Proteostasis Deregulation in Neurodegeneration and Its Link with Stress Granules: Focus on the Scaffold and Ribosomal Protein RACK1
Abstract
1. Introduction
2. Translation Impairment in Neurodegeneration
2.1. Ribosome Dysfunction and Impaired Protein Synthesis
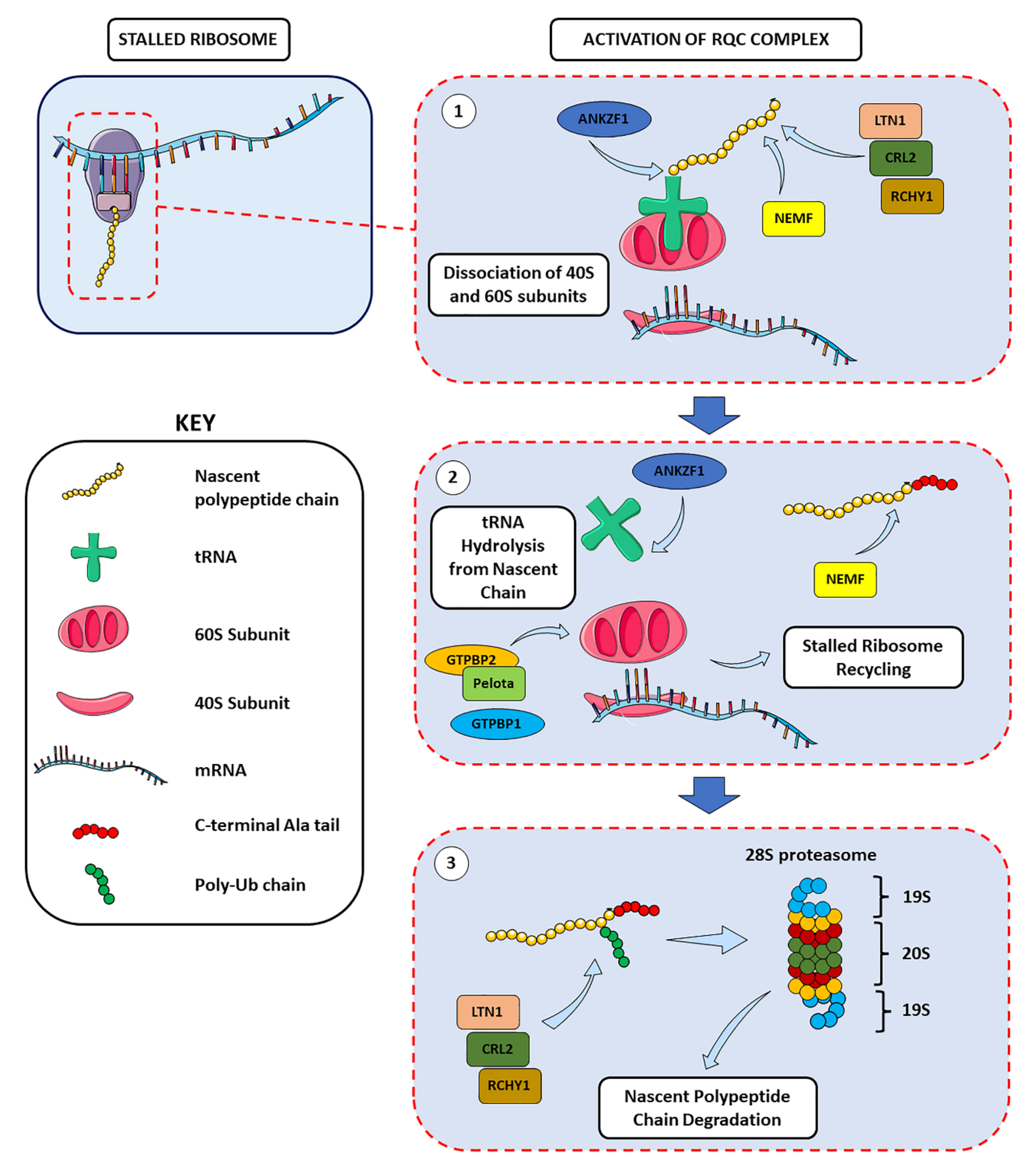
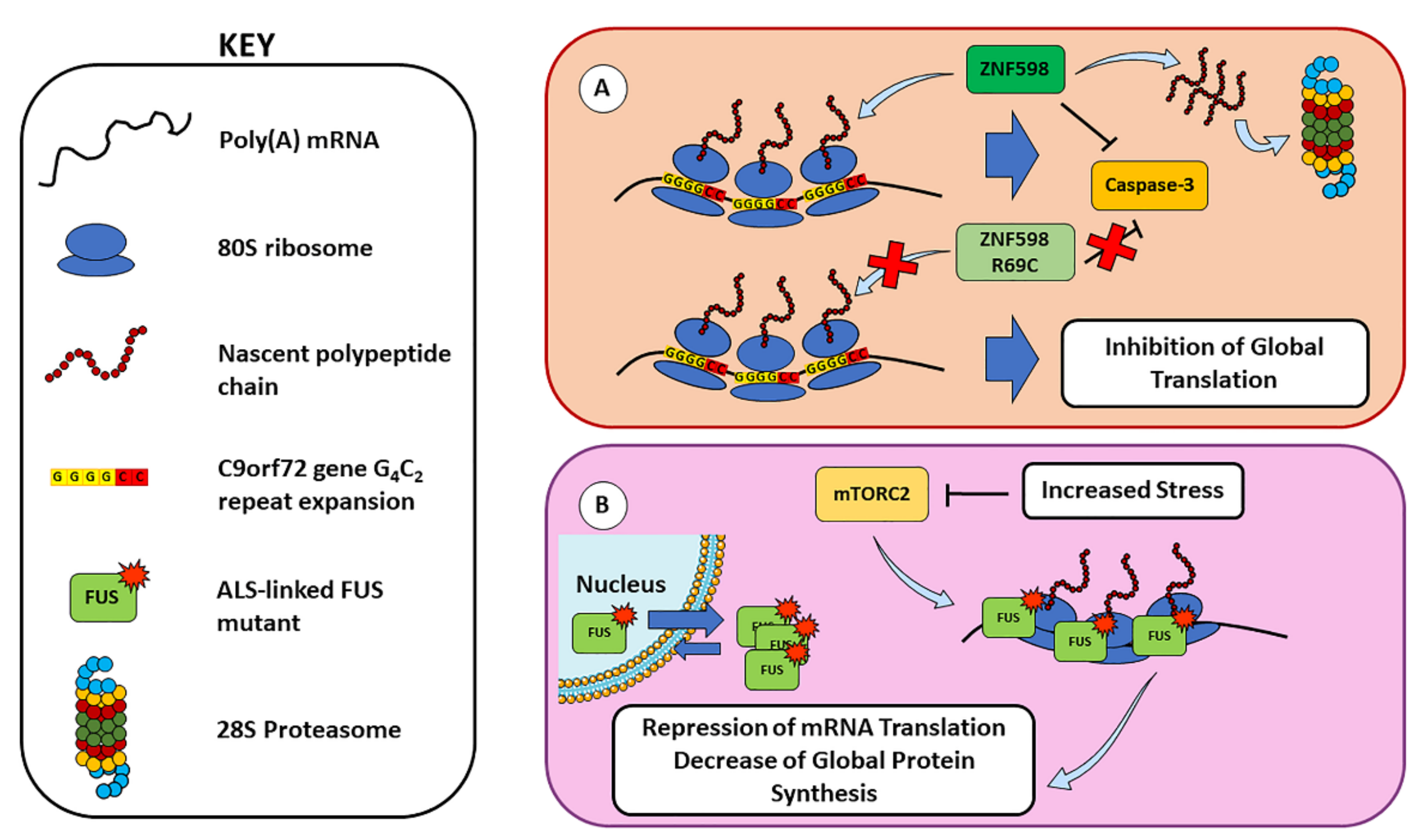
2.2. Ribosome Stalling and Ribosome-Associated Quality Control
2.3. Protein Quality Control and Proteostasis Regulation
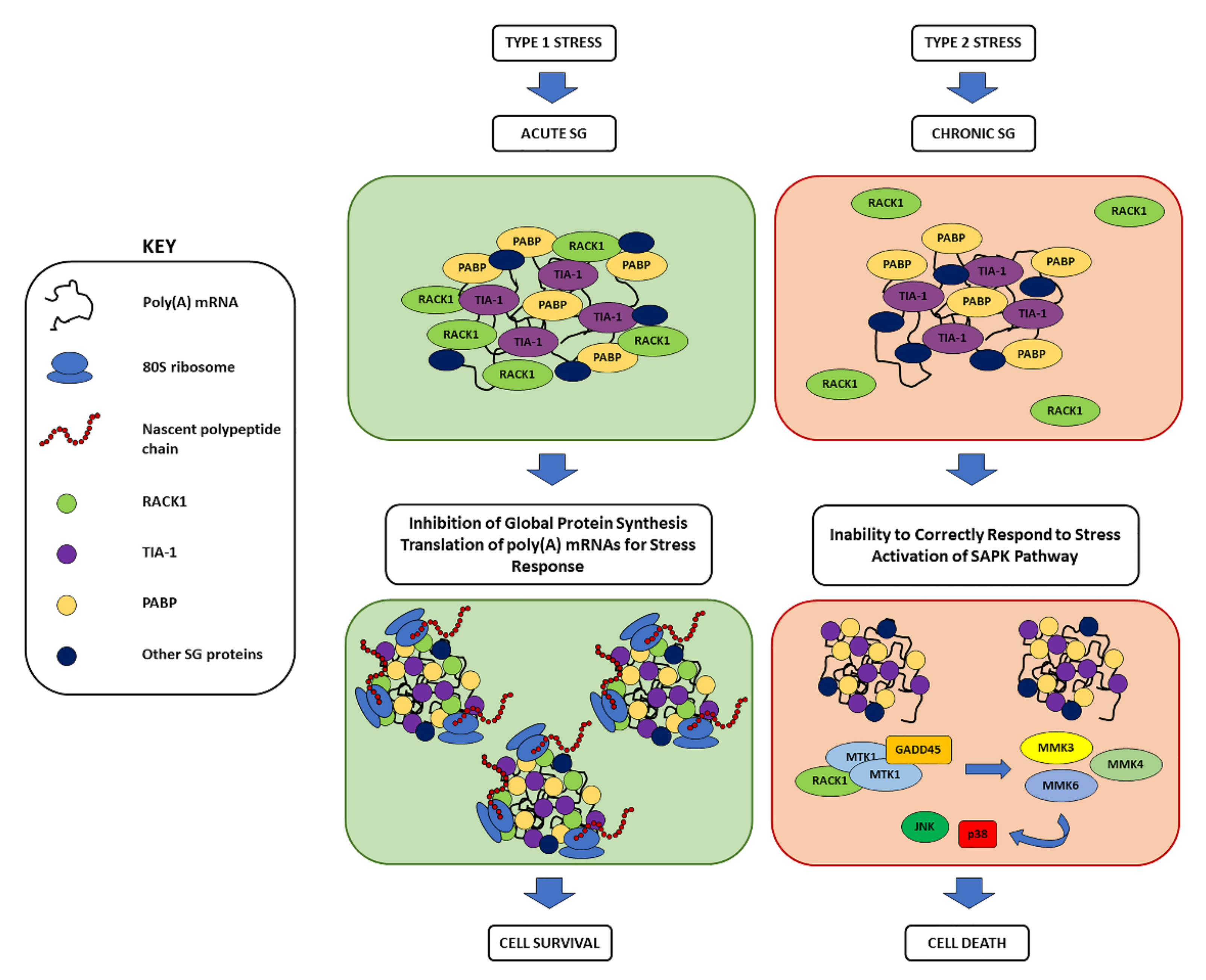
3. Overview on Stress Granules: Function, Composition, Assembly, and Their Role in Neurodegeneration
4. Receptor for Activated C Kinase 1
4.1. RACK1 Role in Translation and in Neuronal Biology
4.2. Role of RACK1 in SGs
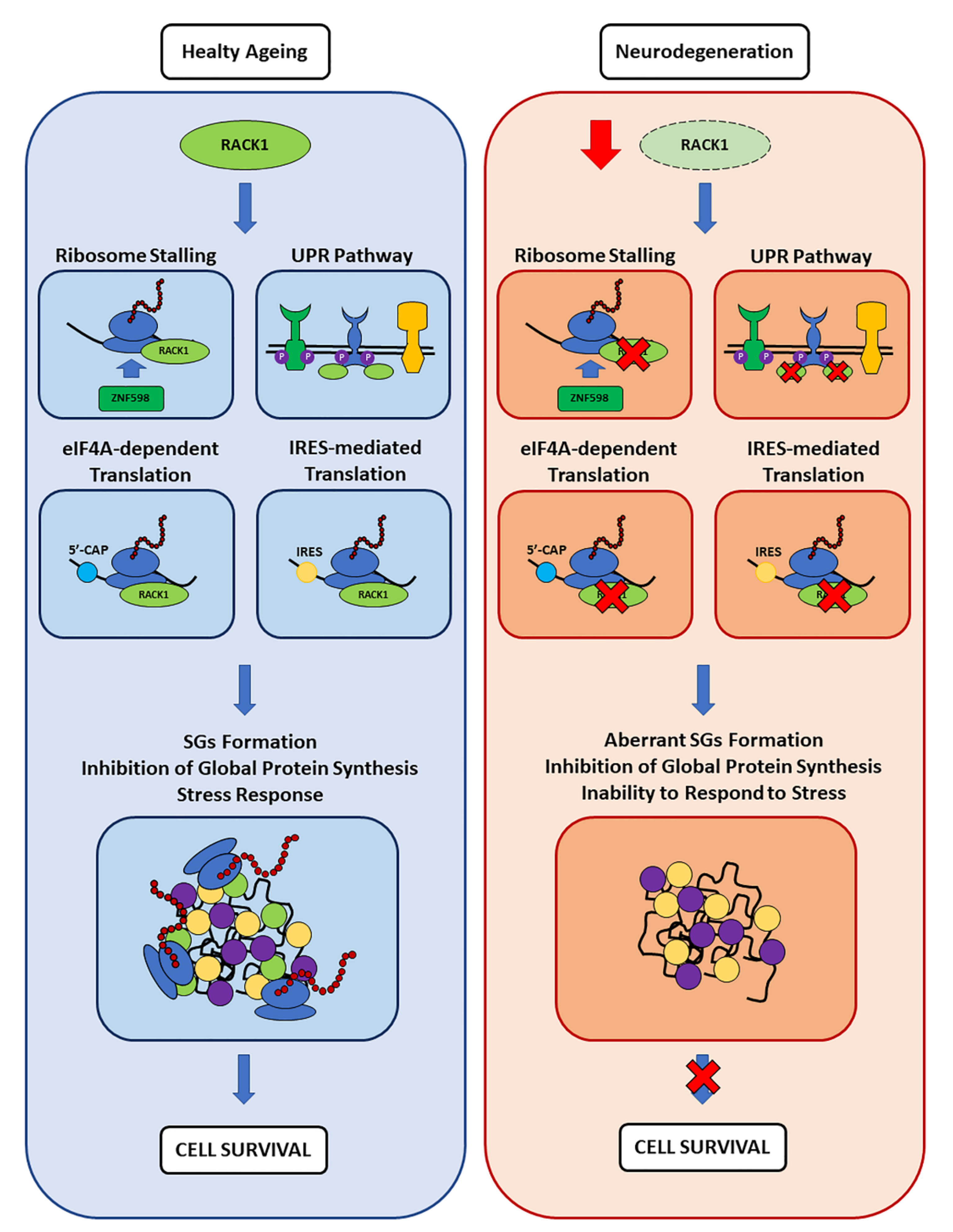
4.3. SGs and RACK1 in Neurodegeneration
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef] [PubMed]
- Tillement, J.-P.; Lecanu, L.; Papadopoulos, V. Amyloidosis and Neurodegenerative Diseases: Current Treatments and New Pharmacological Options. Pharmacology 2009, 85, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Soto, C.; Pritzkow, S. Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat. Neurosci. 2018, 21, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Wolozin, B.; Ivanov, P. Stress granules and neurodegeneration. Nat. Rev. Neurosci. 2019, 20, 649–666. [Google Scholar] [CrossRef] [PubMed]
- An, W.-L.; Cowburn, R.F.; Li, L.; Braak, H.; Alafuzoff, I.; Iqbal, K.; Iqbal, I.-G.; Winblad, B.; Pei, J.-J. Up-Regulation of Phosphorylated/Activated p70 S6 Kinase and Its Relationship to Neurofibrillary Pathology in Alzheimer’s Disease. Am. J. Pathol. 2003, 163, 591–607. [Google Scholar] [CrossRef]
- Li, J.; Zhu, M.; Manning-Bog, A.B.; Di Monte, D.A.; Fink, A.L. Dopamine and L-dopa disaggregate amyloid fibrils: Implications for Parkinson’s and Alzheimer’s disease. FASEB J. 2004, 18, 962–964. [Google Scholar] [CrossRef]
- Ding, Q.; Markesbery, W.R.; Chen, Q.; Li, F.; Keller, J. Ribosome Dysfunction Is an Early Event in Alzheimer’s Disease. J. Neurosci. 2005, 25, 9171–9175, Erratum in: J. Neurosci. 2006, 26, 3077. [Google Scholar] [CrossRef]
- Mills, E.W.; Green, R. Ribosomopathies: There’s strength in numbers. Science 2017, 358, eaan2755. [Google Scholar] [CrossRef]
- Wang, D.-S.; Bennett, D.A.; Mufson, E.J.; Mattila, P.; Cochran, E.; Dickson, D.W. Contribution of changes in ubiquitin and myelin basic protein to age-related cognitive decline. Neurosci. Res. 2003, 48, 93–100. [Google Scholar] [CrossRef]
- Beckelman, B.C.; Yang, W.; Kasica, N.P.; Zimmermann, H.R.; Zhou, X.; Keene, C.D.; Ryazanov, A.G.; Ma, T. Genetic reduction of eEF2 kinase alleviates pathophysiology in Alzheimer’s disease model mice. J. Clin. Investig. 2019, 129, 820–833. [Google Scholar] [CrossRef]
- Meier, S.; Bell, M.; Lyons, D.N.; Rodriguez-Rivera, J.; Ingram, A.; Fontaine, S.N.; Mechas, E.; Chen, J.; Wolozin, B.; LeVine, H.; et al. Pathological Tau Promotes Neuronal Damage by Impairing Ribosomal Function and Decreasing Protein Synthesis. J. Neurosci. 2016, 36, 1001–1007. [Google Scholar] [CrossRef]
- Evans, H.T.; Taylor, D.; Kneynsberg, A.; Bodea, L.-G.; Götz, J. Altered ribosomal function and protein synthesis caused by tau. Acta Neuropathol. Commun. 2021, 9, 110. [Google Scholar] [CrossRef] [PubMed]
- Nyhus, C.; Pihl, M.; Hyttel, P.; Hall, V. Evidence for nucleolar dysfunction in Alzheimer’s disease. Rev. Neurosci. 2019, 30, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Smith, M.A.; Zhu, X.; Baus, D.; Merrick, W.C.; Tartakoff, A.M.; Hattier, T.; Harris, P.L.; Siedlak, S.L.; Fujioka, H.; et al. Ribosomal RNA in Alzheimer Disease Is Oxidized by Bound Redox-active Iron. J. Biol. Chem. 2005, 280, 20978–20986. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Dimayuga, E.; Markesbery, W.R.; Keller, J.N. Proteasome inhibition increases DNA and RNA oxidation in astrocyte and neuron cultures. J. Neurochem. 2004, 91, 1211–1218. [Google Scholar] [CrossRef]
- Nunomura, A.; Moreira, P.I.; Castellani, R.J.; Lee, H.-G.; Zhu, X.; Smith, M.A.; Perry, G. Oxidative Damage to RNA in Aging and Neurodegenerative Disorders. Neurotox. Res. 2012, 22, 231–248. [Google Scholar] [CrossRef]
- Poulsen, H.E.; Specht, E.; Broedbaek, K.; Henriksen, T.; Ellervik, C.; Mandrup-Poulsen, T.; Tonnesen, M.; Nielsen, P.E.; Andersen, H.U.; Weimann, A. RNA modifications by oxidation: A novel disease mechanism? Free Radic. Biol. Med. 2012, 52, 1353–1361. [Google Scholar] [CrossRef]
- Boccaletto, P.; Machnicka, M.A.; Purta, E.; Piątkowski, P.; Baginski, B.; Wirecki, T.K.; De Crécy-Lagard, V.; Ross, R.; Limbach, P.A.; Kotter, A.; et al. MODOMICS: A database of RNA modification pathways. 2017 update. Nucleic Acids Res. 2018, 46, D303–D307. [Google Scholar] [CrossRef]
- Boccaletto, P.; Magnus, M.; Almeida, C.; Żyła, A.; Astha, A.; Pluta, R.; Baginski, B.; Jankowska, E.; Dunin-Horkawicz, S.; Wirecki, T.; et al. RNArchitecture: A database and a classification system of RNA families, with a focus on structural information. Nucleic Acids Res. 2017, 46, D202–D205. [Google Scholar] [CrossRef]
