [CrossRef]
- Zhao, M.; Momma, S.; Delfani, K.; Carlen, M.; Cassidy, R.M.; Johansson, C.B.; Brismar, H.; Shupliakov, O.; Frisen, J.; Janson, A.M. Evidence for neurogenesis in the adult mammalian substantia nigra. Proc Natl. Acad. Sci. USA 2003, 100, 7925–7930. [Google Scholar] [CrossRef]
- Jhaveri, D.J.; Tedoldi, A.; Hunt, S.; Sullivan, R.; Watts, N.R.; Power, J.M.; Bartlett, P.F.; Sah, P. Evidence for newly generated interneurons in the basolateral amygdala of adult mice. Mol. Psychiatry 2018, 23, 521–532. [Google Scholar] [CrossRef]
- Magavi, S.S.; Leavitt, B.R.; Macklis, J.D. Induction of neurogenesis in the neocortex of adult mice. Nature 2000, 405, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Batailler, M.; Droguerre, M.; Baroncini, M.; Fontaine, C.; Prevot, V.; Migaud, M. DCX-expressing cells in the vicinity of the hypothalamic neurogenic niche: A comparative study between mouse, sheep, and human tissues. J. Comp. Neurol. 2014, 522, 1966–1985. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Bittman, E.L. Olfactory bulb cells generated in adult male golden hamsters are specifically activated by exposure to estrous females. Horm. Behav. 2002, 4, 343–350. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mohr, M.A.; Sisk, C.L. Pubertally born neurons and glia are functionally integrated into limbic and hypothalamic circuits of the male Syrian hamster. Proc. Natl. Acad. Sci. USA 2013, 110, 4792–4797. [Google Scholar] [CrossRef]
- Fowler, C.D.; Liu, Y.; Ouimet, C.; Wang, Z. The effects of social environment on adult neurogenesis in the female prairie vole. J. Neurobiol. 2002, 51, 115–128. [Google Scholar] [CrossRef]
- Fowler, C.D.; Johnson, F.; Wang, Z. Estrogen regulation of cell proliferation and distribution of estrogen receptor-alpha in the brains of adult female prairie and meadow voles. J. Comp. Neurol. 2005, 489, 166–179. [Google Scholar] [CrossRef]
- Castro, A.E.; Young, L.J.; Camacho, F.J.; Paredes, R.G.; Diaz, N.F.; Portillo, W. Effects of Mating and Social Exposure on Cell Proliferation in the Adult Male Prairie Vole (Microtus ochrogaster). Neural. Plast. 2020, 2020, 8869669. [Google Scholar] [CrossRef]
- Jara, N.; Cifuentes, M.; Martínez, F.; Salazar, K.; Nualart, F. Cytoarchitecture, Proliferative Activity and Neuroblast Migration in the Subventricular Zone and Lateral Ventricle Extension of the Adult Guinea Pig Brain. Stem Cells 2016, 34, 2574–2586. [Google Scholar] [CrossRef]
- Altman, J.; Das, G.D. Postnatal neurogenesis in the guinea-pig. Nature 1967, 214, 1098–1101. [Google Scholar] [CrossRef]
- He, X.; Zhang, X.M.; Wu, J.; Fu, J.; Mou, L.; Lu, D.H.; Cai, Y.; Luo, X.G.; Pan, A.; Yan, X.X. Olfactory experience modulates immature neuron development in postnatal and adult guinea pig piriform cortex. Neuroscience 2014, 259, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Amrein, I.; Becker, A.S.; Engler, S.; Huang, S.H.; Müller, J.; Slomianka, L.; Oosthuizen, M.K. Adult neurogenesis and its anatomical context in the hippocampus of three mole-rat species. Front. Neuroanat. 2014, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Peragine, D.E.; Simpson, J.A.; Mooney, S.J.; Lovern, M.B.; Holmes, M.M. Social regulation of adult neurogenesis in a eusocial mammal. Neuroscience 2014, 268, 10–20. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oosthuizen, M.K. Exploratory behaviour, memory and neurogenesis in the social Damaraland mole-rat (Fukomys damarensis). J. Exp. Biol. 2020, 223, jeb221093. [Google Scholar] [CrossRef]
