Abstract
Astrocytes are non-excitable cells in the CNS that can cause life-threatening astrocytoma tumors when they transform to cancerous cells. Perturbed homeostasis of the neurotransmitter glutamate is associated with astrocytoma tumor onset and progression, but the factors that govern this phenomenon are less known. Herein, we review possible mechanisms by which glutamate may act in facilitating the growth of projections in astrocytic cells. This review discusses the similarities and differences between the morphology of astrocytes and astrocytoma cells, and the role that dysregulation in glutamate and calcium signaling plays in the aberrant morphology of astrocytoma cells. Converging reports suggest that ionotropic glutamate receptors and voltage-gated calcium channels expressed in astrocytes may be responsible for the abnormal filopodiagenesis or process extension leading to astrocytoma cells’ infiltration throughout the brain.
1. Introduction
Tumor progression in the brain is different and more aggressive than that in other tissues [1,2]. Indeed, the average life expectancy for patients suffering from high-grade gliomas is only 12–16 months. This short timeline is due to aggressive diffusion, adaptive resistance against chemotherapy, and a high probability of local recurrence after surgery [3]. Cancerous cells arising from glia, astrocytes in particular, are naturally prone to extending cell projections and can infiltrate tissue and spread to distant brain regions. This infiltrative property of transformed astrocytes is thought to be one mechanism by which astrocytoma cells resist chemo- and radiotherapy treatments, or reoccur after ablative surgery [4,5,6,7]. Identifying the stimuli and receptors that contribute to the aberrant morphology of astrocytoma cells might offer means to intervene, slow, or prevent brain tumor infiltration, thereby decreasing the odds of tumor recurrence post-treatment. While several research efforts aim to shed light on how undesired filopodiagenesis occurs, no consensus has been reached yet.
This review discusses possible mechanisms that govern astrocytoma cell tissue infiltration. It outlines how glutamate dysregulation in the brain may be responsible for the aberrant morphology of transformed astrocytes (Figure 1). The effect of glutamate signaling on the morphology of normal astrocytes versus astrocytoma cells is presented first. This is followed by the discussion of glutamate receptors as candidate initiators of undesired glutamate-dependent morphology such as filopodiagenesis, and the involvement of voltage-gated calcium channels via their associated glutamate-sensitive α2δ subunit. Each section presents the context for current hypotheses, along with knowledge gaps or open questions remaining to be addressed.
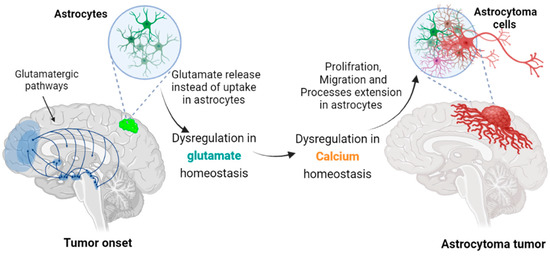
Figure 1.
Transformation of astrocytes leading to astrocytoma cells. In grade II astrocytoma, the mutated astrocytes extend cellular projections that can infiltrate tissue distant from their soma location. Aberrant, excessive glutamate release at synapses is neurotoxic and could allow a tumor cell to spread in the interstitial space liberated by the retracting neurons. An associated glutamate-induced intracellular Ca2+ rise in astrocytoma could also accelerate filopodiagenesis and cancer cell migration.
1.1. Tumors, Glioma and Astrocytoma
Gliomas are the most prevalent primary tumors in the central nervous system [7,8]. The innate ability of glioma cells to use their long processes for invasion, proliferation, and replication, and to enable long-range communication within a network of cancer cells is emerging [4,5,6]. Gliomas are classified according to their parent normal glia: astrocytic tumors, oligodendroglial tumors, oligoastrocytoma tumors, ependymal tumors, and neuronal and mixed neuronal–glial tumors (e.g., ganglioglioma and glioblastoma multiforme) [9,10]. Gliomas are also classified according to their invasiveness as the major determinant of malignancy, starting with grade I tumors that have low proliferative potential and are usually circumscribed to a single region. More infiltrating malignant glioma tumors are classified as grade II (astrocytoma and oligodendroglioma), grade III (anaplastic oligodendroglioma, anaplastic astrocytoma, anaplastic oligoastrocytoma, and anaplastic ependymoma), and grade IV (glioblastoma). [3,7,8]. Grade II astrocytoma cancers are useful models for gaining important biochemical insight, as they arise from transformed astrocytes [11].
1.2. Dynamic Morphology of Astrocytes
Astrocytes extend and retract their fine processes at synaptic contacts [12,13,14,15]. Such dynamic reshaping is influenced by their neighboring cells and the architecture of each brain region [3,4,5,6]. For instance, live confocal imaging of the hippocampus, brainstem, and cortex has demonstrated that astrocytes frequently probe at glutamatergic synapses to eventually enwrap them tightly [16,17,18]. Peripheral astrocytic processes (PAPs) in healthy cells show a clear directional motility preference toward glutamatergic synapses [19,20,21,22]. This suggests that they respond to chemical signals released externally at the synaptic cleft, specifically the neurotransmitter glutamate [23,24,25]. While the malignancy and therapy resistance of astrocytoma tumors correlate strongly with the abnormal morphology of astrocytoma cells, e.g., extra-long processes and excessive network elaboration, the exact cause is still unclear. Dysregulation of glutamate homeostasis has been suggested as a plausible mechanism for the overactive elongation of processes in astrocytoma cells [4,5,6,26].
