Double Deletion of PI3K and PTEN Modifies Lens Postnatal Growth and Homeostasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Generation of Knockout Mice
2.2. Lens Growth Analysis and Photography
2.3. Western Blotting
2.4. 5-Ethynyl-2′-deoxyuridine (EdU) Staining
2.5. Histology
2.6. Statistics
3. Results
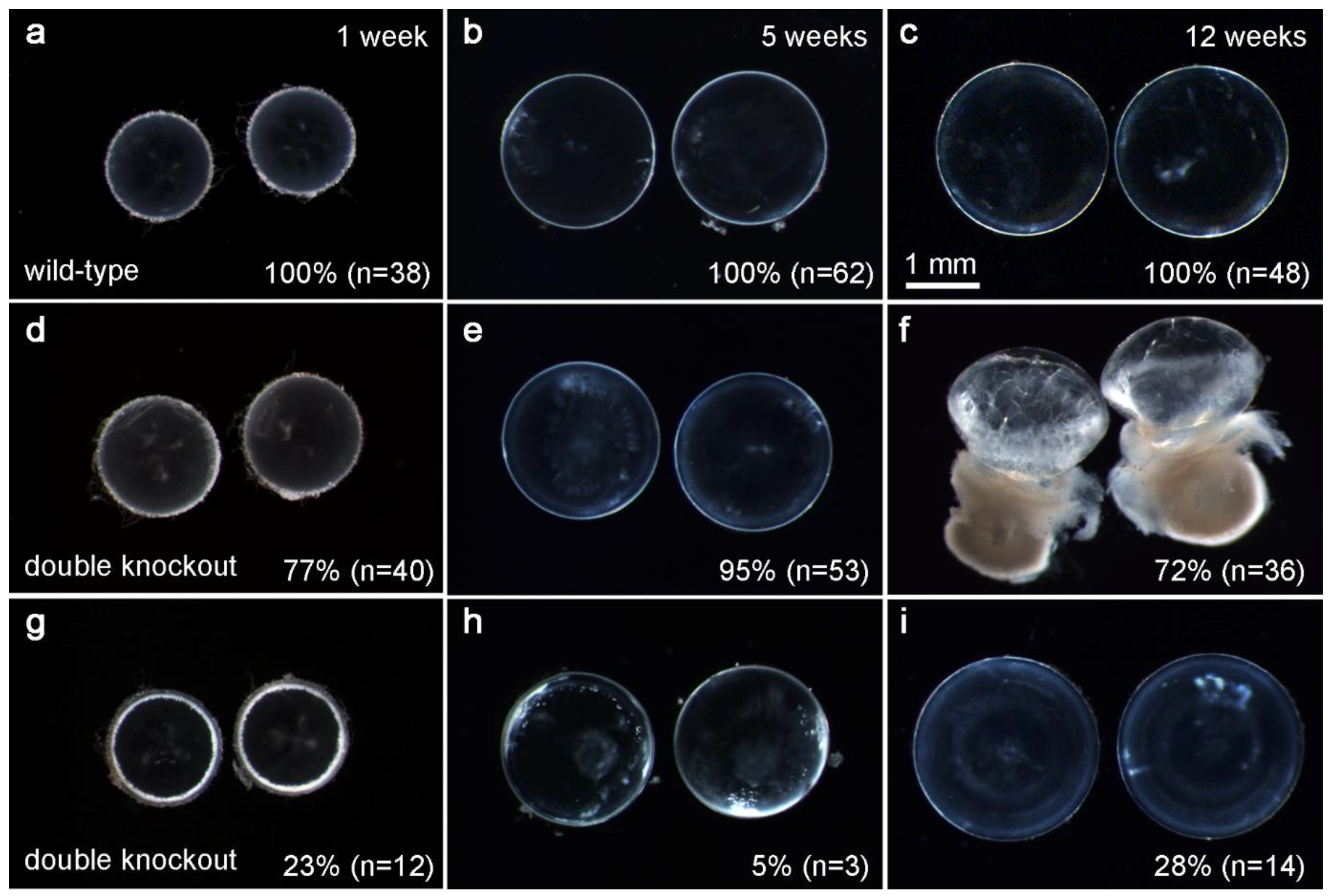
3.1. Lens-Specific Double Deletion of p110α and PTEN Caused Accelerated Cataract and Lens Rupture
3.2. Double Knockout of p110α and PTEN Increased Lens Volume and Accelerated Lens Rupture
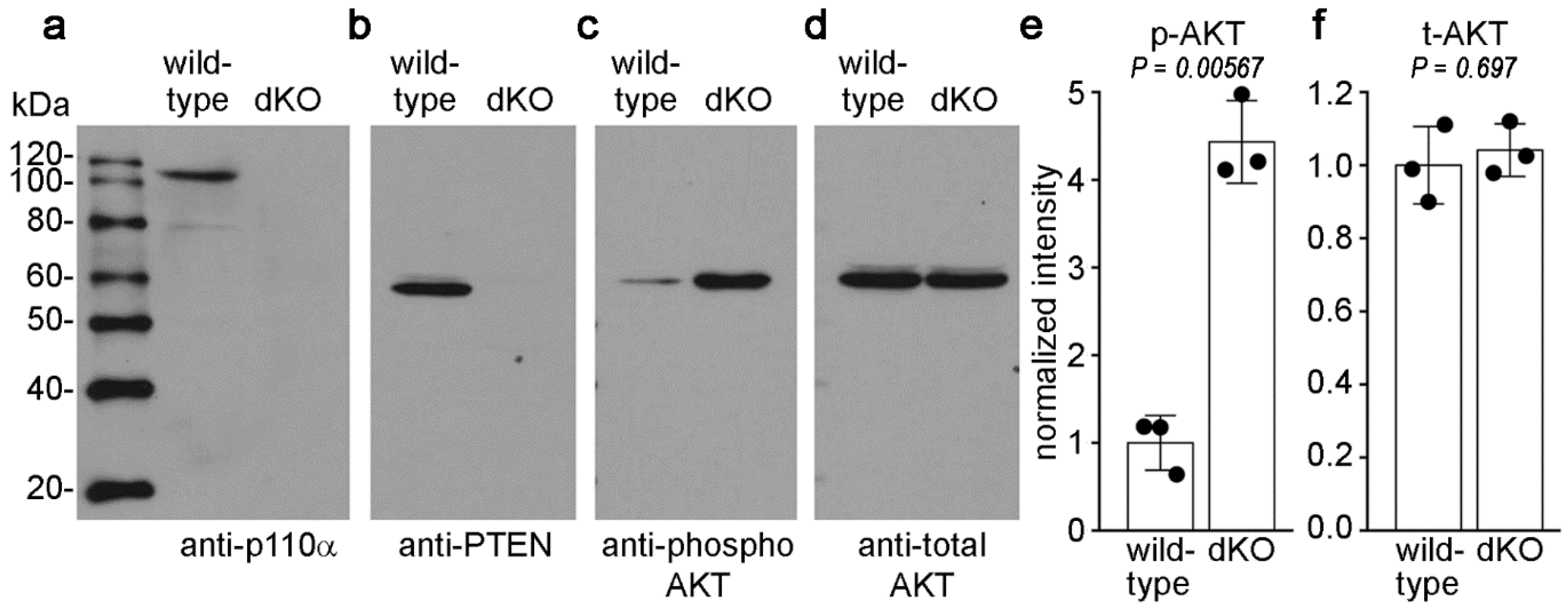
3.3. Double Knockout of p110α and PTEN Increased Levels of Phospho-Akt
3.4. Double Knockout of p110α and PTEN Eliminated the Postnatal Proliferation Defect Present in Single p110α Knockout Lenses
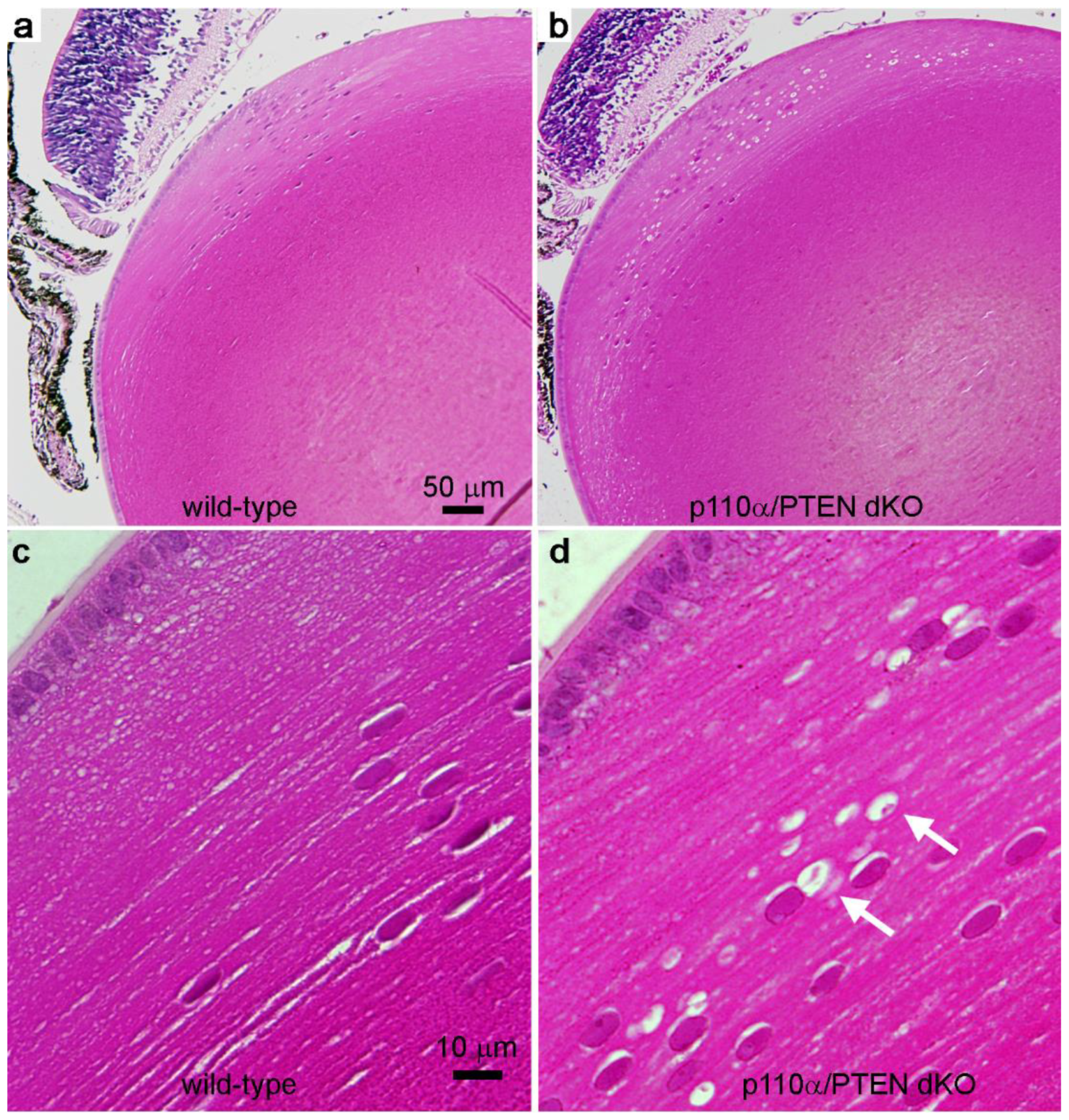
3.5. Double Deletion of p110α/PTEN Produced Histological Abnormalities in the Equatorial Region of the Lens
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ilic, N.; Roberts, T.M. Comparing the roles of the p110alpha and p110beta isoforms of PI3K in signaling and cancer. Curr. Top. Microbiol. Immunol. 2010, 347, 55–77. [Google Scholar] [CrossRef] [PubMed]
- Vanhaesebroeck, B.; Guillermet-Guibert, J.; Graupera, M.; Bilanges, B. The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell Biol. 2010, 11, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Cantley, L.C. The phosphoinositide 3-kinase pathway. Science 2002, 296, 1655–1657. [Google Scholar] [CrossRef]
- Engelman, J.A.; Luo, J.; Cantley, L.C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 2006, 7, 606–619. [Google Scholar] [CrossRef]
- Fruman, D.A.; Meyers, R.E.; Cantley, L.C. Phosphoinositide kinases. Annu. Rev. Biochem. 1998, 67, 481–507. [Google Scholar] [CrossRef]
- Hawkins, P.T.; Anderson, K.E.; Davidson, K.; Stephens, L.R. Signalling through Class I PI3Ks in mammalian cells. Biochem. Soc. Trans. 2006, 34, 647–662. [Google Scholar] [CrossRef]
- Cantley, L.C.; Neel, B.G. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc. Natl. Acad. Sci. USA 1999, 96, 4240–4245. [Google Scholar] [CrossRef]
