Stem Cells from Healthy and Tendinopathic Human Tendons: Morphology, Collagen and Cytokines Expression and Their Response to T3 Thyroid Hormone
Abstract
:1. Introduction
2. Materials and Methods
2.1. TSPCs Extraction
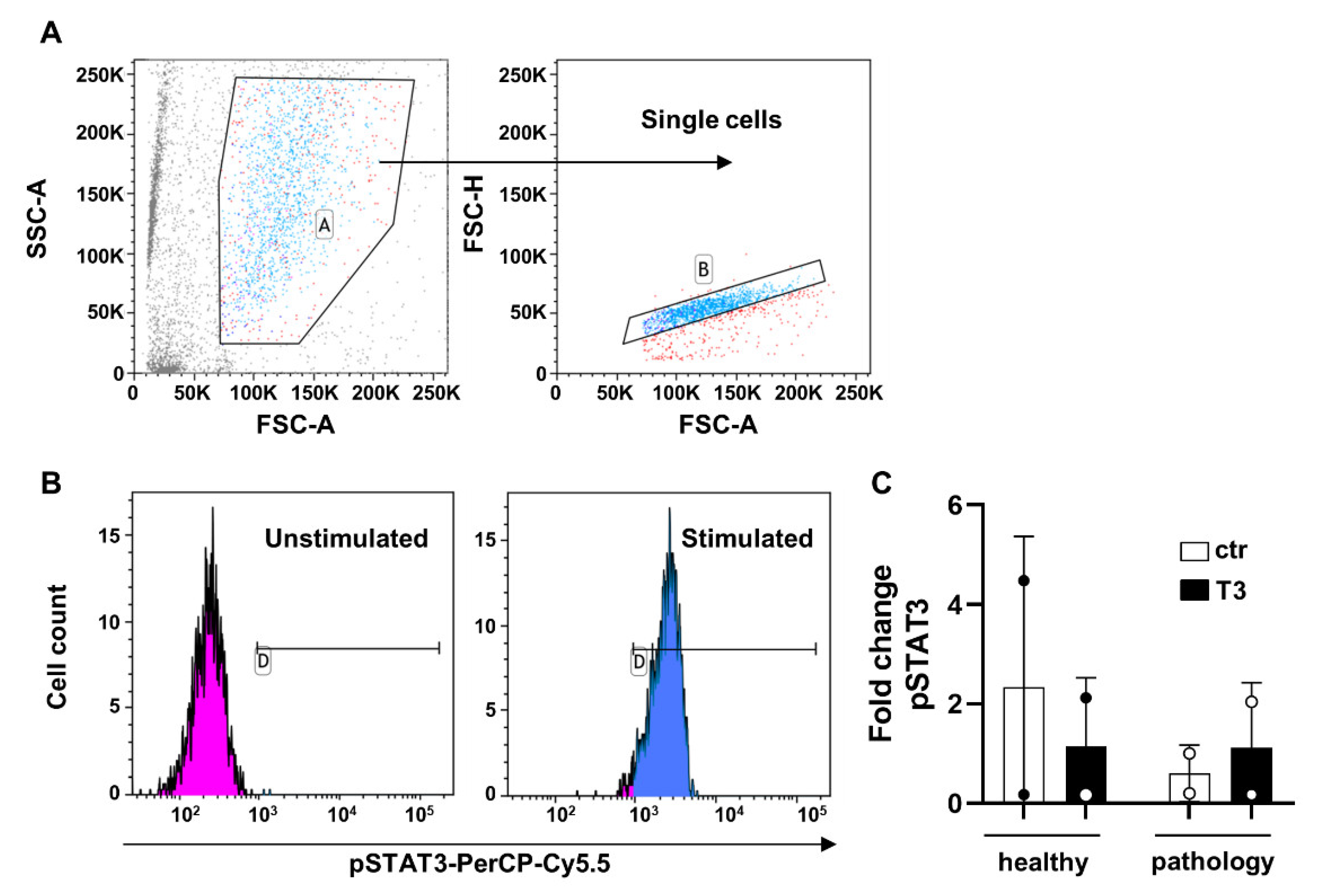
2.2. Flow Cytometry and Gating Strategy
2.3. T3 Treatment
2.4. Brightfield Images
2.5. Total RNA Isolation and Gene Expression by qRT-PCR
2.6. Immunofluorescence Assay
2.7. Immunohistochemical Assay
2.8. Multiplex Immunoassay
2.9. Statistical Analysis
3. Results
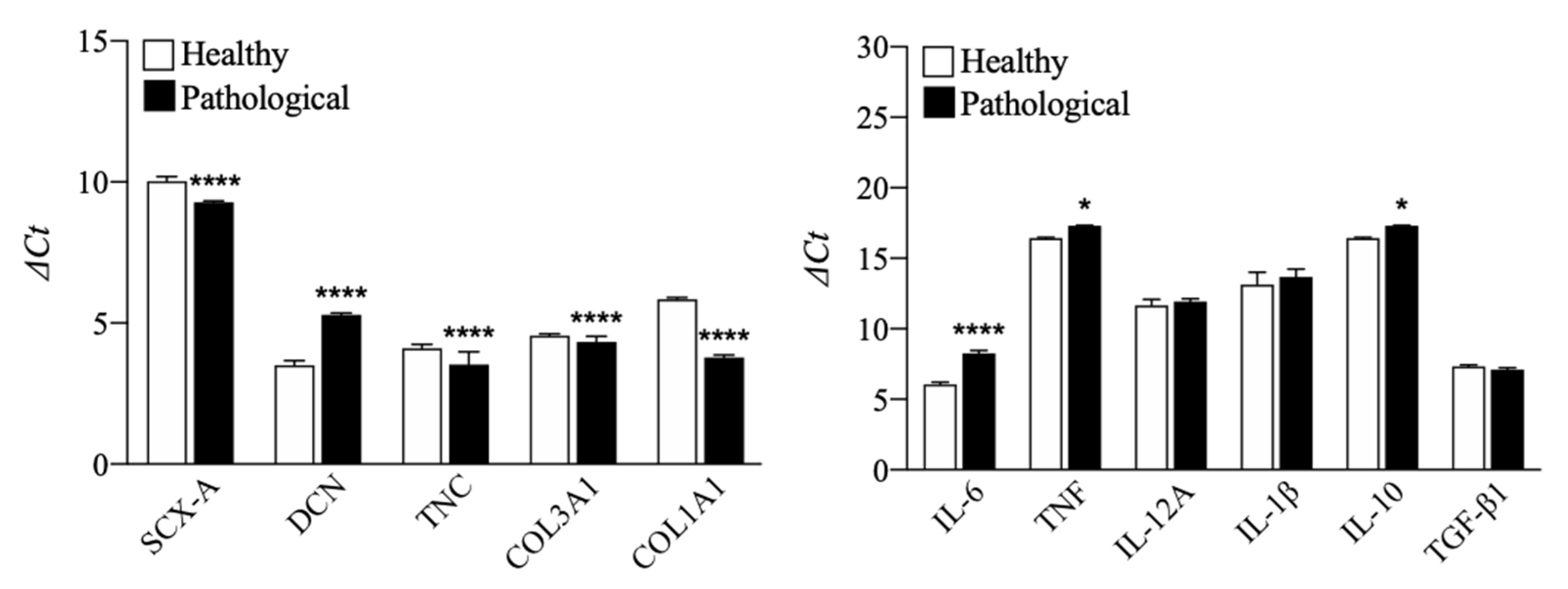
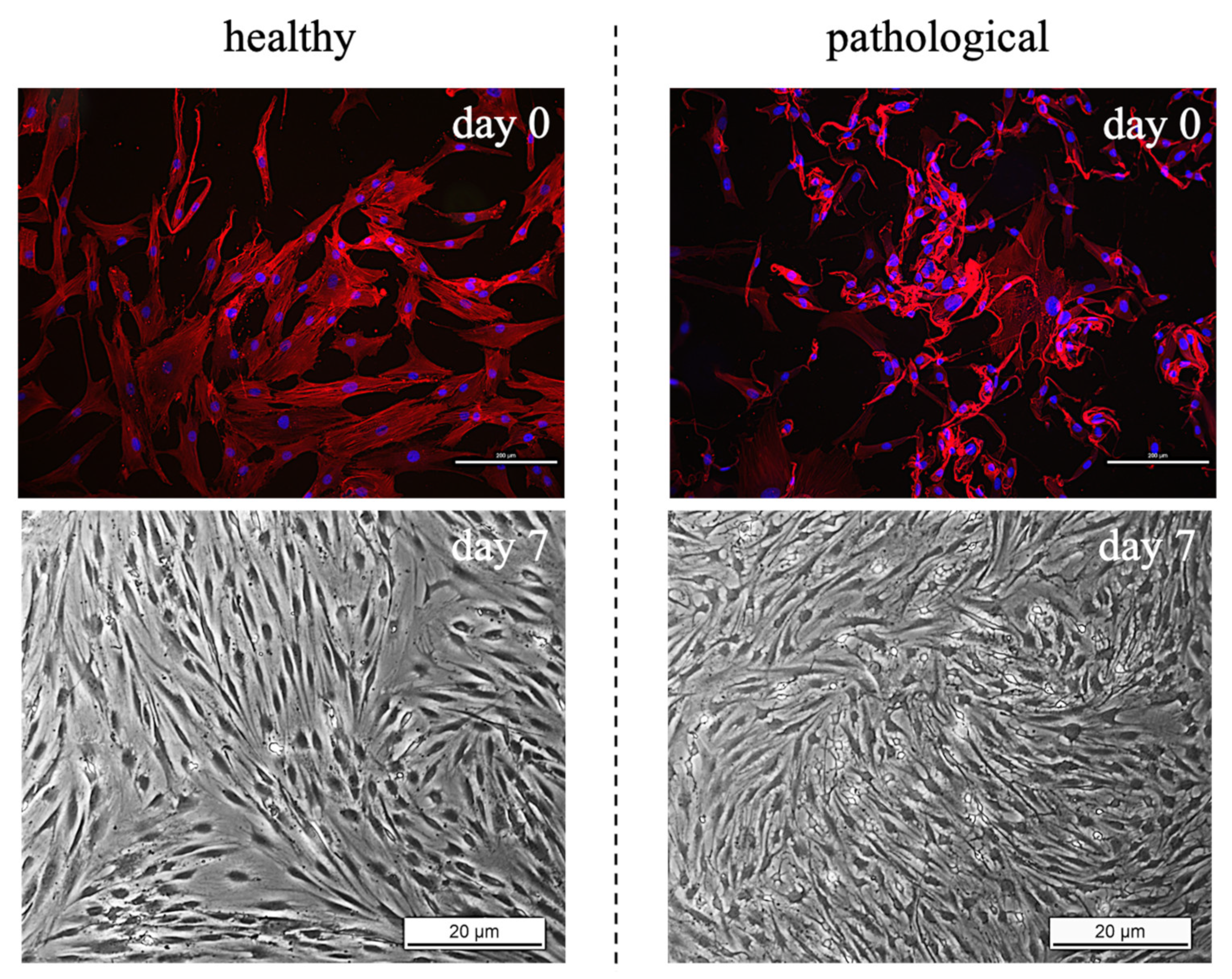
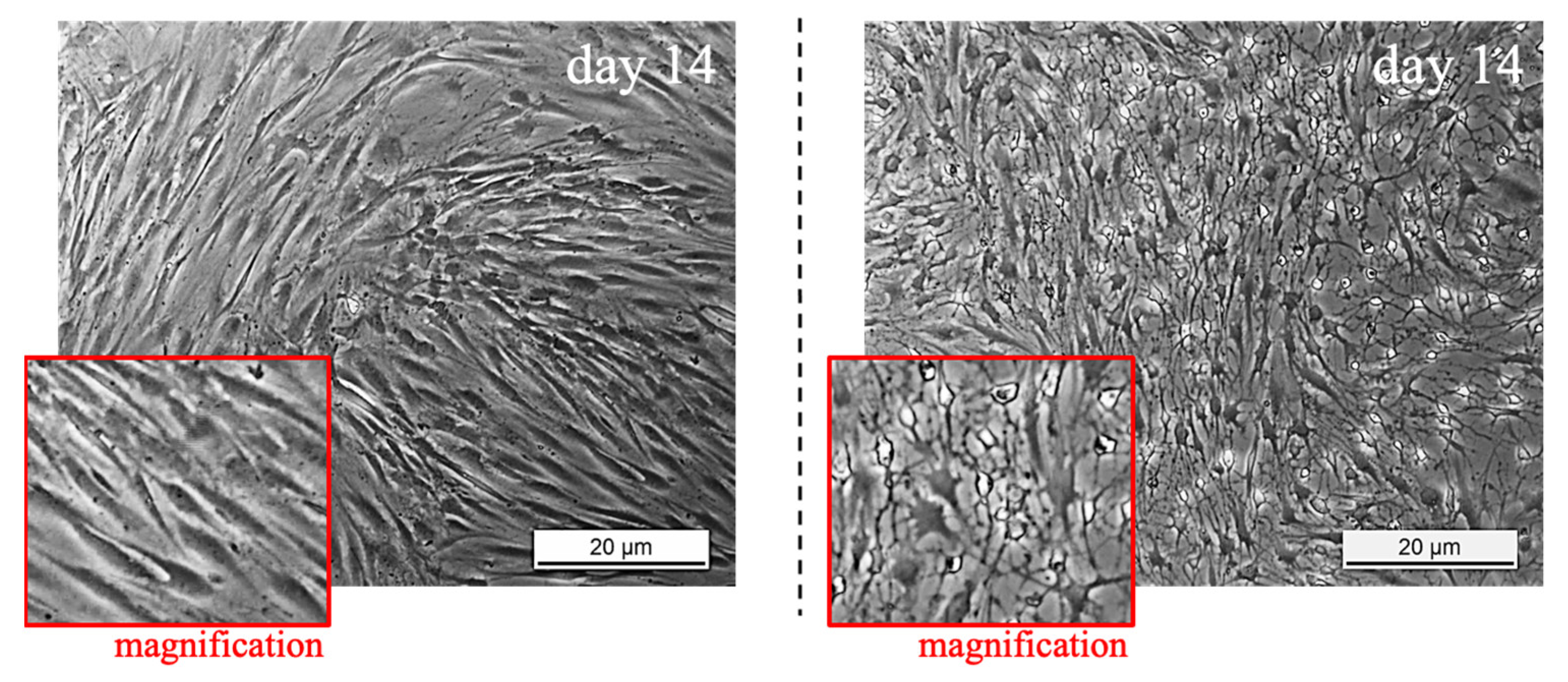
3.1. TSPCs Isolation and Characterization from Healthy and Pathological Tendons
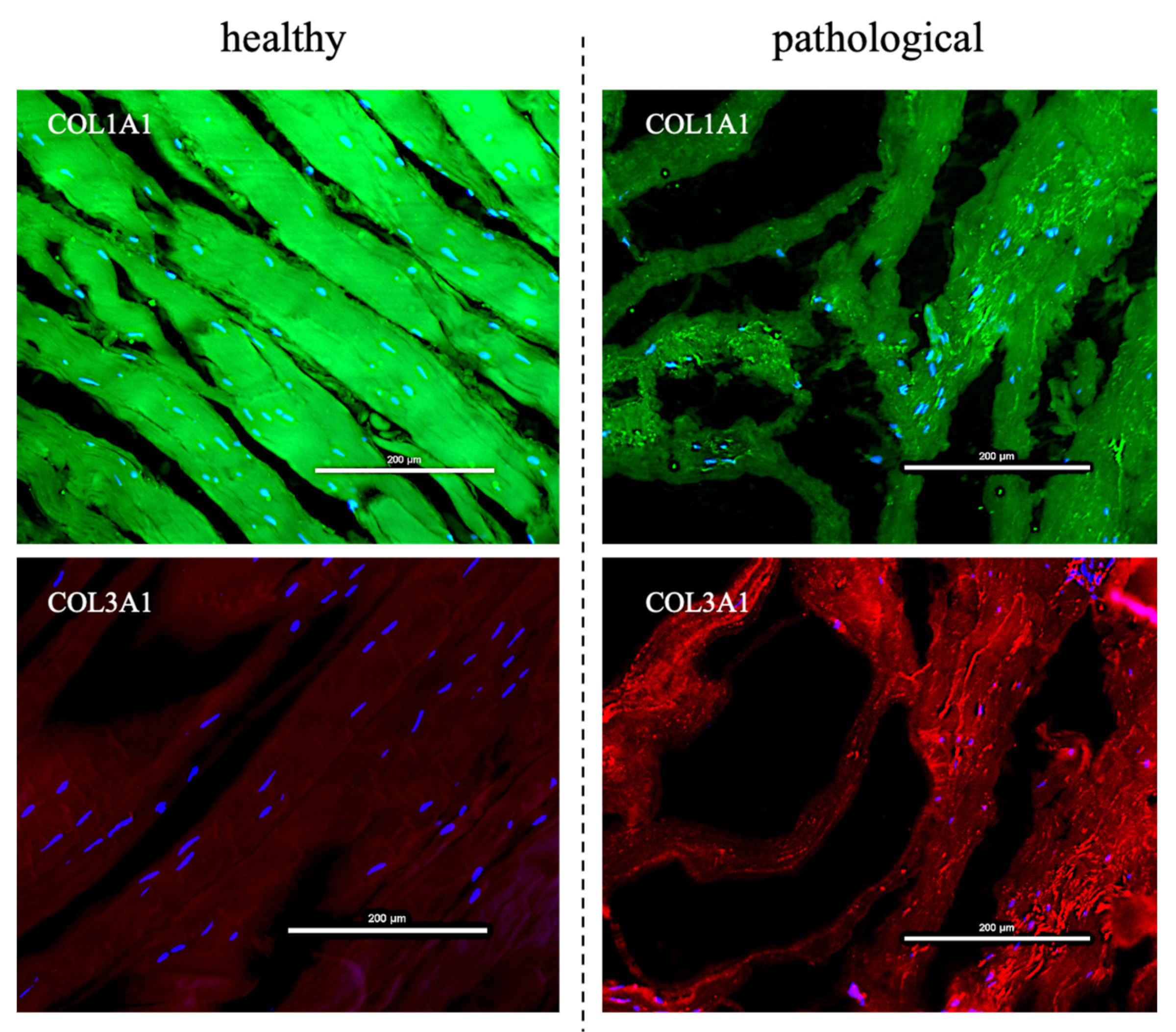
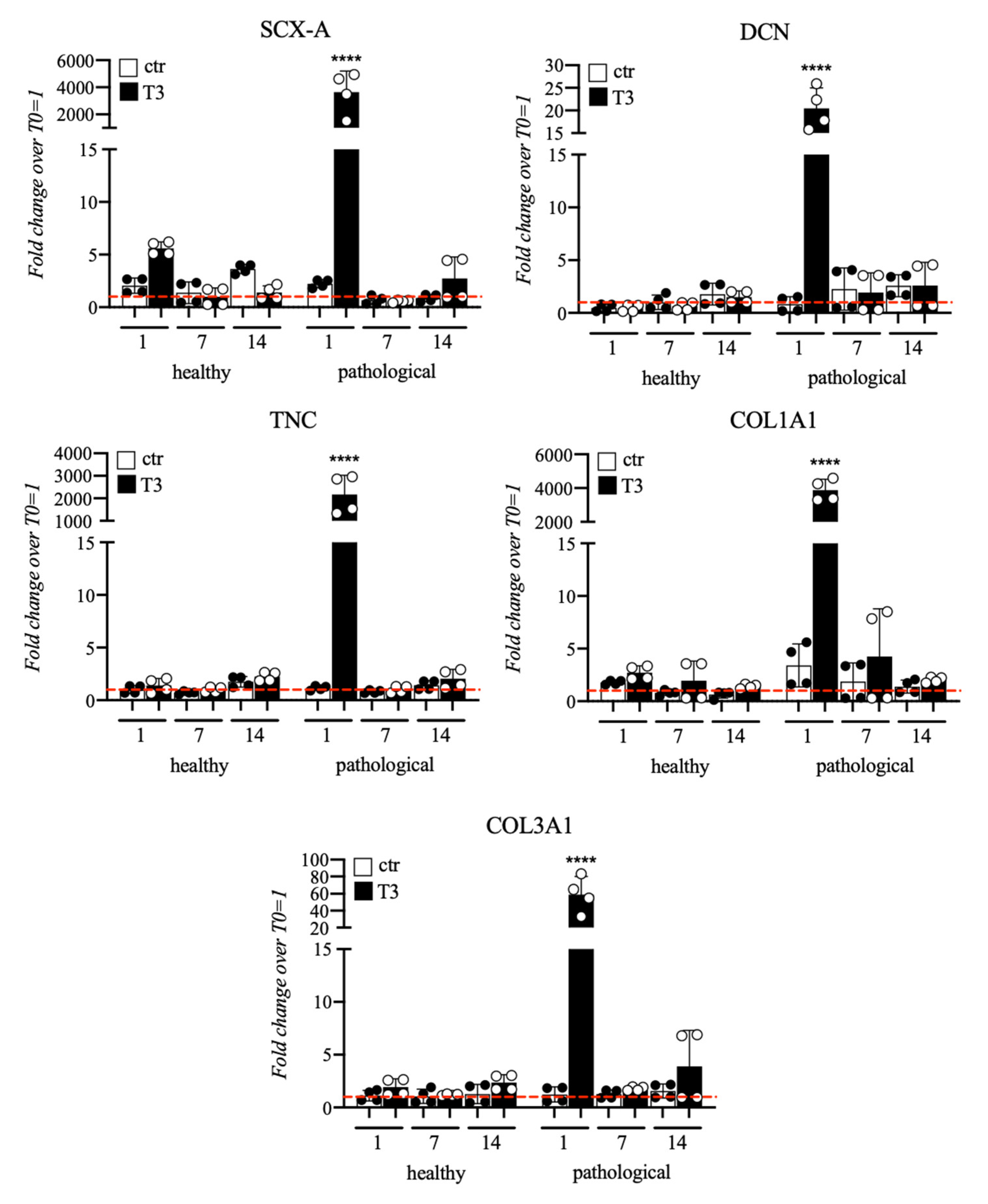
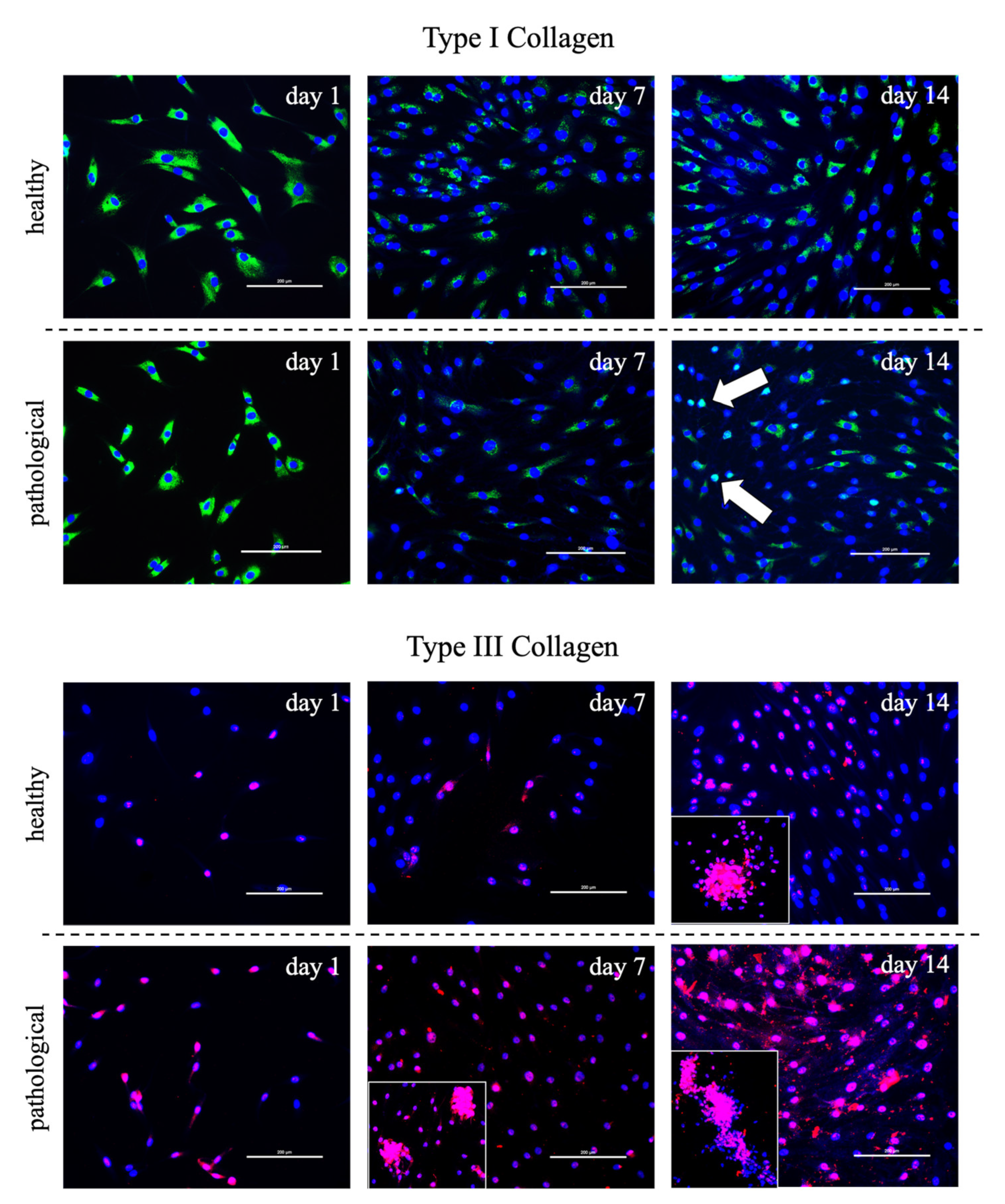
3.2. Evaluation of T3 Hormone Effect on Collagen Production
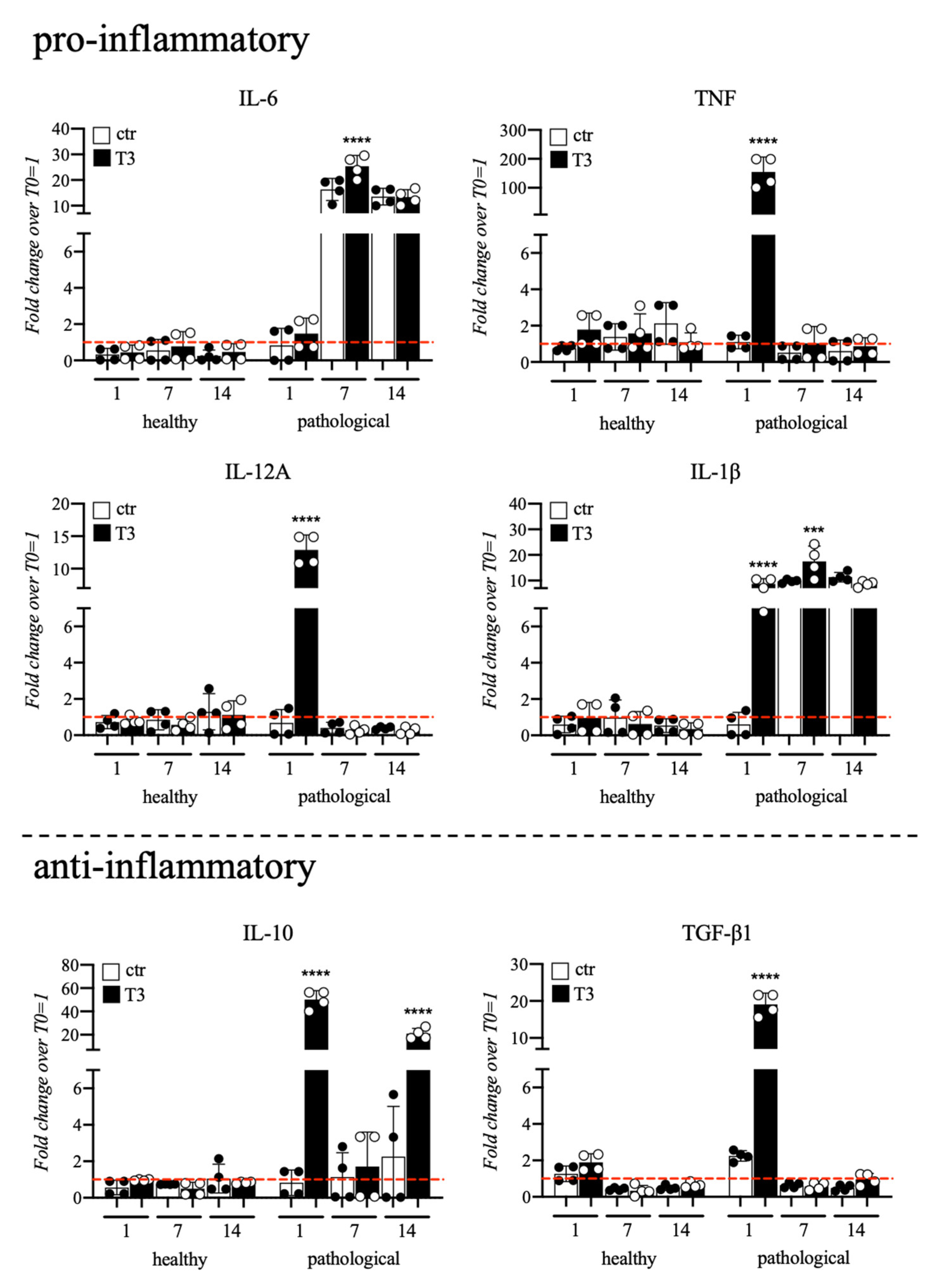
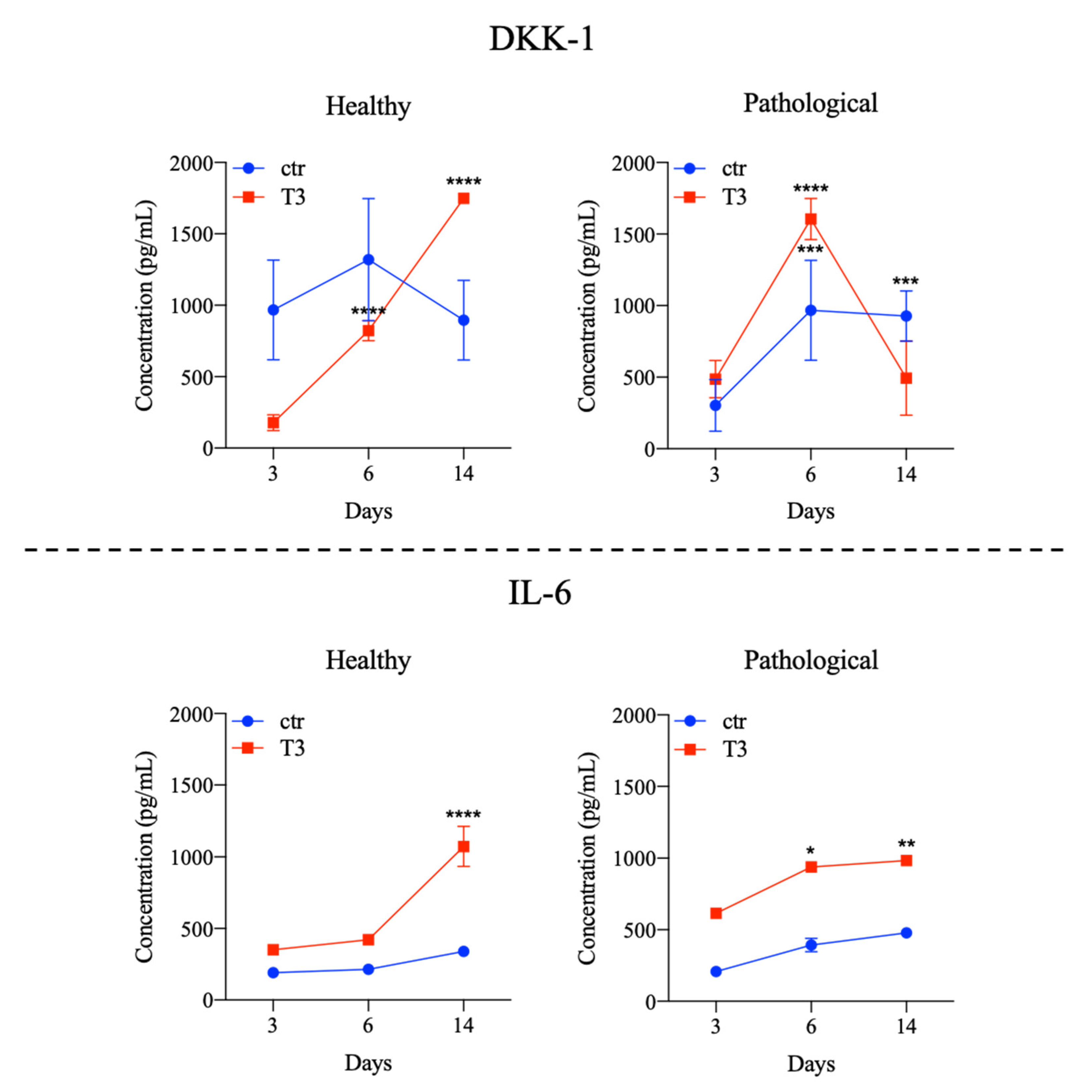
3.3. Evaluation of T3 Hormone Effect on Cytokines Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaux, J.-F.; Forthomme, B.; Goff, C.L.; Crielaard, J.-M.; Croisier, J.-L. Current Opinions on Tendinopathy. J. Sports Sci. Med. 2011, 10, 238–253. [Google Scholar] [PubMed]
- Sharma, P.; Maffulli, N. Tendon Injury and Tendinopathy: Healing and Repair. J. Bone Jt. Surg. 2005, 87, 187–202. [Google Scholar] [CrossRef]
- van Dijk, C.N.; van Sterkenburg, M.N.; Wiegerinck, J.I.; Karlsson, J.; Maffulli, N. Terminology for Achilles Tendon Related Disorders. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Maffulli, N. Biology of Tendon Injury: Healing, Modeling and Remodeling. J. Musculoskelet. Neuronal Interact. 2006, 6, 181–190. [Google Scholar] [PubMed]
- D’Addona, A.; Maffulli, N.; Formisano, S.; Rosa, D. Inflammation in Tendinopathy. Surgeon 2017, 15, 297–302. [Google Scholar] [CrossRef]
- Yao, L.; Bestwick, C.S.; Bestwick, L.A.; Aspden, R.M.; Maffulli, N. Non-Immortalized Human Tenocyte Cultures as a Vehicle for Understanding Cellular Aspects to Tendinopathy. Transl. Med.@ UniSa 2011, 1, 173–194. [Google Scholar]