- Oosthuizen, M.K.; Amrein, I. Trading new neurons for status: Adult hippocampal neurogenesis in eusocial Damaraland mole-rats. Neuroscience 2016, 324, 227–237. [Google Scholar] [CrossRef]
- Luzzati, F.; De Marchis, S.; Fasolo, A.; Peretto, P. Neurogenesis in the caudate nucleus of the adult rabbit. J. Neurosci. 2006, 26, 609–621. [Google Scholar] [CrossRef]
- Siwak-Tapp, C.T.; Head, E.; Muggenburg, B.A.; Milgram, N.W.; Cotman, C.W. Neurogenesis decreases with age in the canine hippocampus and correlates with cognitive function. Neurobiol. Learn. Mem. 2007, 88, 249–259. [Google Scholar] [CrossRef]
- Kamiya, S.; Sawada, K. Immunohistochemical characterization of postnatal changes in cerebellar cortical cytoarchitectures in ferrets. Anat. Rec. 2021, 304, 413–424. [Google Scholar] [CrossRef]
- Chawana, R.; Patzke, N.; Alagaili, A.N.; Bennett, N.C.; Mohammed, O.B.; Kaswera-Kyamakya, C.; Gilissen, E.; Ihunwo, A.O.; Pettigrew, J.D.; Manger, P.R. The Distribution of Ki-67 and Doublecortin Immunopositive Cells in the Brains of Three Microchiropteran Species, Hipposideros fuliginosus, Triaenops persicus, and Asellia tridens. Anat. Rec. 2016, 299, 1548–1560. [Google Scholar] [CrossRef]
- Chawana, R.; Patzke, N.; Kaswera, C.; Gilissen, E.; Ihunwo, A.O.; Manger, P.R. Adult neurogenesis in eight Megachiropteran species. Neuroscience 2013, 244, 159–172. [Google Scholar] [CrossRef]
- Akter, M.; Kaneko, N.; Herranz-Pérez, V.; Nakamura, S.; Oishi, H.; García-Verdugo, J.M.; Sawamoto, K. Dynamic Changes in the Neurogenic Potential in the Ventricular-Subventricular Zone of Common Marmoset during Postnatal Brain Development. Cereb. Cortex 2020, 30, 4092–4109. [Google Scholar] [CrossRef] [PubMed]
- Leuner, B.; Kozorovitskiy, Y.; Gross, C.G.; Gould, E. Diminished adult neurogenesis in the marmoset brain precedes old age. Proc. Natl. Acad. Sci. USA 2007, 104, 17169–17173. [Google Scholar] [CrossRef] [PubMed]
- Marlatt, M.W.; Philippens, I.; Manders, E.; Czéh, B.; Joels, M.; Krugers, H.; Lucassen, P.J. Distinct structural plasticity in the hippocampus and amygdala of the middle-aged common marmoset (Callithrix jacchus). Exp. Neurol. 2011, 230, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Bédard, A.; Lévesque, M.; Bernier, P.J.; Parent, A. The rostral migratory stream in adult squirrel monkeys: Contribution of new neurons to the olfactory tubercle and involvement of the antiapoptotic protein Bcl-2. Eur. J. Neurosci. 2002, 16, 1917–1924. [Google Scholar] [CrossRef] [PubMed]
- Lyons, D.M.; Buckmaster, P.S.; Lee, A.G.; Wu, C.; Mitra, R.; Duffey, L.M.; Buckmaster, C.L.; Her, S.; Patel, P.D.; Schatzberget, A.F. Stress coping stimulates hippocampal neurogenesis in adult monkeys. Proc. Natl. Acad. Sci. USA 2010, 107, 14823–14827. [Google Scholar] [CrossRef]
- Bernier, P.J.; Bedard, A.; Vinet, J.; Levesque, M.; Parent, A. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc. Natl. Acad. Sci. USA 2002, 99, 11464–11469. [Google Scholar] [CrossRef]
- Zhang, X.M.; Cai, Y.; Chu, Y.; Chen, E.Y.; Feng, J.C.; Luo, X.G.; Xiong, K.; Struble, R.G.; Clough, R.W.; Patrylo, P.R.; et al. Doublecortin-expressing cells persist in the associative cerebral cortex and amygdala in aged nonhuman primates. Front. Neuroanat. 2009, 3, 17. [Google Scholar] [CrossRef]