- Waite, K.A.; Eng, C. Protean PTEN: Form and function. Am. J. Hum. Genet. 2002, 70, 829–844. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Ali, S.M.; Sabatini, D.M. Growing roles for the mTOR pathway. Curr. Opin. Cell Biol. 2005, 17, 596–603. [Google Scholar] [CrossRef]
- Martinez, G.; de Iongh, R.U. The lens epithelium in ocular health and disease. Int. J. Biochem. Cell Biol. 2010, 42, 1945–1963. [Google Scholar] [CrossRef]
- Teo, Z.L.; McQueen-Miscamble, L.; Turner, K.; Martinez, G.; Madakashira, B.; Dedhar, S.; Robinson, M.L.; de Iongh, R.U. Integrin linked kinase (ILK) is required for lens epithelial cell survival, proliferation and differentiation. Exp. Eye Res. 2014, 121, 130–142. [Google Scholar] [CrossRef]
- Weber, G.F.; Menko, A.S. Phosphatidylinositol 3-kinase is necessary for lens fiber cell differentiation and survival. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4490–4499. [Google Scholar] [CrossRef][Green Version]
- Sellitto, C.; Li, L.; Vaghefi, E.; Donaldson, P.J.; Lin, R.Z.; White, T.W. The Phosphoinosotide 3-Kinase Catalytic Subunit p110alpha is Required for Normal Lens Growth. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3145–3151. [Google Scholar] [CrossRef][Green Version]
- Sellitto, C.; Li, L.; Gao, J.; Robinson, M.L.; Lin, R.Z.; Mathias, R.T.; White, T.W. AKT activation promotes PTEN hamartoma tumor syndrome-associated cataract development. J. Clin. Investig. 2013, 123, 5401–5409. [Google Scholar] [CrossRef]
- Piatigorsky, J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation 1981, 19, 134–153. [Google Scholar] [CrossRef]
- McAvoy, J.W.; Chamberlain, C.G.; de Iongh, R.U.; Hales, A.M.; Lovicu, F.J. Lens development. Eye 1999, 13 (Pt. 3b), 425–437. [Google Scholar] [CrossRef]
- McAvoy, J.W. Cell division, cell elongation and the co-ordination of crystallin gene expression during lens morphogenesis in the rat. J. Embryol. Exp. Morphol. 1978, 45, 271–281. [Google Scholar] [CrossRef]
- Sikic, H.; Shi, Y.; Lubura, S.; Bassnett, S. A stochastic model of eye lens growth. J. Theor. Biol. 2015, 376, 15–31. [Google Scholar] [CrossRef]
