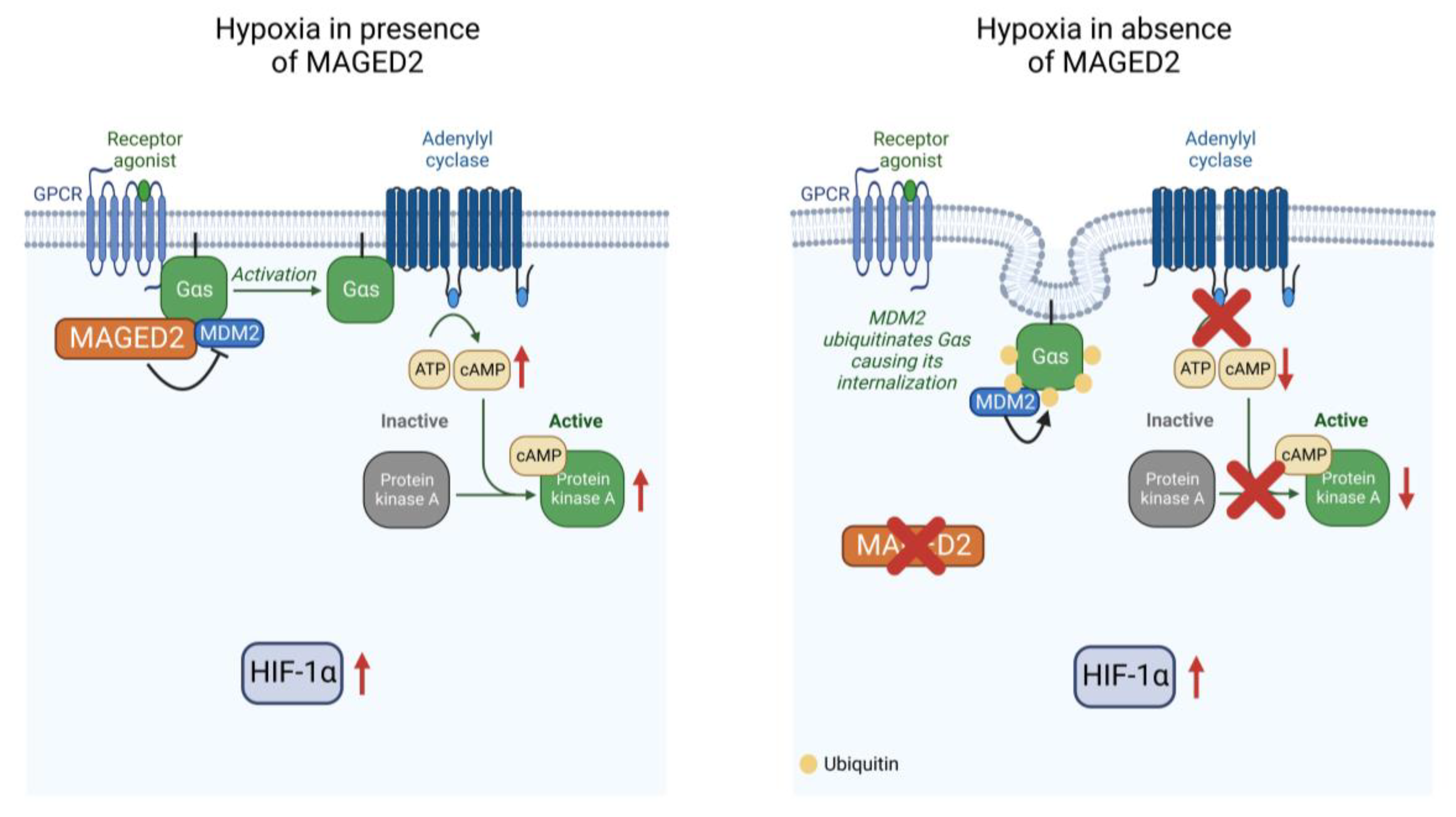
MAGED2 Is Required under Hypoxia for cAMP Signaling by Inhibiting MDM2-Dependent Endocytosis of G-Alpha-S
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmid Constructions and Site Directed Mutagenesis
2.2. Cell Culture
2.3. Chemical Hypoxia
2.4. Physical Hypoxia
2.5. Small Interfering RNA (siRNA) Knockdown and Plasmid Transfection
2.6. Biotinylation
2.7. Immunocytochemistry
2.8. Immunoprecipitation Assay
2.9. Ubiquitination Assay
2.10. Intracellular cAMP Measurement
2.11. PKA Kinase Activity
2.12. Western Blotting
2.13. Statistical Analyses
3. Results
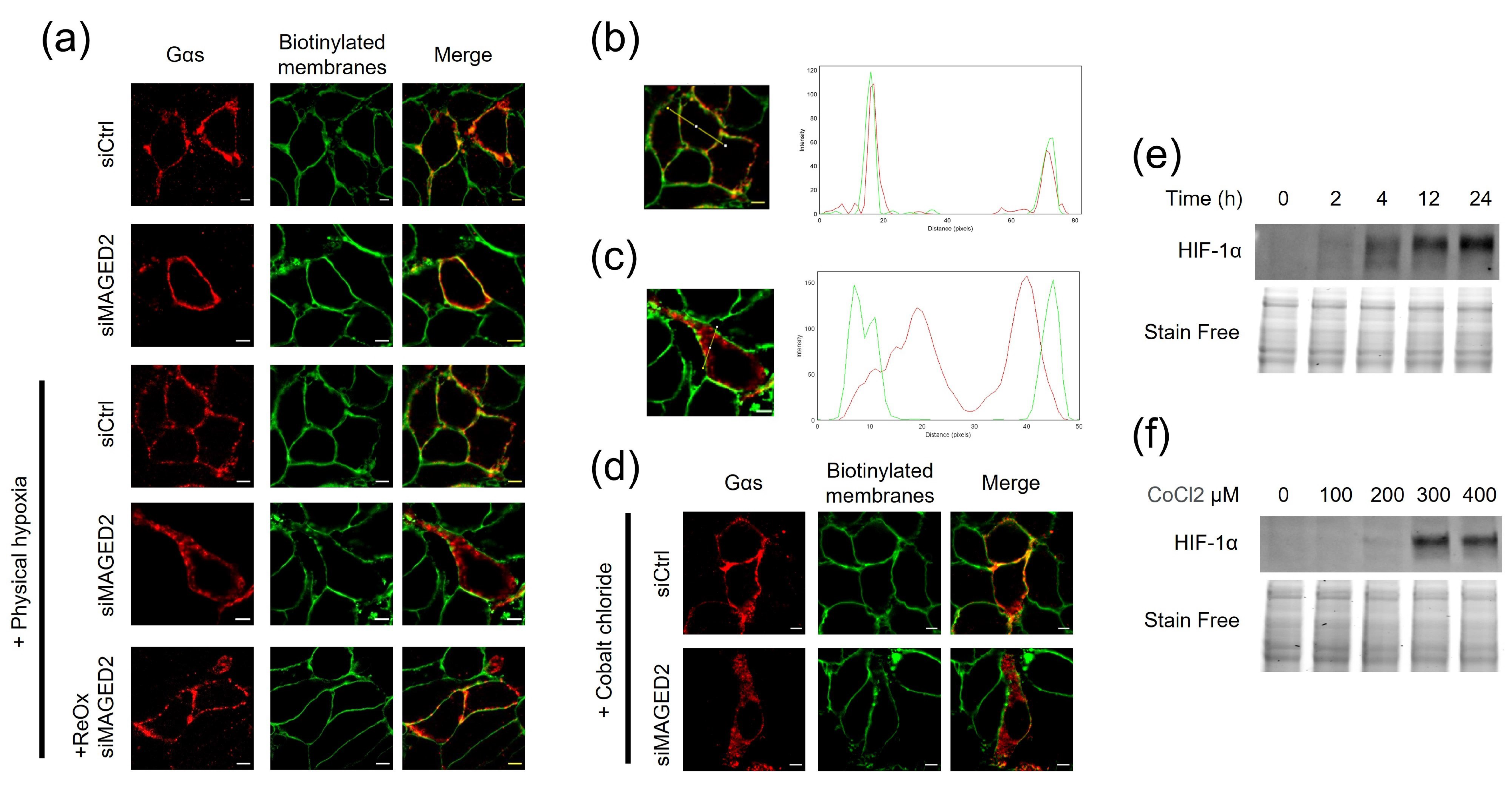
3.1. MAGED2 Is Required for the Expression of Gαs at the Plasma Membrane under Hypoxic Condition
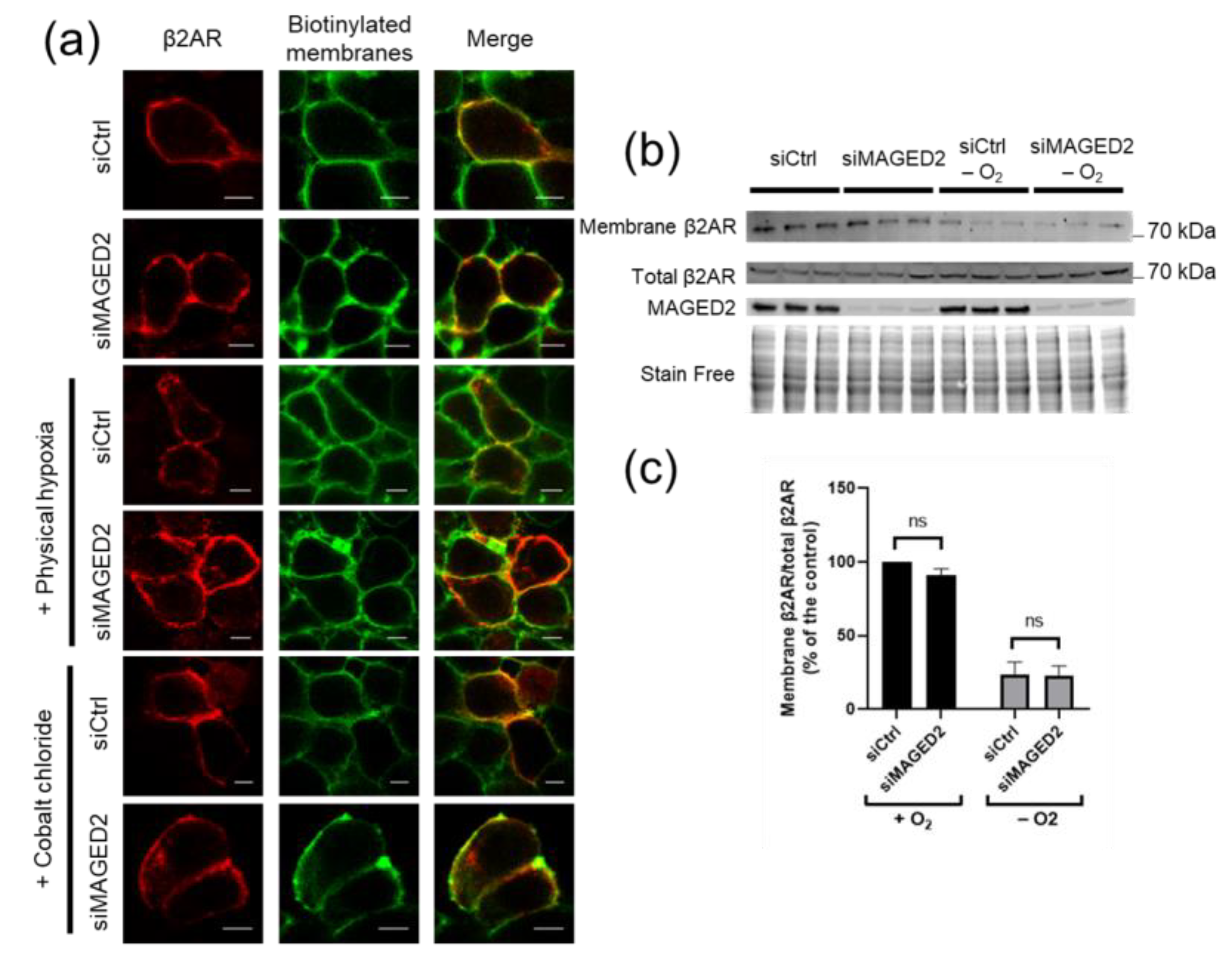
3.2. Expression of the β2-Adrenergic Receptor at the Plasma Membrane Is Independent of MAGED2
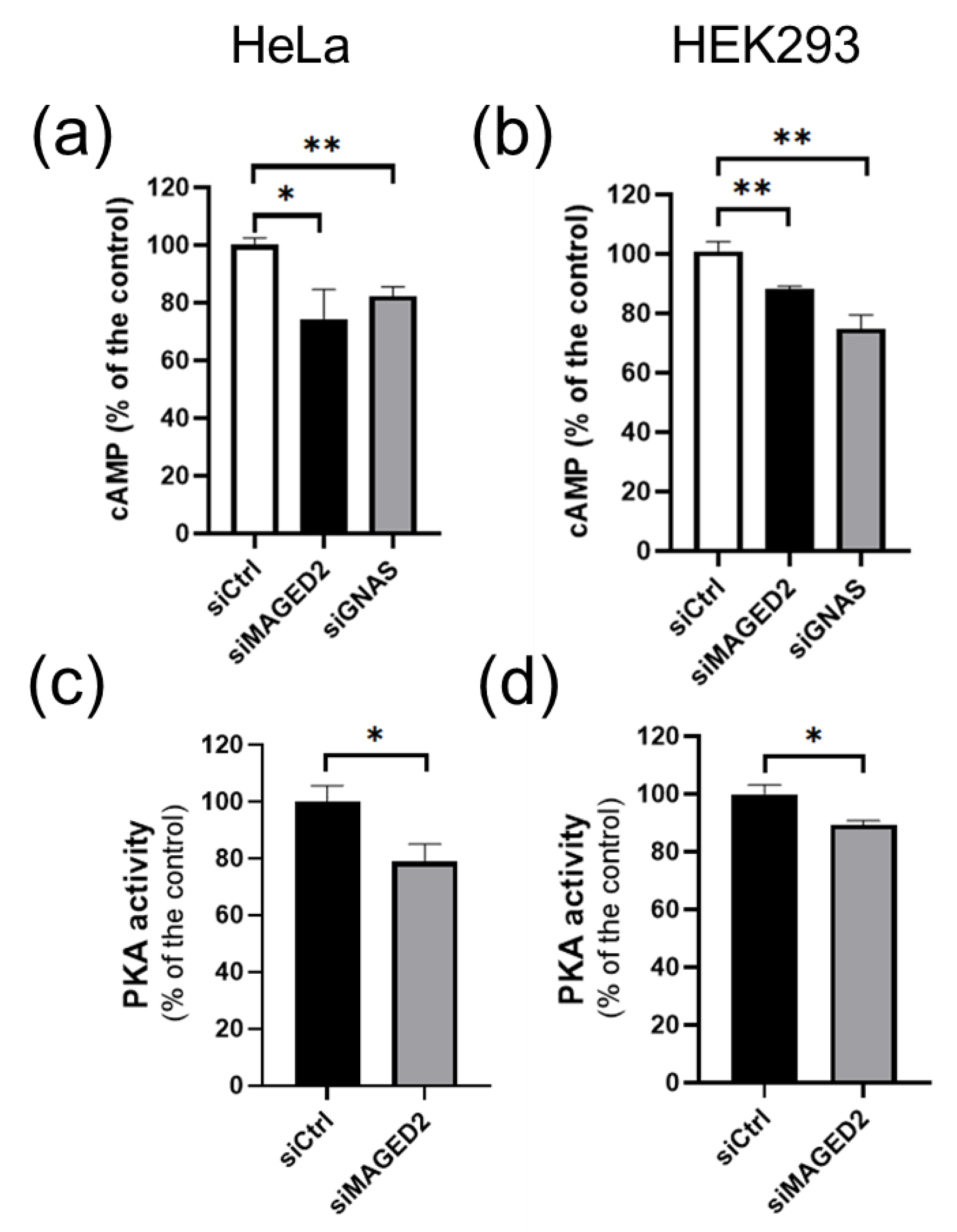
3.3. MAGED2 Is Required for cAMP Generation and PKA Activity under Hypoxia
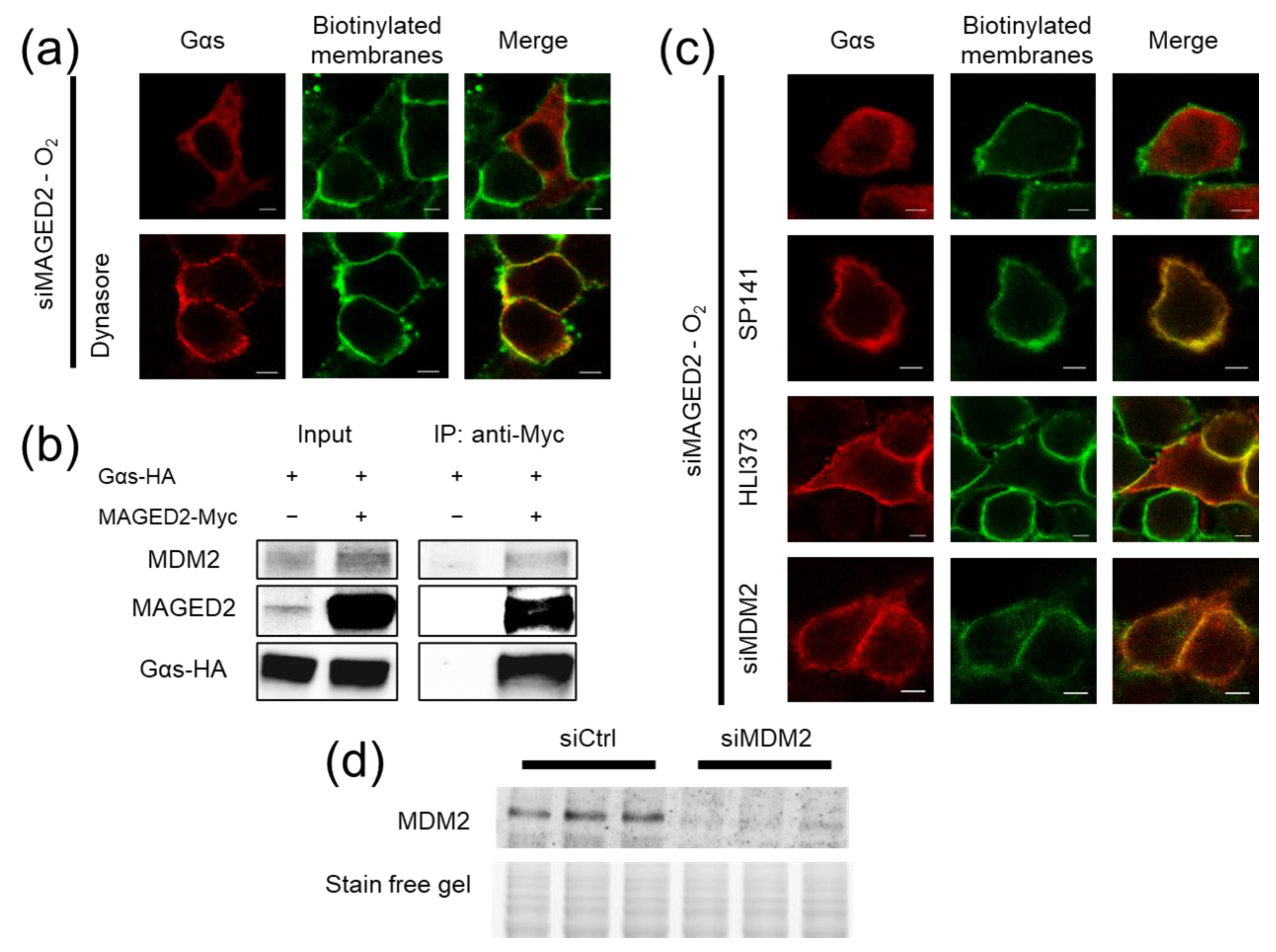
3.4. Under Hypoxia, MAGED2 Depletion Promotes Endocytosis of Gαs by Enhancing Its MDM2-Dependent Ubiquitination