- Gould, E.; Reeves, A.J.; Graziano, M.S.; Gross, C.G. Neurogenesis in the neocortex of adult primates. Science 1999, 286, 548–552. [Google Scholar] [CrossRef]
- Sorrells, S.F.; Paredes, M.F.; Cebrian-Silla, A.; Sandoval, K.; Qi, D.; Kelley, K.W.; James, D.; Mayer, S.; Chang, J.; Auguste, K.I.; et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 2018, 555, 377–381. [Google Scholar] [CrossRef]
- Sorrells, S.F.; Paredes, M.F.; Zhang, Z.; Kang, G.; Pastor-Alonso, O.; Biagiotti, S.; Page, C.E.; Sandoval, K.; Knox, A.; Connolly, A.; et al. Positive Controls in Adults and Children Support That Very Few, If Any, New Neurons Are Born in the Adult Human Hippocampus. J. Neurosci. 2021, 41, 2554–2565. [Google Scholar] [CrossRef]
- Bédard, A.; Parent, A. Evidence of newly generated neurons in the human olfactory bulb. Brain Res. Dev. Brain Res. 2004, 151, 159–168. [Google Scholar] [CrossRef]
- Spalding, K.L.; Bergmann, O.; Alkass, K.; Bernard, S.; Salehpour, M.; Huttner, H.B.; Boström, E.; Westerlund, I.; Vial, C.; Buchholz, B.A.; et al. Dynamics of hippocampal neurogenesis in adult humans. Cell 2013, 153, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Boldrini, M.; Fulmore, C.A.; Tartt, A.N.; Simeon, L.R.; Pavlova, I.; Poposka, V.; Rosoklija, G.B.; Stankov, A.; Arango, V.; Dwork, A.J.; et al. Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell 2018, 22, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Jiménez, E.P.; Terreros-Roncal, J.; Flor-García, M.; Rábano, A.; Llorens-Martín, M. Evidences for Adult Hippocampal Neurogenesis in Humans. J. Neurosci. 2021, 41, 2541–2553. [Google Scholar] [CrossRef] [PubMed]
- Ernst, A.; Alkass, K.; Bernard, S.; Salehpour, M.; Perl, S.; Tisdale, J.; Possnert, G.; Druid, H.; Frisén, J. Neurogenesis in the striatum of the adult human brain. Cell. 2014, 156, 1072–1083. [Google Scholar] [CrossRef]
- Sorrells, S.; Paredes, M.F.; Velmeshev, D.; Herranz-Pérez, V.; Sandoval, K.; Mayer, S.; Chang, E.F.; Insausti, R.; Kriegstein, A.R.; Rubenstein, J.L.; et al. Immature excitatory neurons develop during adolescence in the human amygdala. Nat. Commun. 2019, 10, 2748. [Google Scholar] [CrossRef]
- Ache, B.W.; Young, J.M. Olfaction: Diverse species, conserved principles. Neuron 2005, 48, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Luskin, M.B. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron 1993, 11, 173–189. [Google Scholar] [CrossRef]
- Carleton, A.; Petreanu, L.T.; Lansford, R.; Alvarez-Buylla, A.; Lledo, P.M. Becoming a new neuron in the adult olfactory bulb. Nat. Neurosci. 2003, 6, 507–518. [Google Scholar] [CrossRef]
- Rochefort, C.; Gheusi, G.; Vincent, J.D.; Lledo, P.M. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J. Neurosci. 2002, 22, 2679–2689. [Google Scholar] [CrossRef]
- Sakamoto, M.; Imayoshi, I.; Ohtsuka, T.; Yamaguchi, M.; Mori, K.; Kageyama, R. Continuous neurogenesis in the adult forebrain is required for innate olfactory responses. Proc. Natl. Acad. Sci. USA 2011, 108, 8479–8484. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Ogawa, Y.; Yoshihara, S. A Subtype of Olfactory Bulb Interneurons Is Required for Odor Detection and Discrimination Behaviors. J. Neurosci. 2016, 36, 8210–8227. [Google Scholar] [CrossRef] [PubMed]