1.3. Morphology of Astrocytoma Cells
In contrast to normal astrocytes, astrocytoma cells develop longer, more dynamic and highly elaborated processes [4,5,27,28]. The extension and retraction of their processes are not restricted to synaptic sites; they can infiltrate tissue and create networks that extend beyond their initial location [29,30]. In a tumor, these spreading cells create a fibrillary background that can keep their microenvironment shielded from interventions or treatments such as chemo- and radiotherapy. This infiltration of long filopodia within tissue is what allows a malignant glioma tumor to expand throughout the brain more rapidly than other cancer types [26,31,32].
Understanding how to prevent uncontrolled filopodiagenesis in astrocytoma is an attractive, logical step toward stopping tumor progression. These cells most likely use the same molecular mechanisms as their parent astrocytes, but dysregulation in chemical signaling seems to cause their filopodia to extend abnormally. Filopodia are long, cylindrical cell projections filled with bundles of parallel actin filaments [30]. They occur in several cell types and often act as pathfinders in response to guiding chemical cues [31,32,33,34]. Filopodiagenesis depends on the intracellular release of Ca2+ ions, and it arises from the reorganization of sheet-like actin arrays. The molecular details that underpin the initiation and maintenance of filopodia are just beginning to emerge [33,34,35,36]. The proteins involved in the guided chemical responses of the and/or their structural reorganization have attracted attention. In astrocytoma cells, such promising candidates are glutamate-sensitive ion channels.
2. Glutamate Signaling
Glutamate is the primary mediator of excitatory signals in the CNS; it can be released and sensed by both neurons and astrocytes [37,38,39]. More specifically, astrocytes express several receptors and enzymes that are essential for maintaining glutamate homeostasis at a synaptic cleft [40,41,42,43,44,45]. In healthy synaptic communication, each neuronal synapse is usually enveloped by an astrocytic projection to form a tripartite synapse (Figure 2) [37,43]. The morphology of these ensheathing astrocytic projections is known to be sensitive to glutamate [23,43,46]. In glioma, the infiltrative activity of cancer cells is associated with filopodium extension and elevated glutamate levels. How the phenomenon takes place is still unclear, but several hypotheses point to calcium-dependent events.
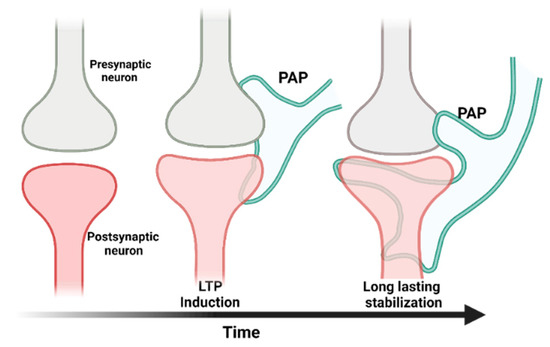
Figure 2.
The tripartite synapse model. A tripartite synapse consists of a pre-synapse (gray), a post-synapse (red), and a perisynaptic astrocyte process (PAP, green). PAPs undergo structural changes in response to long-term potentiation. The growth of post-synaptic dendritic spines is associated with enhanced motility of PAPs, which increases their coverage of synapses and strengthens the synaptic connection [38].
When astrocytes sense a glutamate signal, it is followed by a rapid rise in the intracellular Ca2+ ion concentration, which is required for filopodiagenesis (Figure 3) [40,41,47,48]. Thus, the glutamate receptors that act as Ca2+ ion channels or release Ca2+ from internal sources are prime suspects as potential contributors to the aberrant morphology of astrocytoma cells. The following sections discuss ion channels that may be responsible for the uncontrolled process extension of astrocytoma cells.
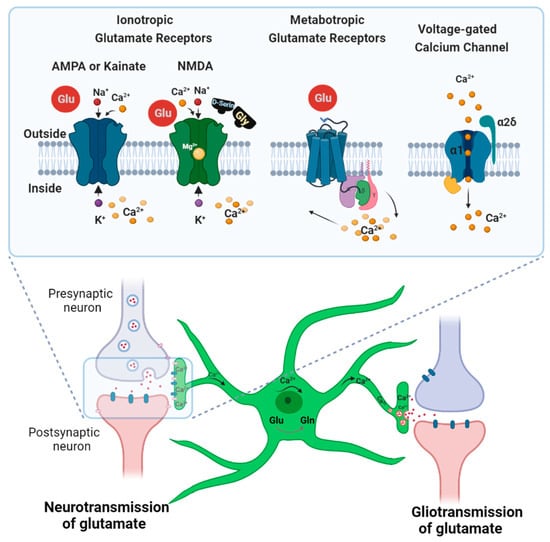
Figure 3.
Scheme outlining glutamate and calcium signaling in astrocytes at a glutamatergic synapse. Astrocytes respond to glutamate stimulation with a strong calcium influx, followed by process extension. Such transient increases in Ca2+ concentration in astrocytes can also cause gliotransmitter release. To prevent excitotoxicity, astrocytes also take up glutamate to recycle it into non-toxic glutamine [49]. Ionotropic (AMPA, KA, and NMDA) and metabotropic glutamate receptors contribute to the intracellular Ca2+ rise in neurons and astrocytes. Voltage-gated calcium channels are also expressed at synapses in astrocytes and play a role in cellular calcium events.
Glutamate Receptors in Astrocytes and Astrocytoma Cells
In astrocytes, the receptors that contribute to Ca2+ events following glutamate stimulation are heterogeneous; their expression profiles vary according to the regions of the brain and stages of development [16,17,48,50]. The two main families of glutamate-sensitive receptors are the ionotropic and metabotropic glutamate receptors (iGluR and mGluR, respectively). iGluRs are ligand-gated ion channels that mediate excitatory neurotransmission upon the binding of glutamate [50,51]. They are well-studied in neurons but not in astrocytes. Three iGluR subfamilies have been found in astrocytes: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs), kainic acid receptors (KARs), and N-methyl-D-aspartate receptors (NMDARs) [52,53,54]. AMPA and KA receptors are usually considered together as non-NMDA receptors due to their structural similarity and their lack of selective antagonists that would allow unequivocal distinction [55,56,57].