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laghmani, K.; Beck, B.B.; Yang, S.-S.; Seaayfan, E.; Wenzel, A.; Reusch, B.; Vitzthum, H.; Priem, D.; Demaretz, S.; Bergmann, K.; et al. Polyhydramnios, Transient Antenatal Bartter’s Syndrome, and MAGED2 Mutations. N. Engl. J. Med. 2016, 374, 1853–1863. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, A.M.; Heymann, M.a.; Teramo, K.a.W.; Barrett, C.T.; Räihä, N.C.R. Studies on the Circulation of the Previable Human Fetus. Pediatric Res. 1971, 5, 452–465. [Google Scholar] [CrossRef]
- Brezis, M.; Rosen, S. Hypoxia of the renal medulla—Its implications for disease. N. Engl. J. Med. 1995, 332, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, W.M.; Schmitt, R.; Rosenberger, C.; Munchenhagen, P.M.; Grone, H.J.; Frei, U.; Warnecke, C.; Bachmann, S.; Wiesener, M.S.; Willam, C.; et al. Expression of hypoxia-inducible transcription factors in developing human and rat kidneys. Kidney Int. 2006, 69, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Hemker, S.L.; Sims-Lucas, S.; Ho, J. Role of hypoxia during nephrogenesis. Pediatr. Nephrol. 2016, 31, 1571–1577. [Google Scholar] [CrossRef]
- Florke Gee, R.R.; Chen, H.; Lee, A.K.; Daly, C.A.; Wilander, B.A.; Fon Tacer, K.; Potts, P.R. Emerging roles of the MAGE protein family in stress response pathways. J. Biol. Chem. 2020, 295, 16121–16155. [Google Scholar] [CrossRef]