- Citeroni, M.R.; Mauro, A.; Ciardulli, M.C.; Di Mattia, M.; El Khatib, M.; Russo, V.; Turriani, M.; Santer, M.; Della Porta, G.; Maffulli, N.; et al. Amnion-Derived Teno-Inductive Secretomes: A Novel Approach to Foster Tendon Differentiation and Regeneration in an Ovine Model. Front. Bioeng. Biotechnol. 2021, 9, 649288. [Google Scholar] [CrossRef]
- Maffulli, N.; Ewen, S.W.B.; Waterston, S.W.; Reaper, J.; Barrass, V. Tenocytes from Ruptured and Tendinopathic Achilles Tendons Produce Greater Quantities of Type III Collagen than Tenocytes from Normal Achilles Tendons: An in Vitro Model of Human Tendon Healing. Am. J. Sports Med. 2000, 28, 499–505. [Google Scholar] [CrossRef]
- Lui, P.P.-Y.; Chan, L.-S.; Lee, Y.-W.; Fu, S.C.; Chan, K.-M. Sustained Expression of Proteoglycans and Collagen Type III/Type I Ratio in a Calcified Tendinopathy Model. Rheumatology 2010, 49, 231–239. [Google Scholar] [CrossRef]
- Jelinsky, S.A.; Rodeo, S.A.; Li, J.; Gulotta, L.V.; Archambault, J.M.; Seeherman, H.J. Regulation of Gene Expression in Human Tendinopathy. BMC Musculoskelet. Disord. 2011, 12, 86. [Google Scholar] [CrossRef]
- Waterston, S.W.; Maffulli, N.; Ewen, S.W. Subcutaneous Rupture of the Achilles Tendon: Basic Science and Some Aspects of Clinical Practice. Br. J. Sports Med. 1997, 31, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Ireland, D.; Harrall, R.; Curry, V.; Holloway, G.; Hackney, R.; Hazleman, B.; Riley, G. Multiple Changes in Gene Expression in Chronic Human Achilles Tendinopathy. Matrix Biol. 2001, 20, 159–169. [Google Scholar] [CrossRef]
- Fearon, A.M.; Cook, J.L.; Smith, P.; Scott, A. Gene expression in tenocytes suggests degenerative tendon tears attempt to heal. Br. J. Sports Med. 2013, 47, e2.44-e2. [Google Scholar] [CrossRef]
- Ciardulli, M.C.; Lovecchio, J.; Scala, P.; Lamparelli, E.P.; Dale, T.P.; Giudice, V.; Giordano, E.; Selleri, C.; Forsyth, N.R.; Maffulli, N.; et al. 3D Biomimetic Scaffold for Growth Factor Controlled Delivery: An In-Vitro Study of Tenogenic Events on Wharton’s Jelly Mesenchymal Stem Cells. Pharmaceutics 2021, 13, 1448. [Google Scholar] [CrossRef] [PubMed]
- Scala, P.; Rehak, L.; Giudice, V.; Ciaglia, E.; Puca, A.A.; Selleri, C.; Della Porta, G.; Maffulli, N. Stem Cell and Macrophage Roles in Skeletal Muscle Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 10867. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Mobasheri, A.; Busch, F.; Aldinger, C.; Stahlmann, R.; Montaseri, A.; Shakibaei, M. Curcumin Modulates Nuclear Factor ΚB (NF-ΚB)-Mediated Inflammation in Human Tenocytes in Vitro. J. Biol. Chem. 2011, 286, 28556–28566. [Google Scholar] [CrossRef]
