The Synergistic Effect of Cyclic Tensile Force and Periodontal Ligament Cell-Laden Calcium Silicate/Gelatin Methacrylate Auxetic Hydrogel Scaffolds for Bone Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Photo-Polymerizable FGelMa
2.2. Synthesis of Calcium Silicate (CS) Powder
2.3. Preparation of CS/FGelMa Bioink
2.4. Characterization of CS/FGelMa Scaffold
2.5. In Vitro Degradation Behaviour of CS/FGelMa Scaffold
2.6. Cell Culture
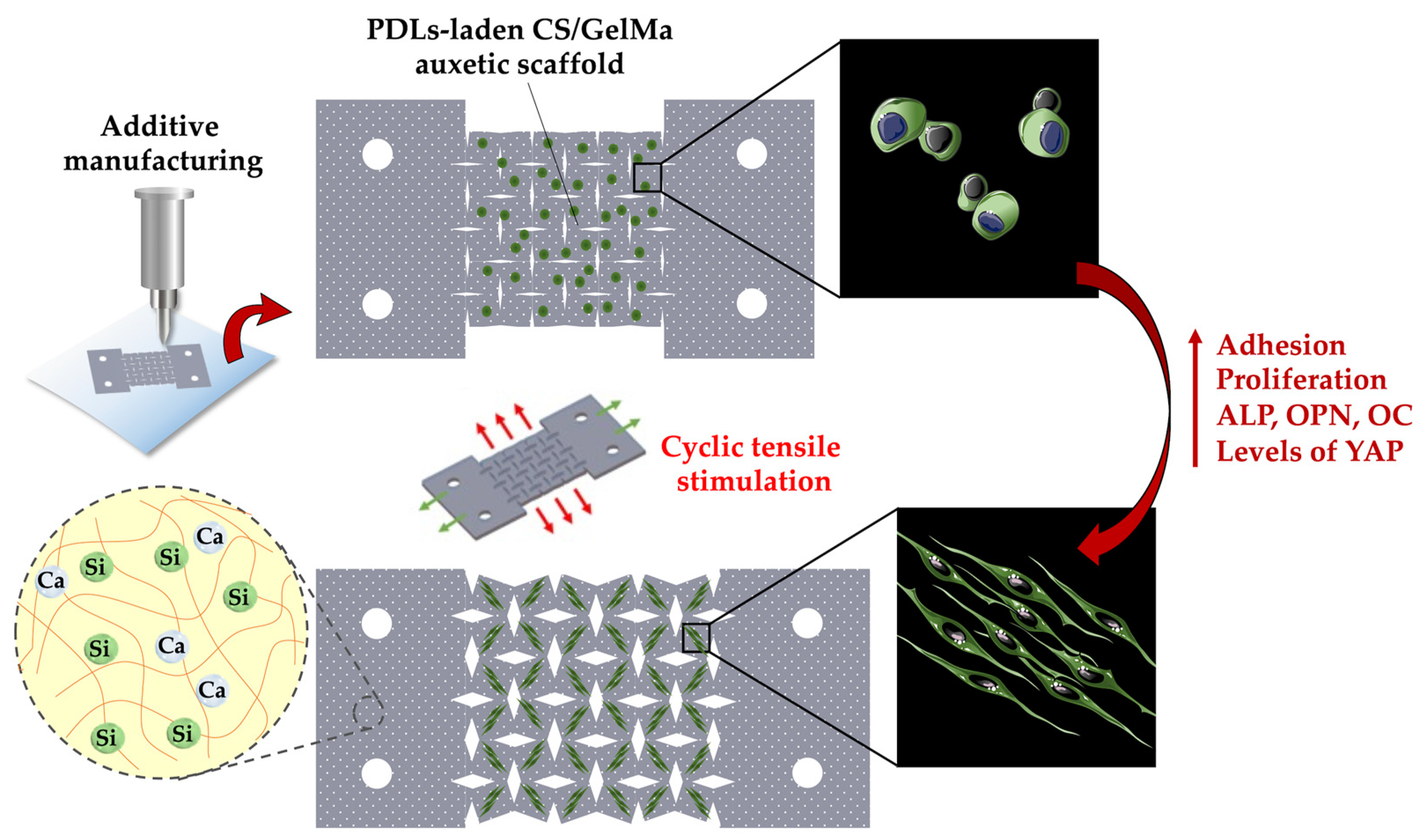
2.7. Fabrication of hPDLs-Laden CS/FGelMa Auxetic Scaffolds and Cyclic Tensile Stimulation
2.8. Cell Viability and Morphology
2.9. Immunofluorescence Staining
2.10. Western Blot
2.11. Osteogenic Markers
2.12. Inhibition of YAP
2.13. Statistical Analyses
3. Results and Discussion
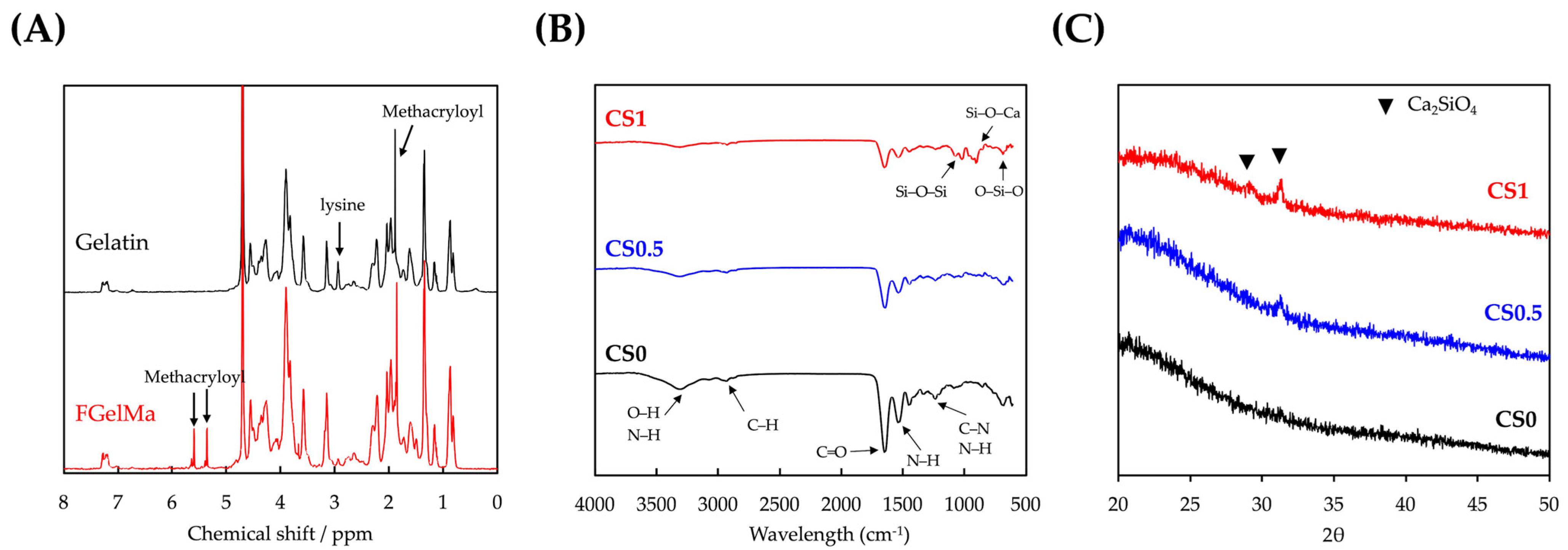
3.1. Characterizations of CS/FGelMa Scaffold
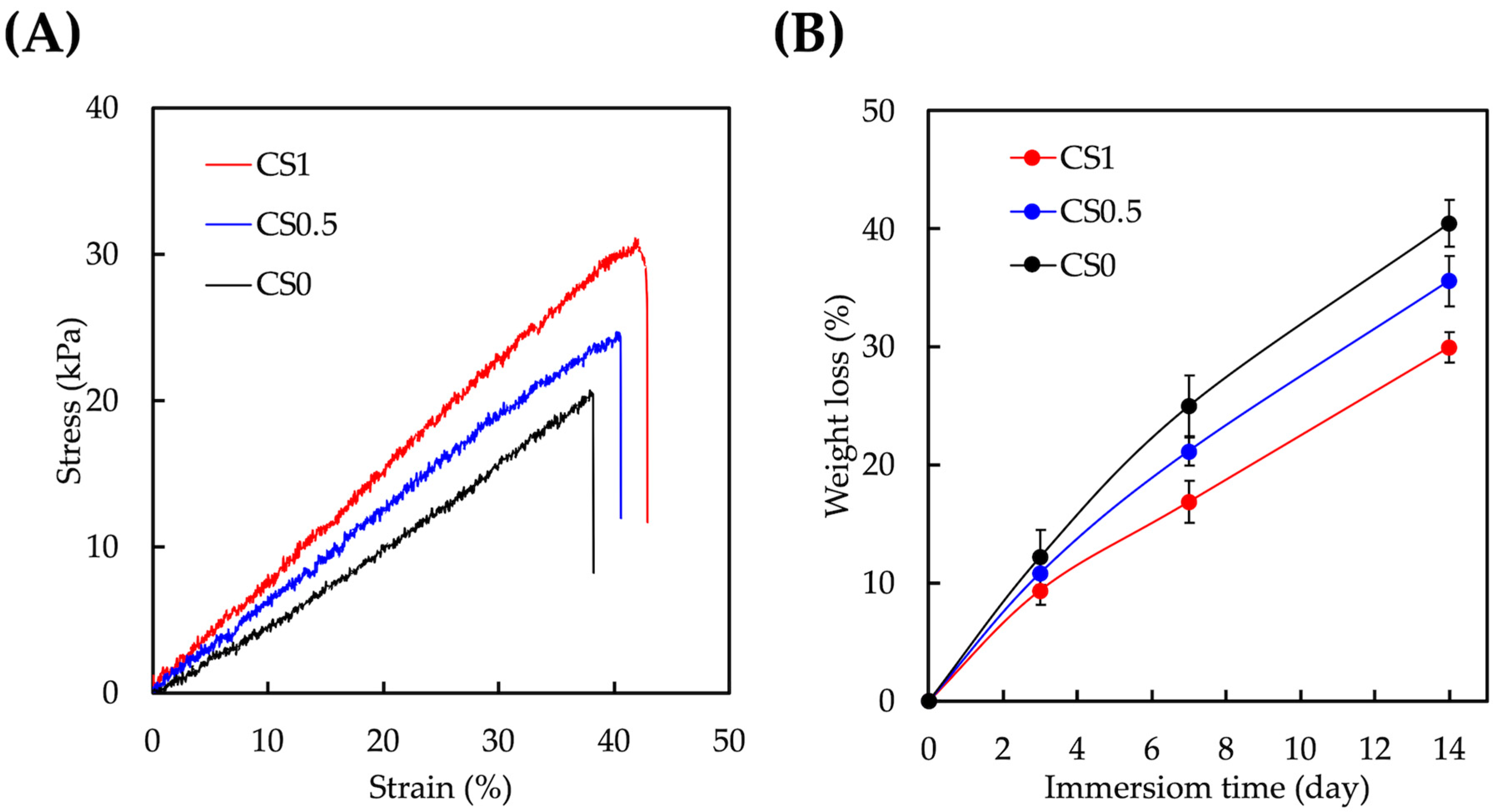
3.2. Mechanical and Degradation Properties of CS/FGelMa Scaffold
3.3. Cell Proliferation and Morphology
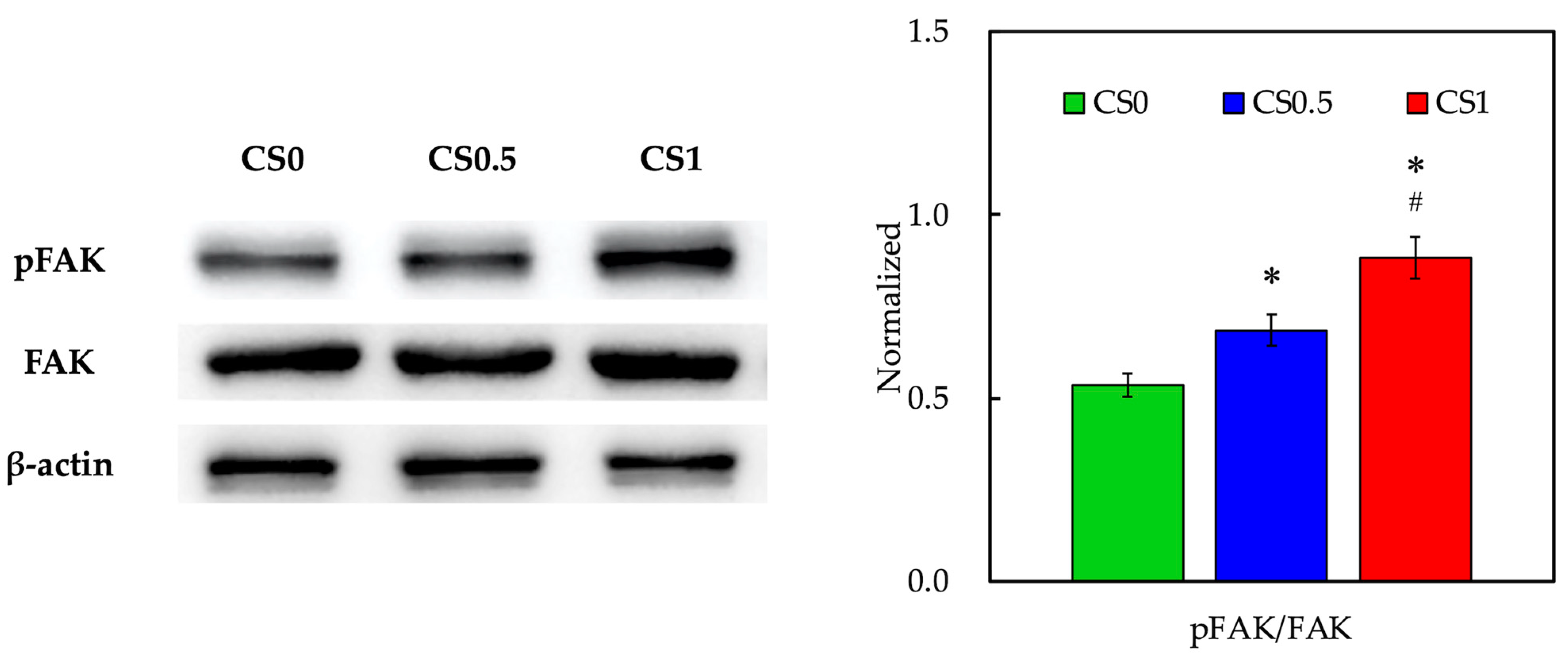
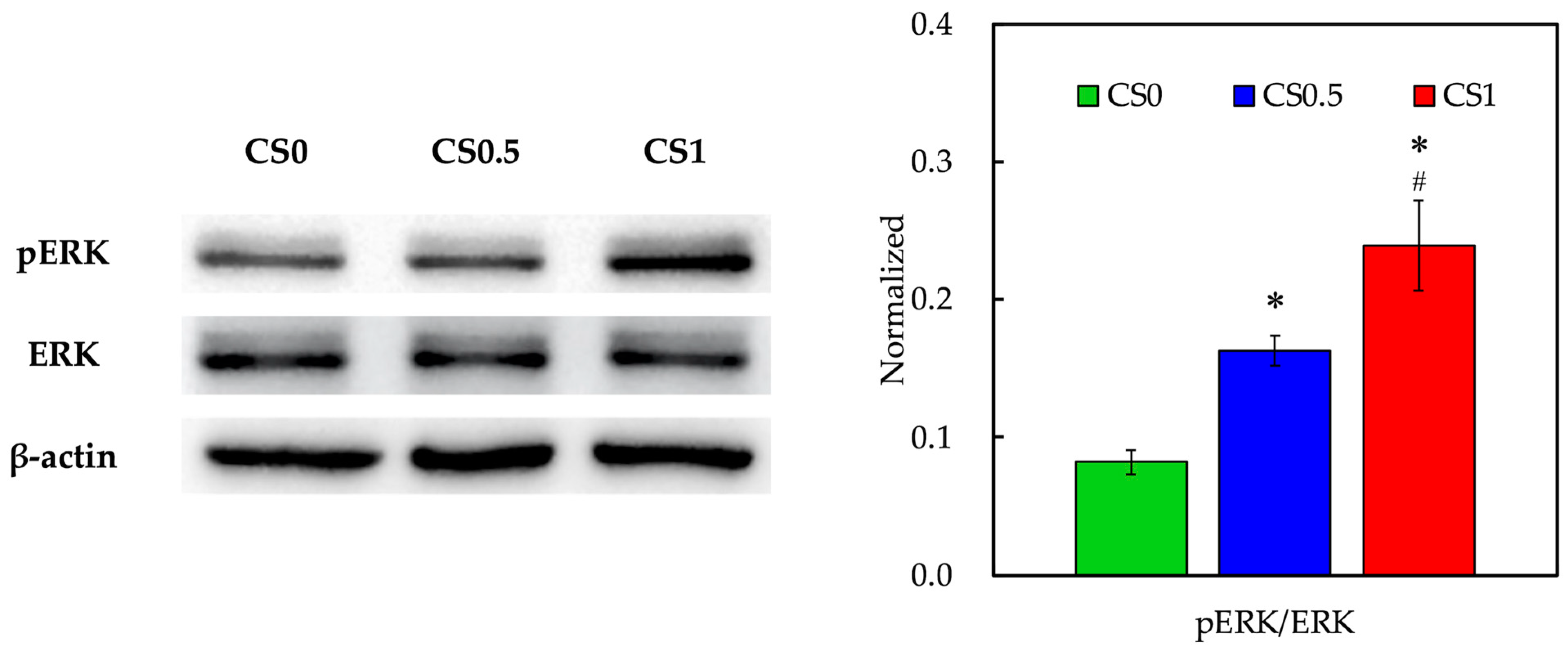
3.4. Biomarker of Adhesion and Proliferation-Related Proteins
3.5. Effect of Cyclic Tensile Stimulation on Cell Proliferation and Morphology
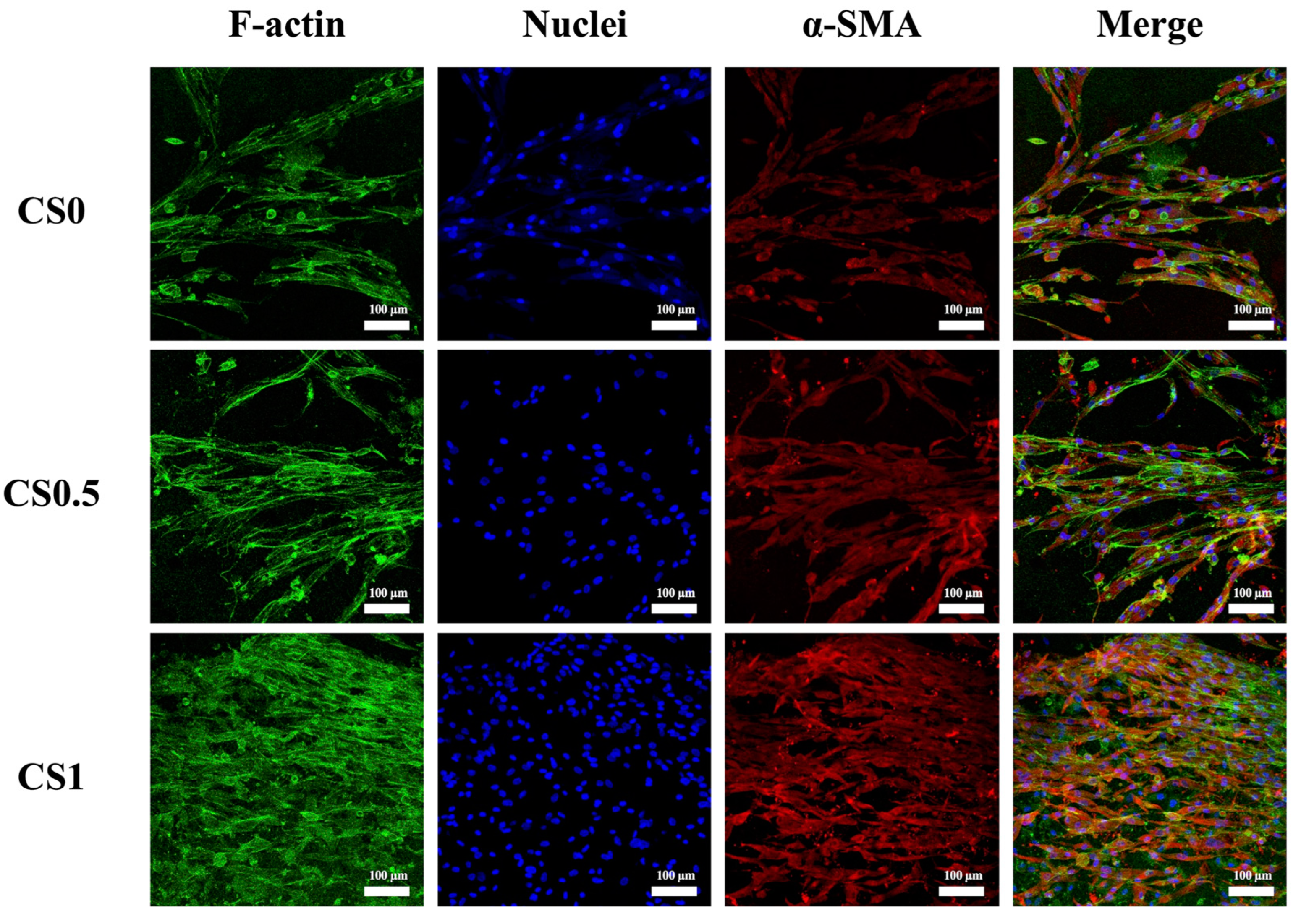
3.6. Effect of Cyclic Tensile Stimulation on α-SMA Expression
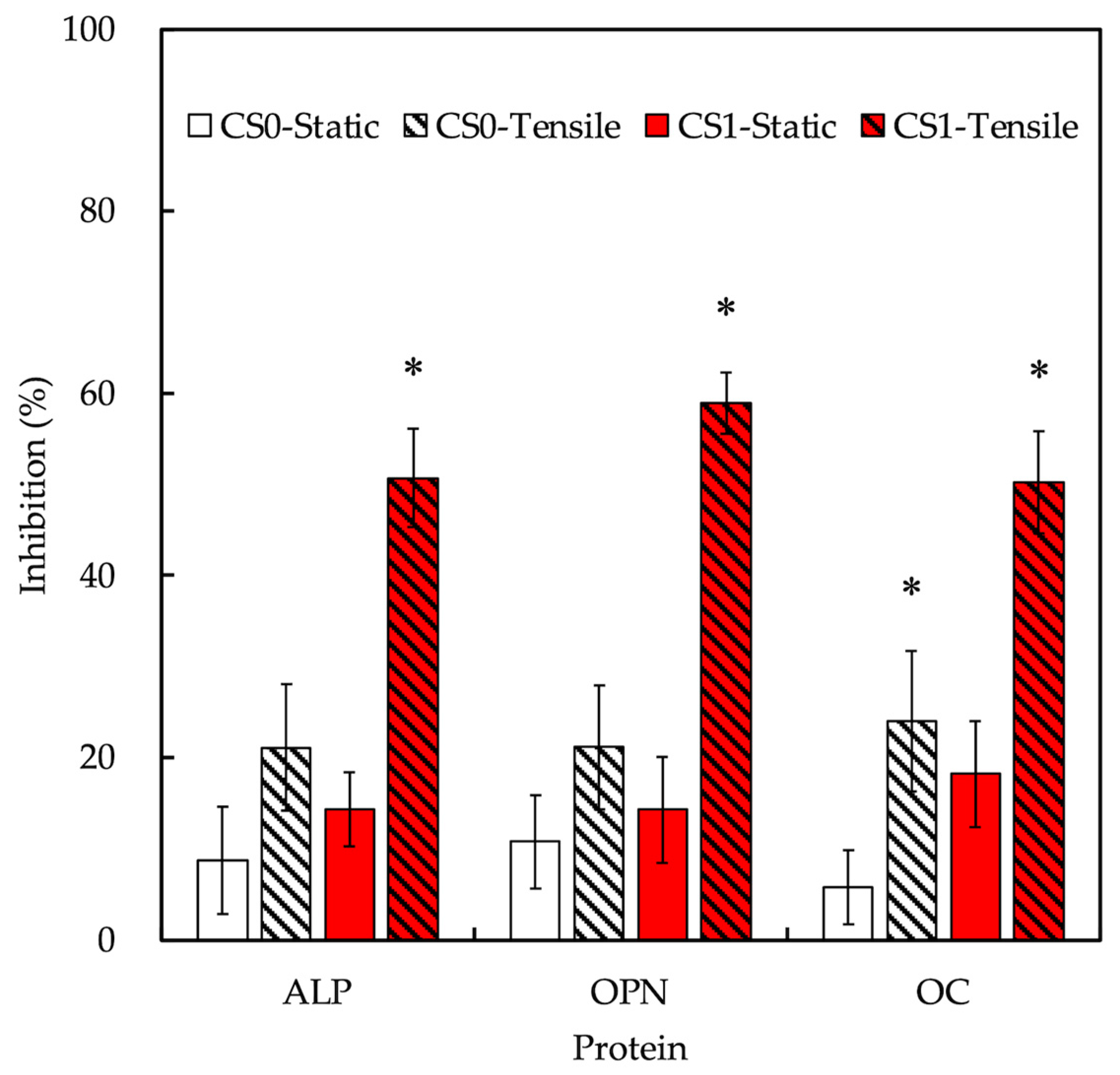
3.7. Effect of Cyclic Tensile Stimulation on Osteogenesis
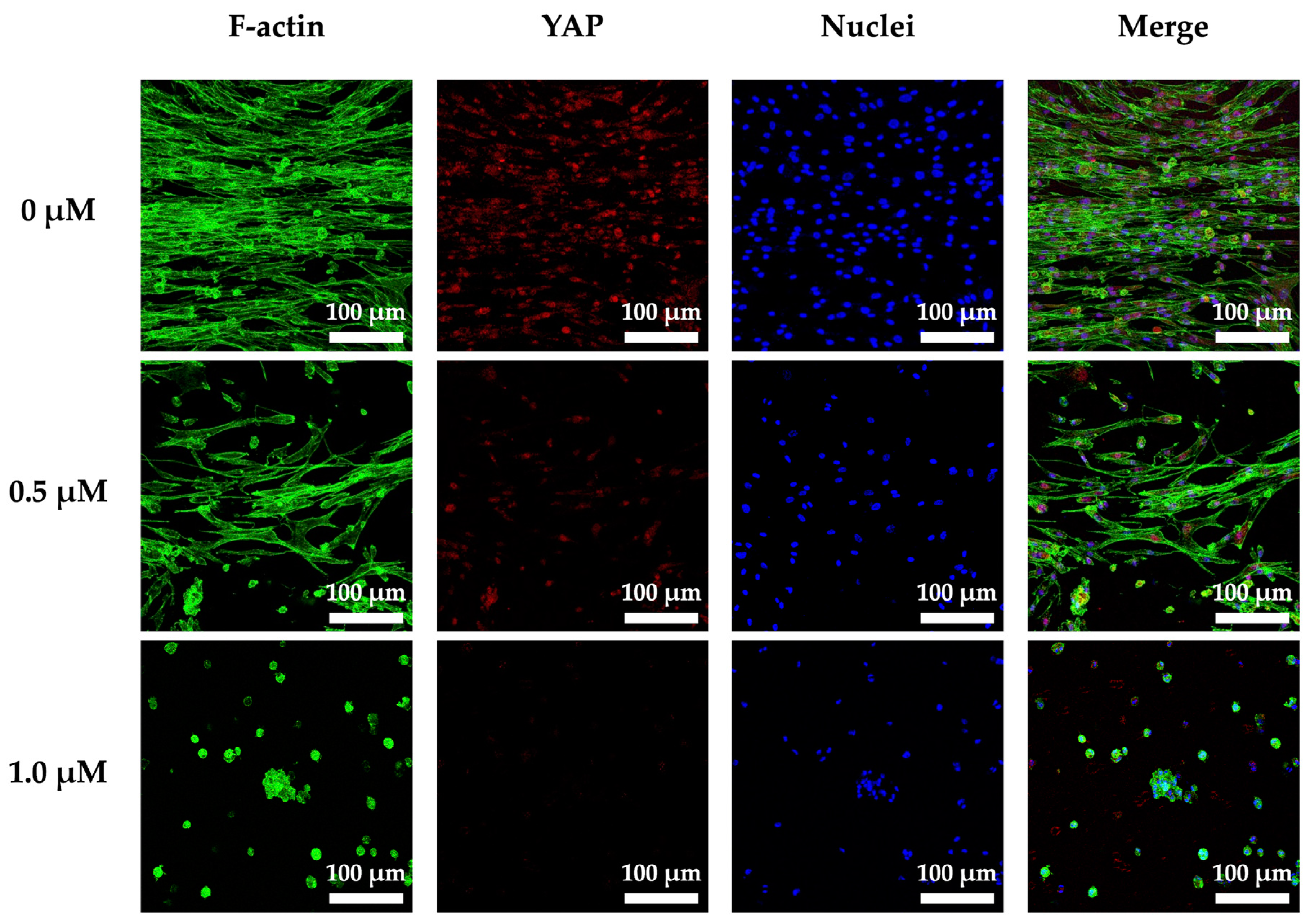
3.8. Cyclic Tensile Stimulated Osteogenic Differentiation of hPDLs through YAP
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thiagarajan, A. In-Vitro 3D Modelling for Charged Particle Therapy-Uncertainties and Opportunities. Adv. Drug Deliv. Rev. 2021, 179, 114018. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Xie, W.; He, Y.; Jiang, Y.; Zhou, Y.; Jing, Y.; Ye, M.; Lin, S.; Wang, W.; Zhang, Z.-Y. Development of Scaffold-Free Micro-Tissues to Accelerate Soft and Hard Tissue Regeneration via Delaying Cellular Senescence and Regulating Inflammation. Appl. Mater. Today 2022, 26, 101370. [Google Scholar] [CrossRef]
