Roles of Cartilage-Resident Stem/Progenitor Cells in Cartilage Physiology, Development, Repair and Osteoarthritis
Abstract
:1. Introduction
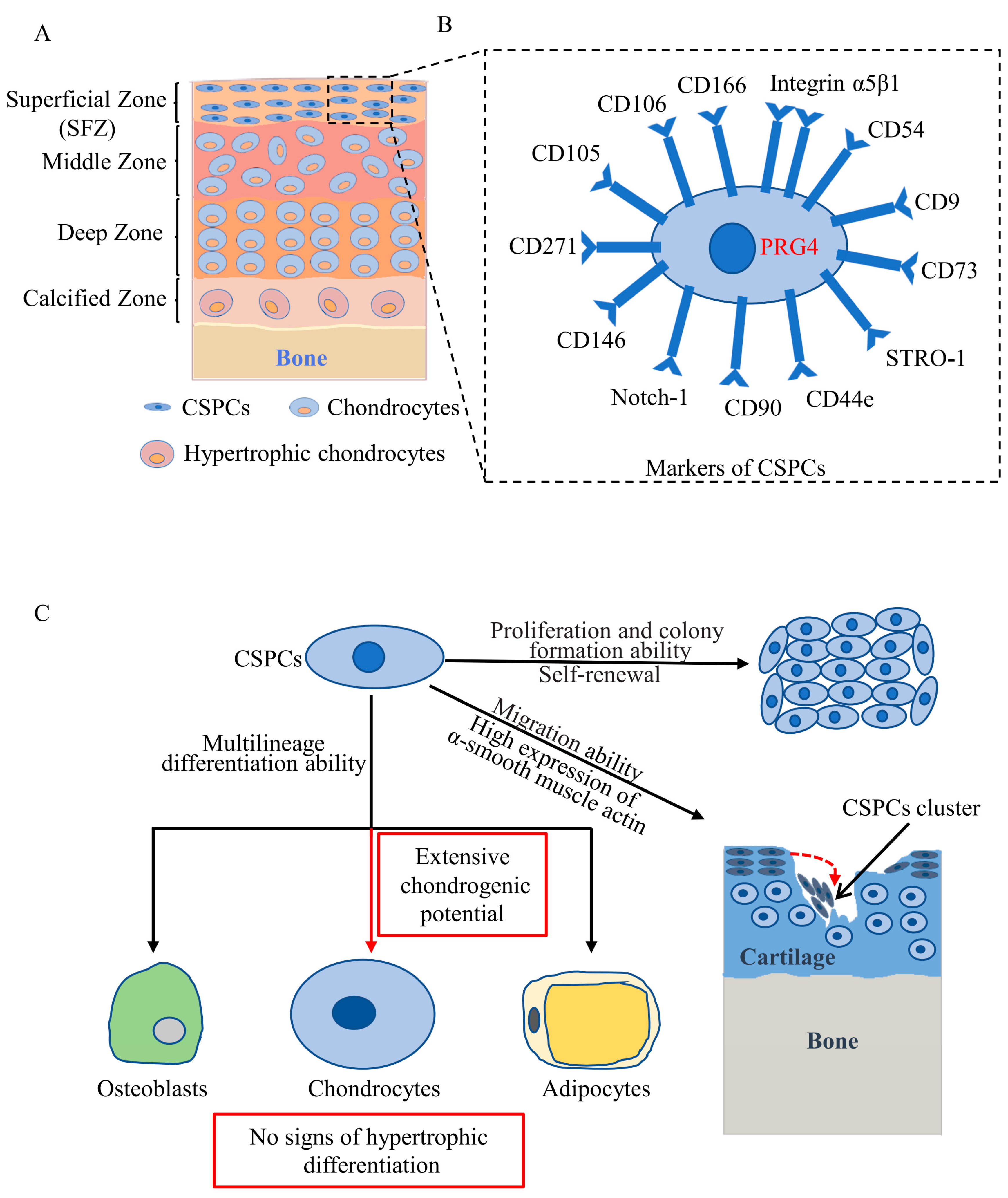
2. Distribution of CSPCs
3. Markers of CSPCs
4. Cytological Features of CSPCs
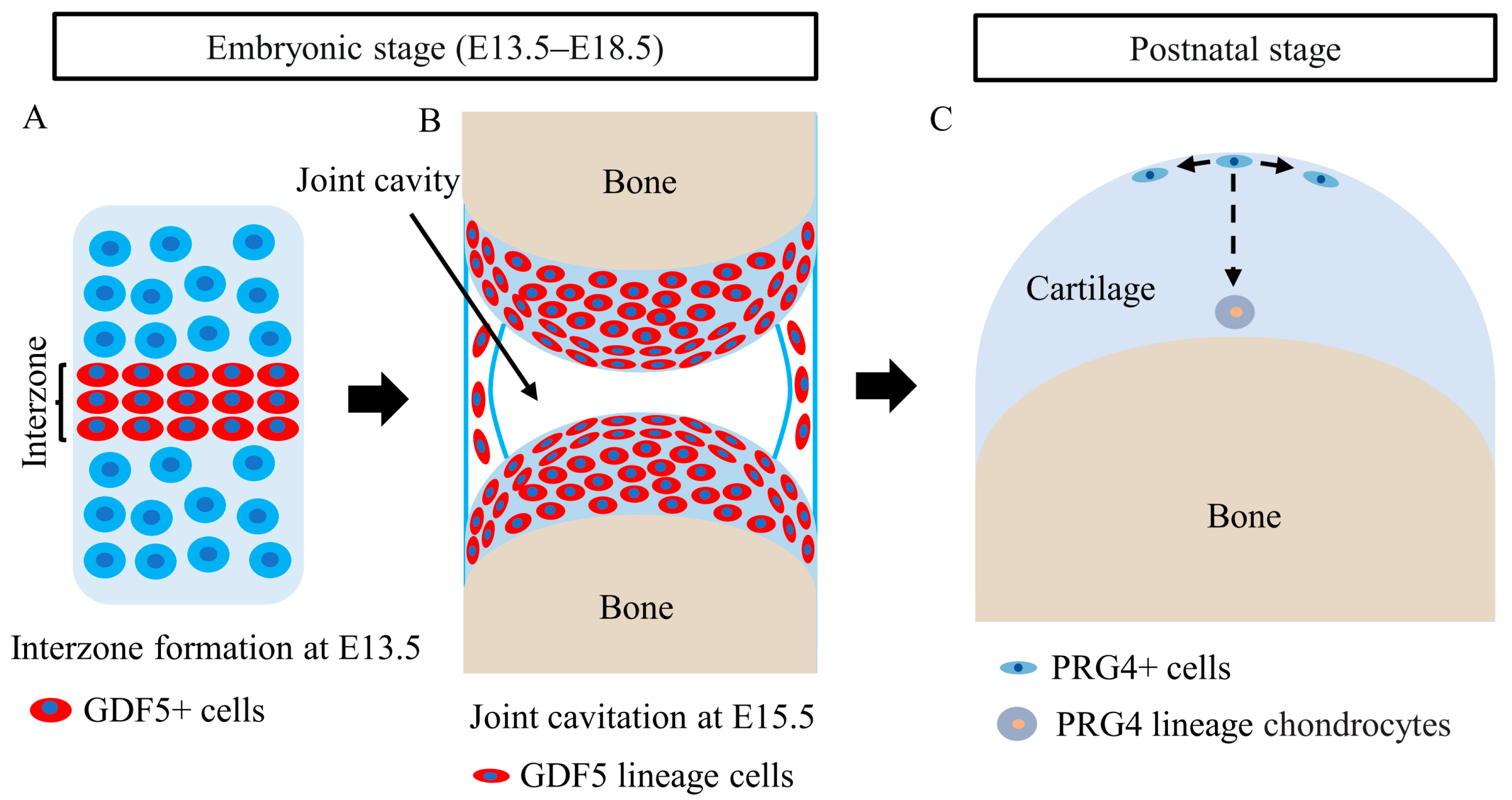
5. Roles of CSPCs in Articular Cartilage Development
6. Roles of CSPCs in Articular Cartilage Repair and OA
7. Signaling Pathways Regulating CSPCs during Articular Cartilage Development and OA
8. Application of CSPCs in Cartilage Regeneration and OA Therapies
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eschweiler, J.; Horn, N.; Rath, B.; Betsch, M.; Baroncini, A.; Tingart, M.; Migliorini, F. The Biomechanics of Cartilage—An Overview. Life 2021, 11, 302. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Sun, H.; Bunpetch, V.; Koh, Y.; Wen, Y.; Wu, D.; Ouyang, H. The regulation of cartilage extracellular matrix homeostasis in joint cartilage degeneration and regeneration. Biomaterials 2021, 268, 120555. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Liu, W.; Sun, C.; Wang, Q.; Yang, W.; Zhang, Z.; Xia, Z.; Shao, Z.; Wang, B. Endogenous Repair and Regeneration of Injured Articular Cartilage: A Challenging but Promising Therapeutic Strategy. Aging Dis. 2021, 12, 886–901. [Google Scholar] [CrossRef]
- Heir, S.; Nerhus, T.K.; Rotterud, J.H.; Loken, S.; Ekeland, A.; Engebretsen, L.; Aroen, A. Focal cartilage defects in the knee impair quality of life as much as severe osteoarthritis: A comparison of knee injury and osteoarthritis outcome score in 4 patient categories scheduled for knee surgery. Am. J. Sports Med. 2010, 38, 231–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Yu, S.; Lin, H.; Bi, R. Expression and function of cartilage-derived pluripotent cells in joint development and repair. Stem Cell Res. Ther. 2020, 11, 111. [Google Scholar] [CrossRef]
- Bunpetch, V.; Wu, H.; Zhang, S.; Ouyang, H. From “Bench to Bedside”: Current Advancement on Large-Scale Production of Mesenchymal Stem Cells. Stem Cells Dev. 2017, 26, 1662–1673. [Google Scholar] [CrossRef]
- Xue, K.; Zhang, X.; Gao, Z.; Xia, W.; Qi, L.; Liu, K. Cartilage progenitor cells combined with PHBV in cartilage tissue engineering. J. Transl. Med. 2019, 17, 104. [Google Scholar] [CrossRef] [Green Version]
- Urlic, I.; Ivkovic, A. Cell Sources for Cartilage Repair-Biological and Clinical Perspective. Cells 2021, 10, 2496. [Google Scholar] [CrossRef]
- Schnabel, M.; Marlovits, S.; Eckhoff, G.; Fichtel, I.; Gotzen, L.; Vecsei, V.; Schlegel, J. Dedifferentiation-associated changes in morphology and gene expression in primary human articular chondrocytes in cell culture. Osteoarthr. Cartil. 2002, 10, 62–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demoor, M.; Ollitrault, D.; Gomez-Leduc, T.; Bouyoucef, M.; Hervieu, M.; Fabre, H.; Lafont, J.; Denoix, J.M.; Audigie, F.; Mallein-Gerin, F.; et al. Cartilage tissue engineering: Molecular control of chondrocyte differentiation for proper cartilage matrix reconstruction. Biochim. Biophys. Acta 2014, 1840, 2414–2440. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.L.; Duchi, S.; Onofrillo, C.; Di Bella, C.; Choong, P.F.M. Adipose-Derived Mesenchymal Stem Cells in the Use of Cartilage Tissue Engineering: The Need for a Rapid Isolation Procedure. Stem Cells Int. 2018, 2018, 8947548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Gao, J.; Mi, L.; Zhang, G.; Zhang, L.; Zhang, N.; Huo, R.; Hu, J.; Xu, K. Synovial membrane mesenchymal stem cells: Past life, current situation, and application in bone and joint diseases. Stem Cell Res. Ther. 2020, 11, 381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Hu, B.; Liu, W.; Wang, P.; Lv, X.; Chen, S.; Liu, H.; Shao, Z. Articular cartilage regeneration: The role of endogenous mesenchymal stem/progenitor cell recruitment and migration. Semin. Arthritis Rheum. 2020, 50, 198–208. [Google Scholar] [CrossRef]