- Grelat, A.; Benoit, L.; Wagner, S.; Moigneu, C.; Lledo, P.M.; Alonso, M. Adult-born neurons boost odor-reward association. Proc. Natl. Acad. Sci. USA 2018, 115, 2514–2519. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Yoshihara, S.; Tsuboi, A. The Functional Role of Olfactory Bulb Granule Cell Subtypes Derived From Embryonic and Postnatal Neurogenesis. Front. Mol. Neurosci. 2018, 11, 229. [Google Scholar] [CrossRef]
- Li, W.L.; Chu, M.W.; Wu, A.; Suzuki, Y.; Imayoshi, I.; Komiyama, T. Adult-born neurons facilitate olfactory bulb pattern separation during task engagement. Elife 2018, 7, e33006. [Google Scholar] [CrossRef]
- Shani-Narkiss, H.; Vinograd, A.; Landau, I.D.; Tasaka, G.; Yayon, N.; Terletsky, S.; Groysman, M.; Maor, I.; Sompolinsky, H.; Mizrahi, A. Young adult-born neurons improve odor coding by mitral cells. Nat. Commun. 2020, 11, 5867. [Google Scholar] [CrossRef]
- Sakamoto, M.; Kageyama, R.; Imayoshi, I. The functional significance of newly born neurons integrated into olfactory bulb circuits. Front. Neurosci. 2014, 8, 121. [Google Scholar] [CrossRef]
- Forest, J.; Chalençon, L.; Midroit, M.; Terrier, C.; Caillé, I.; Sacquet, J.; Benetollo, C.; Martin, K.; Richard, M.; Didier, A.; et al. Role of Adult-Born Versus Preexisting Neurons Born at P0 in Olfactory Perception in a Complex Olfactory Environment in Mice. Cereb. Cortex 2020, 30, 534–549. [Google Scholar] [CrossRef]
- Quiñones-Hinojosa, A.; Sanai, N.; Soriano-Navarro, M.; Gonzalez-Perez, O.; Mirzadeh, Z.; Gil-Perotin, S.; Romero-Rodriguez, R.; Berger, M.S.; García-Verdugo, J.M.; Alvarez-Buylla, A. Cellular composition and cytoarchitecture of the adult human subventricular zone: A niche of neural stem cells. J. Comp. Neurol. 2006, 494, 415–434. [Google Scholar] [CrossRef]
- Weickert, C.S.; Webster, M.J.; Colvin, S.M.; Herman, M.M.; Hyde, T.M.; Weinberger, D.R.; Kleinman, J.E. Localization of epidermal growth factor receptors and putative neuroblasts in human subependymal zone. J. Comp. Neurol. 2000, 423, 359–372. [Google Scholar] [CrossRef]
- Curtis, M.A.; Faull, R.L.; Eriksson, P.S. The effect of neurodegenerative diseases on the subventricular zone. Nat. Rev. Neurosci. 2007, 8, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Sanai, N.; Nguyen, T.; Ihrie, R.A. Corridors of migrating neurons in the human brain and their decline during infancy. Nature 2011, 478, 382–386. [Google Scholar] [CrossRef]
- Santopolo, G.; Magnusson, J.P.; Lindvall, O.; Kokaia, Z.; Frisén, J. Blocking Notch-Signaling Increases Neurogenesis in the Striatum after Stroke. Cells 2020, 9, 1732. [Google Scholar] [CrossRef] [PubMed]
- Tepper, B.; Koguc-Sobolewska, P.; Jaslan, K.; Turlejski, K.; Bartkowska, K.; Djavadian, R. Impaired olfactory neurogenesis affects the performance of olfactory-guided behavior in aged female opossums. Sci. Rep. 2021, 11, 4418. [Google Scholar] [CrossRef]
- Cádiz-Moretti, B.; Abellán-Álvaro, M.; Pardo-Bellver, C.; Martínez-García, F.; Lanuza, E. Afferent and Efferent Connections of the Cortex-Amygdala Transition Zone in Mice. Front. Neuroanat. 2016, 10, 125. [Google Scholar] [CrossRef] [PubMed]