- Doyle, J.M.; Gao, J.; Wang, J.; Yang, M.; Potts, P.R. MAGE-RING protein complexes comprise a family of E3 ubiquitin ligases. Mol. Cell 2010, 39, 963–974. [Google Scholar] [CrossRef]
- Foot, N.; Henshall, T.; Kumar, S. Ubiquitination and the Regulation of Membrane Proteins. Physiol. Rev. 2017, 97, 253–281. [Google Scholar] [CrossRef]
- Huttlin, E.L.; Bruckner, R.J.; Navarrete-Perea, J.; Cannon, J.R.; Baltier, K.; Gebreab, F.; Gygi, M.P.; Thornock, A.; Zarraga, G.; Tam, S.; et al. Dual proteome-scale networks reveal cell-specific remodeling of the human interactome. Cell 2021, 184, 3022–3040.e3028. [Google Scholar] [CrossRef]
- Sassone-Corsi, P. The cyclic AMP pathway. Cold Spring Harb. Perspect. Biol. 2012, 4, a011148. [Google Scholar] [CrossRef]
- Fenton, R.A.; Knepper, M.A. Mouse models and the urinary concentrating mechanism in the new millennium. Physiol. Rev. 2007, 87, 1083–1112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lv, Y.; Zhang, Y.; Gao, H. Regulation of p53 Level by UBE4B in Breast Cancer. PLoS ONE 2014, 9, e90154. [Google Scholar] [CrossRef] [PubMed]
- García-Cano, J.; Sánchez-Tena, S.; Sala-Gaston, J.; Figueras, A.; Viñals, F.; Bartrons, R.; Ventura, F.; Rosa, J.L. Regulation of the MDM2-p53 pathway by the ubiquitin ligase HERC2. Mol. Oncol. 