- Liu, Y.; Shah, K.M.; Luo, J. Strategies for Articular Cartilage Repair and Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 770655. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.N.; Zhu, S.Y.; He, H.C.; Yu, X.; Xu, Y.; He, C.Q. Mesenchymal stromal cell-based therapy for cartilage regeneration in knee osteoarthritis. Stem Cell Res. Ther. 2022, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, G.; Fox, J.; Ashton, B.; Middleton, J. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007, 25, 2739–2749. [Google Scholar] [CrossRef] [Green Version]
- do Amaral, R.; Almeida, H.V.; Kelly, D.J.; O’Brien, F.J.; Kearney, C.J. Infrapatellar Fat Pad Stem Cells: From Developmental Biology to Cell Therapy. Stem Cells Int. 2017, 2017, 6843727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, M.B.; Fischer, M.; Zellner, J.; Berner, A.; Dienstknecht, T.; Prantl, L.; Kujat, R.; Nerlich, M.; Tuan, R.S.; Angele, P. Hypertrophy in mesenchymal stem cell chondrogenesis: Effect of TGF-β isoforms and chondrogenic conditioning. Cells Tissues Organs 2010, 192, 158–166. [Google Scholar] [CrossRef] [Green Version]
- Gawlitta, D.; Farrell, E.; Malda, J.; Creemers, L.B.; Alblas, J.; Dhert, W.J. Modulating endochondral ossification of multipotent stromal cells for bone regeneration. Tissue Eng. Part B Rev. 2010, 16, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Kozhemyakina, E.; Zhang, M.; Ionescu, A.; Ayturk, U.M.; Ono, N.; Kobayashi, A.; Kronenberg, H.; Warman, M.L.; Lassar, A.B. Identification of a Prg4-expressing articular cartilage progenitor cell population in mice. Arthritis Rheumatol. 2015, 67, 1261–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decker, R.S.; Um, H.B.; Dyment, N.A.; Cottingham, N.; Usami, Y.; Enomoto-Iwamoto, M.; Kronenberg, M.S.; Maye, P.; Rowe, D.W.; Koyama, E.; et al. Cell origin, volume and arrangement are drivers of articular cartilage formation, morphogenesis and response to injury in mouse limbs. Dev. Biol. 2017, 426, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Newton, P.T.; Bouderlique, T.; Sejnohova, M.; Zikmund, T.; Kozhemyakina, E.; Xie, M.; Krivanek, J.; Kaiser, J.; Qian, H.; et al. Superficial cells are self-renewing chondrocyte progenitors, which form the articular cartilage in juvenile mice. FASEB J. 2017, 31, 1067–1084. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Tuan, R.S. Origin and function of cartilage stem/progenitor cells in osteoarthritis. Nat. Rev. Rheumatol. 2015, 11, 206–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGonagle, D.; Baboolal, T.G.; Jones, E. Native joint-resident mesenchymal stem cells for cartilage repair in osteoarthritis. Nat. Rev. Rheumatol. 2017, 13, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Kurenkova, A.D.; Medvedeva, E.V.; Newton, P.T.; Chagin, A.S. Niches for Skeletal Stem Cells of Mesenchymal Origin. Front Cell Dev. Biol. 2020, 8, 592. [Google Scholar] [CrossRef]
- Centeno, C.J. Clinical challenges and opportunities of mesenchymal stem cells in musculoskeletal medicine. PM&R 2014, 6, 70–77. [Google Scholar]
