SOCS3 Regulates Dectin-2-Induced Inflammation in PBMCs of Diabetic Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Volunteers
2.2. PBMC Collection
2.3. Cell Stimulation
2.4. Small-Interfering RNA (siRNA) Transfection
2.5. Flow Cytometry—Staining of Cell-Surface Markers
2.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.7. Sandwich Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Western Blotting
2.9. Statistical Analysis
3. Results
3.1. Dectin-2 Expression Is Increased on Monocytes in Diabetic Patients
3.2. Dectin-2 Gene Expression in Monocytes Correlates with HOMA-IR and HbA1c Levels in Diabetic Patients
3.3. Dectin-2 Gene Expression Shows a Negative Association with SOCS3
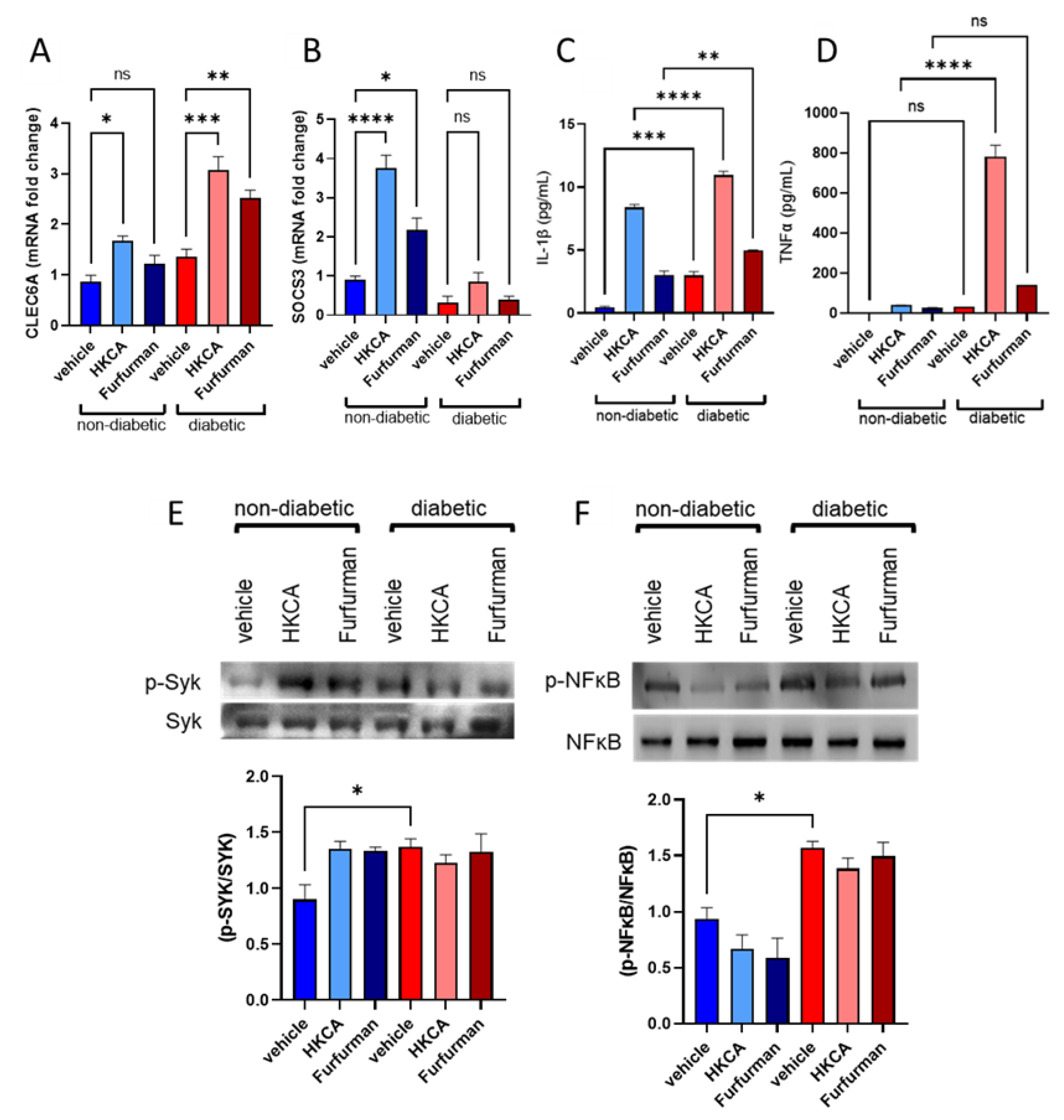
3.4. Dectin-2 Activation Fails to Induce SOCS3 Expression and Suppress Subsequent Inflammatory Responses in the PBMCs of Diabetic Patients
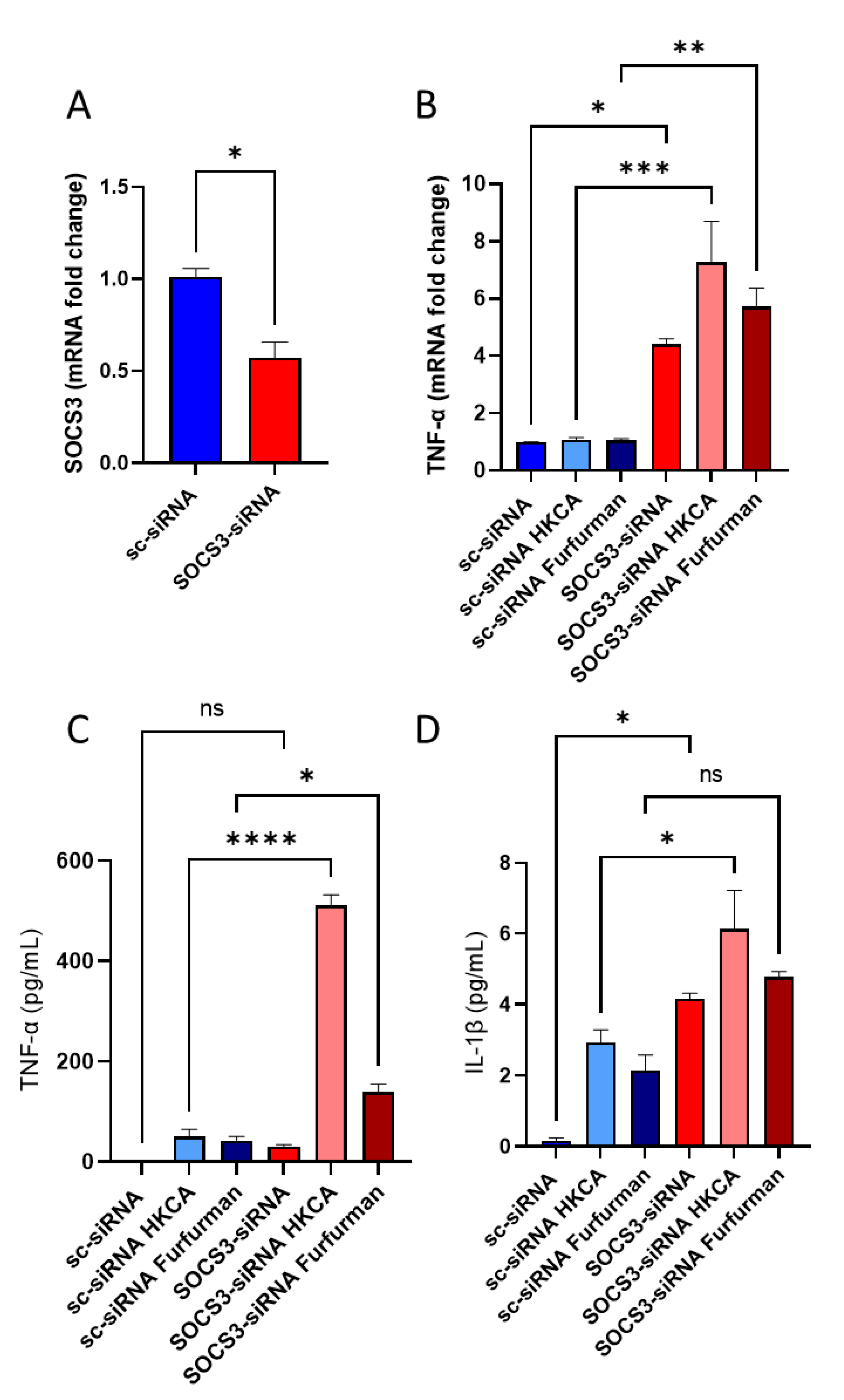
3.5. SOCS3-Deficient PBMCs Display Similar Inflammatory Phenotype of Diabetic PBMCs When Exposed to Dectin-2 Ligand
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The role of inflammation in diabetes: Current concepts and future perspectives. Eur. Cardiol. Rev. 2019, 14, 50. [Google Scholar] [CrossRef] [PubMed]
- Illán-Gómez, F.; Gonzálvez-Ortega, M.; Orea-Soler, I.; Alcaraz-Tafalla, M.S.; Aragón-Alonso, A.; Pascual-Díaz, M.; Pérez-Paredes, M.; Lozano-Almela, M.L. Obesity and inflammation: Change in adiponectin, c-reactive protein, tumour necrosis factor-alpha and interleukin-6 after bariatric surgery. Obes. Surg. 2012, 22, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Gross, O.; Gewies, A.; Finger, K.; Schäfer, M.; Sparwasser, T.; Peschel, C.; Förster, I.; Ruland, J. Card9 controls a non-tlr signalling pathway for innate anti-fungal immunity. Nature 2006, 442, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Underhill, D.M.; Rossnagle, E.; Lowell, C.A.; Simmons, R.M. Dectin-1 activates syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood 2005, 106, 2543. [Google Scholar] [CrossRef]
- Thiem, K.; Hoeke, G.; Zhou, E.; Hijmans, A.; Houben, T.; Boels, M.G.; Mol, I.M.; Lutgens, E.; Shiri-Sverdlov, R.; Bussink, J.; et al. Deletion of haematopoietic dectin-2 or card9 does not protect from atherosclerosis development under hyperglycaemic conditions. Diabetes Vasc. Dis. Res. 2020, 17, 1479164119892140. [Google Scholar] [CrossRef]
- Ozment-Skelton, T.R.; deFluiter, E.A.; Ha, T.; Li, C.; Graves, B.M.; Ferguson, D.A.; Schweitzer, J.B.; Preizsner, J.; Brown, G.D.; Gordon, S.; et al. Leukocyte dectin-1 expression is differentially regulated in fungal versus polymicrobial sepsis. Crit. Care Med. 2009, 37, 1038–1045. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Yamada, S.; Sugamata, M.; Kanauchi, O.; Morita, Y. Dectin-2 mediates phagocytosis of lactobacillus paracasei kw3110 and il-10 production by macrophages. Sci. Rep. 2021, 11, 17737. [Google Scholar] [CrossRef]
- Castoldi, A.; Andrade-Oliveira, V.; Aguiar, C.F.; Amano, M.T.; Lee, J.; Miyagi, M.T.; Latância, M.T.; Braga, T.T.; da Silva, M.B.; Ignácio, A.; et al. Dectin-1 activation exacerbates obesity and insulin resistance in the absence of myd88. Cell Rep. 2017, 19, 2272–2288. [Google Scholar] [CrossRef]