- Xu, H.; Qiu, Y.; Xiong, Z.; Shao, W.; Zhang, Q.; Tang, G. Tracking Mesenchymal Stem Cells with Ir(III) Complex-Encapsulated Nanospheres in Cranium Defect with Postmenopausal Osteoporosis. Mater. Sci. Eng. C 2021, 122, 111842. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, Y.; Zhang, Y.; Wang, T.; Lin, K.; Liu, J. A Polydopamine-Assisted Strontium-Substituted Apatite Coating for Titanium Promotes Osteogenesis and Angiogenesis via FAK/MAPK and PI3K/AKT Signaling Pathways. Biomater. Adv. 2021, 131, 112482. [Google Scholar] [CrossRef]
- Zhou, Y.; Qin, R.; Chen, T.; Zhang, K.; Gui, J. 3D Bioprinting Modified Autologous Matrix-Induced Chondrogenesis (AMIC) Technique for Repair of Cartilage Defects. Mater. Design 2021, 203, 109621. [Google Scholar] [CrossRef]
- Chen, K.Y.; Yao, C.H. Repair of Bone Defects with Gelatin-Based Composites: A Review. Biomedicine 2011, 1, 29–32. [Google Scholar] [CrossRef]
- Wan, M.C.; Qin, W.; Lei, C.; Li, Q.H.; Meng, M.; Fang, M.; Song, W.; Chen, J.H.; Tay, F.; Niu, L.N. Biomaterials from the Sea: Future Building Blocks for Biomedical Applications. Bioact. Mater. 2021, 6, 4255–4285. [Google Scholar] [CrossRef]
- Kargozar, S.; Singh, R.K.; Kim, H.-W.; Baino, F. “Hard” Ceramics for “Soft” Tissue Engineering: Paradox or Opportunity? Acta Biomater. 2020, 115, 1–28. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, C.; Zhang, B.; Hu, J.; Xu, J.; Xue, C.; Bao, S.; Gu, X.; Ding, F.; Yang, Y.; et al. BMSC-Derived Extracellular Matrix Better Optimizes the Microenvironment to Support Nerve Regeneration. Biomaterials 2021, 280, 121251. [Google Scholar] [CrossRef]
- Beca, B.M.; Sun, Y.; Wong, E.; Moraes, C.; Simmons, C.A. Dynamic Bioreactors with Integrated Microfabricated Devices for Mechanobiological Screening. Tissue Eng. Part C Methods 2019, 25, 581–592. [Google Scholar] [CrossRef]
- Yousuf, M.H.; Abuzaid, W.; Alkhader, M. 4D Printed Auxetic Structures with Tunable Mechanical Properties. Addit. Manuf. 2020, 35, 101364. [Google Scholar] [CrossRef]