- Nacher, J.; Crespo, C.; McEwen, B.S. Doublecortin expression in the adult rat telencephalon. Eur. J. Neurosci. 2001, 14, 629–644. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xie, M.-X.; Li, J.-M.; Hu, X.; Patrylo, P.R.; Luo, X.-G.; Cai, Y.; Li, Z.; Yan, X.-X. Prenatal genesis of layer II doublecortin expressing neurons in neonatal and young adult guinea pig cerebral cortex. Front. Neuroanat. 2015, 9, 109. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cameron, H.A.; McKay, R.D. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp. Neurol. 2001, 435, 406–417. [Google Scholar] [CrossRef]
- Snyder, J.S.; Kee, N.; Wojtowicz, J.M. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J. Neurophysiol. 2001, 85, 2423–2431. [Google Scholar] [CrossRef]
- van Praag, H.; Schinder, A.F.; Christie, B.R.; Toni, N.; Palmer, T.D.; Gage, F.H. Functional neurogenesis in the adult hippocampus. Nature 2002, 415, 1030–1034. [Google Scholar] [CrossRef]
- Toni, N.; Laplagne, D.A.; Zhao, C.; Lombardi, G.; Ribak, C.E.; Gage, F.H.; Schinder, A.F. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat. Neurosci. 2008, 11, 901–907. [Google Scholar] [CrossRef]
- Knoth, R.; Singec, I.; Ditter, M.; Pantazis, G.; Capetian, P.; Meyer, R.P.; Horvat, V.; Volk, B.; Kempermann, G. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS ONE 2010, 5, e8809. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, H.G.; Toda, T.; Gage, F.H. Adult Hippocampal Neurogenesis: A Coming-of-Age Story. J. Neurosci. 2018, 38, 10401–10410. [Google Scholar] [CrossRef]
- Kempermann, G.; Gage, F.H.; Aigner, L.; Song, H.; Curtis, M.A.; Thuret, S.; Kuhn, H.G.; Jessberger, S.; Frankland, P.W.; Cameron, H.A.; et al. Human Adult Neurogenesis: Evidence and Remaining Questions. Cell Stem Cell 2018, 23, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Paredes, M.F.; Sorrells, S.F.; Cebrian-Silla, A.; Sandoval, K.; Qi, D.; Kelley, K.W.; James, D.; Mayer, S.; Chang, J.; Auguste, K.I.; et al. Does Adult Neurogenesis Persist in the Human Hippocampus? Cell Stem Cell 2018, 23, 780–781. [Google Scholar] [CrossRef]
- Alam, M.J.; Kitamura, T.; Saitoh, Y.; Ohkawa, N.; Kondo, T.; Inokuchi, K. Adult Neurogenesis Conserves Hippocampal Memory Capacity. J. Neurosci. 2018, 38, 6854–6863. [Google Scholar] [CrossRef] [PubMed]
- Epp, J.R.; Chow, C.; Galea, L.A. Hippocampus-dependent learning influences hippocampal neurogenesis. Front. Neurosci. 2013, 7, 57. [Google Scholar] [CrossRef]
- Suárez-Pereira, I.; Carrión, Á.M. Updating stored memory requires adult hippocampal neurogenesis. Sci. Rep. 2015, 5, 13993. [Google Scholar] [CrossRef]
- Abrous, D.N.; Wojtowicz, J.M. Interaction between Neurogenesis and Hippocampal Memory System: New Vistas. Cold Spring Harb. Perspect. Biol. 2015, 7, a018952. [Google Scholar] [CrossRef]
- Tashiro, A.; Makino, H.; Gage, F.H. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: A critical period during an immature stage. J. Neurosci. 2007, 27, 3252–3259. [Google Scholar] [CrossRef]
- Nilsson, M.; Perfilieva, E.; Johansson, U.; Orwar, O.; Eriksson, P.S. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J. Neurobiol. 1999, 39, 569–578. [Google Scholar] [CrossRef]
- Dupret, D.; Fabre, A.; Dobrossy, M.; Panatier, A.; Rodríguez, J.J.; Lamarque, S.; Lemaire, V.; Oliet, S.H.R.; Piazza, P.-V.; Abrous, D.N. Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS Biol. 2007, 5, e214. [Google Scholar] [CrossRef] [PubMed]