- Levato, R.; Webb, W.R.; Otto, I.A.; Mensinga, A.; Zhang, Y.; van Rijen, M.; van Weeren, R.; Khan, I.M.; Malda, J. The bio in the ink: Cartilage regeneration with bioprintable hydrogels and articular cartilage-derived progenitor cells. Acta Biomater. 2017, 61, 41–53. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, H.E.; Bara, J.J.; Brakspear, K.; Singhrao, S.K.; Archer, C.W. The comparison of equine articular cartilage progenitor cells and bone marrow-derived stromal cells as potential cell sources for cartilage repair in the horse. Vet. J. 2012, 192, 345–351. [Google Scholar] [CrossRef]
- Hayes, A.J.; MacPherson, S.; Morrison, H.; Dowthwaite, G.; Archer, C.W. The development of articular cartilage: Evidence for an appositional growth mechanism. Anat. Embryol. 2001, 203, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, E.B.; Kapfinger, E.; Geiss, J. The structural architecture of adult mammalian articular cartilage evolves by a synchronized process of tissue resorption and neoformation during postnatal development. Osteoarthr. Cartil. 2007, 15, 403–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowthwaite, G.P.; Bishop, J.C.; Redman, S.N.; Khan, I.M.; Rooney, P.; Evans, D.J.; Haughton, L.; Bayram, Z.; Boyer, S.; Thomson, B.; et al. The surface of articular cartilage contains a progenitor cell population. J. Cell Sci. 2004, 117, 889–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, T.; Li, Y.; Gui, C.; Ma, Y.; Ge, Y.; Dai, H.; Zhang, K.; Du, J.; Guo, Y.; Jiang, Y.; et al. Fibronectin Enhances Cartilage Repair by Activating Progenitor Cells Through Integrin alpha5beta1 Receptor. Tissue Eng. Part A 2018, 24, 1112–1124. [Google Scholar] [CrossRef]
- Tong, W.; Geng, Y.; Huang, Y.; Shi, Y.; Xiang, S.; Zhang, N.; Qin, L.; Shi, Q.; Chen, Q.; Dai, K.; et al. In Vivo Identification and Induction of Articular Cartilage Stem Cells by Inhibiting NF-kappaB Signaling in Osteoarthritis. Stem Cells 2015, 33, 3125–3137. [Google Scholar] [CrossRef] [Green Version]
- He, R.; Wang, B.; Cui, M.; Xiong, Z.; Lin, H.; Zhao, L.; Li, Z.; Wang, Z.; Peggrem, S.; Xia, Z.; et al. Link Protein N-Terminal Peptide as a Potential Stimulating Factor for Stem Cell-Based Cartilage Regeneration. Stem Cells Int. 2018, 2018, 3217895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Z.; Hong, M.; Xu, L.; Yang, K.; Li, C.; Sun, T.; Feng, Y.; Zeng, H.; Lu, W.W.; Chiu, K.Y. Prevent action of magnoflorine with hyaluronic acid gel from cartilage degeneration in anterior cruciate ligament transection induced osteoarthritis. Biomed. Pharmacother. 2020, 126, 109733. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, J.; Yang, X.; Jiang, Y.; Gui, J. Intermittent hydrostatic pressure maintains and enhances the chondrogenic differentiation of cartilage progenitor cells cultivated in alginate beads. Dev. Growth Differ. 2016, 58, 180–193. [Google Scholar] [CrossRef]
- Khan, I.M.; Bishop, J.C.; Gilbert, S.; Archer, C.W. Clonal chondroprogenitors maintain telomerase activity and Sox9 expression during extended monolayer culture and retain chondrogenic potential. Osteoarthr. Cartil. 2009, 17, 518–528. [Google Scholar] [CrossRef] [Green Version]
- Marcus, P.; De Bari, C.; Dell’Accio, F.; Archer, C.W. Articular Chondroprogenitor Cells Maintain Chondrogenic Potential but Fail to Form a Functional Matrix When Implanted into Muscles of SCID Mice. Cartilage 2014, 5, 231–240. [Google Scholar]
- Li, L.; Ma, Y.; Li, X.; Li, X.; Bai, C.; Ji, M.; Zhang, S.; Guan, W.; Li, J. Isolation, Culture, and Characterization of Chicken Cartilage Stem/Progenitor Cells. BioMed Res. Int. 2015, 2015, 586290. [Google Scholar] [CrossRef] [Green Version]