- Oliva, F.; Piccirilli, E.; Berardi, A.C.; Tarantino, U.; Maffulli, N. Influence of Thyroid Hormones on Tendon Homeostasis. In Metabolic Influences on Risk for Tendon Disorders; Advances in Experimental Medicine and Biology; Ackermann, P.W., Hart, D.A., Eds.; Springer International Publishing: Cham, Germany, 2016; Volume 920, pp. 133–138. ISBN 9783319339412. [Google Scholar]
- Berardi, A.C.; Oliva, F.; Berardocco, M.; la Rovere, M.; Accorsi, P.; Maffulli, N. Thyroid Hormones Increase Collagen I and Cartilage Oligomeric Matrix Protein (COMP) Expression in Vitro Human Tenocytes. Muscles Ligaments Tendons J. 2014, 4, 285–291. [Google Scholar] [CrossRef]
- Lamparelli, E.P.; Ciardulli, M.C.; Scala, P.; Scognamiglio, M.; Charlier, B.; Di Pietro, P.; Izzo, V.; Vecchione, C.; Maffulli, N.; Della Porta, G. Lipid Nano-Vesicles for Thyroid Hormone Encapsulation: A Comparison between Different Fabrication Technologies, Drug Loading, and an in Vitro Delivery to Human Tendon Stem/Progenitor Cells in 2D and 3D Culture. Int. J. Pharm. 2022, 624, 122007. [Google Scholar] [CrossRef]
- Magnan, B.; Bondi, M.; Pierantoni, S.; Samaila, E. The Pathogenesis of Achilles Tendinopathy: A Systematic Review. Foot Ankle Surg. 2014, 20, 154–159. [Google Scholar] [CrossRef]
- Oliva, F.; Osti, L.; Padulo, J.; Maffulli, N. Epidemiology of the Rotator Cuff Tears: A New Incidence Related to Thyroid Disease. Muscles Ligaments Tendons J. 2014, 4, 309–314. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Yeh, C.-T.; Lin, K.-H. Molecular Functions of Thyroid Hormone Signaling in Regulation of Cancer Progression and Anti-Apoptosis. Int. J. Mol. Sci. 2019, 20, 4986. [Google Scholar] [CrossRef] [PubMed]