- Lovicu, F.J.; McAvoy, J.W. FGF-induced lens cell proliferation and differentiation is dependent on MAPK (ERK1/2) signalling. Development 2001, 128, 5075–5084. [Google Scholar] [CrossRef]
- Garcia, C.M.; Yu, K.; Zhao, H.; Ashery-Padan, R.; Ornitz, D.M.; Robinson, M.L.; Beebe, D.C. Signaling through FGF receptor-2 is required for lens cell survival and for withdrawal from the cell cycle during lens fiber cell differentiation. Dev. Dyn. 2005, 233, 516–527. [Google Scholar] [CrossRef]
- Robinson, M.L. An essential role for FGF receptor signaling in lens development. Semin. Cell Dev. Biol. 2006, 17, 726–740. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Stump, R.; McAvoy, J.W.; Lovicu, F.J. MAPK/ERK1/2 and PI3-kinase signalling pathways are required for vitreous-induced lens fibre cell differentiation. Exp. Eye Res. 2009, 88, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, L.; Patkunanathan, B.; Lynch, O.T.; McAvoy, J.W.; Rasko, J.E.; Lovicu, F.J. Aqueous humour- and growth factor-induced lens cell proliferation is dependent on MAPK/ERK1/2 and Akt/PI3-K signalling. Exp. Eye Res. 2006, 83, 667–678. [Google Scholar] [CrossRef]
- Xie, L.; Overbeek, P.A.; Reneker, L.W. Ras signaling is essential for lens cell proliferation and lens growth during development. Dev. Biol. 2006, 298, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Upadhya, D.; Ogata, M.; Reneker, L.W. MAPK1 is required for establishing the pattern of cell proliferation and for cell survival during lens development. Development 2013, 140, 1573–1582. [Google Scholar] [CrossRef]
- Gong, X.; Wang, X.; Han, J.; Niesman, I.; Huang, Q.; Horwitz, J. Development of cataractous macrophthalmia in mice expressing an active MEK1 in the lens. Investig. Ophthalmol. Vis. Sci. 2001, 42, 539–548. [Google Scholar]
- Shakespeare, T.I.; Sellitto, C.; Li, L.; Rubinos, C.; Gong, X.; Srinivas, M.; White, T.W. Interaction between Connexin50 and mitogen-activated protein kinase signaling in lens homeostasis. Mol. Biol. Cell. 2009, 20, 2582–2592. [Google Scholar] [CrossRef][Green Version]