- Li, L.; Li, H.; He, Y.; Tang, H.; Dong, J.; Chen, X.; Lyu, F.; Dong, Y. Cyclic Pulsation Stress Promotes Bone Formation of Tissue Engineered Laminae through the F-Actin/YAP-1/β-Catenin Signaling Axis. NPJ Regen. Med. 2021, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Chandorkar, Y.; Bastard, C.; Russo, J.D.; Haraszti, T.; Laporte, L.D. Cells Feel the Beat–Temporal Effect of Cyclic Mechanical Actuation on Muscle Cells. Appl. Mater. Today 2022, 27, 101492. [Google Scholar] [CrossRef]
- Guillot-Ferriols, M.; Lanceros-Méndez, S.; Ribelles, J.L.G.; Ferrer, G.G. Electrical Stimulation: Effective Cue to Direct Osteogenic Differentiation of Mesenchymal Stem Cells? Biomater. Adv. 2022, 138, 212918. [Google Scholar] [CrossRef]
- Park, H.; Nazhat, S.N.; Rosenzweig, D.H. Mechanical Activation Drives Tenogenic Differentiation of Human Mesenchymal Stem Cells in Aligned Dense Collagen Hydrogels. Biomaterials 2022, 286, 121606. [Google Scholar] [CrossRef]
- Lai, W.Y.; Chen, Y.J.; Lee, A.K.X.; Lin, Y.H.; Liu, Y.W.; Shie, M.Y. Therapeutic Effects of the Addition of Fibroblast Growth Factor-2 to Biodegradable Gelatin/Magnesium-Doped Calcium Silicate Hybrid 3D-Printed Scaffold with Enhanced Osteogenic Capabilities for Critical Bone Defect Restoration. Biomedicines 2021, 9, 712. [Google Scholar] [CrossRef]
- Kakarla, A.B.; Kong, I.; Turek, I.; Kong, C.; Irving, H. Printable Gelatin, Alginate and Boron Nitride Nanotubes Hydrogel-Based Ink for 3D Bioprinting and Tissue Engineering Applications. Mater. Design 2022, 213, 110362. [Google Scholar] [CrossRef]
- Nelson, M.; Li, S.; Page, S.J.; Shi, X.; Lee, P.D.; Stevens, M.M.; Hanna, J.V.; Jones, J.R. 3D Printed Silica-Gelatin Hybrid Scaffolds of Specific Channel Sizes Promote Collagen Type II, Sox9 and Aggrecan Production from Chondrocytes. Mater. Sci. Eng. C 2021, 123, 111964. [Google Scholar] [CrossRef]
- Otani, Y.; Tabata, Y.; Ikada, Y. Effect of additives on gelation and tissue adhesion of gelatin-poly(L-glutamic acid) mixture. Biomaterials 1998, 19, 2167–2173. [Google Scholar] [CrossRef]
- Lin, Y.T.; Hsu, T.T.; Liu, Y.W.; Kao, C.T.; Huang, T.H. Bidirectional Differentiation of Human-Derived Stem Cells Induced by Biomimetic Calcium Silicate-Reinforced Gelatin Methacrylate Bioink for Odontogenic Regeneration. Biomedicines 2021, 9, 929. [Google Scholar] [CrossRef]