- Campo Verde Arboccó, F.; Persia, F.A.; Hapon, M.B.; Jahn, G.A. Hypothyroidism Decreases JAK/STAT Signaling Pathway in Lactating Rat Mammary Gland. Mol. Cell. Endocrinol. 2017, 450, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Kim, H.; Suh, J.M.; Park, S.J.; You, S.H.; Chung, H.K.; Lee, K.W.; Kwon, O.-Y.; Cho, B.Y.; Kim, Y.K.; et al. Involvement of JAK/STAT (Janus Kinase/Signal Transducer and Activator of Transcription) in the Thyrotropin Signaling Pathway. Mol. Endocrinol. 2000, 14, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xiao, L.; Dai, G.; Lu, P.; Zhang, Y.; Li, Y.; Ni, M.; Rui, Y. Inhibition of JAK-STAT Signaling Pathway Alleviates Age-Related Phenotypes in Tendon Stem/Progenitor Cells. Front. Cell Dev. Biol. 2021, 9, 650250. [Google Scholar] [CrossRef]
- Arvind, V.; Huang, A.H. Reparative and Maladaptive Inflammation in Tendon Healing. Front. Bioeng. Biotechnol. 2021, 9, 719047. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Ehirchiou, D.; Kilts, T.M.; Inkson, C.A.; Embree, M.C.; Sonoyama, W.; Li, L.; Leet, A.I.; Seo, B.-M.; Zhang, L.; et al. Identification of Tendon Stem/Progenitor Cells and the Role of the Extracellular Matrix in Their Niche. Nat. Med. 2007, 13, 1219–1227. [Google Scholar] [CrossRef]
- Huang, Z.; Yin, Z.; Xu, J.; Fei, Y.; Heng, B.C.; Jiang, X.; Chen, W.; Shen, W. Tendon Stem/Progenitor Cell Subpopulations and Their Implications in Tendon Biology. Front. Cell Dev. Biol. 2021, 9, 631272. [Google Scholar] [CrossRef]
- Tan, G.-K.; Pryce, B.A.; Stabio, A.; Brigande, J.V.; Wang, C.; Xia, Z.; Tufa, S.F.; Keene, D.R.; Schweitzer, R. Tgfβ Signaling Is Critical for Maintenance of the Tendon Cell Fate. Elife 2020, 9, e52695. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Ohkawara, B.; Sakai, T.; Ito, M.; Masuda, A.; Ishiguro, N.; Shukunami, C.; Docheva, D.; Ohno, K. Wnt/β-Catenin Signaling Suppresses Expressions of Scx, Mkx, and Tnmd in Tendon-Derived Cells. PLoS ONE 2017, 12, e0182051. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, Y.; Rui, Y.; Xu, H.; Chen, H.; Wang, C.; Teng, G. Therapeutic Roles of Tendon Stem/Progenitor Cells in Tendinopathy. Stem Cells Int. 2016, 2016, 1–14. [Google Scholar] [CrossRef]
- Kharaz, Y.A.; Canty-Laird, E.G.; Tew, S.R.; Comerford, E.J. Variations in Internal Structure, Composition and Protein Distribution between Intra- and Extra-Articular Knee Ligaments and Tendons. J. Anat. 2018, 232, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.L.; Vian, L.; Giudice, V.; Kieltyka, J.; Liu, C.; Fonseca, V.; Gazaniga, N.; Gao, S.; Kajigaya, S.; Young, N.S.; et al. High Throughput PSTAT Signaling Profiling by Fluorescent Cell Barcoding and Computational Analysis. J. Immunol. Methods 2020, 477, 112667. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. QBase Relative Quantification Framework and Software for Management and Automated Analysis of Real-time Quantitative PCR Data. Genome Biol. 2007, 8, R19. [Google Scholar] [CrossRef]
- Qin, S.; Wang, W.; Liu, Z.; Hua, X.; Fu, S.; Dong, F.; Li, A.; Liu, Z.; Wang, P.; Dai, L.; et al. Fibrochondrogenic Differentiation Potential of Tendon-Derived Stem/Progenitor Cells from Human Patellar Tendon. J. Orthop. Transl. 2020, 22, 101–108. [Google Scholar] [CrossRef]
- Tarafder, S.; Chen, E.; Jun, Y.; Kao, K.; Sim, K.H.; Back, J.; Lee, F.Y.; Lee, C.H. Tendon Stem/Progenitor Cells Regulate Inflammation in Tendon Healing via JNK and STAT3 Signaling. FASEB J. 2017, 31, 3991–3998. [Google Scholar] [CrossRef]
- Voleti, P.B.; Buckley, M.R.; Soslowsky, L.J. Tendon Healing: Repair and Regeneration. Annu. Rev. Biomed. Eng. 2012, 14, 47–71. [Google Scholar] [CrossRef]
- Sharma, P.; Maffulli, N. Basic Biology of Tendon Injury and Healing. Surgeon 2005, 3, 309–316. [Google Scholar] [CrossRef]
- Caplan, A.I. Review: Mesenchymal Stem Cells: Cell–Based Reconstructive Therapy in Orthopedics. Tissue Eng. 2005, 11, 1198–1211. [Google Scholar] [CrossRef]
- Song, H.; Yin, Z.; Wu, T.; Li, Y.; Luo, X.; Xu, M.; Duan, L.; Li, J. Enhanced Effect of Tendon Stem/Progenitor Cells Combined With Tendon-Derived Decellularized Extracellular Matrix on Tendon Regeneration. Cell Transplant. 2018, 27, 1634–1643. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Clegg, P.D.; Strassburg, S.; Smith, R.K. Cell Phenotypic Variation in Normal and Damaged Tendons. Int. J. Exp. Pathol. 2007, 88, 227–235. [Google Scholar] [CrossRef]
- Screen, H.R.C.; Berk, D.E.; Kadler, K.E.; Ramirez, F.; Young, M.F. Tendon Functional Extracellular Matrix. J. Orthop. Res. 2015, 33, 793–799. [Google Scholar] [CrossRef]