- Chatterjee, B.; Shen, C.-K.J.; Majumder, P. RNA Modifications and RNA Metabolism in Neurological Disease Pathogenesis. Int. J. Mol. Sci. 2021, 22, 11870. [Google Scholar] [CrossRef]
- Jiang, L.; Lin, W.; Zhang, C.; Ash, P.E.; Verma, M.; Kwan, J.; van Vliet, E.; Yang, Z.; Cruz, A.L.; Boudeau, S.; et al. Interaction of tau with HNRNPA2B1 and N6-methyladenosine RNA mediates the progression of tauopathy. Mol. Cell 2021, 81, 4209–4227.e12. [Google Scholar] [CrossRef] [PubMed]
- Shafik, A.M.; Zhou, H.; Lim, J.; Dickinson, B.; Jin, P. Dysregulated mitochondrial and cytosolic tRNA m1A methylation in Alzheimer’s disease. Hum. Mol. Genet. 2022, 31, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Patassini, S.; Rustogi, N.; Riba-Garcia, I.; Hale, B.D.; Phillips, A.M.; Waldvogel, H.; Haines, R.; Bradbury, P.; Stevens, A.; et al. Regional protein expression in human Alzheimer’s brain correlates with disease severity. Commun. Biol. 2019, 2, 43. [Google Scholar] [CrossRef] [PubMed]
- Chaney, J.L.; Clark, P.L. Roles for Synonymous Codon Usage in Protein Biogenesis. Annu. Rev. Biophys. 2015, 44, 143–166. [Google Scholar] [CrossRef] [PubMed]
- Ingolia, N.T. Ribosome Footprint Profiling of Translation throughout the Genome. Cell 2016, 165, 22–33. [Google Scholar] [CrossRef]
- Buchan, J.R.; Stansfield, I. Halting a cellular production line: Responses to ribosomal pausing during translation. Biol. Cell 2007, 99, 475–487. [Google Scholar] [CrossRef]
- Tsai, C.-J.; Sauna, Z.E.; Kimchi-Sarfaty, C.; Ambudkar, S.V.; Gottesman, M.M.; Nussinov, R. Synonymous Mutations and Ribosome Stalling Can Lead to Altered Folding Pathways and Distinct Minima. J. Mol. Biol. 2008, 383, 281–291. [Google Scholar] [CrossRef]
- Fredrick, K.; Ibba, M. How the Sequence of a Gene Can Tune Its Translation. Cell 2010, 141, 227–229. [Google Scholar] [CrossRef]
- Ishimura, R.; Nagy, G.; Dotu, I.; Zhou, H.; Yang, X.-L.; Schimmel, P.; Senju, S.; Nishimura, Y.; Chuang, J.H.; Ackerman, S.L. Ribosome stalling induced by mutation of a CNS-specific tRNA causes neurodegeneration. Science 2014, 345, 455–459. [Google Scholar] [CrossRef]
- Verma, R.; Reichermeier, K.M.; Burroughs, A.M.; Oania, R.S.; Reitsma, J.M.; Aravind, L.; Deshaies, R.J. Vms1 and ANKZF1 peptidyl-tRNA hydrolases release nascent chains from stalled ribosomes. Nature 2018, 557, 446–451. [Google Scholar] [CrossRef]
- Joazeiro, C.A.P. Mechanisms and functions of ribosome-associated protein quality control. Nat. Rev. Mol. Cell Biol. 2019, 20, 368–383. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.B.; Kigoshi-Tansho, Y.; Sher, R.B.; Ravenscroft, G.; Stauffer, J.E.; Kumar, R.; Yonashiro, R.; Müller, T.; Griffith, C.; Allen, W.; et al. NEMF mutations that impair ribosome-associated quality control are associated with neuromuscular disease. Nat. Commun. 2020, 11, 4625. [Google Scholar] [CrossRef] [PubMed]
- Udagawa, T.; Seki, M.; Okuyama, T.; Adachi, S.; Natsume, T.; Noguchi, T.; Matsuzawa, A.; Inada, T. Failure to Degrade CAT-Tailed Proteins Disrupts Neuronal Morphogenesis and Cell Survival. Cell Rep. 2021, 34, 108599. [Google Scholar] [CrossRef]
- Thrun, A.; Garzia, A.; Kigoshi-Tansho, Y.; Patil, P.R.; Umbaugh, C.S.; Dallinger, T.; Liu, J.; Kreger, S.; Patrizi, A.; Cox, G.A.; et al. Convergence of mammalian RQC and C-end rule proteolytic pathways via alanine tailing. Mol. Cell 2021, 81, 2112–2122.e7. [Google Scholar] [CrossRef] [PubMed]
- Choe, Y.-J.; Park, S.-H.; Hassemer, T.; Körner, R.; Vincenz-Donnelly, L.; Hayer-Hartl, M.; Hartl, F.U. Failure of RQC machinery causes protein aggregation and proteotoxic stress. Nature 2016, 531, 191–195. [Google Scholar] [CrossRef]
- Chu, J.; Hong, N.A.; Masuda, C.A.; Jenkins, B.V.; Nelms, K.A.; Goodnow, C.C.; Glynne, R.J.; Wu, H.; Masliah, E.; Joazeiro, C.A.P.; et al. A mouse forward genetics screen identifies LISTERIN as an E3 ubiquitin ligase involved in neurodegeneration. Proc. Natl. Acad. Sci. USA 2009, 106, 2097–2103. [Google Scholar] [CrossRef]
- Rimal, S.; Li, Y.; Vartak, R.; Geng, J.; Tantray, I.; Li, S.; Huh, S.; Vogel, H.; Glabe, C.; Grinberg, L.T.; et al. Inefficient quality control of ribosome stalling during APP synthesis generates CAT-tailed species that precipitate hallmarks of Alzheimer’s disease. Acta Neuropathol. Commun. 2021, 9, 169. [Google Scholar] [CrossRef]
- Ishimura, R.; Nagy, G.; Dotu, I.; Chuang, J.; Ackerman, S.L. Activation of GCN2 kinase by ribosome stalling links translation elongation with translation initiation. eLife 2016, 5, e14295. [Google Scholar] [CrossRef]
- Terrey, M.; Adamson, S.I.; Gibson, A.L.; Deng, T.; Ishimura, R.; Chuang, J.H.; Ackerman, S.L. GTPBP1 resolves paused ribosomes to maintain neuronal homeostasis. eLife 2020, 9, e62731. [Google Scholar] [CrossRef]
- Mori, K.; Gotoh, S.; Yamashita, T.; Uozumi, R.; Kawabe, Y.; Tagami, S.; Kamp, F.; Nuscher, B.; Edbauer, D.; Haass, C.; et al. The porphyrin TMPyP4 inhibits elongation during the noncanonical translation of the FTLD/ALS-associated GGGGCC repeat in the C9orf72 gene. J. Biol. Chem. 2021, 297, 101120. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.; Kim, J.-H.; Lee, J.; Park, H.; Lim, C. ZNF598 co-translationally titrates poly(GR) protein implicated in the pathogenesis of C9ORF72-associated ALS/FTD. Nucleic Acids Res. 2021, 49, 11294–11311. [Google Scholar] [CrossRef] [PubMed]