- Tronel, S.; Fabre, A.; Charrier, V.; Oliet, S.H.; Gage, F.H.; Abrous, D.N. Spatial learning sculpts the dendritic arbor of adult-born hippocampal neurons. Proc. Natl. Acad. Sci. USA 2010, 107, 7963–7968. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.S.; Hong, N.S.; McDonald, R.J.; Wojtowicz, J.M. A role for adult neurogenesis in spatial long-term memory. Neuroscience 2005, 130, 843–852. [Google Scholar] [CrossRef]
- Leuner, B.; Gould, E.; Shors, T.J. Is there a link between adult neurogenesis and learning? Hippocampus 2006, 16, 216–224. [Google Scholar] [CrossRef]
- Groves, J.O.; Leslie, I.; Huang, G.-J.; McHugh, S.B.; E Taylor, A.; Mott, R.; Munafo, M.; Bannerman, D.M.; Flint, J. Ablating adult neurogenesis in the rat has no effect on spatial processing: Evidence from a novel pharmacogenetic model. PLoS Genet. 2013, 9, e1003718. [Google Scholar] [CrossRef]
- Tepper, B.; Aniszewska, A.; Bartkowska, K.; Grochocka, L.; Turlejski, K.; Djavadian, R. Aged Opossums Show Alterations in Spatial Learning Behavior and Reduced Neurogenesis in the Dentate Gyrus. Front. Neurosci. 2019, 13, 1210. [Google Scholar] [CrossRef]

| Order/Family | Species | Gestation | Eyes Opening | Lifespan | Postnatal Developmental Neurogenesis | Adult Neurogenesis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OB | DG | CER | OB/SVZ | DG | CER | PIR | Other | |||||
| Didelphimorphia/Didelphidae | Gray short-tailed opossum | 14–15 days | P35–P37 | 2.5 yrs | up to P28 [2,28] | P1-P155 [29] | 5 mth-2 yrs [65] | 6.5–21.5 mth [65] | up to P270 | |||
| Dasyuromorphia/Dasyuridae | Fat-tailed dunnart | 13–16 days | P45 | 1–2 yrs | 4–24 mth [66] | |||||||
| Didrotodontia/Phalangeridae | Brush-tailed possum | 18 days | P110 | 13 yrs | P5–P82 [3] | up to 3 mth [4] | ||||||
| Diprotodontia/Macropodidae | Tammar wallaby | 25–28 days | P140 | 11–14 yrs | up to P25 [54] | |||||||
| Quokka wallaby | 28 days | P110 | 8–15 yrs | P20-P85 [50] | ||||||||
| Euliptyphla/Erinaceidae | White-breasted hedgehog | 35 days | P21 | 3–5 yrs | AD [80] | AD [80] | AD [80] | |||||
| Eulipotyhla/Talpidae | European mole | 30 days | P22 | 2–3 yrs | AD [80] | AD [80] | AD [80] | |||||
| Eulipotphla/Soricidae | Hottentot golden mole | AD [79] | AD [79] | AD [79] | CTX [79] | |||||||
| Giant other shrew | 22 days | P20–P24 | 5 yrs | AD [76] | AD [76] | AD [76] | OT, EPN [76] | |||||
| Pygmy shrew, Common shrew | 22–24 days | P20–P24 | 1 yr | 1 yr [74] | 5 mth [74] | |||||||
| Greater white-toothed shrew, Eurasian water shrew, African giant shrew, Asian house shrew | 20–31 days | P20–P24 | 1.5–3 yrs | AD [75] | AD [75] | |||||||
| Rodentia/Muridae | Rats (Long Evans, Sprague-Dawley, Wistar, Fischer 344, Brown Norway wild rats) | 21–23 days | P13–P15 | 2 yrs | P0-P10 [87] | P6-P15 [6,88] up to P14 [89] | up to P21 [63] | AD [90] | 6–21 mth [9,67] 8–9 wk [88] | AD [91] | HTH 2 mth [92,93] CTX, STR 9–10 wk [94] BrSt AD [95] | |
| Mice (C57BL/6, CD1, BALB/c, ICR, A/J, FVB, C3H/HeJ, 129/SvJ, DBA/1, DBA/2) | 19–21 days | P10–P13 | 1–1.5 yrs | P0-P20 [53,96] | up to P20 [48,49,97] | up to P15 [55] | 3–4 mth [98] 2 mth [99] | 3–4 mth [98,100] | AD [101] | SN 2–20 mth [102] BLA 2–4 mth [103] CTX, STR 2–4 mth [98,104] HTH 3–4 mth [105] | ||
| Rodentia/Cricetidae | Syrian hamster | 16 days | P12–P14 | 2–3 yrs | 2.5 mth [106] | P28-P49 [107] | BLA, HTH P28-P49 [107] | |||||
| Meadow vole, Prairie vole | 21 days | P14 | 3–16 mth | 3–5 mth [108,109,110] | 3–5 mth [108,109,110] | CTX, CP, BLA, THT 3–5 mth [108,109] | ||||||