Calcium-permeable AMPARs have been identified in glioma cells as well as in astrocytes from the forebrain, the neocortex, and Bergmann glial cells of the cerebellar cortex [43,46]. They have been shown to be involved in glutamate-mediated proliferative signals in glioma cells [11,58]. It is still unknown whether they also contribute to the morphological response of astrocytoma cells upon exposure to glutamate.
Astrocytes and astrocytoma cells also express kainic acid receptors (KARs), but their function remains largely unexplored [23,58,59]. Since astrocytoma progression is known to be affected by perturbed glutamate homeostasis [60,61,62,63], our lab investigated the relationship between the glutamate stimulation of astrocytoma cells and their aberrant morphology [64,65]. Glutamate was found to trigger the rapid extension of processes in astrocytoma cells (as observed in their parent astrocytes). We also found that glutamate receptors sensitive to kainic acid (GluK) are involved in filopodiagenesis [23,24,43]. This selective activation of KARs was sufficient to cause filopodium extension in astrocytoma cells, suggesting for the first time that KARs play a direct role in glutamate-induced filopodiagenesis.
Some astrocytoma cells have been reported to express subunits of NMDARs; however, the absence of the NR1 subunit makes these proteins unfunctional [58]. Despite having been identified in normal astrocytes from a few regions of the brain, NMDAR’s activity is barely detectable due to the high polarization of the astrocytes’ membranes (resting potential: –80 mV) [16,17,44,63,66]. Only in cultured cortical astrocytes have active NMDARs been observed [67].
Astrocytes stimulated with glutamate show a sharp rise in intracellular Ca2+ concentration rise that depends on metabotropic glutamate receptor 5 (mGluR5) [68,69]. These G protein-coupled receptors release calcium from internal stores via an IP3-mediated pathway by activating Gq and phospholipase C [42,45,68,69]. However, whether mGluR5 is essential for astrocytic glutamate signaling is still an unanswered question, as literature reports vary. For instance, glutamate-dependent calcium signaling was found to involve mGluR5 in juvenile hippocampal astrocytes, but mGluR5 agonists failed to induce the same calcium cascade in astrocyte soma from the adult brain [43,48]. Similarly, glutamate released by mossy fibers of the mature hippocampus was shown to induce only partial calcium signals via mGluR5 in astrocytes [70,71].
Thus far, ionotropic glutamate receptors sensitive to AMPA and KA have shown the most direct correlation with triggering filopodiagenesis in astrocytoma cells. It should be noted that astrocytomas also express mGluRs whose function remains unidentified [58].
3. Calcium Influx in Astrocytoma
The presence and role of calcium influx in astrocytes have often been debated over the past decades, but the phenomenon is now generally accepted [72,73]. Consequently, modifying the calcium influx in glioma and astrocytoma is viewed as a potential therapeutic avenue for treating brain cancers [74]. The proteins that govern the calcium influx in astrocytoma have not been unambiguously established. However, the repeated observation of glutamate-dependent calcium signaling events presents a possible means by which to stifle astrocytoma progression.
3.1. Glutamate Signaling vs. Intracellular Calcium
Instead of sequestering and recycling excess glutamate like normal astrocytes, astrocytoma cells increase the glutamate concentration at synaptic contacts [3,60,61,62,63,75,76]. This glutamate accumulation can be caused by the lack of Na+-dependent glutamate uptake [75,77] or by the hyperactive release of glutamate [37,43,61]. Such a local excess of neurotransmitters leads to a Ca2+ rise in astrocytoma that creates a positive feedback loop and amplifies glutamate release [3,75]. The resulting overstimulation of neurons is excitotoxic—the associated uncontrolled intracellular Ca2+ can cause their apoptosis [75,78,79,80].
The glutamate receptors responsible for the large Ca2+ rise in astrocytoma cells have not yet been identified. As described above, we recently reported that glutamate receptors sensitive to kainic acid are involved in filopodiagenesis. Intriguingly, we also found that CaV1.2 voltage-gated calcium ion channels may also participate in filopodiagenesis in astrocytoma cells [64,65,81,82].
3.2. Glutamate Signaling and Votage-Gated Calcium Channels
The possibility that external glutamate stimulation can lead to the activation of CaV channels in astrocytoma raises several mechanistic questions that are only starting to be investigated. In the CNS, high-voltage CaVs were thought to be exclusive to excitable cells such as neurons (i.e., the CaV1 calcium channel family) [83], but they are now known to also be expressed in non-excitable cells such as astrocytes and astrocytoma cells [84,85]. Functional CaVs are composed of a pore-forming α1 subunit that conducts Ca2+ ions, an internal β subunit, and an extracellular α2δ subunit [76].
The function of CaV1s is not limited to excitability in neurons: they help to regulate ion homeostasis [86,87,88], and in non-excitable cells, CaV1s were shown to contribute to non-electrical events that require a high volume of Ca2+ input, such as actin cytoskeleton reorganization [33,34,89]. For instance, calcium “hotspots” arising from CaV1 clusters promote morphological changes in neuronal growth cones [90,91,92].
The greater frequency of opening CaV channels observed during filopodiagenesis suggests their involvement in filopodium formation [33]. Indeed, calcium signaling has been shown to regulate filopodiagenesis via a network of signaling proteins such as Ca2+-activated K+ channels (BK) coupled to voltage-gated calcium channels (CaVs) [33,93]. Moreover, pharmacological CaV blockers have been shown to reduce glioma cell proliferation and infiltration in the brain [94]. Paradoxically, CaV agonists did not enhance tumor growth and did not increase intracellular Ca2+ concentrations [95,96,97,98].