- Sévigny, M.; Julien, I.B.; Venkatasubramani, J.P.; Hui, J.B.; Dutchak, P.A.; Sephton, C.F. FUS contributes to mTOR-dependent inhibition of translation. J. Biol. Chem. 2020, 295, 18459–18473. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J.; Minton, A. Protein aggregation in crowded environments. Biol. Chem. 2006, 387, 485–497. [Google Scholar] [CrossRef] [PubMed]
- White, D.A.; Buell, A.K.; Knowles, T.P.J.; Welland, M.E.; Dobson, C.M. Protein Aggregation in Crowded Environments. J. Am. Chem. Soc. 2010, 132, 5170–5175. [Google Scholar] [CrossRef] [PubMed]
- Cha-Molstad, H.; Kwon, Y.T.; Kim, B.Y. Amino-terminal arginylation as a degradation signal for selective autophagy. BMB Rep. 2015, 48, 487–488. [Google Scholar] [CrossRef]
- Cha-Molstad, H.; Sung, K.S.; Hwang, J.; Kim, K.A.; Yu, J.E.; Yoo, Y.D.; Jang, J.M.; Han, D.H.; Molstad, M.; Kim, J.G.; et al. Amino-terminal arginylation targets endoplasmic reticulum chaperone BiP for autophagy through p62 binding. Nat. Cell Biol. 2015, 17, 917–929. [Google Scholar] [CrossRef]
- Dice, J.F.; Terlecky, S.R.; Chiang, H.L.; Olson, T.S.; Isenman, L.D.; Short-Russell, S.R.; Freundlieb, S.; Terlecky, L.J. A selective pathway for degradation of cytosolic proteins by lysosomes. Semin. Cell Biol. 1990, 1, 449–455. [Google Scholar]
- Eldridge, A.G.; O’Brien, T. Therapeutic strategies within the ubiquitin proteasome system. Cell Death Differ. 2009, 17, 4–13. [Google Scholar] [CrossRef]
- Wyatt, A.R.; Yerbury, J.J.; Dabbs, R.A.; Wilson, M.R. Roles of Extracellular Chaperones in Amyloidosis. J. Mol. Biol. 2012, 421, 499–516. [Google Scholar] [CrossRef]
- Wyttenbach, A. Role of Heat Shock Proteins During Polyglutamine Neurodegeneration: Mechanisms and Hypothesis. J. Mol. Neurosci. 2004, 23, 69–96. [Google Scholar] [CrossRef]
- Moreno-Gonzalez, I.; Soto, C. Misfolded protein aggregates: Mechanisms, structures and potential for disease transmission. Semin. Cell Dev. Biol. 2011, 22, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Valastyan, J.S.; Lindquist, S. Mechanisms of protein-folding diseases at a glance. Dis. Model. Mech. 2014, 7, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Majd, R.M.; Mayeli, M.; Rahmani, F. Pathogenesis and promising therapeutics of Alzheimer disease through eIF2α pathway and correspondent kinases. Metab. Brain Dis. 2020, 35, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Martinez, N.W.; Gómez, F.E.; Matus, S. The Potential Role of Protein Kinase R as a Regulator of Age-Related Neurodegeneration. Front. Aging Neurosci. 2021, 13, 638208. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C.; Saxena, S. ER stress and the unfolded protein response in neurodegeneration. Nat. Rev. Neurol. 2017, 13, 477–491. [Google Scholar] [CrossRef]
- Ni, H.; Rui, Q.; Xu, Y.; Zhu, J.; Gao, F.; Dang, B.; Li, D.; Gao, R.; Chen, G. RACK1 upregulation induces neuroprotection by activating the IRE1-XBP1 signaling pathway following traumatic brain injury in rats. Exp. Neurol. 2018, 304, 102–113. [Google Scholar] [CrossRef]
- Liu, D.; Liu, X.; Zhou, T.; Yao, W.; Zhao, J.; Zheng, Z.; Jiang, W.; Wang, F.; Aikhionbare, F.O.; Hill, D.L.; et al. IRE1–RACK1 axis orchestrates ER stress preconditioning-elicited cytoprotection from ischemia/reperfusion injury in liver. J. Mol. Cell Biol. 2015, 8, 144–156. [Google Scholar] [CrossRef]
- Mateju, D.; Franzmann, T.M.; Patel, A.; Kopach, A.; Boczek, E.E.; Maharana, S.; Lee, H.O.; Carra, S.; Hyman, A.A.; Alberti, S. An aberrant phase transition of stress granules triggered by misfolded protein and prevented by chaperone function. EMBO J. 2017, 36, 1669–1687. [Google Scholar] [CrossRef]
- Ganassi, M.; Mateju, D.; Bigi, I.; Mediani, L.; Poser, I.; Lee, H.O.; Seguin, S.J.; Morelli, F.F.; Vinet, J.; Leo, G.; et al. A Surveillance Function of the HSPB8-BAG3-HSP70 Chaperone Complex Ensures Stress Granule Integrity and Dynamism. Mol. Cell 2016, 63, 796–810. [Google Scholar] [CrossRef]
- Turakhiya, A.; Meyer, S.R.; Marincola, G.; Böhm, S.; Vanselow, J.T.; Schlosser, A.; Hofmann, K.; Buchberger, A. ZFAND1 Recruits p97 and the 26S Proteasome to Promote the Clearance of Arsenite-Induced Stress Granules. Mol. Cell 2018, 70, 906–919.e7. [Google Scholar] [CrossRef]
- Mandrioli, J.; Mediani, L.; Alberti, S.; Carra, S. ALS and FTD: Where RNA metabolism meets protein quality control. Semin. Cell Dev. Biol. 2019, 99, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Pascual, C.; Klionsky, D.J. Autophagy: Machinery and regulation. Microb. Cell 2016, 3, 588–596. [Google Scholar] [CrossRef]
- Bartoletti, M.; Bosco, D.A.; Da Cruz, S.; Lagier-Tourenne, C.; Liachko, N.; Markmiller, S.; Webster, K.M.; Wharton, K.A. Phenotypic Suppression of ALS/FTD-Associated Neurodegeneration Highlights Mechanisms of Dysfunction. J. Neurosci. 2019, 39, 8217–8224. [Google Scholar] [CrossRef]