2020, 14, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Hu, L.A.; Miller, W.E.; Ringstad, N.; Hall, R.A.; Pitcher, J.A.; DeCamilli, P.; Lefkowitz, R.J. Identification of the endophilins (SH3p4/p8/p13) as novel binding partners for the β1-adrenergic receptor. Proc. Natl. Acad. Sci. USA 1999, 96, 12559–12564. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Sánchez, J.; Chánez-Cárdenas, M.E. The use of cobalt chloride as a chemical hypoxia model. J. Appl. Toxicol. 2019, 39, 556–570. [Google Scholar] [CrossRef] [PubMed]
- Kido, A.; Yoshitani, K.; Shimizu, T.; Akahane, M.; Fujii, H.; Tsukamoto, S.; Kondo, Y.; Honoki, K.; Imano, M.; Tanaka, Y. Effect of mesenchymal stem cells on hypoxia-induced desensitization of β2-adrenergic receptors in rat osteosarcoma cells. Oncol. Lett. 2012, 4, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Cheong, H.I.; Asosingh, K.; Stephens, O.R.; Queisser, K.A.; Xu, W.; Willard, B.; Hu, B.; Dermawan, J.K.T.; Stark, G.R.; Naga Prasad, S.V.; et al. Hypoxia sensing through β-adrenergic receptors. JCI Insight 2016, 1, e90240. [Google Scholar] [CrossRef]
- Haupt, Y.; Maya, R.; Kazaz, A.; Oren, M. Mdm2 promotes the rapid degradation of p53. Nature 1997, 387, 296–299. [Google Scholar] [CrossRef]