- Zhou, Q.; Ren, X.; Oberoi, M.K.; Bedar, M.; Caprini, R.M.; Dewey, M.J.; Kolliopoulos, V.; Yamaguchi, D.T.; Harley, B.A.C.; Lee, J.C. β-Catenin Limits Osteogenesis on Regenerative Materials in a Stiffness-Dependent Manner. Adv. Healthc. Mater. 2021, 10, 2101467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, X.; Guo, P.; Li, X.; He, Z.; Li, Z.; Stoddart, M.J.; Grad, S.; Tian, W.; Chen, D.; et al. Effect of Cyclic Mechanical Loading on Immunoinflammatory Microenvironment in Biofabricating Hydroxyapatite Scaffold for Bone Regeneration. Bioact. Mater. 2021, 6, 3097–3108. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.T.; Chiu, Y.C.; Lee, A.K.X.; Lin, Y.H.; Huang, T.H.; Liu, Y.C.; Shie, M.Y. The Synergistic Effects of Xu Duan Combined Sr-Contained Calcium Silicate/Poly-ε-Caprolactone Scaffolds for the Promotion of Osteogenesis Marker Expression and the Induction of Bone Regeneration in Osteoporosis. Mater. Sci. Eng. C 2021, 119, 111629. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Shimizu, N.; Shibata, Y.; Abiko, Y. Effects of Different Magnitudes of Tension-Force on Alkaline Phosphatase Activity in Periodontal Ligament Cells. J. Dent. Res. 1996, 75, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Kojima, T.; Nagasawa, T.; Kobayashi, H.; Ishikawa, I. Novel Isolation of Alkaline Phosphatase-Positive Subpopulation from Periodontal Ligament Fibroblasts. J. Periodontol. 2003, 74, 780–786. [Google Scholar] [CrossRef]
- Chen, Y.W.; Wang, K.; Ho, C.C.; Kao, C.T.; Ng, H.Y.; Shie, M.Y. Cyclic Tensile Stimulation Enrichment of Schwann Cell-Laden Auxetic Hydrogel Scaffolds towards Peripheral Nerve Tissue Engineering. Mater. Design 2020, 195, 108982. [Google Scholar] [CrossRef]
- Chen, Y.C.; Lin, R.Z.; Qi, H.; Yang, Y.; Bae, H.; Melero-Martin, J.M.; Khademhosseini, A. Functional Human Vascular Network Generated in Photocrosslinkable Gelatin Methacrylate Hydrogels. Adv. Funct. Mater. 2012, 22, 2027–2039. [Google Scholar] [CrossRef] [Green Version]
- Ding, Z.; Xi, W.; Ji, M.; Chen, H.; Zhang, Q.; Yan, Y. Developing a Biodegradable Tricalcium Silicate/Glucono-Delta-Lactone/Calcium Sulfate Dihydrate Composite Cement with High Preliminary Mechanical Property for Bone Filling. Mater. Sci. Eng. C 2021, 119, 111621. [Google Scholar] [CrossRef]