While studying glutamate signaling, our team observed a CaV-channel-dependent morphological response in astrocytoma cells [82]. The stimulation of U118 astrocytoma cells with glutamate caused rapid filopodiagenesis. Selectively blocking the pore-forming α1 subunit of CaVs only reduced the extension of processes. In contrast, using antagonists selective for the extracellular α2δ subunit of CaVs completely inhibited filopodiagenesis [65,82]. Moreover, we observed a rapid translocation and redistribution of high-voltage CaVs at the membrane when astrocytoma cells were exposed to glutamate [81]. These observations suggest that the morphological response to glutamate may involve the extracellular α2δ subunit of CaVs in astrocytoma cells. Whether α2δ exerts its effect by modulating Ca2+ or other pathways remains to be elucidated. It should be noted that, while the α2δ protein was first discovered with CaVs, reports are emerging of its association with other proteins such as NMDA or BK channels as well [99,100].
The complex interplay between glutamate exposure and CaV activation has been reported in neurons but not in astrocytes [101]. However, the expression of functional voltage-gated Ca2+ channels as well as glutamate receptors in astrocytes and astrocytoma cells has been established [43,82,84,102,103]. Hypotheses are starting to converge to involve undesired filopodiagenesis triggered by the possible binding of excess glutamate to CaV’s α2δ subunit. However, much more work is needed to control for other Ca2+-mediated pathways.
4. Concluding Remarks
Studies on tumor progression have shown that the abnormal accumulation of extracellular glutamate at the synaptic cleft leads to disrupted Ca2+ homeostasis. Most reports studying filopodiagenesis caused by cellular calcium signaling in astrocytoma have focused on glutamate receptors. In contrast, voltage-gated calcium channels have been much less investigated [11,48,62,104]. Empirical data now suggest that the rapid process extension of astrocytoma cells associated with glutamate signaling may involve kainic acid receptors (KARs) and/or CaV1 voltage-gated calcium channels. This arguably simplistic view will likely gain complexity as more data become available.
Several questions remain to be answered on what governs the astrocytic morphological changes induced by glutamate signaling. For instance, what is the function of kainic acid receptors (KARs) expressed in astrocytes and astrocytoma cells? Are there cases where AMPA receptors initiate filopodiagenesis? What is the role of other mGluRs expressed in astrocytoma cells? How do CaVs influence glioma tumor growth? How does the extracellular α2δ subunit of CaVs contribute to the morphological response in astrocytoma? Does α2δ exert its effect by modulating Ca2+ directly, or by other pathways? Are there more glutamate receptors responsible for the uncontrolled Ca2+ rise in astrocytoma cells?
Taken together, the accumulating evidence for a glutamate-dependent morphological response of astrocytoma cells points to new research avenues for the role of glutamate signaling in the progression and malignancy of astrocytoma tumors. This review aimed to gather, in one place, the open questions that arise when one surveys the latest developments. We hope that the discussion will stimulate more investigations toward promising approaches to overcoming therapy resistance and the infiltration of astrocytoma tumors within the brain.
Author Contributions
Conceptualization, writing, review, and editing: M.T. and F.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in part by the Discovery Grant program of the National Science and Engineering Research Council of Canada (NSERC).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Streitberger, K.J.; Lilaj, L.; Schrank, F.; Braun, J.; Hoffmann, K.T.; Reiss-Zimmermann, M.; Käs, J.A.; Sack, I. How tissue fluidity influences brain tumor progression. Proc. Natl. Acad. Sci. USA 2020, 117, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Lee, K.C.; Khan, A.; Erisnor, G.; Wang, H.Y. Pathway analysis of glutamate-mediated, calcium-related signaling in glioma progression. Biochem. Pharmacol. 2020, 176, 113814. [Google Scholar] [CrossRef] [PubMed]
- Osswald, M.; Jung, E.; Sahm, F.; Solecki, G.; Venkataramani, V.; Blaes, J.; Weil, S.; Horstmann, H.; Wiestler, B.; Syed, M.; et al. Brain tumour cells interconnect to a functional and resistant network. Nature 2015, 528, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Osswald, M.; Solecki, G.; Wick, W.; Winkler, F. A malignant cellular network in gliomas: Potential clinical implications. Neuro Oncol. 2016, 18, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Weil, S.; Osswald, M.; Solecki, G.; Grosch, J.; Jung, E.; Lemke, D.; Ratliff, M.; Hänggi, D.; Wick, W.; Winkler, F. Tumor microtubes convey resistance to surgical lesions and chemotherapy in gliomas. Neuro Oncol. 2017, 19, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Smith-Cohn, M.; Cohen, A.L.; Colman, H. Glioma subclassifications and their clinical significance. Neurotherapeutics 2017, 14, 284–297. [Google Scholar] [CrossRef]
- Alcantara Llaguno, S.R.; Parada, L.F. Cell of origin of glioma: Biological and clinical implications. Br. J. Cancer 2016, 115, 1445–1450. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 who classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114, 97. [Google Scholar] [CrossRef]