- Williams, R.; Khan, I.M.; Richardson, K.; Nelson, L.; McCarthy, H.E.; Analbelsi, T.; Singhrao, S.K.; Dowthwaite, G.P.; Jones, R.E.; Baird, D.M.; et al. Identification and clonal characterisation of a progenitor cell sub-population in normal human articular cartilage. PLoS ONE 2010, 5, e13246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ustunel, I.; Ozenci, A.M.; Sahin, Z.; Ozbey, O.; Acar, N.; Tanriover, G.; Celik-Ozenci, C.; Demir, R. The immunohistochemical localization of notch receptors and ligands in human articular cartilage, chondroprogenitor culture and ultrastructural characteristics of these progenitor cells. Acta Histochem. 2008, 110, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Vinod, E.; Kachroo, U.; Ozbey, O.; Sathishkumar, S.; Boopalan, P. Comparison of human articular chondrocyte and chondroprogenitor cocultures and monocultures: To assess chondrogenic potential and markers of hypertrophy. Tissue Cell 2019, 57, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Vinod, E.; Kachroo, U.; Rebekah, G.; Yadav, B.K.; Ramasamy, B. Characterization of human articular chondrocytes and chondroprogenitors derived from non-diseased and osteoarthritic knee joints to assess superiority for cell-based therapy. Acta Histochem. 2020, 122, 151588. [Google Scholar] [CrossRef] [PubMed]
- Grogan, S.P.; Miyaki, S.; Asahara, H.; D’Lima, D.D.; Lotz, M.K. Mesenchymal progenitor cell markers in human articular cartilage: Normal distribution and changes in osteoarthritis. Arthritis Res. Ther. 2009, 11, R85. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Zheng, H.; Buckwalter, J.A.; Martin, J.A. Single cell sorting identifies progenitor cell population from full thickness bovine articular cartilage. Osteoarthr. Cartil. 2014, 22, 1318–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Cai, Y.; Zhang, W.; Yin, Z.; Hu, C.; Tong, T.; Lu, P.; Zhang, S.; Neculai, D.; Tuan, R.S.; et al. Human Cartilage-Derived Progenitor Cells from Committed Chondrocytes for Efficient Cartilage Repair and Regeneration. Stem Cells Transl. Med. 2016, 5, 733–744. [Google Scholar] [CrossRef]
- Zhang, K.; Shi, J.; Li, Y.; Jiang, Y.; Tao, T.; Li, W.; Gui, J. Chondrogenic cells respond to partial-thickness defects of articular cartilage in adult rats: An in vivo study. J. Mol. Histol. 2016, 47, 249–258. [Google Scholar] [CrossRef]
- Alsalameh, S.; Amin, R.; Gemba, T.; Lotz, M. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 2004, 50, 1522–1532. [Google Scholar] [CrossRef] [PubMed]
- Pretzel, D.; Linss, S.; Rochler, S.; Endres, M.; Kaps, C.; Alsalameh, S.; Kinne, R.W. Relative percentage and zonal distribution of mesenchymal progenitor cells in human osteoarthritic and normal cartilage. Arthritis Res. Ther. 2011, 13, R64. [Google Scholar] [CrossRef] [Green Version]
- Su, X.; Zuo, W.; Wu, Z.; Chen, J.; Wu, N.; Ma, P.; Xia, Z.; Jiang, C.; Ye, Z.; Liu, S.; et al. CD146 as a new marker for an increased chondroprogenitor cell sub-population in the later stages of osteoarthritis. J. Orthop. Res. 2015, 33, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Zhao, Z.D.; Wang, Q.; Li, Z.L.; Huang, Y.; Zhao, S.; Hu, W.; Liang, J.W.; Li, P.L.; Wang, H.; et al. Biological potential alterations of migratory chondrogenic progenitor cells during knee osteoarthritic progression. Arthritis Res. Ther. 2020, 22, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fickert, S.; Fiedler, J.; Brenner, R.E. Identification of subpopulations with characteristics of mesenchymal progenitor cells from human osteoarthritic cartilage using triple staining for cell surface markers. Arthritis Res. Ther. 2004, 6, R422–R432. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.; Yang, L.; Chang, H.; Dai, G.; Wang, F.; Duan, X.; Guo, L.; Zhang, Y.; Chen, G. Wnt/beta-catenin signaling regulates the proliferation and differentiation of mesenchymal progenitor cells through the p53 pathway. PLoS ONE 2014, 9, e97283. [Google Scholar]