- Gaharwar, U.S.; Kumar, S.; Rajamani, P. Iron Oxide Nanoparticle-Induced Hematopoietic and Immunological Response in Rats. RSC Adv. 2020, 10, 35753–35764. [Google Scholar] [CrossRef]
- Yoon, H.J.; Shin, S.R.; Cha, J.M.; Lee, S.-H.; Kim, J.-H.; Do, J.T.; Song, H.; Bae, H. Cold Water Fish Gelatin Methacryloyl Hydrogel for Tissue Engineering Application. PLoS ONE 2016, 11, e0163902. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.K.X.; Lin, Y.H.; Tsai, C.H.; Chang, W.T.; Lin, T.L.; Shie, M.Y. Digital Light Processing Bioprinted Human Chondrocyte-Laden Poly (γ-Glutamic Acid)/Hyaluronic Acid Bio-Ink towards Cartilage Tissue Engineering. Biomedicines 2021, 9, 714. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Lee, K.X.; Ho, C.C.; Fang, M.J.; Kuo, T.Y.; Shie, M.Y. The Effects of a 3D-Printed Magnesium-/Strontium-Doped Calcium Silicate Scaffold on Regulation of Bone Regeneration via Dual-Stimulation of the AKT and WNT Signaling Pathways. Biomater. Adv. 2022, 135, 112660. [Google Scholar] [CrossRef] [PubMed]
- Tien, N.; Lee, J.J.; Lee, K.X.; Lin, Y.H.; Chen, J.X.; Kuo, T.Y.; Shie, M.Y. Additive Manufacturing of Caffeic Acid-Inspired Mineral Trioxide Aggregate/Poly-ε-Caprolactone Scaffold for Regulating Vascular Induction and Osteogenic Regeneration of Dental Pulp Stem Cells. Cells 2021, 10, 2911. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Chiu, Y.C.; Lee, A.K.X.; Lin, Y.A.; Lin, P.Y.; Shie, M.Y. Biofabrication of Gingival Fibroblast Cell-Laden Collagen/Strontium-Doped Calcium Silicate 3D-Printed Bi-Layered Scaffold for Osteoporotic Periodontal Regeneration. Biomedicines 2021, 9, 431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, W.; Ma, H.; Qin, C.; Chen, J.; Wu, C. Spindle-like Zinc Silicate Nanoparticles Accelerating Innervated and Vascularized Skin Burn Wound Healing. Adv. Healthc. Mater. 2022, 11, 2102359. [Google Scholar] [CrossRef]
- Shie, M.Y.; Ding, S.J. Integrin Binding and MAPK Signal Pathways in Primary Cell Responses to Surface Chemistry of Calcium Silicate Cements. Biomaterials 2013, 34, 6589–6606. [Google Scholar] [CrossRef]