- Girnita, L.; Girnita, A.; Larsson, O. Mdm2-dependent ubiquitination and degradation of the insulin-like growth factor 1 receptor. Proc. Natl. Acad. Sci. USA 2003, 100, 8247–8252. [Google Scholar] [CrossRef]
- Huttlin, E.L.; Ting, L.; Bruckner, R.J.; Gebreab, F.; Gygi, M.P.; Szpyt, J.; Tam, S.; Zarraga, G.; Colby, G.; Baltier, K.; et al. The BioPlex Network: A Systematic Exploration of the Human Interactome. Cell 2015, 162, 425–440. [Google Scholar] [CrossRef]
- Huttlin, E.L.; Bruckner, R.J.; Paulo, J.A.; Cannon, J.R.; Ting, L.; Baltier, K.; Colby, G.; Gebreab, F.; Gygi, M.P.; Parzen, H.; et al. Architecture of the human interactome defines protein communities and disease networks. Nature 2017, 545, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Yang, Y.; Zhang, G.; Wang, C.; Wang, D.; Wu, M.; Mei, Y. Regulation of the Mdm2–p53 pathway by the ubiquitin E3 ligase MARCH7. EMBO Rep. 2018, 19, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Gao, M.H.; Miyanohara, A.; Hammond, H.K. Gαq reduces cAMP production by decreasing Gαs protein abundance. Biochem. Biophys. Res. Commun. 2008, 377, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Gao, X.; Ren, J.; Jin, C.; Xue, Y. BDM-PUB: Computational Prediction of Protein Ubiquitination Sites with a Bayesian Discriminant Method. Available online: http://bdmpub.biocuckoo.org/prediction.php (accessed on 1 June 2021).