- Cortez-Espinosa, N.; García-Hernández, M.H.; Reynaga-Hernández, E.; Cortés-García, J.D.; Corral-Fernández, N.E.; Rodríguez-Rivera, J.G.; Bravo-Ramírez, A.; González-Amaro, R.; Portales-Pérez, D.P. Abnormal expression and function of dectin-1 receptor in type 2 diabetes mellitus patients with poor glycemic control (hba1c > 8%). Metabolism 2012, 61, 1538–1546. [Google Scholar] [CrossRef]
- Al-Rashed, F.; Ahmad, Z.; Snider, A.J.; Thomas, R.; Kochumon, S.; Melhem, M.; Sindhu, S.; Obeid, L.M.; Al-Mulla, F.; Hannun, Y.A.; et al. Ceramide kinase regulates tnf-α-induced immune responses in human monocytic cells. Sci. Rep. 2021, 11, 8259. [Google Scholar] [CrossRef]
- Kircheis, R.; Kichler, A.; Wallner, G.; Kursa, M.; Ogris, M.; Felzmann, T.; Buchberger, M.; Wagner, E. Coupling of cell-binding ligands to polyethylenimine for targeted gene delivery. Gene Ther. 1997, 4, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Al-Rashed, F.; Kochumon, S.; Usmani, S.; Sindhu, S.; Ahmad, R. Pam3csk4 induces mmp-9 expression in human monocytic thp-1 cells. Cell. Physiol. Biochem. 2017, 41, 1993–2003. [Google Scholar] [CrossRef] [PubMed]
- Sobah, M.L.; Liongue, C.; Ward, A.C. Socs proteins in immunity, inflammatory diseases, and immune-related cancer. Front. Med. 2021, 8, 1532. [Google Scholar] [CrossRef] [PubMed]
- Cramer, A.; Galvão, I.; Venturini de Sá, N.; Gaio, P.; Fernanda de Melo Oliveira, N.; Rates Gonzaga Santos, M.; Henrique Campolina-Silva, G.; Vinicius Santos Valiate, B.; Rezende Souza, F.; Dantas Cassali, G.; et al. Role of suppressor of cytokine signaling 2 during the development and resolution of an experimental arthritis. Cell. Immunol. 