- Corsi, L.; Mescola, A.; Alessandrini, A. Glutamate receptors and glioblastoma multiforme: An old “route” for new perspectives. Int. J. Mol. Sci. 2019, 20, 1796. [Google Scholar] [CrossRef] [PubMed]
- Lim-Fat, M.J.; Wen, P.Y. Glioma progression through synaptic activity. Nat. Rev. Neurol. 2020, 16, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.J.; Barres, B.A. Signaling between glia and neurons: Focus on synaptic plasticity. Curr. Opin. Neurobiol. 2005, 15, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Bernardinelli, Y.; Randall, J.; Janett, E.; Nikonenko, I.; König, S.; Jones, E.V.; Flores, C.E.; Murai, K.K.; Bochet, C.G.; Holtmaat, A.; et al. Activity-dependent structural plasticity of perisynaptic astrocytic domains promotes excitatory synapse stability. Curr. Biol. 2014, 24, 1679–1688. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.J.; Barres, B.A. Glia—More than just brain glue. Nature 2009, 457, 675–677. [Google Scholar] [CrossRef]
- Chung, W.-S.; Allen, N.J.; Eroglu, C. Astrocytes control synapse formation, function, and elimination. Cold Spring Harb. Perspect. Biol. 2015, 7, a020370. [Google Scholar] [CrossRef] [PubMed]
- Matyash, V.; Kettenmann, H. Heterogeneity in astrocyte morphology and physiology. Brain Res. Rev. 2010, 63, 2–10. [Google Scholar] [CrossRef]
- Farmer, W.T.; Murai, K. Resolving astrocyte heterogeneity in the cns. Front. Cell. Neurosci. 2017, 11, 300. [Google Scholar] [CrossRef]
- Stogsdill, J.A.; Ramirez, J.; Liu, D.; Kim, Y.H.; Baldwin, K.T.; Enustun, E.; Ejikeme, T.; Ji, R.R.; Eroglu, C. Astrocytic neuroligins control astrocyte morphogenesis and synaptogenesis. Nature 2017, 551, 192–197. [Google Scholar] [CrossRef]
- Haber, M.; Zhou, L.; Murai, K.K. Cooperative astrocyte and dendritic spine dynamics at hippocampal excitatory synapses. J. Neurosci. 2006, 26, 8881–8891. [Google Scholar] [CrossRef]
- Santello, M.; Toni, N.; Volterra, A. Astrocyte function from information processing to cognition and cognitive impairment. Nat. Neurosci. 2019, 22, 154–166. [Google Scholar] [CrossRef]
- Hirrlinger, J.; Hülsmann, S.; Kirchhoff, F. Astroglial processes show spontaneous motility at active synaptic terminals in situ. Eur. J. Neurosci. 2004, 20, 2235–2239. [Google Scholar] [CrossRef] [PubMed]
- Perez-Alvarez, A.; Navarrete, M.; Covelo, A.; Martin, E.D.; Araque, A. Structural and functional plasticity of astrocyte processes and dendritic spine interactions. J. Neurosci. 2014, 34, 12738–12744. [Google Scholar] [CrossRef] [PubMed]
- Cornell-Bell, A.H.; Prem, T.G.; Smith, S.J. The excitatory neurotransmitter glutamate causes filopodia formation in cultured hippocampal astrocytes. Glia 1990, 3, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Cornell-Bell, A.H.; Thomas, P.G.; Caffrey, J.M. Ca2+ and filopodial responses to glutamate in cultured astrocytes and neurons. Can. J. Physiol. Pharmacol. 1992, 70, S206–S218. [Google Scholar] [CrossRef] [PubMed]
- Aumann, G.; Friedländer, F.; Thümmler, M.; Keil, F.; Brunkhorst, R.; Korf, H.-W.; Derouiche, A. Quantifying filopodia in cultured astrocytes by an algorithm. Neurochem. Res. 2017, 42, 1795–1809. [Google Scholar] [CrossRef] [PubMed]
- Venkataramani, V.; Tanev, D.I.; Strahle, C.; Studier-Fischer, A.; Fankhauser, L.; Kessler, T.; Körber, C.; Kardorff, M.; Ratliff, M.; Xie, R.; et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 2019, 573, 532–538. [Google Scholar] [CrossRef]
- Weingart, J.D.; McGirt, M.J.; Brem, H. High-grade astrocytoma/glioblastoma. In Oncology of CNS Tumors; Springer: Berlin/Heidelberg, Germany, 2010; pp. 147–161. [Google Scholar] [CrossRef]
- Vargová, L.; Homola, A.; Zámečník, J.; Tichý, M.; Beneš, V.; Syková, E. Diffusion parameters of the extracellular space in human gliomas. Glia 2003, 42, 77–88. [Google Scholar] [CrossRef]
- Heller, J.P.; Rusakov, D.A. Morphological plasticity of astroglia: Understanding synaptic microenvironment. Glia 2015, 63, 2133. [Google Scholar] [CrossRef]
- Ozcan, A.S. Filopodia: A rapid structural plasticity substrate for fast learning. Front. Synaptic Neurosci. 2017, 9, 12. [Google Scholar] [CrossRef]
- Fulga, T.A.; Rørth, P. Invasive cell migration is initiated by guided growth of long cellular extensions. Nat. Cell Biol. 2002, 4, 715–719. [Google Scholar] [CrossRef]
- Yee, K.T.; Simon, H.H.; Tessier-Lavigne, M.; O’Leary, D.D.M. Extension of long leading processes and neuronal migration in the mammalian brain directed by the chemoattractant netrin-1. Neuron 1999, 24, 607–622. [Google Scholar] [CrossRef]
- Heckman, C.A.; Plummer, H.K. Filopodia as sensors. Cell Signal. 2013, 25, 2298–2311. [Google Scholar] [CrossRef] [PubMed]
- Faix, J.; Rottner, K. The making of filopodia. Curr. Opin. Cell Biol. 2006, 18, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Mejillano, M.R.; Kojima, S.-I.; Applewhite, D.A.; Gertler, F.B.; Svitkina, T.M.; Borisy, G.G. Lamellipodial versus filopodial mode of the actin nanomachinery: Pivotal role of the filament barbed end. Cell 2004, 118, 363–373. [Google Scholar] [CrossRef]
- Tabatabaee, M.; Menard, F. The α2δ1 protein—Voltage-gated calcium channel subunit or neuronal signal transducer? In Proceedings of the Cold Spring Harbor Symposia on Glia in Health and Disease, Virtual Meeting.