- Pajala, A.; Melkko, J.; Leppilahti, J.; Ohtonen, P.; Soini, Y.; Risteli, J. Tenascin-C and Type I and III Collagen Expression in Total Achilles Tendon Rupture. An Immunohistochemical Study. Histol. Histopathol. 2009, 24, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Doral, M.N.; Alam, M.; Bozkurt, M.; Turhan, E.; Atay, O.A.; Dönmez, G.; Maffulli, N. Functional Anatomy of the Achilles Tendon. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.R.S.; Trentin, A.G.; Rosenthal, D.; Chagas, C.; Neto, V.M. Thyroid Hormone Induces Protein Secretion and Morphological Changes in Astroglial Cells with an Increase in Expression of Glial Fibrillary Acidic Protein. J. Endocrinol. 1997, 154, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves Trentin, A.; Alcantara Gomes, F.C.; Souza Lina, F.R.; Moura Neto, V. Thyroid Hormone Acting on Astrocytes in Culture. In Vitro Cell. Dev. Biol. Anim. 1998, 34, 280–282. [Google Scholar] [CrossRef]
- Farwell, A.P.; Dubord-Tomasetti, S.A. Thyroid Hormone Regulates the Extracellular Organization of Laminin on Astrocytes. Endocrinology 1999, 140, 5014–5021. [Google Scholar] [CrossRef]
- Farwell, A.P.; Dubord-Tomasetti, S.A. Thyroid Hormone Regulates the Expression of Laminin in the Developing Rat Cerebellum. Endocrinology 1999, 140, 4221–4227. [Google Scholar] [CrossRef]
- di Giacomo, V.; Berardocco, M.; Gallorini, M.; Oliva, F.; Colosimo, A.; Cataldi, A.; Maffulli, N.; Berardi, A.C. Combined Supplementation of Ascorbic Acid and Thyroid Hormone T3 Affects Tenocyte Proliferation. The Effect of Ascorbic Acid in the Production of Nitric Oxide. Muscles Ligaments Tendons J. 2017, 7, 11–18. [Google Scholar] [CrossRef]
- Sterling, K.; Bellabarba, D.; Newman, E.S.; Brenner, M.A. Determination of Triiodothyronine Concentration in Human Serum. J. Clin. Investig. 1969, 48, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Gil-Ibañez, P.; García-García, F.; Dopazo, J.; Bernal, J.; Morte, B. Global Transcriptome Analysis of Primary Cerebrocortical Cells: Identification of Genes Regulated by Triiodothyronine in Specific Cell Types. Cereb. Cortex 2015, 27, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.C.; Anderson, G.; Forrest, D.; Galton, V.A.; Gereben, B.; Kim, B.W.; Kopp, P.A.; Liao, X.H.; Obregon, M.J.; Peeters, R.P.; et al. American Thyroid Association Guide to Investigating Thyroid Hormone Economy and Action in Rodent and Cell Models: Report of the American Thyroid Association Task Force on Approaches and Strategies to Investigate Thyroid Hormone Economy and Action. Thyroid 2014, 24, 88–168. [Google Scholar] [CrossRef] [PubMed]