2022, 372, 104476. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.; Manchanda, M.; Gupta, S.; Singla, R.; Behera, D.; Das, G.; Natarajan, K. Toll-like receptor 2 and dc-signr1 differentially regulate suppressors of cytokine signaling 1 in dendritic cells during mycobacterium tuberculosis infection. J. Biol. Chem. 2009, 284, 25532–25541. [Google Scholar] [CrossRef]
- Eberle, M.E.; Dalpke, A.H. Dectin-1 stimulation induces suppressor of cytokine signaling 1, thereby modulating TLR signaling and T cell responses. J. Immunol. 2012, 188, 5644–5654. [Google Scholar] [CrossRef]
- Geijtenbeek, T.B.H.; Gringhuis, S.I. Signalling through c-type lectin receptors: Shaping immune responses. Nat. Rev. Immunol. 2009, 9, 465. [Google Scholar] [CrossRef]
- Yi, Y.S.; Son, Y.J.; Ryou, C.; Sung, G.H.; Kim, J.H.; Cho, J.Y. Functional roles of syk in macrophage-mediated inflammatory responses. Mediat. Inflamm. 2014, 2014, 270302. [Google Scholar] [CrossRef]
- de Vries, H.S.; Plantinga, T.S.; van Krieken, J.H.; Stienstra, R.; van Bodegraven, A.A.; Festen, E.A.M.; Weersma, R.K.; Crusius, J.B.A.; Linskens, R.K.; Joosten, L.A.B.; et al. Genetic association analysis of the functional c.714t>g polymorphism and mucosal expression of dectin-1 in inflammatory bowel disease. PLoS ONE 2009, 4, e7818. [Google Scholar] [CrossRef]
- Ren, A.; Li, Z.; Zhang, X.; Deng, R.; Ma, Y. Inhibition of dectin-1 on dendritic cells prevents maturation and prolongs murine islet allograft survival. J. Inflamm. Res. 2021, 14, 63–73. [Google Scholar] [CrossRef]