- Chen, Y.; Wang, D.-D.; Wu, Y.-P.; Su, D.; Zhou, T.-Y.; Gai, R.-H.; Fu, Y.-Y.; Zheng, L.; He, Q.-J.; Zhu, H.; et al. MDM2 promotes epithelial-mesenchymal transition and metastasis of ovarian cancer SKOV3 cells. Br. J. Cancer 2017, 117, 1192–1201. [Google Scholar] [CrossRef]
- Wedegaertner, P.B.; Bourne, H.R.; von Zastrow, M. Activation-induced subcellular redistribution of Gs alpha. Mol. Biol. Cell 1996, 7, 1225–1233. [Google Scholar] [CrossRef]
- Ferrandon, S.; Feinstein, T.N.; Castro, M.; Wang, B.; Bouley, R.; Potts, J.T.; Gardella, T.J.; Vilardaga, J.P. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat. Chem. Biol. 2009, 5, 734–742. [Google Scholar] [CrossRef]
- Feinstein, T.N.; Yui, N.; Webber, M.J.; Wehbi, V.L.; Stevenson, H.P.; King, J.D., Jr.; Hallows, K.R.; Brown, D.; Bouley, R.; Vilardaga, J.P. Noncanonical control of vasopressin receptor type 2 signaling by retromer and arrestin. J. Biol. Chem. 2013, 288, 27849–27860. [Google Scholar] [CrossRef]
- Li, X.; Letourneau, D.; Holleran, B.; Leduc, R.; Lavigne, P.; Lavoie, C. Galphas protein binds ubiquitin to regulate epidermal growth factor receptor endosomal sorting. Proc. Natl. Acad. Sci. USA 2017, 114, 13477–13482. [Google Scholar] [CrossRef]
- Rosciglione, S.; Theriault, C.; Boily, M.O.; Paquette, M.; Lavoie, C. Galphas regulates the post-endocytic sorting of G protein-coupled receptors. Nat. Commun. 2014, 5, 4556. [Google Scholar] [CrossRef]
- Saini, D.K.; Chisari, M.; Gautam, N. Shuttling and translocation of heterotrimeric G proteins and Ras. Trends Pharmacol. Sci. 2009, 30, 278–286. [Google Scholar] [CrossRef]
- Thiyagarajan, M.M.; Bigras, E.; Van Tol, H.H.; Hébert, T.E.; Evanko, D.S.; Wedegaertner, P.B. Activation-induced subcellular redistribution of G alpha(s) is dependent upon its unique N-terminus. Biochemistry 2002, 41, 9470–9484. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.A.; Yu, J.Z.; Donati, R.J.; Rasenick, M.M. Beta-adrenergic receptor stimulation promotes G alpha s internalization through lipid rafts: A study in living cells. Mol. Pharmacol. 2005, 67, 1493–1504. [Google Scholar] [CrossRef] [PubMed]
- Hynes, T.R.; Mervine, S.M.; Yost, E.A.; Sabo, J.L.; Berlot, C.H. Live cell imaging of Gs and the beta2-adrenergic receptor demonstrates that both alphas and beta1gamma7 internalize upon stimulation and exhibit similar trafficking patterns that differ from that of the beta2-adrenergic receptor. J. Biol. Chem. 2004, 279, 44101–44112. [Google Scholar] [CrossRef]
- Yu, J.Z.; Rasenick, M.M. Real-time visualization of a fluorescent G(alpha)(s): Dissociation of the activated G protein from plasma membrane. Mol. Pharmacol. 2002, 61, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Song, X.; Wang, J.; Guo, C.; Li, Y.; Li, B.; Zhang, Y.; Yin, Y. Cancer-testis antigen MAGE-C2 binds Rbx1 and inhibits ubiquitin ligase-mediated turnover of cyclin E. Oncotarget 2015, 6, 42028–42039. [Google Scholar] [CrossRef][Green Version]
- Marcar, L.; Ihrig, B.; Hourihan, J.; Bray, S.E.; Quinlan, P.R.; Jordan, L.B.; Thompson, A.M.; Hupp, T.R.; Meek, D.W. MAGE-A Cancer/Testis Antigens Inhibit MDM2 Ubiquitylation Function and Promote Increased Levels of MDM4. PLoS ONE 2015, 10, e0127713. [Google Scholar] [CrossRef]
- Lefkowitz, R.J.; Rajagopal, K.; Whalen, E.J. New roles for beta-arrestins in cell signaling: Not just for seven-transmembrane receptors. Mol. Cell 2006, 24, 643–652. [Google Scholar] [CrossRef]
- Sorensen, A.; Sinding, M.; Peters, D.A.; Petersen, A.; Frokjaer, J.B.; Christiansen, O.B.; Uldbjerg, N. Placental oxygen transport estimated by the hyperoxic placental BOLD MRI response. Physiol. Rep. 2015, 3, e12582. [Google Scholar] [CrossRef]
- Ammon, H.P.T.; Muller, A.B. Forskolin—From an Ayurvedic Remedy to a Modern Agent. Planta Med. 1985, 51, 473–477. [Google Scholar] [CrossRef]