- Araque, A.; Parpura, V.; Sanzgiri, R.P.; Haydon, P.G. Tripartite synapses: Glia, the unacknowledged partner. Trends Neurosci. 1999, 22, 208–215. [Google Scholar] [CrossRef]
- Schiweck, J.; Eickholt, B.J.; Murk, K. Important shapeshifter: Mechanisms allowing astrocytes to respond to the changing nervous system during development, injury and disease. Front. Cell. Neurosci. 2018, 12, 261. [Google Scholar] [CrossRef]
- Nedergaard, M. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science 1994, 263, 1768–1771. [Google Scholar] [CrossRef]
- Bazargani, N.; Attwell, D. Astrocyte calcium signaling: The third wave. Nat. Neurosci. 2016, 19, 182–189. [Google Scholar] [CrossRef]
- Iino, M.; Goto, K.; Kakegawa, W.; Okado, H.; Sudo, M.; Ishiuchi, S.; Miwa, A.; Takayasu, Y.; Saito, I.; Tsuzuki, K.; et al. Glia-synapse interaction through ca2+-permeable AMPA receptors in Bergmann glia. Science 2001, 292, 926–929. [Google Scholar] [CrossRef]
- Lavialle, M.; Aumann, G.; Anlauf, E.; Pröls, F.; Arpin, M.; Derouiche, A. Structural plasticity of perisynaptic astrocyte processes involves ezrin and metabotropic glutamate receptors. Proc. Natl. Acad. Sci. USA 2011, 108, 12915–12919. [Google Scholar] [CrossRef]
- Sun, W.; McConnell, E.; Pare, J.-F.; Xu, Q.; Chen, M.; Peng, W.; Lovatt, D.; Han, X.; Smith, Y.; Nedergaard, M. Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science 2013, 339, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Oberheim, N.A.; Wang, X.; Goldman, S.; Nedergaard, M. Astrocytic complexity distinguishes the human brain. Trends Neurosci. 2006, 29, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Willard, S.S.; Koochekpour, S. Glutamate, glutamate receptors, and downstream signaling pathways. Int. J. Biol. Sci. 2013, 9, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.A.; Adamson, D.C. Neuronal-astrocyte metabolic interactions: Understanding the transition into abnormal astrocytoma metabolism. J. Neuropathol. Exp. Neurol. 2011, 70, 167–176. [Google Scholar] [CrossRef]
- Brand-Schieber, E.; Lowery, S.; Werner, P. Select ionotropic glutamate AMPAF46/kainate receptors are expressed at the astrocyte-vessel interface. Brain Res. 2004, 1007, 178–182. [Google Scholar] [CrossRef]
- Rose, C.R.; Felix, L.; Zeug, A.; Dietrich, D.; Reiner, A.; Henneberger, C. Astroglial glutamate signaling and uptake in the hippocampus. Front. Mol. Neurosci. 2018, 10, 451. [Google Scholar] [CrossRef]
- Hayashi, M.K. Structure-function relationship of transporters in the glutamate–glutamine cycle of the central nervous system. Int. J. Mol. Sci. 2018, 19, 1177. [Google Scholar] [CrossRef]
- Allen, N.J.; Eroglu, C. Cell biology of astrocyte-synapse interactions. Neuron 2017, 96, 697–708. [Google Scholar] [CrossRef]
- Zhou, Y.; Danbolt, N.C. Glutamate as a neurotransmitter in the healthy brain. J. Neural Transm. 2014, 121, 799–817. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Kirchhoff, F. Glutamate-mediated neuronal-glial transmission. In Journal of Anatomy; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 651–660. [Google Scholar]
- Swanson, G.T.; Sakai, R. Ligands for ionotropic glutamate receptors. Prog. Mol. Subcell. Biol. 2009, 46, 123–157. [Google Scholar] [CrossRef]
- Tabatabaee, M.S.; Tian, Z.; Gibon, J.; Menard, F. Aminooxadiazolyl kainic acid reveals that kainic acid receptors contribute to astrocytoma glutamate signaling. bioRxiv 2021, 1–7. [Google Scholar] [CrossRef]
- Alt, A.; Weiss, B.; Ogden, A.M.; Knauss, J.L.; Oler, J.; Ho, K.; Large, T.H.; Bleakman, D. Pharmacological characterization of glutamatergic agonists and antagonists at recombinant human homomeric and heteromeric kainate receptors in vitro. Neuropharmacology 2004, 46, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Matute, C. Therapeutic potential of kainate receptors. CNS Neurosci. Ther. 2011, 17, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Lerma, J. Roles and rules of kainate receptors in synaptic transmission. Nat. Rev. Neurosci. 2003, 4, 481–495. [Google Scholar] [CrossRef]
- Stepulak, A.; Rola, R.; Polberg, K. Glutamate and its receptors in cancer. J. Neural Transm. 2014, 121, 933–944. [Google Scholar] [CrossRef]
- Vargas, J.R.; Koji Takahashi, D.; Thomson, K.E.; Wilcox, K.S. The expression of kainate receptor subunits in hippocampal astrocytes after experimentally induced status epilepticus. J. Neuropathol. Exp. Neurol. 2013, 72, 919–932. [Google Scholar] [CrossRef]
- Rosati, A.; Marconi, S.; Pollo, B.; Tomassini, A.; Lovato, L.; Maderna, E.; Maier, K.; Schwartz, A.; Rizzuto, N.; Padovani, A.; et al. Epilepsy in glioblastoma multiforme: Correlation with glutamine synthetase levels. J. Neurooncol. 2009, 93, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.C.; Sontheimer, H. Glioma cells release excitotoxic concentrations of glutamate. Cancer Res. 1999, 59, 4383–4391. [Google Scholar] [PubMed]