- Oliva, F.; Maffulli, N.; Gissi, C.; Veronesi, F.; Calciano, L.; Fini, M.; Brogini, S.; Gallorini, M.; Antonetti Lamorgese Passeri, C.; Bernardini, R.; et al. Combined Ascorbic Acid and T3 Produce Better Healing Compared to Bone Marrow Mesenchymal Stem Cells in an Achilles Tendon Injury Rat Model: A Proof of Concept Study. J. Orthop. Surg. Res. 2019, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Alberton, P.; Popov, C.; Prägert, M.; Kohler, J.; Shukunami, C.; Schieker, M.; Docheva, D. Conversion of Human Bone Marrow-Derived Mesenchymal Stem Cells into Tendon Progenitor Cells by Ectopic Expression of Scleraxis. Stem Cells Dev. 2012, 21, 846–858. [Google Scholar] [CrossRef]
- Hsieh, C.-F.; Alberton, P.; Loffredo-Verde, E.; Volkmer, E.; Pietschmann, M.; Müller, P.; Schieker, M.; Docheva, D. Scaffold-Free Scleraxis-Programmed Tendon Progenitors Aid in Significantly Enhanced Repair of Full-Size Achilles Tendon Rupture. Nanomedicine 2016, 11, 1153–1167. [Google Scholar] [CrossRef]
- Fu, S.-C.; Chan, K.-M.; Rolf, C.G. Increased Deposition of Sulfated Glycosaminoglycans in Human Patellar Tendinopathy. Clin. J. Sport Med. 2007, 17, 129–134. [Google Scholar] [CrossRef]
- Ali, O.J. Immunolocalization of Decorin, a Small Leucin-Rich Proteoglycan, in the Normal and Injured Horse Tendon. Iraqi J. Vet. Sci. 2021, 35, 465–471. [Google Scholar] [CrossRef]
- Riley, G.P.; Harrall, R.L.; Cawston, T.E.; Hazleman, B.L.; Mackie, E.J. Tenascin-C and Human Tendon Degeneration. Am. J. Pathol. 1996, 149, 933–943. [Google Scholar]
- Wang, Z.-C.; Zhao, W.-Y.; Cao, Y.; Liu, Y.-Q.; Sun, Q.; Shi, P.; Cai, J.-Q.; Shen, X.Z.; Tan, W.-Q. The Roles of Inflammation in Keloid and Hypertrophic Scars. Front. Immunol. 2020, 11, 603187. [Google Scholar] [CrossRef]
- Kuivaniemi, H.; Tromp, G. Type III Collagen (COL3A1): Gene and Protein Structure, Tissue Distribution, and Associated Diseases. Gene 2019, 707, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Stallmach, A.; Schuppan, D.; Riese, H.H.; Matthes, H.; Riecken, E.O. Increased Collagen Type III Synthesis by Fibroblasts Isolated from Strictures of Patients with Crohn’s Disease. Gastroenterology 1992, 102, 1920–1929. [Google Scholar] [CrossRef]