- Kim, J.L.; Leucht, P.; Luppen, C.; Park, Y.; Beggs, H.; Damsky, C.; Helms, J. Reconciling the roles of FAK in osteoblast differentiation, osteoclast remodeling, and bone regeneration. Bone 2007, 41, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Shie, M.Y.; Ding, S.J.; Chang, H.C. The Role of Silicon in Osteoblast-like Cell Proliferation and Apoptosis. Acta Biomater. 2011, 7, 2604–2614. [Google Scholar] [CrossRef]
- Chandran, S.V.; Vairamani, M.; Selvamurugan, N. Osteostimulatory Effect of Biocomposite Scaffold Containing Phytomolecule Diosmin by Integrin/FAK/ERK Signaling Pathway in Mouse Mesenchymal Stem Cells. Sci. Rep. 2019, 9, 11900. [Google Scholar] [CrossRef]
- Chen, Y.J.; Kuo, Y.R.; Yang, K.D.; Wang, C.J.; Chen, S.M.S.; Huang, H.C.; Yang, Y.J.; Sun, Y.C.; Wang, F.S. Activation of Extracellular Signal-Regulated Kinase (ERK) and P38 Kinase in Shock Wave-Promoted Bone Formation of Segmental Defect in Rats. Bone 2004, 34, 466–477. [Google Scholar] [CrossRef]
- Mestre, R.; Patiño, T.; Barceló, X.; Anand, S.; Pérez-Jiménez, A.; Sánchez, S. Force Modulation and Adaptability of 3D-bioprinted Biological Actuators Based on Skeletal Muscle Tissue. Adv. Mater. Technol. 2018, 4, 1800631. [Google Scholar] [CrossRef] [Green Version]
- Vining, K.H.; Mooney, D.J. Mechanical Forces Direct Stem Cell Behaviour in Development and Regeneration. Nat. Rev. Mol. Cell Biol. 2017, 18, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, S.D.; Buckley, C.T.; Steward, A.J.; Kelly, D.J. European Society of Biomechanics S.M. Perren Award 2012: The External Mechanical Environment Can Override the Influence of Local Substrate in Determining Stem Cell Fate. J. Biomech. 2012, 45, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Sang, J.; Fu, J. On Human Pluripotent Stem Cell Control: The Rise of 3D Bioengineering and Mechanobiology. Biomaterials 2015, 52, 26–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Yang, R.; Zhou, Y. Mechanobiology of Periodontal Ligament Stem Cells in Orthodontic Tooth Movement. Stem Cells Int. 2018, 2018, 6531216. [Google Scholar] [CrossRef] [Green Version]