- Le, A.C.; Musil, L.S. A novel role for FGF and extracellular signal-regulated kinase in gap junction-mediated intercellular communication in the lens. J. Cell Biol. 2001, 154, 197–216. [Google Scholar] [CrossRef]
- Le, A.C.; Musil, L.S. FGF signaling in chick lens development. Dev. Biol. 2001, 233, 394–411. [Google Scholar] [CrossRef]
- Iyengar, L.; Wang, Q.; Rasko, J.E.; McAvoy, J.W.; Lovicu, F.J. Duration of ERK1/2 phosphorylation induced by FGF or ocular media determines lens cell fate. Differentiation 2007, 75, 662–668. [Google Scholar] [CrossRef]
- Chaffee, B.R.; Hoang, T.V.; Leonard, M.R.; Bruney, D.G.; Wagner, B.D.; Dowd, J.R.; Leone, G.; Ostrowski, M.C.; Robinson, M.L. FGFR and PTEN signaling interact during lens development to regulate cell survival. Dev. Biol. 2016, 410, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yang, Y.; Rizo, C.M.; Overbeek, P.A.; Robinson, M.L. Insertion of a Pax6 consensus binding site into the alphaA-crystallin promoter acts as a lens epithelial cell enhancer in transgenic mice. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1930–1939. [Google Scholar]
- Lu, Z.; Jiang, Y.P.; Wang, W.; Xu, X.H.; Mathias, R.T.; Entcheva, E.; Ballou, L.M.; Cohen, I.S.; Lin, R.Z. Loss of cardiac phosphoinositide 3-kinase p110 alpha results in contractile dysfunction. Circulation 2009, 120, 318–325. [Google Scholar] [CrossRef]
- Suzuki, A.; Yamaguchi, M.T.; Ohteki, T.; Sasaki, T.; Kaisho, T.; Kimura, Y.; Yoshida, R.; Wakeham, A.; Higuchi, T.; Fukumoto, M.; et al. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity 2001, 14, 523–534. [Google Scholar] [PubMed]
- Lam, P.T.; Padula, S.L.; Hoang, T.V.; Poth, J.E.; Liu, L.; Liang, C.; LeFever, A.S.; Wallace, L.M.; Ashery-Padan, R.; Riggs, P.K.; et al. Considerations for the use of Cre recombinase for conditional gene deletion in the mouse lens. Hum. Genom. 2019, 13, 10. [Google Scholar] [CrossRef]
- DeRosa, A.M.; Mese, G.; Li, L.; Sellitto, C.; Brink, P.R.; Gong, X.; White, T.W. The cataract causing Cx50-S50P mutant inhibits Cx43 and intercellular communication in the lens epithelium. Exp. Cell Res. 2009, 315, 1063–1075. [Google Scholar] [CrossRef]