- Xia, Z.; Ma, P.; Wu, N.; Su, X.; Chen, J.; Jiang, C.; Liu, S.; Chen, W.; Ma, B.; Yang, X.; et al. Altered function in cartilage derived mesenchymal stem cell leads to OA-related cartilage erosion. Am. J. Transl. Res. 2016, 8, 433–446. [Google Scholar] [PubMed]
- Kachroo, U.; Ramasamy, B.; Vinod, E. Evaluation of CD49e as a distinguishing marker for human articular cartilage derived chondroprogenitors. Knee 2020, 27, 833–837. [Google Scholar] [CrossRef]
- De Luca, P.; Kouroupis, D.; Vigano, M.; Perucca-Orfei, C.; Kaplan, L.; Zagra, L.; de Girolamo, L.; Correa, D.; Colombini, A. Human Diseased Articular Cartilage Contains a Mesenchymal Stem Cell-Like Population of Chondroprogenitors with Strong Immunomodulatory Responses. J. Clin. Med. 2019, 8, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schminke, B.; Frese, J.; Bode, C.; Goldring, M.B.; Miosge, N. Laminins and Nidogens in the Pericellular Matrix of Chondrocytes: Their Role in Osteoarthritis and Chondrogenic Differentiation. Am. J. Pathol. 2016, 186, 410–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rikkers, M.; Korpershoek, J.V.; Levato, R.; Malda, J.; Vonk, L.A. The clinical potential of articular cartilage-derived progenitor cells: A systematic review. NPJ Regen. Med. 2022, 7, 2. [Google Scholar] [CrossRef]
- Carluccio, S.; Martinelli, D.; Palama, M.E.F.; Pereira, R.C.; Benelli, R.; Guijarro, A.; Cancedda, R.; Gentili, C. Progenitor Cells Activated by Platelet Lysate in Human Articular Cartilage as a Tool for Future Cartilage Engineering and Reparative Strategies. Cells 2020, 9, 3390. [Google Scholar] [CrossRef] [Green Version]
- Fellows, C.R.; Williams, R.; Davies, I.R.; Gohil, K.; Baird, D.M.; Fairclough, J.; Rooney, P.; Archer, C.W.; Khan, I.M. Characterisation of a divergent progenitor cell sub-populations in human osteoarthritic cartilage: The role of telomere erosion and replicative senescence. Sci. Rep. 2017, 7, 41421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, L.; McCarthy, H.E.; Fairclough, J.; Williams, R.; Archer, C.W. Evidence of a Viable Pool of Stem Cells within Human Osteoarthritic Cartilage. Cartilage 2014, 5, 203–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, A.C.; Spector, M. Distribution of chondrocytes containing alpha-smooth muscle actin in human articular cartilage. J. Orthop. Res. 2000, 18, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Koelling, S.; Kruegel, J.; Irmer, M.; Path, J.R.; Sadowski, B.; Miro, X.; Miosge, N. Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell 2009, 4, 324–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seol, D.; McCabe, D.J.; Choe, H.; Zheng, H.; Yu, Y.; Jang, K.; Walter, M.W.; Lehman, A.D.; Ding, L.; Buckwalter, J.A.; et al. Chondrogenic progenitor cells respond to cartilage injury. Arthritis Rheum. 2012, 64, 3626–3637. [Google Scholar] [CrossRef] [Green Version]
- Joos, H.; Wildner, A.; Hogrefe, C.; Reichel, H.; Brenner, R.E. Interleukin-1 beta and tumor necrosis factor alpha inhibit migration activity of chondrogenic progenitor cells from non-fibrillated osteoarthritic cartilage. Arthritis Res. Ther. 2013, 15, R119. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.C.; Lin, T.H.; Hsu, C.C.; Yeh, M.L. Restoring Osteochondral Defects through the Differentiation Potential of Cartilage Stem/Progenitor Cells Cultivated on Porous Scaffolds. Cells 2021, 10, 3536. [Google Scholar] [CrossRef] [PubMed]