- Saeidi, A.; Soltani, M.; Daraei, A.; Nohbaradar, H.; Haghighi, M.M.; Khosravi, N.; Johnson, K.E.; Laher, I.; Hackney, A.C.; Vandusseldorp, T.A.; et al. The effects of aerobic-resistance training and broccoli supplementation on plasma dectin-1 and insulin resistance in males with type 2 diabetes. Nutrients 2021, 13, 3144. [Google Scholar] [CrossRef] [PubMed]
- Reid, D.M.; Montoya, M.; Taylor, P.R.; Borrow, P.; Gordon, S.; Brown, G.D.; Wong, S.Y.C. Expression of the β-glucan receptor, dectin-1, on murine leukocytes in situ correlates with its function in pathogen recognition and reveals potential roles in leukocyte interactions. J. Leukoc. Biol. 2004, 76, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Karumuthil-Melethil, S.; Perez, N.; Li, R.; Vasu, C. Induction of innate immune response through toll-like receptor 2 and dectin 1 prevents type 1 diabetes. J. Immunol. 2008, 181, 8323. [Google Scholar] [CrossRef] [PubMed]
- Suchy, D.; Łabuzek, K.; Machnik, G.; Kozłowski, M.; Okopień, B. Socs and diabetes—Ups and downs of a turbulent relationship. Cell Biochem. Funct. 2013, 31, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Shichita, T.; Yu, Q.; Yoshida, R.; Hashimoto, M.; Okamoto, F.; Torisu, T.; Nakaya, M.; Kobayashi, T.; Takaesu, G.; et al. Suppression of socs3 expression in the pancreatic beta-cell leads to resistance to type 1 diabetes. Biochem. Biophys. Res. Commun. 2007, 359, 952–958. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Q.; Soderland, C.; Steinle, J.J. Tnfα and socs3 regulate irs-1 to increase retinal endothelial cell apoptosis. Cell. Signal. 2012, 24, 1086. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Lin, C.; Chen, R.; Luo, L.; Huang, J.; Liu, H.; Chen, W.; Xu, J.; Yu, H.; Ding, Y. Association analysis of socs3, jak2 and stat3 gene polymorphisms and genetic susceptibility to type 2 diabetes mellitus in chinese population. Diabetol. Metab. Syndr. 2022, 14, 1086–1092. [Google Scholar] [CrossRef]
- Torisu, T.; Sato, N.; Yoshiga, D.; Kobayashi, T.; Yoshioka, T.; Mori, H.; Iida, M.; Yoshimura, A. The dual function of hepatic socs3 in insulin resistance in vivo. Genes Cells 2007, 12, 143–154. [Google Scholar] [CrossRef]
- Liu, X.; Qian, X.; Tu, R.; Mao, Z.; Huo, W.; Zhang, H.; Jiang, J.; Zhang, X.; Tian, Z.; Li, Y.; et al. Socs3 methylation mediated the effect of sedentary time on type 2 diabetes mellitus: The henan rural cohort study. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 634–643. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X.; Wei, D.; Kang, N.; Nie, L.; Liu, P.; Fan, K.; Zhang, L.; Hou, J.; Li, X.; et al. The mediation role of socs3 methylation in the effect of serum testosterone on type 2 diabetes. J. Diabetes 2021, 13, 701–712. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Rodrigues, M.E.; Henriques, M. Candida sp. Infections in patients with diabetes mellitus. J. Clin. Med. 2019, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Lao, M.; Li, C.; Li, J.; Chen, D.; Ding, M.; Gong, Y. Opportunistic invasive fungal disease in patients with type 2 diabetes mellitus from southern china: Clinical features and associated factors. J. Diabetes Investig. 2020, 11, 731. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, L.; Jha, G.; Malasevskaia, I.; Goud, H.K.; Hassan, A. The interplay between sugar and yeast infections: Do diabetics have a greater predisposition to develop oral and vulvovaginal candidiasis? Cureus 2021, 13, e13407. [Google Scholar] [CrossRef] [PubMed]