- Jiang, L.; Ash, P.E.A.; Maziuk, B.F.; Ballance, H.I.; Boudeau, S.; Al Abdullatif, A.; Orlando, M.; Petrucelli, L.; Ikezu, T.; Wolozin, B. TIA1 regulates the generation and response to toxic tau oligomers. Acta Neuropathol. 2018, 137, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Apicco, D.J.; Zhang, C.; Maziuk, B.; Jiang, L.; Ballance, H.I.; Boudeau, S.; Ung, C.; Li, H.; Wolozin, B. Dysregulation of RNA Splicing in Tauopathies. Cell Rep. 2019, 29, 4377–4388.e4. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi, H.; Stochaj, U. Cytoplasmic stress granules: Dynamic modulators of cell signaling and disease. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2017, 1863, 884–895. [Google Scholar] [CrossRef]
- Thomas, M.G.; Loschi, M.; Desbats, M.A.; Boccaccio, G.L. RNA granules: The good, the bad and the ugly. Cell. Signal. 2011, 23, 324–334. [Google Scholar] [CrossRef]
- Anderson, P.; Kedersha, N.; Ivanov, P. Stress granules, P-bodies and cancer. Biochim. Biophys. Acta 2014, 1849, 861–870. [Google Scholar] [CrossRef]
- Arimoto, K.; Fukuda, H.; Imajoh-Ohmi, S.; Saito, H.; Takekawa, M. Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK pathways. Nat. Cell Biol. 2008, 10, 1324–1332. [Google Scholar] [CrossRef]
- Reineke, L.C.; Neilson, J.R. Differences between acute and chronic stress granules, and how these differences may impact function in human disease. Biochem. Pharmacol. 2018, 162, 123–131. [Google Scholar] [CrossRef]
- Kedersha, N.; Ivanov, P.; Anderson, P. Stress granules and cell signaling: More than just a passing phase? Trends Biochem. Sci. 2013, 38, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Pothof, J.; Verkaik, N.S.; Hoeijmakers, J.H.; Van Gent, D.C. MicroRNA responses and stress granule formation modulate the DNA damage response. Cell Cycle 2009, 8, 3462–3468. [Google Scholar] [CrossRef] [PubMed]
- Aulas, A.; Velde, C.V. Alterations in stress granule dynamics driven by TDP-43 and FUS: A link to pathological inclusions in ALS? Front. Cell. Neurosci. 2015, 9, 423. [Google Scholar] [CrossRef]
- Leung, A.K.; Todorova, T.; Ando, Y.; Chang, P. Poly(ADP-ribose) regulates post-transcriptional gene regulation in the cytoplasm. RNA Biol. 2012, 9, 542–548. [Google Scholar] [CrossRef]
- Adeli, K. Translational control mechanisms in metabolic regulation: Critical role of RNA binding proteins, microRNAs, and cytoplasmic RNA granules. Am. J. Physiol. Metab. 2011, 301, E1051–E1064. [Google Scholar] [CrossRef]
- Mahboubi, H.; Kodiha, M.; Stochaj, U. Automated Detection and Quantification of Granular Cell Compartments. Microsc. Microanal. 2013, 19, 617–628. [Google Scholar] [CrossRef]
- Wolozin, B. Regulated protein aggregation: Stress granules and neurodegeneration. Mol. Neurodegener. 2012, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.; Kedersha, N. Stress granules: The Tao of RNA triage. Trends Biochem. Sci. 2008, 33, 141–150. [Google Scholar] [CrossRef]
- Jain, S.; Wheeler, J.R.; Walters, R.W.; Agrawal, A.; Barsic, A.; Parker, R. ATPase-Modulated Stress Granules Contain a Diverse Proteome and Substructure. Cell 2016, 164, 487–498. [Google Scholar] [CrossRef]
- Bounedjah, O.; Desforges, B.; Wu, T.-D.; Pioche-Durieu, C.; Marco, S.; Hamon, L.; Curmi, P.A.; Guerquin-Kern, J.-L.; Pietrement, O.; Pastré, D. Free mRNA in excess upon polysome dissociation is a scaffold for protein multimerization to form stress granules. Nucleic Acids Res. 2014, 42, 8678–8691. [Google Scholar] [CrossRef]
- Buchan, J.R. mRNP granules. RNA Biol. 2014, 11, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Gilks, N.; Kedersha, N.; Ayodele, M.; Shen, L.; Stoecklin, G.; Dember, L.M.; Anderson, P. Stress Granule Assembly Is Mediated by Prion-like Aggregation of TIA-1. Mol. Biol. Cell 2004, 15, 5383–5398. [Google Scholar] [CrossRef] [PubMed]
- Buchan, J.R.; Kolaitis, R.-M.; Taylor, J.P.; Parker, R. Eukaryotic Stress Granules Are Cleared by Autophagy and Cdc48/VCP Function. Cell 2013, 153, 1461–1474. [Google Scholar] [CrossRef]
- Walters, R.W.; Muhlrad, D.; Garcia, J.; Parker, R. Differential effects of Ydj1 and Sis1 on Hsp70-mediated clearance of stress granules in Saccharomyces cerevisiae. RNA 2015, 21, 1660–1671. [Google Scholar] [CrossRef]
- Kroschwald, S.; Maharana, S.; Mateju, D.; Malinovska, L.; Nüske, E.; Poser, I.; Richter, D.; Alberti, S. Promiscuous interactions and protein disaggregases determine the material state of stress-inducible RNP granules. eLife 2015, 4, e06807. [Google Scholar] [CrossRef]
- Vanderweyde, T.; Yu, W.H.; Varnum, M.; Liu-Yesucevitz, L.; Citro, A.; Ikezu, T.; Duff, K.; Wolozin, B. Contrasting Pathology of the Stress Granule Proteins TIA-1 and G3BP in Tauopathies. J. Neurosci. 2012, 32, 8270–8283. [Google Scholar] [CrossRef] [PubMed]
- Vanderweyde, T.; Apicco, D.J.; Youmans-Kidder, K.; Ash, P.E.A.; Cook, C.; da Rocha, E.L.; Jansen-West, K.; Frame, A.A.; Citro, A.; Leszyk, J.D.; et al. Interaction of tau with the RNA-Binding Protein TIA1 Regulates tau Pathophysiology and Toxicity. Cell Rep. 2016, 15, 1455–1466. [Google Scholar] [CrossRef]
- Buoso, E.; Galasso, M.; Ronfani, M.; Papale, A.; Galbiati, V.; Eberini, I.; Marinovich, M.; Racchi, M.; Corsini, E. The scaffold protein RACK1 is a target of endocrine disrupting chemicals (EDCs) with important implication in immunity. Toxicol. Appl. Pharmacol. 2017, 325, 37–47. [Google Scholar] [CrossRef]