- Kukolj, T.; Trivanović, D.; Djordjević, I.O.; Mojsilović, S.; Krstić, J.; Obradović, H.; Janković, S.; Santibanez, J.F.; Jauković, A.; Bugarski, D. Lipopolysaccharide Can Modify Differentiation and Immunomodulatory Potential of Periodontal Ligament Stem Cells via ERK1,2 Signaling. J. Cell Physiol. 2018, 233, 447–462. [Google Scholar] [CrossRef]
- Iwasaki, K.; Komaki, M.; Akazawa, K.; Nagata, M.; Yokoyama, N.; Watabe, T.; Morita, I. Spontaneous Differentiation of Periodontal Ligament Stem Cells into Myofibroblast during Ex Vivo Expansion. J. Cell Physiol. 2019, 234, 20377–20391. [Google Scholar] [CrossRef]
- He, Y.; Xu, H.; Xiang, Z.; Yu, H.; Xu, L.; Guo, Y.; Tian, Y.; Shu, R.; Yang, X.; Xue, C.; et al. YAP Regulates Periodontal Ligament Cell Differentiation into Myofibroblast Interacted with RhoA/ROCK Pathway. J. Cell Physiol. 2019, 234, 5086–5096. [Google Scholar] [CrossRef]
- Nakamura, T.; Yamashita, M.; Ikegami, K.; Suzuki, M.; Yanagita, M.; Kitagaki, J.; Kitamura, M.; Murakami, S. Autophagy Facilitates Type I Collagen Synthesis in Periodontal Ligament Cells. Sci. Rep. 2021, 11, 1291. [Google Scholar] [CrossRef]
- Enríquez, Á.; Libring, S.; Field, T.C.; Jimenez, J.; Lee, T.; Park, H.; Satoski, D.; Wendt, M.K.; Calve, S.; Tepole, A.B.; et al. High-throughput Magnetic Actuation Platform for Evaluating the Effect of Mechanical Force on 3D Tumor Microenvironment. Adv. Funct. Mater. 2020, 31, 2005021. [Google Scholar] [CrossRef]
- Tantilertanant, Y.; Niyompanich, J.; Everts, V.; Supaphol, P.; Pavasant, P.; Sanchavanakit, N. Cyclic Tensile Force Stimulates BMP9 Synthesis and in Vitro Mineralization by Human Periodontal Ligament Cells. J. Cell Physiol. 2019, 234, 4528–4539. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Zhou, N.; Feng, Y.; Yang, X. Cyclic Tensile Stress Promotes Osteogenic Differentiation of Adipose Stem Cells via ERK and P38 Pathways. Stem Cell Res. 2019, 37, 101433. [Google Scholar] [CrossRef] [PubMed]