- Lyons, S.A.; Chung, W.J.; Weaver, A.K.; Ogunrinu, T.; Sontheimer, H. Autocrine glutamate signaling promotes glioma cell invasion. Cancer Res. 2007, 67, 9463–9471. [Google Scholar] [CrossRef]
- Takano, T.; Lin, J.H.C.; Arcuino, G.; Gao, Q.; Yang, J.; Nedergaard, M. Glutamate release promotes growth of malignant gliomas. Nat. Med. 2001, 7, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Tabatabaee, M.S.; Edemann, S.; Gibon, J.; Menard, F. Optical Control of Ca2+-Mediated Morphological Response in Glial Cells with Visible Light Using a Photocaged Kainoid. ChemRxiv 2020, 1–6. [Google Scholar] [CrossRef]
- Tabatabaee, M. Glutamate Induced Morphological Response in Astrocytoma Cells. Ph.D. Thesis, The University of British Columbia, Vancouver, BC, Canada, 2021. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Kirchhoff, F. NMDA receptors in glia. Neuroscientist 2007, 13, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Lalo, U.; Pankratov, Y.; Kirchhoff, F.; North, R.A.; Verkhratsky, A. NMDA receptors mediate neuron-to-glia signaling in mouse cortical astrocytes. J. Neurosci. 2006, 26, 2673–2683. [Google Scholar] [CrossRef] [PubMed]
- Panatier, A.; Robitaille, R. Astrocytic mglur5 and the tripartite synapse. Neuroscience 2016, 323, 29–34. [Google Scholar] [CrossRef]
- Panatier, A.; Vallée, J.; Haber, M.; Murai, K.K.; Lacaille, J.C.; Robitaille, R. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell 2011, 146, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Bernardinelli, Y.; Muller, D.; Nikonenko, I. Astrocyte-synapse structural plasticity. Neural Plast. 2014, 2014, 232105. [Google Scholar] [CrossRef] [PubMed]
- Haustein, M.D.; Kracun, S.; Lu, X.-H.; Shih, T.; Jackson-Weaver, O.; Tong, X.; Xu, J.; Yang, X.W.; O’Dell, T.J.; Marvin, J.S.; et al. Conditions and constraints for astrocyte calcium signaling in the hippocampal mossy fiber pathway. Neuron 2014, 82, 413–429. [Google Scholar] [CrossRef] [PubMed]
- Agulhon, C.; Petravicz, J.; McMullen, A.B.; Sweger, E.J.; Minton, S.K.; Taves, S.R.; Casper, K.B.; Fiacco, T.A.; McCarthy, K.D. What is the role of astrocyte calcium in neurophysiology? Neuron 2008, 59, 932–946. [Google Scholar] [CrossRef]
- Ahmadpour, N.; Kantroo, M.; Stobart, J.L. Extracellular calcium influx pathways in astrocyte calcium microdomain physiology. Biomolecules 2021, 11, 1467. [Google Scholar] [CrossRef]
- Maklad, A.; Sharma, A.; Azimi, I. Calcium signaling in brain cancers: Roles and therapeutic targeting. Cancers 2019, 11, 145. [Google Scholar] [CrossRef]
- de Groot, J.; Sontheimer, H. Glutamate and the biology of gliomas. Glia 2011, 59, 1181–1189. [Google Scholar] [CrossRef]
- van Lith, S.A.M.; Navis, A.C.; Verrijp, K.; Niclou, S.P.; Bjerkvig, R.; Wesseling, P.; Tops, B.; Molenaar, R.; van Noorden, C.J.F.; Leenders, W.P.J. Glutamate as chemotactic fuel for diffuse glioma cells: Are they glutamate suckers? Biochim. Biophys. Acta-Rev. Cancer 2014, 1846, 66–74. [Google Scholar] [CrossRef]
- Ye, Z.C.; Rothstein, J.D.; Sontheimer, H. Compromised glutamate transport in human glioma cells: Reduction-mislocalization of sodium-dependent glutamate transporters and enhanced activity of cystine-glutamate exchange. J. Neurosci. 1999, 19, 10767–10777. [Google Scholar] [CrossRef] [PubMed]
- Hamadi, A.; Giannone, G.; Takeda, K.; Rondé, P. Glutamate involvement in calcium-dependent migration of astrocytoma cells. Cancer Cell Int. 2014, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.W. Glutamate neurotoxicity in cortical cell culture is calcium dependent. Neurosci. Lett. 1985, 58, 293–297. [Google Scholar] [CrossRef]
- Mattson, M.P. Excitotoxicity. In Stress: Physiology, Biochemistry, and Pathology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 125–134. [Google Scholar] [CrossRef]
- Tabatabaee, M.S.; Kerkovius, J.; Menard, F. Design of an imaging probe to monitor real-time redistribution of l-type voltage-gated calcium channels in astrocytic glutamate signaling. Mol. Imaging Biol. 2021, 23, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaee, M.S.; Menard, F. L-type voltage-gated calcium channel modulators inhibit glutamate-induced morphology changes in u118-mg astrocytoma cells. Cell. Mol. Neurobiol. 2020, 40, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Striessnig, J.; Bolz, H.J.; Koschak, A. Channelopathies in cav1.1, cav1.3, and ca v1.4 voltage-gated l-type Ca2+ channels. Pflugers Arch. Eur. J. Physiol. 2010, 460, 361–374. [Google Scholar] [CrossRef]
- Latour, I.; Hamid, J.; Beedle, A.M.; Zamponi, G.W.; Macvicar, B.A. Expression of voltage-gated ca2+ channel subtypes in cultured astrocytes. Glia 2003, 41, 347–353. [Google Scholar] [CrossRef]
- Cheli, V.T.; Santiago González, D.A.; Smith, J.; Spreuer, V.; Murphy, G.G.; Paez, P.M. L-type voltage-operated calcium channels contribute to astrocyte activation in vitro. Glia 2016, 64, 1396–1415. [Google Scholar] [CrossRef]