- Racchi, M.; Buoso, E.; Ronfani, M.; Serafini, M.M.; Galasso, M.; Lanni, C.; Corsini, E. Role of Hormones in the Regulation of RACK1 Expression as a Signaling Checkpoint in Immunosenescence. Int. J. Mol. Sci. 2017, 18, 1453. [Google Scholar] [CrossRef]
- Buoso, E.; Masi, M.; Galbiati, V.; Maddalon, A.; Iulini, M.; Kenda, M.; Dolenc, M.S.; Marinovich, M.; Racchi, M.; Corsini, E. Effect of estrogen-active compounds on the expression of RACK1 and immunological implications. Arch. Toxicol. 2020, 94, 2081–2095. [Google Scholar] [CrossRef]
- Buoso, E.; Masi, M.; Racchi, M.; Corsini, E. Endocrine-Disrupting Chemicals’ (EDCs) Effects on Tumour Microenvironment and Cancer Progression: Emerging Contribution of RACK1. Int. J. Mol. Sci. 2020, 21, 9229. [Google Scholar] [CrossRef] [PubMed]
- Buoso, E.; Kenda, M.; Masi, M.; Linciano, P.; Galbiati, V.; Racchi, M.; Dolenc, M.S.; Corsini, E. Effects of Bisphenols on RACK1 Expression and Their Immunological Implications in THP-1 Cells. Front. Pharmacol. 2021, 12, 743991. [Google Scholar] [CrossRef] [PubMed]
- Buoso, E.; Ronfani, M.; Galasso, M.; Ventura, D.; Corsini, E.; Racchi, M. Cortisol-induced SRSF3 expression promotes GR splicing, RACK1 expression and breast cancer cells migration. Pharmacol. Res. 2019, 143, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Buoso, E.; Masi, M.; Long, A.; Chiappini, C.; Travelli, C.; Govoni, S.; Racchi, M. Ribosomes as a nexus between translation and cancer progression: Focus on ribosomal Receptor for Activated C Kinase 1 (RACK1) in breast cancer. J. Cereb. Blood Flow Metab. 2020, 179, 2813–2828. [Google Scholar] [CrossRef]
- Masi, M.; Garattini, E.; Bolis, M.; Di Marino, D.; Maraccani, L.; Morelli, E.; Grolla, A.A.; Fagiani, F.; Corsini, E.; Travelli, C.; et al. OXER1 and RACK1-associated pathway: A promising drug target for breast cancer progression. Oncogenesis 2020, 9, 105. [Google Scholar] [CrossRef]
- Masi, M.; Racchi, M.; Travelli, C.; Corsini, E.; Buoso, E. Molecular Characterization of Membrane Steroid Receptors in Hormone-Sensitive Cancers. Cells 2021, 10, 2999. [Google Scholar] [CrossRef]
- Duff, D.; Long, A. Roles for RACK1 in cancer cell migration and invasion. Cell. Signal. 2017, 35, 250–255. [Google Scholar] [CrossRef]
- Buoso, E.; Galasso, M.; Serafini, M.M.; Ronfani, M.; Lanni, C.; Corsini, E.; Racchi, M. Transcriptional regulation of RACK1 and modulation of its expression: Role of steroid hormones and significance in health and aging. Cell. Signal. 2017, 35, 264–271. [Google Scholar] [CrossRef]
- Battainia, F.; Pascaleb, A.; Lucchib, L.; Pasinetti, G.; Govoni, S. Protein Kinase C Anchoring Deficit in Postmortem Brains of Alzheimer’s Disease Patients. Exp. Neurol. 1999, 159, 559–564. [Google Scholar] [CrossRef]
- Battaini, F.; Pascale, A. Protein Kinase C Signal Transduction Regulation in Physiological and Pathological Aging. Ann. N. Y. Acad. Sci. 2005, 1057, 177–192. [Google Scholar] [CrossRef]
- Buoso, E.; Biundo, F.; Lanni, C.; Aiello, S.; Grossi, S.; Schettini, G.; Govoni, S.; Racchi, M. Modulation of Rack-1/PKCβII Signalling By Soluble AβPPα in SH-SY5Y Cells. Curr. Alzheimer Res. 2013, 10, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Brivio, P.; Buoso, E.; Masi, M.; Gallo, M.T.; Gruca, P.; Lason, M.; Litwa, E.; Papp, M.; Fumagalli, F.; Racchi, M.; et al. The coupling of RACK1 with the beta isoform of the glucocorticoid receptor promotes resilience to chronic stress exposure. Neurobiol. Stress 2021, 15, 100372. [Google Scholar] [CrossRef] [PubMed]
- He, D.-Y.; Neasta, J.; Ron, D. Epigenetic Regulation of BDNF Expression via the Scaffolding Protein RACK1. J. Biol. Chem. 2010, 285, 19043–19050. [Google Scholar] [CrossRef] [PubMed]
- Buoso, E.; Biundo, F.; Lanni, C.; Schettini, G.; Govoni, S.; Racchi, M. AβPP Intracellular C-Terminal Domain Function is Related to its Degradation Processes. J. Alzheimer’s Dis. 2012, 30, 393–405. [Google Scholar] [CrossRef]
- Adams, D.R.; Ron, D.; A Kiely, P. RACK1, A multifaceted scaffolding protein: Structure and function. Cell Commun. Signal. 2011, 9, 22. [Google Scholar] [CrossRef]
- Sengupta, J.; Nilsson, J.; Gursky, R.; Spahn, C.M.T.; Nissen, P.; Frank, J. Identification of the versatile scaffold protein RACK1 on the eukaryotic ribosome by cryo-EM. Nat. Struct. Mol. Biol. 2004, 11, 957–962. [Google Scholar] [CrossRef]
- Ceci, M.; Gaviraghi, C.; Gorrini, C.; Sala, L.A.; Offenhauser, N.; Marchisio, P.C.; Biffo, S. Release of eIF6 (p27BBP) from the 60S subunit allows 80S ribosome assembly. Nature 2003, 426, 579–584. [Google Scholar] [CrossRef]
- Klinge, S.; Voigts-Hoffmann, F.; Leibundgut, M.; Arpagaus, S.; Ban, N. Crystal Structure of the Eukaryotic 60 S Ribosomal Subunit in Complex with Initiation Factor 6. Science 2011, 334, 941–948. [Google Scholar] [CrossRef]
- Miluzio, A.; Beugnet, A.; Volta, V.; Biffo, S. Eukaryotic initiation factor 6 mediates a continuum between 60S ribosome biogenesis and translation. EMBO Rep. 2009, 10, 459–465. [Google Scholar] [CrossRef]