- Thankam, F.G.; Roesch, Z.K.; Dilisio, M.F.; Radwan, M.M.; Kovilam, A.; Gross, R.M.; Agrawal, D.K. Association of Inflammatory Responses and ECM Disorganization with HMGB1 Upregulation and NLRP3 Inflammasome Activation in the Injured Rotator Cuff Tendon. Sci. Rep. 2018, 8, 8918. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Mathai, S.K.; Murray, L.A.; Russell, T.; Reilkoff, R.; Chen, Q.; Gulati, M.; Elias, J.A.; Bucala, R.; Gan, Y.; et al. Local Apoptosis Promotes Collagen Production by Monocyte-Derived Cells in Transforming Growth Factor Β1-Induced Lung Fibrosis. Fibrogenesis Tissue Repair 2011, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Niida, A.; Hiroko, T.; Kasai, M.; Furukawa, Y.; Nakamura, Y.; Suzuki, Y.; Sugano, S.; Akiyama, T. DKK1, a Negative Regulator of Wnt Signaling, Is a Target of the β-Catenin/TCF Pathway. Oncogene 2004, 23, 8520–8526. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciardulli, M.C.; Scala, P.; Giudice, V.; Santoro, A.; Selleri, C.; Oliva, F.; Maffulli, N.; Porta, G.D. Stem Cells from Healthy and Tendinopathic Human Tendons: Morphology, Collagen and Cytokines Expression and Their Response to T3 Thyroid Hormone. Cells 2022, 11, 2545. https://doi.org/10.3390/cells11162545
Ciardulli MC, Scala P, Giudice V, Santoro A, Selleri C, Oliva F, Maffulli N, Porta GD. Stem Cells from Healthy and Tendinopathic Human Tendons: Morphology, Collagen and Cytokines Expression and Their Response to T3 Thyroid Hormone. Cells. 2022; 11(16):2545. https://doi.org/10.3390/cells11162545
Chicago/Turabian StyleCiardulli, Maria Camilla, Pasqualina Scala, Valentina Giudice, Antonietta Santoro, Carmine Selleri, Francesco Oliva, Nicola Maffulli, and Giovanna Della Porta. 2022. "Stem Cells from Healthy and Tendinopathic Human Tendons: Morphology, Collagen and Cytokines Expression and Their Response to T3 Thyroid Hormone" Cells 11, no. 16: 2545. https://doi.org/10.3390/cells11162545
APA StyleCiardulli, M. C., Scala, P., Giudice, V., Santoro, A., Selleri, C., Oliva, F., Maffulli, N., & Porta, G. D. (2022). Stem Cells from Healthy and Tendinopathic Human Tendons: Morphology, Collagen and Cytokines Expression and Their Response to T3 Thyroid Hormone. Cells, 11(16), 2545. https://doi.org/10.3390/cells11162545