- White, T.W.; Goodenough, D.A.; Paul, D.L. Targeted ablation of connexin50 in mice results in microphthalmia and zonular pulverulent cataracts. J. Cell Biol. 1998, 143, 815–825. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar]
- Wiley, L.A.; Shui, Y.B.; Beebe, D.C. Visualizing lens epithelial cell proliferation in whole lenses. Mol. Vis. 2010, 16, 1253–1259. [Google Scholar]
- Hoang, T.V.; Kumar, P.K.; Sutharzan, S.; Tsonis, P.A.; Liang, C.; Robinson, M.L. Comparative transcriptome analysis of epithelial and fiber cells in newborn mouse lenses with RNA sequencing. Mol. Vis. 2014, 20, 1491–1517. [Google Scholar]
- Shi, Y.; De Maria, A.; Lubura, S.; Sikic, H.; Bassnett, S. The penny pusher: A cellular model of lens growth. Investig. Ophthalmol. Vis. Sci. 2015, 56, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Harding, C.V.; Hughes, W.L.; Bond, V.P.; Schork, P. Autoradiographic localization of tritiated thymidine in wholemount preparations of lens epithelium. Arch. Ophthalmol. 1960, 63, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Graw, J. Mouse models of congenital cataract. Eye 1999, 13 (Pt. 3b), 438–444. [Google Scholar] [CrossRef]
- Foster, F.S.; Zhang, M.; Duckett, A.S.; Cucevic, V.; Pavlin, C.J. In vivo imaging of embryonic development in the mouse eye by ultrasound biomicroscopy. Invest. Ophthalmol. Vis. Sci. 2003, 44, 2361–2366. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mu, J.; Slevin, J.C.; Qu, D.; McCormick, S.; Adamson, S.L. In vivo quantification of embryonic and placental growth during gestation in mice using micro-ultrasound. Reprod. Biol. Endocrinol. 2008, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Brewitt, B.; Clark, J.I. Growth and transparency in the lens, an epithelial tissue, stimulated by pulses of PDGF. Science 1988, 242, 777–779. [Google Scholar] [CrossRef]
- Brewitt, B.; Teller, D.C.; Clark, J.I. Periods of oscillatory growth in developing ocular lens correspond with cell cycle times. J. Cell Physiol. 1992, 150, 586–592. [Google Scholar] [CrossRef]