- Gringhuis, S.I.; den Dunnen, J.; Litjens, M.; van der Vlist, M.; Wevers, B.; Bruijns, S.C.M.; Geijtenbeek, T.B.H. Dectin-1 directs t helper cell differentiation by controlling noncanonical nf-κb activation through raf-1 and syk. Nat. Immunol. 2009, 10, 203–213. [Google Scholar] [CrossRef]
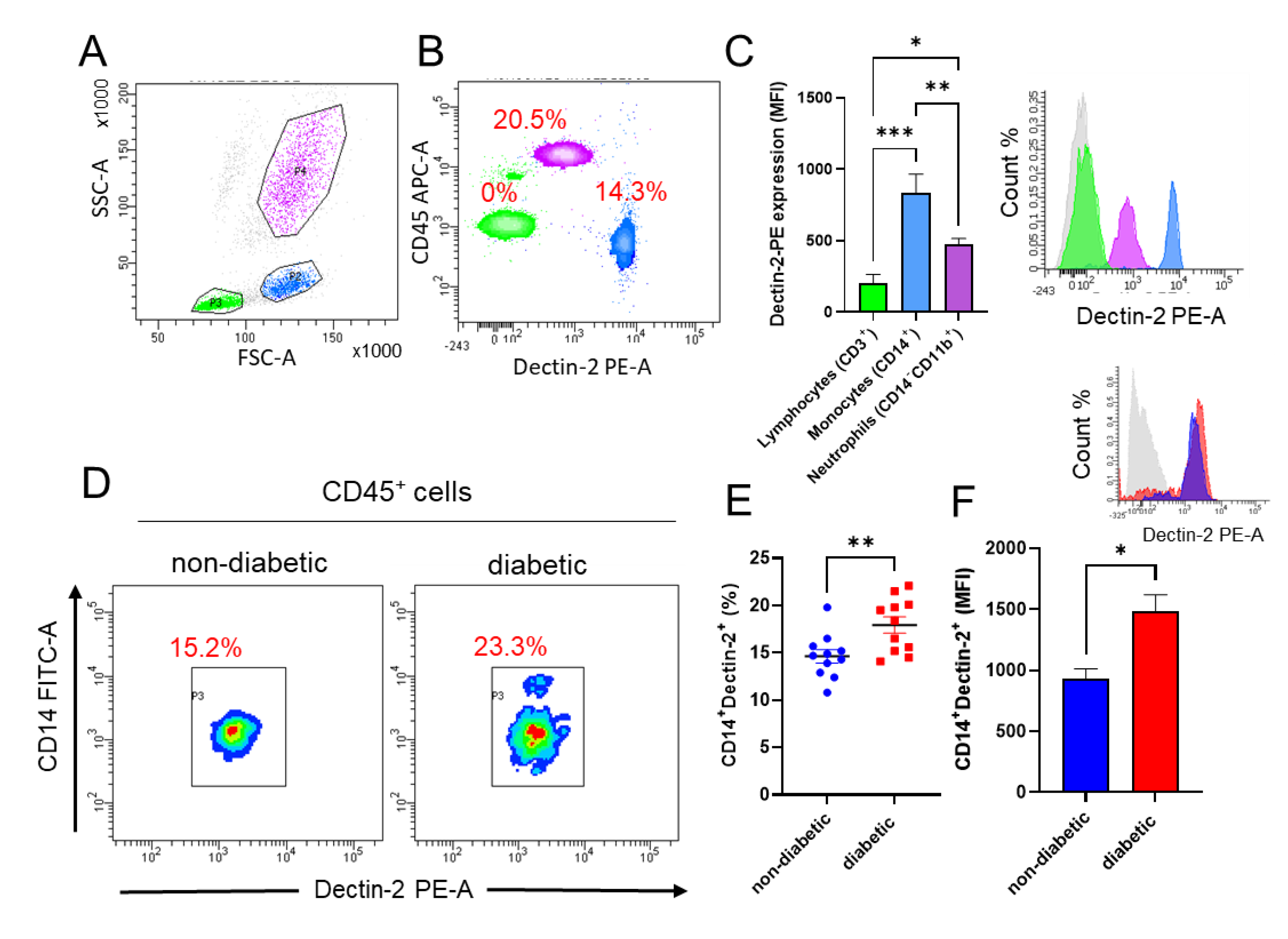
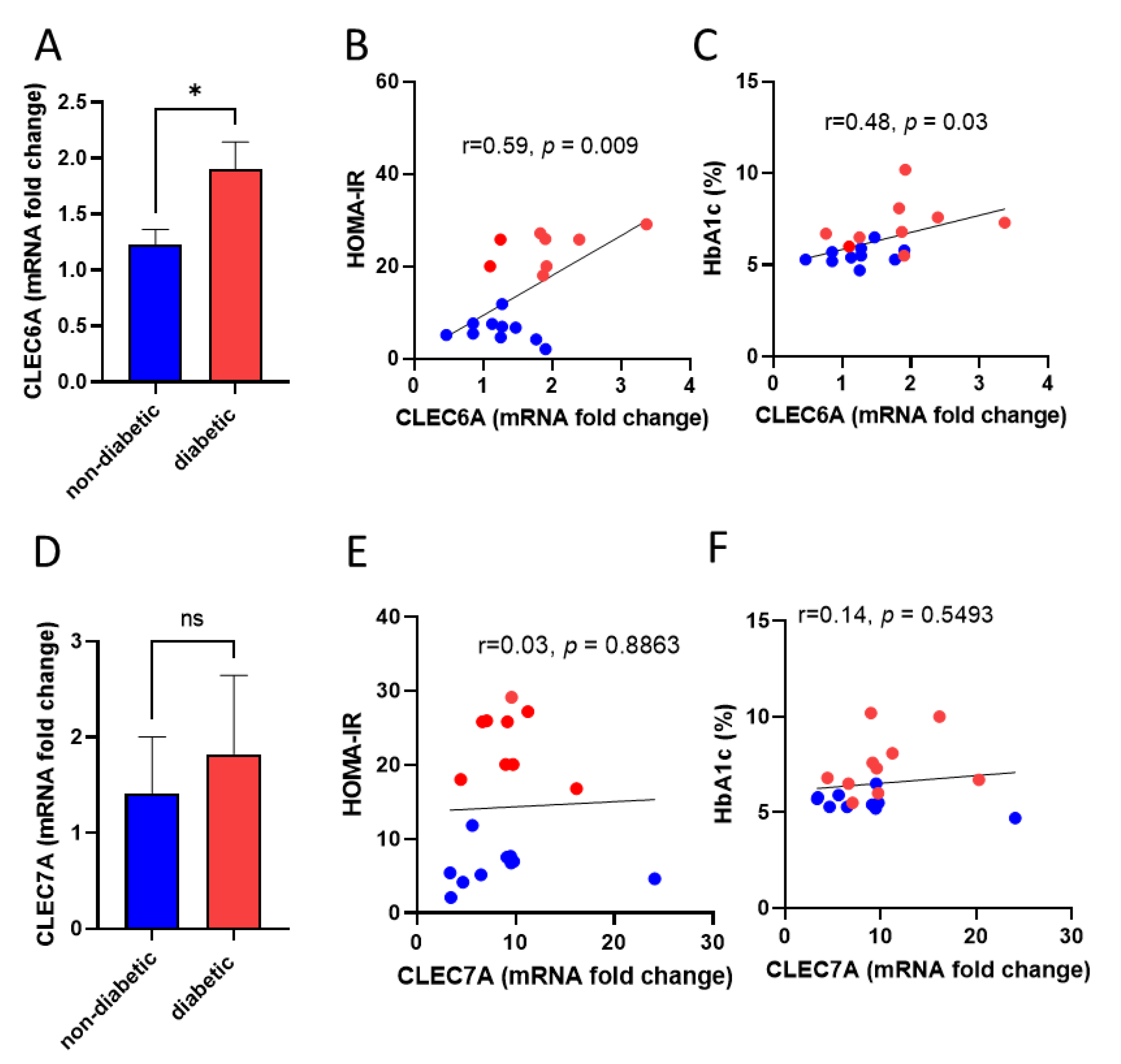
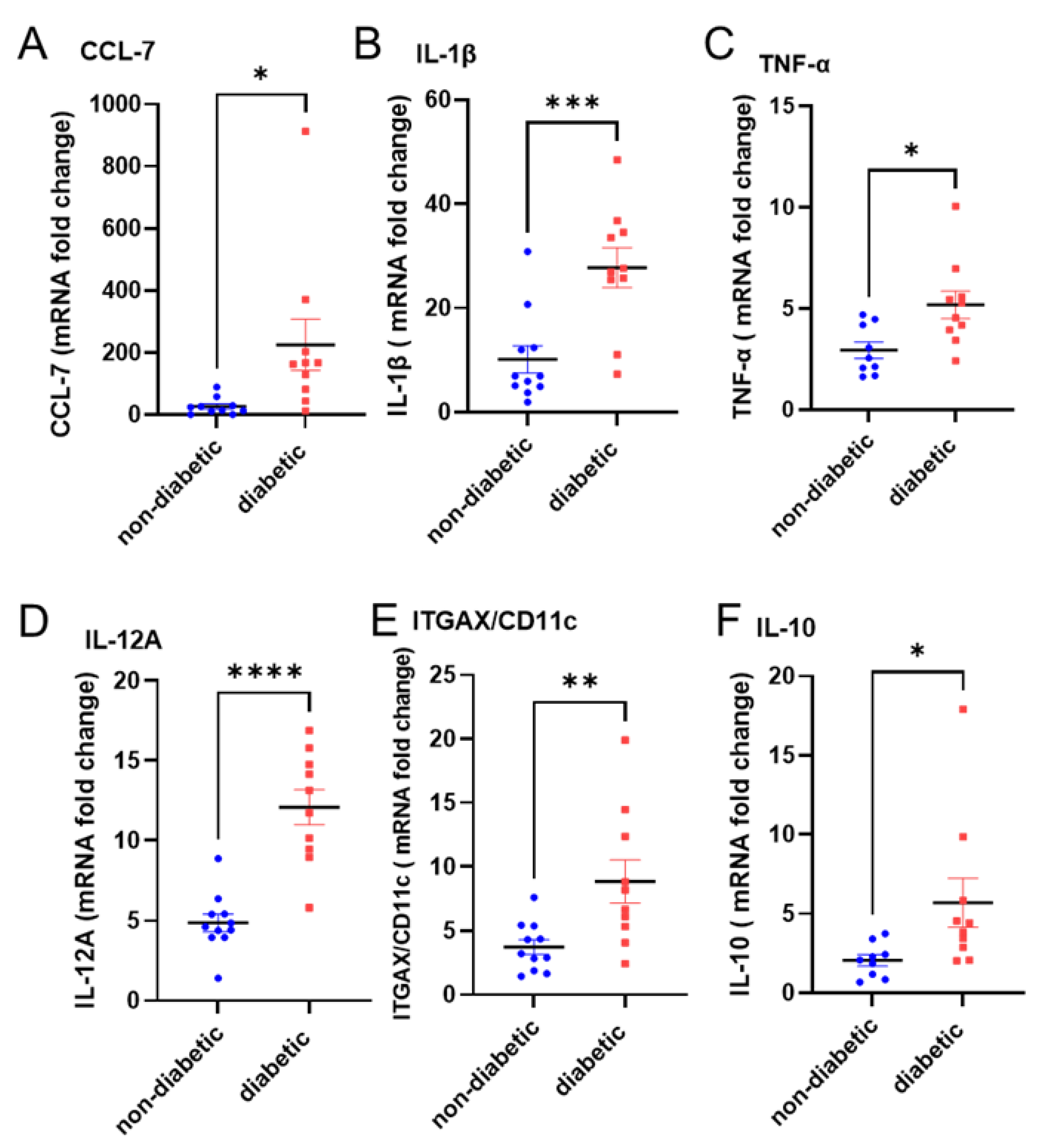
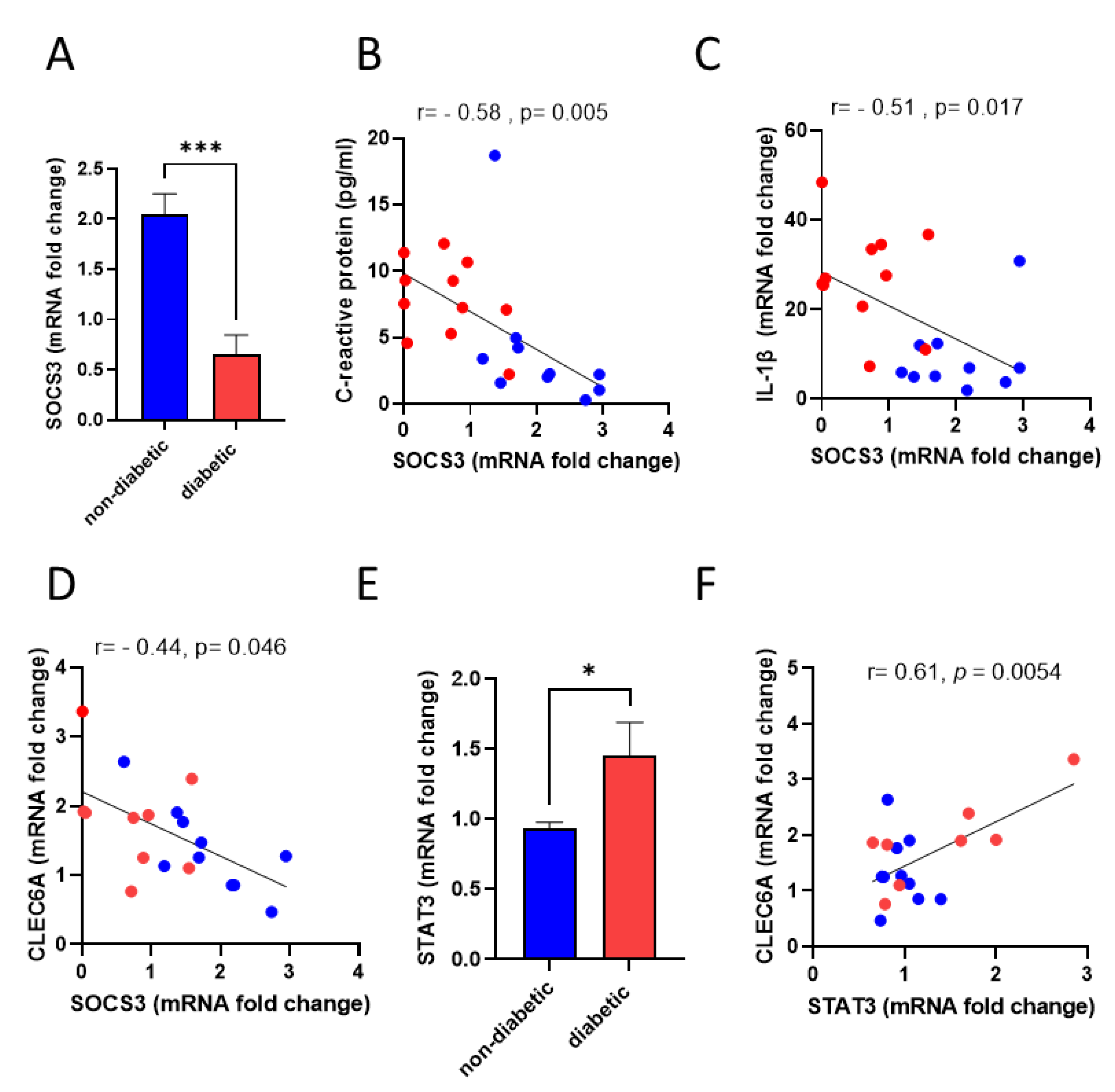
| Characteristic | Non-Diabetic n = 11 (6M/5F) | Diabetic n = 10 (3M/7F) | p Value |
|---|---|---|---|
| Age (years) | 44.2 ± 11.5 | 48 ± 12.5 | 0.3035 |
| Weight (kg) | 86.8 ± 18.6 | 89.1 ± 12.5 | 0.7454 |
| Height (cm) | 1.6 ± 0.08 | 1.69 ± 0.06 | 0.9365 |
| BMI (kg/m2) | 30.4 ± 6.2 | 31.1 ± 3.4 | 0.7455 |
| Waist circumference(inch) | 103.5 ± 17.9 | 102.5 ± 8.12 | 0.8950 |
| Hip circumference (inch) | 107.0 ± 12.4 | 114.8 ± 10.8 | 0.1903 |
| Fat weight (kg) | 34.4 ± 7.4 | 36.9 ± 5.0 | 0.3245 |
| Blood work | Non-Diabetic n = 11 (6M/5F) | Diabetic n = 10 (3M/7F) | p Value |
|---|---|---|---|
| BP/ systolic (mmHg) | 123.5 ± 13.4 | 132 ± 10.3 | 0.1630 |
| BP/diastolic (mmHg) | 81.1 ± 9.1 | 82 ± 12.2 | 0.9745 |
| HR | 77 ± 21.4 | 114.8 ± 14.4 | 0.6057 |
| Triglycerides (mmol/L) | 1.4 ± 0.86 | 1.5 ± 0.68 | 0.6333 |
| Total cholesterol (mmol/L) | 4.8 ± 0.77 | 5.0 ± 0.79 | 0.4856 |