- Zamponi, G.W.; Striessnig, J.; Koschak, A.; Dolphin, A.C. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol. Rev. 2015, 67, 821–870. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, S.; Ishii, Y.; Horigane, S.I.; Suzuki, K.; Ohkura, M.; Nakai, J.; Fujii, H.; Takemoto-Kimura, S.; Bito, H. A critical neurodevelopmental role for l-type voltage-gated calcium channels in neurite extension and radial migration. J. Neurosci. 2018, 38, 5551–5566. [Google Scholar] [CrossRef]
- Zuccotti, A.; Clementi, S.; Reinbothe, T.; Torrente, A.; Vandael, D.H.; Pirone, A. Structural and functional differences between l-type calcium channels: Crucial issues for future selective targeting. Trends Pharmacol. Sci. 2011, 32, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Jacquemet, G.; Baghirov, H.; Georgiadou, M.; Sihto, H.; Peuhu, E.; Cettour-janet, P.; Kronqvist, P.; Joensuu, H.; Ivaska, J.; He, T.; et al. L-type calcium channels regulate filopodia stability and cancer cell invasion downstream of integrin signalling. Nat. Commun. 2016, 7, 13297. [Google Scholar] [CrossRef] [PubMed]
- Tam, T.; Mathews, E.; Snutch, T.P.; Schafer, W.R. Voltage-gated calcium channels direct neuronal migration in caenorhabditis elegans. Dev. Biol. 2000, 226, 104–117. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zatkova, M.; Reichova, A.; Bacova, Z.; Strbak, V.; Kiss, A.; Bakos, J. Neurite outgrowth stimulated by oxytocin is modulated by inhibition of the calcium voltage-gated channels. Cell. Mol. Neurobiol. 2018, 38, 371–378. [Google Scholar] [CrossRef]
- Silver, R.A.; Lamb, A.G.; Bolsover, S.R. Calcium hotspots caused by l-channel clustering promote morphological changes in neuronal growth cones. Nature 1990, 343, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, X.; Gui, P.; Wu, J.; Sheng, J.Z.; Ling, S.; Braun, A.P.; Davis, G.E.; Davis, M.J. A5β1 integrin engagement increases large conductance, Ca2+-activated K+ channel current and Ca2+ sensitivity through c-src-mediated channel phosphorylation. J. Biol. Chem. 2010, 285, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Phan, N.N.; Wang, C.Y.; Chen, C.F.; Sun, Z.; Lai, M.D.; Lin, Y.C. Voltage-gated calcium channels: Novel targets for cancer therapy. Oncol. Lett. 2017, 14, 2059–2074. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Delgado, G.; Felix, R. Emerging role of cav 1.2 channels in proliferation and migration in distinct cancer cell lines. Oncology 2017, 93, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Reardon, D.A.; Rich, J.N.; Friedman, H.S.; Bigner, D.D. Recent advances in the treatment of malignant astrocytoma. J. Clin. Oncol. 2006, 24, 1253–1265. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Sayeed, M.M.; Wurster, R.D. Inhibition of cell growth and intracellular Ca2+ mobilization in human brain tumor cells by Ca2+ channel antagonists. Mol. Chem. Neuropathol. 1994, 22, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Kunert-Radek, J.; Stepien, H.; Radek, A.; Lyson, K.; Pawlikowski, M. Inhibitory effect of calcium channel blockers on proliferation of human glioma cells in vitro. Acta Neurol. Scand. 1989, 79, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.X.; Gadotti, V.M.; Souza, I.A.; Chen, L.; Zamponi, G.W. BK potassium channels suppress cavα2δ subunit function to reduce inflammatory and neuropathic pain. Cell Rep. 2018, 22, 1956–1964. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, L.; Chen, S.R.; Chen, H.; Xie, J.D.; Sirrieh, R.E.; MacLean, D.M.; Zhang, Y.; Zhou, M.H.; Jayaraman, V.; et al. The α2δ-1-nmda receptor complex is critically involved in neuropathic pain development and gabapentin therapeutic actions. Cell Rep. 2018, 22, 2455–2468. [Google Scholar] [CrossRef]
- Youn, D.; Gerber, G.; Sather, W.A. Ionotropic glutamate receptors and voltage-gated ca2+ channels in long-term potentiation of spinal dorsal horn synapses and pain hypersensitivity. Neural Plast. 2013, 2013, 654257. [Google Scholar] [CrossRef]
- Zamora, N.N.; Cheli, V.T.; Santiago González, D.A.; Wan, R.; Paez, P.M. Deletion of voltage-gated calcium channels in astrocytes during demyelination reduces brain inflammation and promotes myelin regeneration in mice. J. Neurosci. 2020, 40, 3332–3347. [Google Scholar] [CrossRef] [PubMed]
- MacVicar, B.A. Voltage-dependent calcium channels in glial cells author (s): B a macvicar published by: American association for the advancement of science stable url. Science 1984, 226, 1345–1347. Available online: http://www.jstor.org/stable/1693335 (accessed on 17 May 2022). [CrossRef]
- Savaskan, N.E.; Seufert, S.; Hauke, J.; Trankle, T.; Eyupoglu, I.E.; Hahnen, E. Dissection of mitogenic and neurodegenerative actions of cystine and glutamate in malignant gliomas. Oncogene 2011, 30, 43–53. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).