| Rodentia/Caviidae | Guinea pig | 65–68 days | Born with open eyes | 4–5 yrs | up to P30 [68] | 6, 12 mth [111] | 1 yr [68,112] | 12–14 mth [113] | ||||
| Rodentia/Bathyergidae | Highveld mole-rat, Cape mole-rat, Naked mole-rat, Damaraland mole-rat | 70 days | P14 | 6–15 yrs | 1 yr [114] 2–9 yrs [115] AD [116,117] | 2–9 yrs [115] | BLA, 2–9 yrs [115] | |||||
| Rodentia/Leporidae | New Zeland white rabbit | 31 days | P7 | 9 yrs | P10 [6] | 1–3 yrs [6] | CN, AD [118] | |||||
| Hyracoidea/Procaviidae | Rock hyrax | 200 days | Born with open eyes | 8–12 yrs | AD [79] | AD [79] | AD [79] | CTX, AD [79] | ||||
| Macrosce-lidea/Macroscelididae | Eastern rock sengi, Four-toed sengi | 40–60 days | Born with open eyes | 4–6 yrs | AD [79] | AD [79] | AD [79] | CTX, AD [79] | ||||
| Artiodactyla/Bovidae | Ilede-France sheep | 147 days | Born with open eyes | 10–12 yrs | AD [73] | AD [73] | HTH, 18–24 mth [105] | |||||
| Carnivora/Felidae | Domestic cat | 64 days | P7–P10 | 12–18 yrs | 18–24 mth [71] | CTX, EC 18–24 mth [71] | ||||||
| Carnivora/Canidae | Domestic dog | 61 days | P10–P14 | 10–13 yrs | 2–6 yrs [70,119] | |||||||
| Carnivora/Mustelidae | Ferret | 42 days | P32 | 5–12 yrs | P4, P21 [120] | |||||||
| Scandentia/Tupaiidae | Tree shrew | 46 days | P21 | 12 yrs | 2 mth-6 yrs [78] | AD [77] | 2 mth -6 yrs [78] | BLA 2 mth-6 yrs [78] | ||||
| Chiroptera | Microchiroptera bats | 44–180 days | P1–P2 | 20 yrs | AD [121] | AD [81] | AD [121] | AC, BLA [121] | ||||
| Megachiroptera bats | 4–6 m | P1–P2 | 20 yrs | AD [122] | AD [82,122] | AD [122] | AD [122] | BrSt, Tectum, AD [122] | ||||
| Primates/(New World monkey) Callitrichidae | Common marmoset | 151 days | Born with open eyes | 12 yrs | up to P30 [123] | 1.5–7 yrs [124] 4 yrs [123,125] | CTX, CC, AMG 4 yrs [125] | |||||
| Primates/(New World monkey) Cebidae | Squirrel monkey | 160–170 days | Born with open eyes | 21 yrs | 4–6 yrs [126] | 7–10 yrs [127] | 3-6 yrs [128] | CTX, BLA 3-6 yrs [128] | ||||
| Primates/(Old World monkey) Cercopithecidae | Rhesus monkey | 165 days | Born with open eyes | 30 yrs | 12, 21, 31 yrs [129] | CTX, BLA 12, 21, 31 yrs [129]] | ||||||
| Macaque monkey | 162 days | E125 | 25 yrs | 5–23 yrs [84] | 6-12 yrs [128] | CTX AD [130], BLA6-12 yrs [128] | ||||||
| Primates/Hominidae | Humans | 280 days | E195 | 75–80 yrs | 3 wk-1 yr [131,132] up to 5 mth [132] | 16-69 yrs [133] | 23–72 yrs [85] AD [134,135,136] 14–79 yrs [135] | STR 3–79 yrs [137] AMG 24–67 yrs [138] | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartkowska, K.; Tepper, B.; Turlejski, K.; Djavadian, R. Postnatal and Adult Neurogenesis in Mammals, Including Marsupials. Cells 2022, 11, 2735. https://doi.org/10.3390/cells11172735
Bartkowska K, Tepper B, Turlejski K, Djavadian R. Postnatal and Adult Neurogenesis in Mammals, Including Marsupials. Cells. 2022; 11(17):2735. https://doi.org/10.3390/cells11172735
Chicago/Turabian StyleBartkowska, Katarzyna, Beata Tepper, Krzysztof Turlejski, and Ruzanna Djavadian. 2022. "Postnatal and Adult Neurogenesis in Mammals, Including Marsupials" Cells 11, no. 17: 2735. https://doi.org/10.3390/cells11172735
APA StyleBartkowska, K., Tepper, B., Turlejski, K., & Djavadian, R. (2022). Postnatal and Adult Neurogenesis in Mammals, Including Marsupials. Cells, 11(17), 2735. https://doi.org/10.3390/cells11172735