- Moro, A.; Driscoll, T.P.; Boraas, L.C.; Armero, W.; Kasper, D.M.; Baeyens, N.; Jouy, C.; Mallikarjun, V.; Swift, J.; Ahn, S.J.; et al. MicroRNA-Dependent Regulation of Biomechanical Genes Establishes Tissue Stiffness Homeostasis. Nat. Cell Biol. 2019, 21, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Liu, W.; Cui, Y.; Liu, Y.; Du, W.; Zheng, L.; Pi, C.; Zhang, D.; Xie, J.; Zhou, X. Biomaterial Stiffness Guides Cross-Talk between Chondrocytes: Implications for a Novel Cellular Response in Cartilage Tissue Engineering. ACS Biomater. Sci. Eng. 2020, 6, 4476–4489. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Digabel, J.L.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in Mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef]
- Chen, L.; Wu, C.; Wei, D.; Chen, S.; Xiao, Z.; Zhu, H.; Luo, H.; Sun, J.; Fan, H. Biomimetic Mineralized Microenvironment Stiffness Regulated BMSCs Osteogenic Differentiation through Cytoskeleton Mediated Mechanical Signaling Transduction. Mater. Sci. Eng. C 2021, 119, 111613. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-J.; Ng, H.-Y.; Lin, Y.-H.; Lin, T.-J.; Kao, C.-T.; Shie, M.-Y. The Synergistic Effect of Cyclic Tensile Force and Periodontal Ligament Cell-Laden Calcium Silicate/Gelatin Methacrylate Auxetic Hydrogel Scaffolds for Bone Regeneration. Cells 2022, 11, 2069. https://doi.org/10.3390/cells11132069
Lee J-J, Ng H-Y, Lin Y-H, Lin T-J, Kao C-T, Shie M-Y. The Synergistic Effect of Cyclic Tensile Force and Periodontal Ligament Cell-Laden Calcium Silicate/Gelatin Methacrylate Auxetic Hydrogel Scaffolds for Bone Regeneration. Cells. 2022; 11(13):2069. https://doi.org/10.3390/cells11132069
Chicago/Turabian StyleLee, Jian-Jr, Hooi-Yee Ng, Yen-Hong Lin, Ting-Ju Lin, Chia-Tze Kao, and Ming-You Shie. 2022. "The Synergistic Effect of Cyclic Tensile Force and Periodontal Ligament Cell-Laden Calcium Silicate/Gelatin Methacrylate Auxetic Hydrogel Scaffolds for Bone Regeneration" Cells 11, no. 13: 2069. https://doi.org/10.3390/cells11132069
APA StyleLee, J.-J., Ng, H.-Y., Lin, Y.-H., Lin, T.-J., Kao, C.-T., & Shie, M.-Y. (2022). The Synergistic Effect of Cyclic Tensile Force and Periodontal Ligament Cell-Laden Calcium Silicate/Gelatin Methacrylate Auxetic Hydrogel Scaffolds for Bone Regeneration. Cells, 11(13), 2069. https://doi.org/10.3390/cells11132069