- Gallo, S.; Manfrini, N. Working hard at the nexus between cell signaling and the ribosomal machinery: An insight into the roles of RACK1 in translational regulation. Translation 2015, 3, e1120382. [Google Scholar] [CrossRef]
- Dobrikov, M.I.; Dobrikova, E.Y.; Gromeier, M. Ribosomal RACK1:Protein Kinase C βII Modulates Intramolecular Interactions between Unstructured Regions of Eukaryotic Initiation Factor 4G (eIF4G) That Control eIF4E and eIF3 Binding. Mol. Cell. Biol. 2018, 38, e00306-18. [Google Scholar] [CrossRef] [PubMed]
- Gallo, S.; Ricciardi, S.; Manfrini, N.; Pesce, E.; Oliveto, S.; Calamita, P.; Mancino, M.; Maffioli, E.; Moro, M.; Crosti, M.; et al. RACK1 Specifically Regulates Translation through Its Binding to Ribosomes. Mol. Cell. Biol. 2018, 38, e00230-18. [Google Scholar] [CrossRef] [PubMed]
- Rollins, M.G.; Shasmal, M.; Meade, N.; Astar, H.; Shen, P.S.; Walsh, D. Negative charge in the RACK1 loop broadens the translational capacity of the human ribosome. Cell Rep. 2021, 36, 109663. [Google Scholar] [CrossRef] [PubMed]
- Kuroha, K.; Akamatsu, M.; Dimitrova, L.; Itoh, T.; Kato, Y.; Shirahige, K.; Inada, T. Receptor for activated C kinase 1 stimulates nascent polypeptide-dependent translation arrest. EMBO Rep. 2010, 11, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Sundaramoorthy, E.; Leonard, M.; Mak, R.; Liao, J.; Fulzele, A.; Bennett, E.J. ZNF598 and RACK1 Regulate Mammalian Ribosome-Associated Quality Control Function by Mediating Regulatory 40S Ribosomal Ubiquitylation. Mol. Cell 2017, 65, 751–760.e4. [Google Scholar] [CrossRef]
- Habelhah, H.; Shah, K.; Huang, L.; Ostareck-Lederer, A.; Burlingame, A.L.; Shokat, K.M.; Hentze, M.; Ronai, Z. ERK phosphorylation drives cytoplasmic accumulation of hnRNP-K and inhibition of mRNA translation. Nat. Cell Biol. 2001, 3, 325–330. [Google Scholar] [CrossRef]
- Angenstein, F.; Evans, A.M.; Settlage, R.E.; Moran, S.T.; Ling, S.-C.; Klintsova, A.Y.; Shabanowitz, J.; Hunt, N.F.; Greenough, W.T. A Receptor for Activated C Kinase Is Part of Messenger Ribonucleoprotein Complexes Associated with PolyA-mRNAs in Neurons. J. Neurosci. 2002, 22, 8827–8837. [Google Scholar] [CrossRef]
- Welshhans, K.; Kershner, L. RACK1 regulates neural development. Neural Regen. Res. 2017, 12, 1036–1039. [Google Scholar] [CrossRef]
- Demarco, R.S.; Lundquist, E.A. RACK-1 Acts with Rac GTPase Signaling and UNC-115/abLIM in Caenorhabditis elegans Axon Pathfinding and Cell Migration. PLoS Genet. 2010, 6, e1001215. [Google Scholar] [CrossRef]
- Dwane, S.; Durack, E.; O’Connor, R.; Kiely, P.A. RACK1 promotes neurite outgrowth by scaffolding AGAP2 to FAK. Cell. Signal. 2014, 26, 9–18. [Google Scholar] [CrossRef]
- Kershner, L.; Welshhans, K. RACK1 is necessary for the formation of point contacts and regulates axon growth. Dev. Neurobiol. 2017, 77, 1038–1056. [Google Scholar] [CrossRef]
- Pastore, D.; Pacifici, F.; Dave, K.R.; Palmirotta, R.; Bellia, A.; Pasquantonio, G.; Guadagni, F.; Donadel, G.; Di Daniele, N.; Abete, P.; et al. Age-Dependent Levels of Protein Kinase Cs in Brain: Reduction of Endogenous Mechanisms of Neuroprotection. Int. J. Mol. Sci. 2019, 20, 3544. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Z.; Takekawa, M.; Ge, Q.; Saito, H. Activation of MTK1/MEKK4 by GADD45 through Induced N-C Dissociation and Dimerization-Mediated trans Autophosphorylation of the MTK1 Kinase Domain. Mol. Cell. Biol. 2007, 27, 2765–2776. [Google Scholar] [CrossRef] [PubMed]
- Takekawa, M.; Saito, H. A Family of Stress-Inducible GADD45-like Proteins Mediate Activation of the Stress-Responsive MTK1/MEKK4 MAPKKK. Cell 1998, 95, 521–530. [Google Scholar] [CrossRef]
- Mita, H.; Tsutsui, J.; Takekawa, M.; Witten, E.A.; Saito, H. Regulation of MTK1/MEKK4 Kinase Activity by Its N-Terminal Autoinhibitory Domain and GADD45 Binding. Mol. Cell. Biol. 2002, 22, 4544–4555. [Google Scholar] [CrossRef] [PubMed]
- Kaehler, C.; Isensee, J.; Hucho, T.; Lehrach, H.; Krobitsch, S. 5-Fluorouracil affects assembly of stress granules based on RNA incorporation. Nucleic Acids Res. 2014, 42, 6436–6447. [Google Scholar] [CrossRef]
- Wang, J.; Gan, Y.; Cao, J.; Dong, X.; Ouyang, W. Pathophysiology of stress granules: An emerging link to diseases (Review). Int. J. Mol. Med. 2022, 49, 44. [Google Scholar] [CrossRef]
- Dewey, C.M.; Cenik, B.; Sephton, C.F.; Johnson, B.A.; Herz, J.; Yu, G. TDP-43 aggregation in neurodegeneration: Are stress granules the key? Brain Res. 2012, 1462, 16–25. [Google Scholar] [CrossRef]
- Bentmann, E.; Haass, C.; Dormann, D. Stress granules in neurodegeneration—Lessons learnt from TAR DNA binding protein of 43 kDa and fused in sarcoma. FEBS J. 2013, 280, 4348–4370. [Google Scholar] [CrossRef]
- Ohn, T.; Kedersha, N.; Hickman, T.; Tisdale, S.; Anderson, P. A functional RNAi screen links O-GlcNAc modification of ribosomal proteins to stress granule and processing body assembly. Nat Cell Biol. 2008, 10, 1224–1231. [Google Scholar] [CrossRef]