- Chijimatsu, R.; Saito, T. Mechanisms of synovial joint and articular cartilage development. Cell Mol. Life Sci. 2019, 76, 3939–3952. [Google Scholar] [CrossRef]
- Koyama, E.; Shibukawa, Y.; Nagayama, M.; Sugito, H.; Young, B.; Yuasa, T.; Okabe, T.; Ochiai, T.; Kamiya, N.; Rountree, R.B.; et al. A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis. Dev. Biol. 2008, 316, 62–73. [Google Scholar] [CrossRef] [Green Version]
- Shwartz, Y.; Viukov, S.; Krief, S.; Zelzer, E. Joint Development Involves a Continuous Influx of Gdf5-Positive Cells. Cell Rep. 2016, 15, 2577–2587. [Google Scholar] [CrossRef] [Green Version]
- Feng, C.; Chan, W.C.W.; Lam, Y.; Wang, X.; Chen, P.; Niu, B.; Ng, V.C.W.; Yeo, J.C.; Stricker, S.; Cheah, K.S.E.; et al. Lgr5 and Col22a1 Mark Progenitor Cells in the Lineage toward Juvenile Articular Chondrocytes. Stem Cell Rep. 2019, 13, 713–729. [Google Scholar] [CrossRef]
- Rhee, D.K.; Marcelino, J.; Baker, M.; Gong, Y.; Smits, P.; Lefebvre, V.; Jay, G.D.; Stewart, M.; Wang, H.; Warman, M.L.; et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J. Clin. Investig. 2005, 115, 622–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chagin, A.S.; Medvedeva, E.V. Regenerative medicine: Cartilage stem cells identified, but can they heal? Nat. Rev. Rheumatol. 2017, 13, 522–524. [Google Scholar] [CrossRef]
- Garcia-Arnandis, I.; Guillen, M.I.; Castejon, M.A.; Gomar, F.; Alcaraz, M.J. Haem oxygenase-1 down-regulates high mobility group box 1 and matrix metalloproteinases in osteoarthritic synoviocytes. Rheumatology 2010, 49, 854–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishima, Y.; Lotz, M. Chemotaxis of human articular chondrocytes and mesenchymal stem cells. J. Orthop. Res. 2008, 26, 1407–1412. [Google Scholar] [CrossRef]
- Beekhuizen, M.; Gierman, L.M.; van Spil, W.E.; Van Osch, G.J.; Huizinga, T.W.; Saris, D.B.; Creemers, L.B.; Zuurmond, A.M. An explorative study comparing levels of soluble mediators in control and osteoarthritic synovial fluid. Osteoarthr. Cartil. 2013, 21, 918–922. [Google Scholar] [CrossRef] [Green Version]
- Riegger, J.; Palm, H.G.; Brenner, R.E. The functional role of chondrogenic stem/progenitor cells: Novel evidence for immunomodulatory properties and regenerative potential after cartilage injury. Eur. Cell Mater. 2018, 36, 110–127. [Google Scholar] [CrossRef] [PubMed]
- Lotz, M.K.; Otsuki, S.; Grogan, S.P.; Sah, R.; Terkeltaub, R.; D’Lima, D. Cartilage cell clusters. Arthritis Rheum. 2010, 62, 2206–2218. [Google Scholar] [CrossRef] [Green Version]
- Roelofs, A.J.; Zupan, J.; Riemen, A.H.; Kania, K.; Ansboro, S.; White, N.; Clark, S.M.; De Bari, C. Joint morphogenetic cells in the adult mammalian synovium. Nat. Commun. 2017, 8, 15040. [Google Scholar] [CrossRef] [Green Version]
- Saito, T. The superficial zone of articular cartilage. Inflamm. Regen. 2022, 42, 14. [Google Scholar] [CrossRef]
- Maenohara, Y.; Chijimatsu, R.; Tachibana, N.; Uehara, K.; Xuan, F.; Mori, D.; Murahashi, Y.; Nakamoto, H.; Oichi, T.; Chang, S.H.; et al. Lubricin Contributes to Homeostasis of Articular Cartilage by Modulating Differentiation of Superficial Zone Cells. J. Bone Miner. Res. 2021, 36, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, N.; Carames, B.; Ronfani, L.; Ulmer, U.; Komiya, S.; Bianchi, M.E.; Lotz, M. Aging-related loss of the chromatin protein HMGB2 in articular cartilage is linked to reduced cellularity and osteoarthritis. Proc. Natl. Acad. Sci. USA 2009, 106, 1181–1186. [Google Scholar] [CrossRef] [Green Version]
- Xuan, F.; Yano, F.; Mori, D.; Chijimatsu, R.; Maenohara, Y.; Nakamoto, H.; Mori, Y.; Makii, Y.; Oichi, T.; Taketo, M.M.; et al. Wnt/beta-catenin signaling contributes to articular cartilage homeostasis through lubricin induction in the superficial zone. Arthritis Res. Ther. 2019, 21, 247. [Google Scholar] [CrossRef] [Green Version]