- Giannone, A.A.; Li, L.; Sellitto, C.; White, T.W. Physiological Mechanisms Regulating Lens Transport. Front. Physiol. 2021, 12, 818649. [Google Scholar] [CrossRef]
- Mathias, R.T.; Kistler, J.; Donaldson, P. The lens circulation. J. Membr. Biol. 2007, 216, 1–16. [Google Scholar] [CrossRef]
- Gao, J.; Sun, X.; White, T.W.; Delamere, N.A.; Mathias, R.T. Feedback Regulation of Intracellular Hydrostatic Pressure in Surface Cells of the Lens. Biophys. J. 2015, 109, 1830–1839. [Google Scholar] [CrossRef]
- Delamere, N.A.; Shahidullah, M.; Mathias, R.T.; Gao, J.; Sun, X.; Sellitto, C.; White, T.W. Signaling Between TRPV1/TRPV4 and Intracellular Hydrostatic Pressure in the Mouse Lens. Investig. Ophthalmol. Vis. Sci. 2020, 61, 58. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, J.; Li, L.; Sellitto, C.; Mathias, R.T.; Donaldson, P.J.; White, T.W. The Ciliary Muscle and Zonules of Zinn Modulate Lens Intracellular Hydrostatic Pressure Through Transient Receptor Potential Vanilloid Channels. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4416–4424. [Google Scholar] [CrossRef]
- Lu, Z.; Wu, C.Y.; Jiang, Y.P.; Ballou, L.M.; Clausen, C.; Cohen, I.S.; Lin, R.Z. Suppression of phosphoinositide 3-kinase signaling and alteration of multiple ion currents in drug-induced long QT syndrome. Sci. Transl. Med. 2012, 4, 131ra150. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Dennis, A.T.; Obejero-Paz, C.; Overholt, J.L.; Heredia-Moya, J.; Kirk, K.L.; Ficker, E. Oxidative inactivation of the lipid phosphatase phosphatase and tensin homolog on chromosome ten (PTEN) as a novel mechanism of acquired long QT syndrome. J. Biol. Chem. 2011, 286, 2843–2852. [Google Scholar] [CrossRef]
- Takeuchi, K.; Gertner, M.J.; Zhou, J.; Parada, L.F.; Bennett, M.V.; Zukin, R.S. Dysregulation of synaptic plasticity precedes appearance of morphological defects in a Pten conditional knockout mouse model of autism. Proc. Natl. Acad. Sci. USA 2013. [Google Scholar] [CrossRef]