- Teng, Y.; Zhang, M.-Q.; Wang, W.; Liu, L.-T.; Zhou, L.-M.; Miao, S.-K.; Wan, L.-H. Compound danshen tablet ameliorated aβ25-35-induced spatial memory impairment in mice via rescuing imbalance between cytokines and neurotrophins. BMC Complement. Altern. Med. 2014, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Tu, M.; Du, Y.; Li, J.; Pang, Y.; Dong, Z. Nicotine Promotes AβPP Nonamyloidogenic Processing via RACK1-Dependent Activation of PKC in SH-SY5Y-AβPP695 Cells. J. Alzheimer’s Dis. 2020, 75, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Van der Zee, E.; Palm, I.; O’Connor, M.; Maizels, E.; Hunzicker-Dunn, M.; Disterhoft, J. Aging-related alterations in the distribution of Ca2+-dependent PKC isoforms in rabbit hippocampus. Hippocampus 2004, 14, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Coronas-Samano, G.; Baker, K.L.; Tan, W.J.T.; Ivanova, A.V.; Verhagen, J.V. Fus1 KO Mouse As a Model of Oxidative Stress-Mediated Sporadic Alzheimer’s Disease: Circadian Disruption and Long-Term Spatial and Olfactory Memory Impairments. Front. Aging Neurosci. 2016, 8, 268. [Google Scholar] [CrossRef]
- Shimohama, S.; Kamiya, S.; Taniguchi, T.; Kimura, J. Intracellular Receptors for Activated C-Kinase in the Postmortem Human Brain: No Alteration in Alzheimer Disease. Alzheimer Dis. Assoc. Disord. 1998, 12, 384–386. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, X.; Song, Y.; Zhang, Y.; Zhou, L.; Wan, L. Deficit of RACK1 contributes to the spatial memory impairment via upregulating BECLIN1 to induce autophagy. Life Sci. 2016, 151, 115–121. [Google Scholar] [CrossRef]
- Liu, W.; Dou, F.; Feng, J.; Yan, Z. RACK1 is involved in β-amyloid impairment of muscarinic regulation of GABAergic transmission. Neurobiol. Aging 2011, 32, 1818–1826. [Google Scholar] [CrossRef]
- Ma, X.-H.; Zhong, P.; Gu, Z.; Feng, J.; Yan, Z. Muscarinic Potentiation of GABAAReceptor Currents Is Gated by Insulin Signaling in the Prefrontal Cortex. J. Neurosci. 2003, 23, 1159–1168. [Google Scholar] [CrossRef]
- Zhong, P.; Gu, Z.; Wang, X.; Jiang, H.; Feng, J.; Yan, Z. Impaired Modulation of GABAergic Transmission by Muscarinic Receptors in a Mouse Transgenic Model of Alzheimer’s Disease. J. Biol. Chem. 2003, 278, 26888–26896. [Google Scholar] [CrossRef]
- Krabbe, G.; Halle, A.; Matyash, V.; Rinnenthal, J.L.; Eom, G.D.; Bernhardt, U.; Miller, K.R.; Prokop, S.; Kettenmann, H.; Heppner, F.L. Functional Impairment of Microglia Coincides with Beta-Amyloid Deposition in Mice with Alzheimer-Like Pathology. PLoS ONE 2013, 8, e60921. [Google Scholar] [CrossRef]
- Hickman, S.E.; Allison, E.K.; El Khoury, J. Microglial Dysfunction and Defective β-Amyloid Clearance Pathways in Aging Alzheimer’s Disease Mice. J. Neurosci. 2008, 28, 8354–8360. [Google Scholar] [CrossRef] [PubMed]
- Mosher, K.I.; Wyss-Coray, T. Microglial dysfunction in brain aging and Alzheimer’s disease. Biochem. Pharmacol. 2014, 88, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Geahlen, R.L. Stress Granules Modulate SYK to Cause Microglial Cell Dysfunction in Alzheimer’s Disease. eBioMedicine 2015, 2, 1785–1798. [Google Scholar] [CrossRef] [PubMed]
- Gasset-Rosa, F.; Lu, S.; Yu, H.; Chen, C.; Melamed, Z.; Guo, L.; Shorter, J.; Da Cruz, S.; Cleveland, D.W. Cytoplasmic TDP-43 De-mixing Independent of Stress Granules Drives Inhibition of Nuclear Import, Loss of Nuclear TDP-43, and Cell Death. Neuron 2019, 102, 339–357.e7. [Google Scholar] [CrossRef]
- Shahheydari, H.; Ragagnin, A.; Walker, A.; Toth, R.P.; Vidal, M.; Jagaraj, C.J.; Perri, E.R.; Konopka, A.; Sultana, J.M.; Atkin, J.D. Protein Quality Control and the Amyotrophic Lateral Sclerosis/Frontotemporal Dementia Continuum. Front. Mol. Neurosci. 2017, 10, 119. [Google Scholar] [CrossRef]
- Khalfallah, Y.; Kuta, R.; Grasmuck, C.; Prat, A.; Durham, H.D.; Velde, C.V. TDP-43 regulation of stress granule dynamics in neurodegenerative disease-relevant cell types. Sci. Rep. 2018, 8, 7551. [Google Scholar] [CrossRef]
- Rossi, S.; Cozzolino, M.; Carrì, M.T. Old versus New Mechanisms in the Pathogenesis of ALS. Brain Pathol. 2016, 26, 276–286. [Google Scholar] [CrossRef]
- Lagier-Tourenne, C.; Cleveland, D.W. Rethinking ALS: The FUS about TDP-43. Cell 2009, 136, 1001–1004. [Google Scholar] [CrossRef]
- Lagier-Tourenne, C.; Polymenidou, M.; Cleveland, D.W. TDP-43 and FUS/TLS: Emerging roles in RNA processing and neurodegeneration. Hum. Mol. Genet. 2010, 19, R46–R64. [Google Scholar] [CrossRef]
- Buratti, E. Multiple roles of TDP-43 in gene expression, splicing regulation, and human disease. Front. Biosci. 2008, 13, 867–878. [Google Scholar] [CrossRef]
- Fiesel, F.C.; Voigt, A.; Weber, S.S.; Van den Haute, C.; Waldenmaier, A.; Görner, K.; Walter, M.; Anderson, M.L.; Kern, J.V.; Rasse, T.M.; et al. Knockdown of transactive response DNA-binding protein (TDP-43) downregulates histone deacetylase 6. EMBO J. 2010, 29, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Scardigli, R.; La Regina, F.; Murray, M.E.; Romano, N.; Dickson, D.W.; Wolozin, B.; Cattaneo, A.; Ceci, M. Increased cytoplasmic TDP-43 reduces global protein synthesis by interacting with RACK1 on polyribosomes. Hum. Mol. Genet. 2017, 26, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Ramaswami, M.; Taylor, J.P.; Parker, R. Altered Ribostasis: RNA-Protein Granules in Degenerative Disorders. Cell 2013, 154, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Baradaran-Heravi, Y.; Van Broeckhoven, C.; van der Zee, J. Stress granule mediated protein aggregation and underlying gene defects in the FTD-ALS spectrum. Neurobiol. Dis. 2019, 134, 104639. [Google Scholar] [CrossRef] [PubMed]