- Tan, Q.Y.; Wang, Q.; Kuang, L.; Zhang, J.; Peng, X.; Liang, S.; Liu, M.; Chen, H.; Chen, S.; Luo, X.; et al. TGF-β/Alk5 signaling prevents osteoarthritis initiation via regulating the senescence of articular cartilage stem cells. J. Cell Physiol. 2021, 236, 5278–5292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Mani, S.B.; He, Y.; Hall, A.M.; Xu, L.; Li, Y.; Zurakowski, D.; Jay, G.D.; Warman, M.L. Induced superficial chondrocyte death reduces catabolic cartilage damage in murine posttraumatic osteoarthritis. J. Clin. Investig. 2016, 126, 2893–2902. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, W.; Wu, W.; Chen, M.; Ji, T.; Xu, H.; Wang, Y. Pin1-mediated regulation of articular cartilage stem/progenitor cell aging. Tissue Cell 2022, 76, 101765. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara, R.; Ohta, Y.; Yuasa, T.; Kondo, N.; Hoang, T.; Addya, S.; Fortina, P.; Pacifici, M.; Iwamoto, M.; Enomoto-Iwamoto, M. Roles of β-catenin signaling in phenotypic expression and proliferation of articular cartilage superficial zone cells. Lab. Investig. 2011, 91, 1739–1752. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.; Ma, X.; Tong, W.; Doyran, B.; Sun, Z.; Wang, L.; Zhang, X.; Zhou, Y.; Badar, F.; Chandra, A.; et al. EGFR signaling is critical for maintaining the superficial layer of articular cartilage and preventing osteoarthritis initiation. Proc. Natl. Acad. Sci. USA 2016, 113, 14360–14365. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Luo, L.; Gui, T.; Yu, F.; Yan, L.; Yao, L.; Zhong, L.; Yu, W.; Han, B.; Patel, J.M.; et al. Targeting cartilage EGFR pathway for osteoarthritis treatment. Sci. Transl. Med. 2021, 13, eabb3946. [Google Scholar] [CrossRef]
- Wang, Q.; Tan, Q.Y.; Xu, W.; Qi, H.B.; Chen, D.; Zhou, S.; Ni, Z.H.; Kuang, L.; Guo, J.Y.; Huang, J.L.; et al. Cartilage-specific deletion of Alk5 gene results in a progressive osteoarthritis-like phenotype in mice. Osteoarthr. Cartil. 2017, 25, 1868–1879. [Google Scholar] [CrossRef] [Green Version]
- Matsuzaki, T.; Alvarez-Garcia, O.; Mokuda, S.; Nagira, K.; Olmer, M.; Gamini, R.; Miyata, K.; Akasaki, Y.; Su, A.I.; Asahara, H.; et al. FoxO transcription factors modulate autophagy and proteoglycan 4 in cartilage homeostasis and osteoarthritis. Sci. Transl. Med. 2018, 10, eaan0746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.H.; Gao, Y.; Jadhav, U.; Hung, H.H.; Holton, K.M.; Grodzinsky, A.J.; Shivdasani, R.A.; Lassar, A.B. Creb5 establishes the competence for Prg4 expression in articular cartilage. Commun. Biol. 2021, 4, 332. [Google Scholar] [CrossRef]
- Delve, E.; Co, V.; Regmi, S.C.; Parreno, J.; Schmidt, T.A.; Kandel, R.A. YAP/TAZ regulates the expression of proteoglycan 4 and tenascin C in superficial-zone chondrocytes. Eur. Cell Mater. 2020, 39, 48–64. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, L.; Chen, Y.; Xiong, Z.; Li, J.; Xu, P.; Pan, Z.; Zhang, H.; Chen, Z.; Xue, K.; et al. The in vivo chondrogenesis of cartilage stem/progenitor cells from auricular cartilage and the perichondrium. Am. J. Transl. Res. 2019, 11, 2855–2865. [Google Scholar]
- Xue, K.; Jiang, Y.; Zhang, X.; Wu, J.; Qi, L.; Liu, K. Hypoxic ADSCs-derived EVs promote the proliferation and chondrogenic differentiation of cartilage stem/progenitor cells. Adipocyte 2021, 10, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.J.; Gardner, O.F.; Williams, R.; Alini, M.; Archer, C.W.; Stoddart, M.J. Human Articular Cartilage Progenitor Cells Are Responsive to Mechanical Stimulation and Adenoviral-Mediated Overexpression of Bone-Morphogenetic Protein 2. PLoS ONE 2015, 10, e0136229. [Google Scholar] [CrossRef] [PubMed]