| HDL cholesterol (mmol/L) | 1.1 ± 0.14 | 1.2 ± 0.31 | 0.5354 |
| Fasting glucose (mmol/L) | 5.2 ± 0.48 | 7.8 ± 1.6 | 0.0001 |
| Insulin Con. (mu/L) | 5.1 ± 1.6 | 59.3 ± 40.2 | 0.0004 |
| HOMA-IR | 3.4 ± 3.6 | 23.1 ± 4.4 | <0.0001 |
| HbA1c (%) | 5.5 ± 0.48 | 7.4 ± 1.5 | 0.0015 |
| CRP (pg/mL) | 3.9 ± 5.6 | 7.4 ± 2.8 | <0.0001 |
| Antibody | Host/Isotype | Cat # | Manufacturer |
|---|---|---|---|
| Anti-CD3- PE-Cy7 | IgG1 | 563423 | BD Pharmingen™ |
| Anti-CD45 VioBlue | IgG2a | 130-113-684 | Miltenyi Biotec |
| Anti-CD14- FITC | IgG2a | 555397 | BD Pharmingen™ |
| Anti-CD11b (D12)-APC | IgG2a | 340936 | BD Biosciences |
| Dectin-2/CLEC6A PE | IgG1 | FAB3114P | R&D systems |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haider, M.J.A.; Albaqsumi, Z.; Al-Mulla, F.; Ahmad, R.; Al-Rashed, F. SOCS3 Regulates Dectin-2-Induced Inflammation in PBMCs of Diabetic Patients. Cells 2022, 11, 2670. https://doi.org/10.3390/cells11172670
Haider MJA, Albaqsumi Z, Al-Mulla F, Ahmad R, Al-Rashed F. SOCS3 Regulates Dectin-2-Induced Inflammation in PBMCs of Diabetic Patients. Cells. 2022; 11(17):2670. https://doi.org/10.3390/cells11172670
Chicago/Turabian StyleHaider, Mohammed J. A., Zahraa Albaqsumi, Fahd Al-Mulla, Rasheed Ahmad, and Fatema Al-Rashed. 2022. "SOCS3 Regulates Dectin-2-Induced Inflammation in PBMCs of Diabetic Patients" Cells 11, no. 17: 2670. https://doi.org/10.3390/cells11172670
APA StyleHaider, M. J. A., Albaqsumi, Z., Al-Mulla, F., Ahmad, R., & Al-Rashed, F. (2022). SOCS3 Regulates Dectin-2-Induced Inflammation in PBMCs of Diabetic Patients. Cells, 11(17), 2670. https://doi.org/10.3390/cells11172670