- Godard, M.P.; Johnson, B.A.; Richmond, S.R. Body Composition and Hormonal Adaptations Associated with Forskolin Consumption in Overweight and Obese Men. Obes. Res. 2005, 13, 1335–1343. [Google Scholar] [CrossRef]
- Henderson, S.; Magu, B.; Rasmussen, C.; Lancaster, S.; Kerksick, C.; Smith, P.; Melton, C.; Cowan, P.; Greenwood, M.; Earnest, C.; et al. Effects of coleus forskohlii supplementation on body composition and hematological profiles in mildly overweight women. J. Int. Soc. Sports Nutr. 2005, 2, 54–62. [Google Scholar] [CrossRef]
- González-Sánchez, R.; Trujillo, X.; Trujillo-Hernández, B.; Vásquez, C.; Huerta, M.; Elizalde, A. Forskolin versus Sodium Cromoglycate for Prevention of Asthma Attacks: A Single-blinded Clinical Trial. J. Int. Med. Res. 2006, 34, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Staniak, M.; Czopek, K.; Stepien, A.; Dua, K.; Wadhwa, R.; Chellappan, D.K.; Sytar, O.; Brestic, M.; Bhat, N.G.; et al. The Therapeutic Potential of the Labdane Diterpenoid Forskolin. Appl. Sci. 2019, 9, 4089. [Google Scholar] [CrossRef]
- Fon Tacer, K.; Montoya, M.C.; Oatley, M.J.; Lord, T.; Oatley, J.M.; Klein, J.; Ravichandran, R.; Tillman, H.; Kim, M.; Connelly, J.P.; et al. MAGE cancer-testis antigens protect the mammalian germline under environmental stress. Sci. Adv. 2019, 5, eaav4832. [Google Scholar] [CrossRef] [PubMed]
- Kidd, M.; Modlin, I.M.; Mane, S.M.; Camp, R.L.; Eick, G.; Latich, I. The role of genetic markers—NAP1L1, MAGE-D2, and MTA1—In defining small-intestinal carcinoid neoplasia. Ann. Surg. Oncol. 2006, 13, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Kanda, M.; Murotani, K.; Tanaka, H.; Miwa, T.; Umeda, S.; Tanaka, C.; Kobayashi, D.; Hayashi, M.; Hattori, N.; Suenaga, M.; et al. A novel dual-marker expression panel for easy and accurate risk stratification of patients with gastric cancer. Cancer Med. 2018, 7, 2463–2471. [Google Scholar] [CrossRef]
- Chung, F.Y.; Cheng, T.L.; Chang, H.J.; Chiu, H.H.; Huang, M.Y.; Chang, M.S.; Chen, C.C.; Yang, M.J.; Wang, J.Y.; Lin, S.R. Differential gene expression profile of MAGE family in taiwanese patients with colorectal cancer. J. Surg. Oncol. 2010, 102, 148–153. [Google Scholar] [CrossRef]
- Tsai, J.R.; Chong, I.W.; Chen, Y.H.; Yang, M.J.; Sheu, C.C.; Chang, H.C.; Hwang, J.J.; Hung, J.Y.; Lin, S.R. Differential expression profile of MAGE family in non-small-cell lung cancer. Lung Cancer 2007, 56, 185–192. [Google Scholar] [CrossRef]
- Jing, X.; Yang, F.; Shao, C.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 2019, 18, 157. [Google Scholar] [CrossRef]
- Rohwer, N.; Zasada, C.; Kempa, S.; Cramer, T. The growing complexity of HIF-1alpha’s role in tumorigenesis: DNA repair and beyond. Oncogene 2013, 32, 3569–3576. [Google Scholar] [CrossRef]
- O’Hayre, M.; Vázquez-Prado, J.; Kufareva, I.; Stawiski, E.W.; Handel, T.M.; Seshagiri, S.; Gutkind, J.S. The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nat. Rev. Cancer 2013, 13, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Tirosh, A.; Jin, D.X.; De Marco, L.; Laitman, Y.; Friedman, E. Activating genomic alterations in the Gs alpha gene (GNAS) in 274694 tumors. Genes Chromosomes Cancer 2020, 59, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Huang, L.; Luo, C.; Liu, Y.; Niu, J. A Case Report and Literature Review of a Novel Mutation in the MAGED2 Gene of a Patient With Severe Transient Polyhydramnios. Front. Pediatrics 2021, 9, 778814. [Google Scholar] [CrossRef] [PubMed]
- Karousis, E.D.; Mühlemann, O. The broader sense of nonsense. Trends Biochem. Sci. 2022. [Google Scholar] [CrossRef] [PubMed]

| Reagent or Resource | Source | Identifier |
|---|---|---|
| Antibodies | ||
| Anti-HIF-1α rabbit | Cell Signaling | 14179 |
| Anti-MAGED2 rabbit | This paper | |
| Anti-beta 2 Adrenergic Receptor antibody | Abcam | ab182136 |
| Anti-Gαs | Sigma Aldrich | 06-237 |
| Anti-HA tag mouse | Thermo Fisher Scientific | 26183 |