- Mancini, I.A.D.; Schmidt, S.; Brommer, H.; Pouran, B.; Schäfer, S.; Tessmar, J.; Mensinga, A.; van Rijen, M.H.P.; Groll, J.; Blunk, T.; et al. A composite hydrogel-3D printed thermoplast osteochondral anchor as example for a zonal approach to cartilage repair: In vivo performance in a long-term equine model. Biofabrication 2020, 12, 035028. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Pan, P.; Qin, L.; Wang, X.; Chen, X.; Deng, Y.; Zhang, X. Nanoengineered hydrogels as 3D biomimetic extracellular matrix with injectable and sustained delivery capability for cartilage regeneration. Bioact. Mater. 2023, 19, 487–498. [Google Scholar] [CrossRef]
- Jay, G.D.; Elsaid, K.A.; Kelly, K.A.; Anderson, S.C.; Zhang, L.; Teeple, E.; Waller, K.; Fleming, B.C. Prevention of cartilage degeneration and gait asymmetry by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis Rheum. 2012, 64, 1162–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Hu, P.; Liu, T.; Li, Z.; Huang, Y.; Liao, J.; Hamid, M.R.; Wen, L.; Wang, T.; Mo, C.; et al. Kartogenin hydrolysis product 4-aminobiphenyl distributes to cartilage and mediates cartilage regeneration. Theranostics 2019, 9, 7108–7121. [Google Scholar] [CrossRef]
- Afewerki, S.; Sheikhi, A.; Kannan, S.; Ahadian, S.; Khademhosseini, A. Gelatin-polysaccharide composite scaffolds for 3D cell culture and tissue engineering: Towards natural therapeutics. Bioeng. Transl. Med. 2019, 4, 96–115. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, V.; Tsianaka, A.; Alessio, N.; Grubel, J.; Cammarota, M.; Tovar, G.E.M.; Southan, A.; Schiraldi, C. Evaluation of novel biomaterials for cartilage regeneration based on gelatin methacryloyl interpenetrated with extractive chondroitin sulfate or unsulfated biotechnological chondroitin. J. Biomed. Mater. Res. A 2022, 110, 1210–1223. [Google Scholar] [CrossRef] [PubMed]
- Stellavato, A.; Tirino, V.; de Novellis, F.; Della Vecchia, A.; Cinquegrani, F.; De Rosa, M.; Papaccio, G.; Schiraldi, C. Biotechnological Chondroitin a Novel Glycosamminoglycan with Remarkable Biological Function on Human Primary Chondrocytes. J. Cell Biochem. 2016, 117, 2158–2169. [Google Scholar] [CrossRef] [Green Version]
- Russo, R.; Vassallo, V.; Stellavato, A.; Valletta, M.; Cimini, D.; Pedone, P.V.; Schiraldi, C.; Chambery, A. Differential Secretome Profiling of Human Osteoarthritic Synoviocytes Treated with Biotechnological Unsulfated and Marine Sulfated Chondroitins. Int. J. Mol. Sci. 2020, 21, 3746. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Wang, W.; Liu, D.; Liao, D. Roles of Cartilage-Resident Stem/Progenitor Cells in Cartilage Physiology, Development, Repair and Osteoarthritis. Cells 2022, 11, 2305. https://doi.org/10.3390/cells11152305
Xu W, Wang W, Liu D, Liao D. Roles of Cartilage-Resident Stem/Progenitor Cells in Cartilage Physiology, Development, Repair and Osteoarthritis. Cells. 2022; 11(15):2305. https://doi.org/10.3390/cells11152305
Chicago/Turabian StyleXu, Wei, Wei Wang, Da Liu, and Dongfa Liao. 2022. "Roles of Cartilage-Resident Stem/Progenitor Cells in Cartilage Physiology, Development, Repair and Osteoarthritis" Cells 11, no. 15: 2305. https://doi.org/10.3390/cells11152305
APA StyleXu, W., Wang, W., Liu, D., & Liao, D. (2022). Roles of Cartilage-Resident Stem/Progenitor Cells in Cartilage Physiology, Development, Repair and Osteoarthritis. Cells, 11(15), 2305. https://doi.org/10.3390/cells11152305