- Weston, M.C.; Chen, H.; Swann, J.W. Multiple roles for mammalian target of rapamycin signaling in both glutamatergic and GABAergic synaptic transmission. J. Neurosci. 2012, 32, 11441–11452. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Turner, S.D.; Hinton, B.T. Alteration of transporter activities in the epididymides of infertile initial segment-specific Pten knockout mice. Biol. Reprod. 2018, 99, 536–545. [Google Scholar] [CrossRef]
- Morani, F.; Phadngam, S.; Follo, C.; Titone, R.; Aimaretti, G.; Galetto, A.; Alabiso, O.; Isidoro, C. PTEN regulates plasma membrane expression of glucose transporter 1 and glucose uptake in thyroid cancer cells. J. Mol. Endocrinol. 2014, 53, 247–258. [Google Scholar] [CrossRef]
- Tang, X.; Powelka, A.M.; Soriano, N.A.; Czech, M.P.; Guilherme, A. PTEN, but not SHIP2, suppresses insulin signaling through the phosphatidylinositol 3-kinase/Akt pathway in 3T3-L1 adipocytes. J. Biol. Chem. 2005, 280, 22523–22529. [Google Scholar] [CrossRef]
- Huang, F.F.; Zhang, L.; Wu, D.S.; Yuan, X.Y.; Yu, Y.H.; Zhao, X.L.; Chen, F.P.; Zeng, H. PTEN regulates BCRP/ABCG2 and the side population through the PI3K/Akt pathway in chronic myeloid leukemia. PLoS ONE 2014, 9, e88298. [Google Scholar] [CrossRef]
- Huang, Z.; Hu, Z.; Xie, P.; Liu, Q. Tyrosine-mutated AAV2-mediated shRNA silencing of PTEN promotes axon regeneration of adult optic nerve. PLoS ONE 2017, 12, e0174096. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sellitto, C.; Li, L.; White, T.W. Double Deletion of PI3K and PTEN Modifies Lens Postnatal Growth and Homeostasis. Cells 2022, 11, 2708. https://doi.org/10.3390/cells11172708
Sellitto C, Li L, White TW. Double Deletion of PI3K and PTEN Modifies Lens Postnatal Growth and Homeostasis. Cells. 2022; 11(17):2708. https://doi.org/10.3390/cells11172708
Chicago/Turabian StyleSellitto, Caterina, Leping Li, and Thomas W. White. 2022. "Double Deletion of PI3K and PTEN Modifies Lens Postnatal Growth and Homeostasis" Cells 11, no. 17: 2708. https://doi.org/10.3390/cells11172708
APA StyleSellitto, C., Li, L., & White, T. W. (2022). Double Deletion of PI3K and PTEN Modifies Lens Postnatal Growth and Homeostasis. Cells, 11(17), 2708. https://doi.org/10.3390/cells11172708