| V5-Tag antibody | Bio-rad | MCA1360GA |
| Monoclonal ANTI-FLAG® M2 antibody produced in mouse | Sigma-Aldrich | F3165 |
| Goat anti-Mouse IgG (H + L), Alexa Fluor Plus 555 | Thermo Fisher Scientific | A32727 |
| Streptavidin, Alexa Fluor™ 488 conjugate | Thermo Fisher Scientific | S11223 |
| StarBright Blue 520 Goat Anti-Rabbit IgG | Bio-rad | 12005869 |
| StarBright Blue 700 Goat Anti-Mouse IgG | Bio-rad | 12004158 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| EZ-Link™ Sulfo-NHS-LC-Biotin | Thermo Fisher Scientific | 21335 |
| Critical Commercial Assays | ||
| PepTag® Non-Radioactive Protein Kinase Assays | Promega | V5340 |
| cAMP Assay Kit (Competitive ELISA) | abcam | Ab133051 |
| Q5® Site-Directed Mutagenesis Kit | New England Biolabs’ | E0554S |
| QuikChange Multi Site-Directed Mutagenesis Kit | Agilent Technologies | 200515 |
| Experimental Models: Cell Lines | ||
| HEK293 | ATCC | CRL1573 |
| HeLa | Gift from Dr. Vijay Renigunta | |
| Oligonucleotides | ||
| ON-TARGETplus Non-targeting Control Pool | Dharmacon | D-001810-10-05 |
| UGGUUUACAUGUCGACUAA | ||
| UGGUUUACAUGUUGUGUGA | ||
| UGGUUUACAUGUUUUCUGA | ||
| UGGUUUACAUGUUUUCCUA | ||
| ON-TARGETplus Human MAGED2 siRNA—SMARTpool | Dharmacon | L-017284-01-0005 |
| GGACGAAGCUGAUAUCGGA | ||
| GCUAAAGACCAGACGAAGA | ||
| AGGCGAUGGAAGCGGAUUU | ||
| GAAAAGGACAGUAGCUCGA | ||
| ON-TARGETplus Human GNAS siRNA—SMARTpool | Dharmacon | L-010825-00-0005 |
| GCAAGUGGAUCCAGUGCUU | ||
| GCAUGCACCUUCGUCAGUA | ||
| AUGAGGAUCCUGCAUGUUA | ||
| CAACCAAAGUGCAGGACAU | ||
| MDM2 siRNA | Dharmacon | |
| GACAAAGAAGAGAGUGUGG | [12] | |
| GAAGUUAUUAAAGUCUGUU | [13] | |
| GNAS from short to long isoform primer | Sigma-Aldrich | |
| GCTGCAAGGAGCAACAGCGATGGTGAGAAGGCAACCAAAG | ||
| CTGCGGGTCCTCTTCGCCGCCCTCTCCATTAAACCCATTAAC | ||
| GNAS from HA to V5 tag primer | Sigma-Aldrich | |
| CTGCTGGGCCTGGATAGCACCTAAACTCGAGTCTAGAGCGGCC | ||
| CGGGTTCGGAATCGGTTTGCCAGAGCCTCCACCCCCGAG | ||
| GNAS 5X lysine mutation primer | Sigma-Aldrich | |
| TGAGGCCAACAAAAAGATCGAGAGGCAGCTGCAGAA | ||
| GGTGCTGGAGAATCTGGTAGAAGCACCATTGTGAAG | ||
| GGAGCAACAGCGATGGTGAGAGGGCAACCAAAG | ||
| AGCAAGATCTGCTCGCTGAGAGAGTCCTTGCTG | ||
| GAAAGTCCTTGCTGGGAGATCGAAGATTGAGGACT | ||
| Recombinant DNA | ||
| G protein alpha S/GNAS cDNA ORF Clone, Human, C-HA tag | Sino Biological Inc. | HG12069-CY |
| pcDNA3 Flag beta-2-adrenergic-receptor | Gift from Robert Lefkowitz | [14] |
| Single Ubiquitin HA tag | Gift from Professor Hemmo Meyer | |
| Software and Algorithms | ||
| ImageJ | Schneider et al., 2012 | https://imagej.nih.gov/ij/, accessed on 22 July 2022 |
| GraphPad Prism 8 | GraphPad | |
| EndNote X9 | Clarivate Analytics | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seaayfan, E.; Nasrah, S.; Quell, L.; Kleim, M.; Weber, S.; Meyer, H.; Laghmani, K.; Kömhoff, M. MAGED2 Is Required under Hypoxia for cAMP Signaling by Inhibiting MDM2-Dependent Endocytosis of G-Alpha-S. Cells 2022, 11, 2546. https://doi.org/10.3390/cells11162546
Seaayfan E, Nasrah S, Quell L, Kleim M, Weber S, Meyer H, Laghmani K, Kömhoff M. MAGED2 Is Required under Hypoxia for cAMP Signaling by Inhibiting MDM2-Dependent Endocytosis of G-Alpha-S. Cells. 2022; 11(16):2546. https://doi.org/10.3390/cells11162546
Chicago/Turabian StyleSeaayfan, Elie, Sadiq Nasrah, Lea Quell, Maja Kleim, Stefanie Weber, Hemmo Meyer, Kamel Laghmani, and Martin Kömhoff. 2022. "MAGED2 Is Required under Hypoxia for cAMP Signaling by Inhibiting MDM2-Dependent Endocytosis of G-Alpha-S" Cells 11, no. 16: 2546. https://doi.org/10.3390/cells11162546
APA StyleSeaayfan, E., Nasrah, S., Quell, L., Kleim, M., Weber, S., Meyer, H., Laghmani, K., & Kömhoff, M. (2022). MAGED2 Is Required under Hypoxia for cAMP Signaling by Inhibiting MDM2-Dependent Endocytosis of G-Alpha-S. Cells, 11(16), 2546. https://doi.org/10.3390/cells11162546