- Lanni, C.; Masi, M.; Racchi, M.; Govoni, S. Cancer and Alzheimer’s disease inverse relationship: An age-associated diverging derailment of shared pathways. Mol. Psychiatry 2020, 26, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J.; Chern, Y. Contribution of Energy Dysfunction to Impaired Protein Translation in Neurodegenerative Diseases. Front. Cell. Neurosci. 2021, 15, 668500. [Google Scholar] [CrossRef]
- Zukin, R.S.; Richter, J.D.; Bagni, C. Signals, synapses, and synthesis: How new proteins control plasticity. Front. Neural Circuits 2009, 3, 14. [Google Scholar] [CrossRef][Green Version]
- Biundo, F.; Del Prete, D.; Zhang, H.; Arancio, O.; D’Adamio, L. A role for tau in learning, memory and synaptic plasticity. Sci. Rep. 2018, 8, 3184. [Google Scholar] [CrossRef]
- Biundo, F.; D’Abramo, C.; Tambini, M.D.; Zhang, H.; Del Prete, D.; Vitale, F.; Giliberto, L.; Arancio, O.; D’Adamio, L. Abolishing Tau cleavage by caspases at Aspartate421 causes memory/synaptic plasticity deficits and pre-pathological Tau alterations. Transl. Psychiatry 2017, 7, e1198. [Google Scholar] [CrossRef]
- Halliday, M.; Radford, H.; Zents, K.A.M.; Molloy, C.; Moreno, J.A.; Verity, N.C.; Smith, E.; Ortori, C.A.; Barrett, D.A.; Bushell, M.; et al. Repurposed drugs targeting eIF2α-P-mediated translational repression prevent neurodegeneration in mice. Brain J. Neurol. 2017, 140, 1768–1783. [Google Scholar] [CrossRef]
- Moreno, J.A.; Halliday, M.; Molloy, C.; Radford, H.; Verity, N.; Axten, J.M.; Ortori, C.A.; Willis, A.E.; Fischer, P.M.; Barrett, D.A.; et al. Oral Treatment Targeting the Unfolded Protein Response Prevents Neurodegeneration and Clinical Disease in Prion-Infected Mice. Sci. Transl. Med. 2013, 5, 206ra138. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Kong, Q.; Shan, X.; Tian, G.; Ilieva, H.; Cleveland, D.W.; Rothstein, J.D.; Borchelt, D.R.; Wong, P.C.; Lin, C.-L.G. Messenger RNA Oxidation Occurs Early in Disease Pathogenesis and Promotes Motor Neuron Degeneration in ALS. PLoS ONE 2008, 3, e2849. [Google Scholar] [CrossRef] [PubMed]
- Gambino, G.; Allegra, M.; Sardo, P.; Attanzio, A.; Tesoriere, L.; Livrea, M.A.; Ferraro, G.; Carletti, F. Brain Distribution and Modulation of Neuronal Excitability by Indicaxanthin From Opuntia Ficus Indica Administered at Nutritionally-Relevant Amounts. Front. Aging Neurosci. 2018, 10, 133. [Google Scholar] [CrossRef]
- Nuzzo, D.; Scordino, M.; Scurria, A.; Giardina, C.; Giordano, F.; Meneguzzo, F.; Mudò, G.; Pagliaro, M.; Picone, P.; Attanzio, A.; et al. Protective, Antioxidant and Antiproliferative Activity of Grapefruit IntegroPectin on SH-SY5Y Cells. Int. J. Mol. Sci. 2021, 22, 9368. [Google Scholar] [CrossRef] [PubMed]
- Mi, J.; He, Y.; Yang, J.; Zhou, Y.; Zhu, G.; Wu, A.; Liu, W.; Sang, Z. Development of naringenin-O-carbamate derivatives as multi-target-directed liagnds for the treatment of Alzheimer’s disease. Bioorganic Med. Chem. Lett. 2022, 60, 128574. [Google Scholar] [CrossRef] [PubMed]
- Araki, W.; Kametani, F. Protection against Amyloid-β Oligomer Neurotoxicity by Small Molecules with Antioxidative Properties: Potential for the Prevention of Alzheimer’s Disease Dementia. Antioxidants 2022, 11, 132. [Google Scholar] [CrossRef] [PubMed]
- Amirbeigiarab, S.; Kiani, P.; Sanchez, A.V.; Krisp, C.; Kazantsev, A.; Fester, L.; Schlüter, H.; Ignatova, Z. Invariable stoichiometry of ribosomal proteins in mouse brain tissues with aging. Proc. Natl. Acad. Sci. USA 2019, 116, 22567–22572, Erratum in: Proc. Natl. Acad. Sci. USA 2019, 116, 24907. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masi, M.; Attanzio, A.; Racchi, M.; Wolozin, B.; Borella, S.; Biundo, F.; Buoso, E. Proteostasis Deregulation in Neurodegeneration and Its Link with Stress Granules: Focus on the Scaffold and Ribosomal Protein RACK1. Cells 2022, 11, 2590. https://doi.org/10.3390/cells11162590
Masi M, Attanzio A, Racchi M, Wolozin B, Borella S, Biundo F, Buoso E. Proteostasis Deregulation in Neurodegeneration and Its Link with Stress Granules: Focus on the Scaffold and Ribosomal Protein RACK1. Cells. 2022; 11(16):2590. https://doi.org/10.3390/cells11162590
Chicago/Turabian StyleMasi, Mirco, Alessandro Attanzio, Marco Racchi, Benjamin Wolozin, Sofia Borella, Fabrizio Biundo, and Erica Buoso. 2022. "Proteostasis Deregulation in Neurodegeneration and Its Link with Stress Granules: Focus on the Scaffold and Ribosomal Protein RACK1" Cells 11, no. 16: 2590. https://doi.org/10.3390/cells11162590
APA StyleMasi, M., Attanzio, A., Racchi, M., Wolozin, B., Borella, S., Biundo, F., & Buoso, E. (2022). Proteostasis Deregulation in Neurodegeneration and Its Link with Stress Granules: Focus on the Scaffold and Ribosomal Protein RACK1. Cells, 11(16), 2590. https://doi.org/10.3390/cells11162590








