CYP1A1, VEGFA and Adipokine Responses of Human Adipocytes Co-exposed to PCB126 and Hypoxia
Abstract
1. Introduction
2. Methods and Materials
2.1. Human Subcutaneous Preadipocyte Culture
2.2. Human Adipocytes’ Exposure to PCB126 and Hypoxia
2.3. Adipokine Quantification
2.4. RNA Extraction and Quantification by qPCR
2.5. Statistical Analysis
3. Results
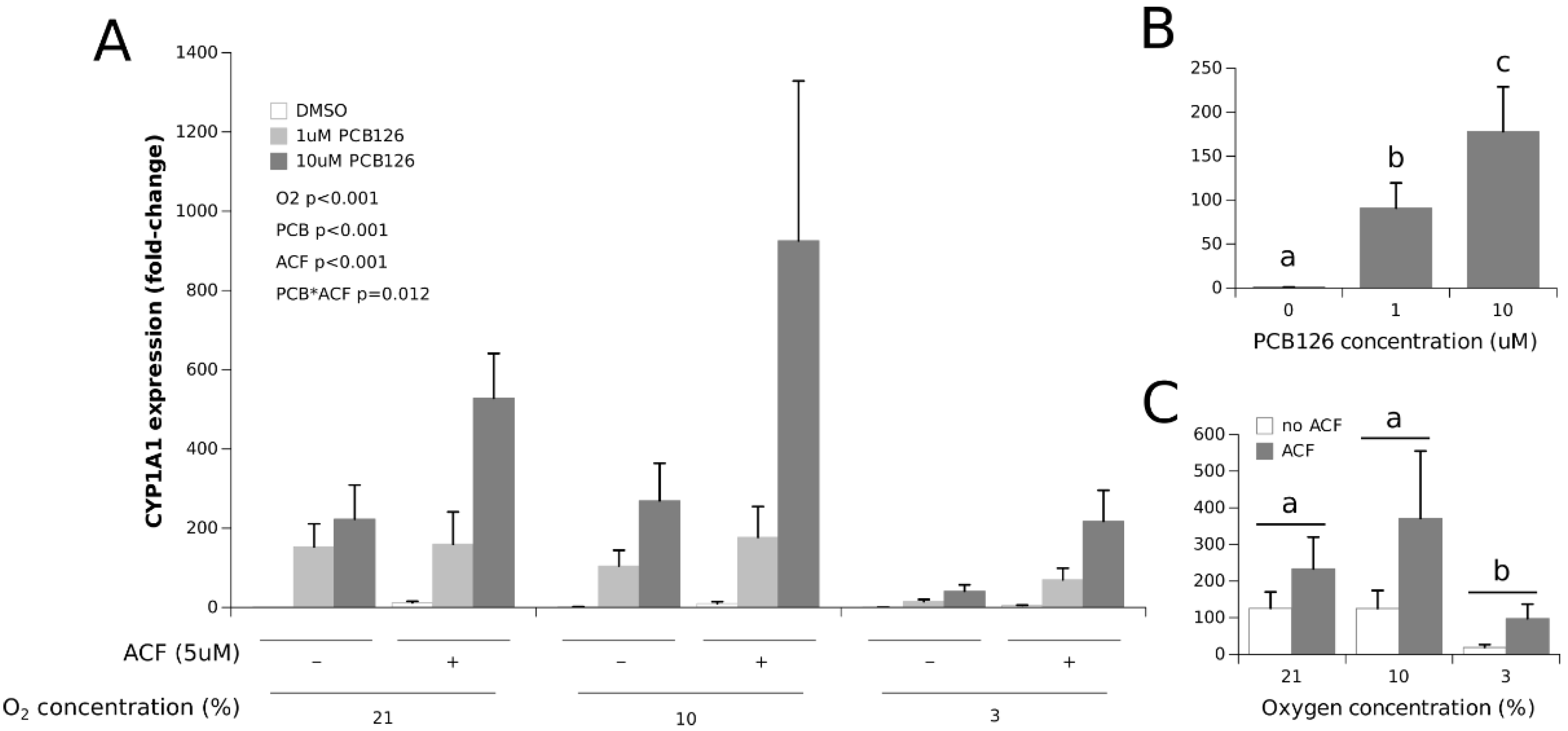
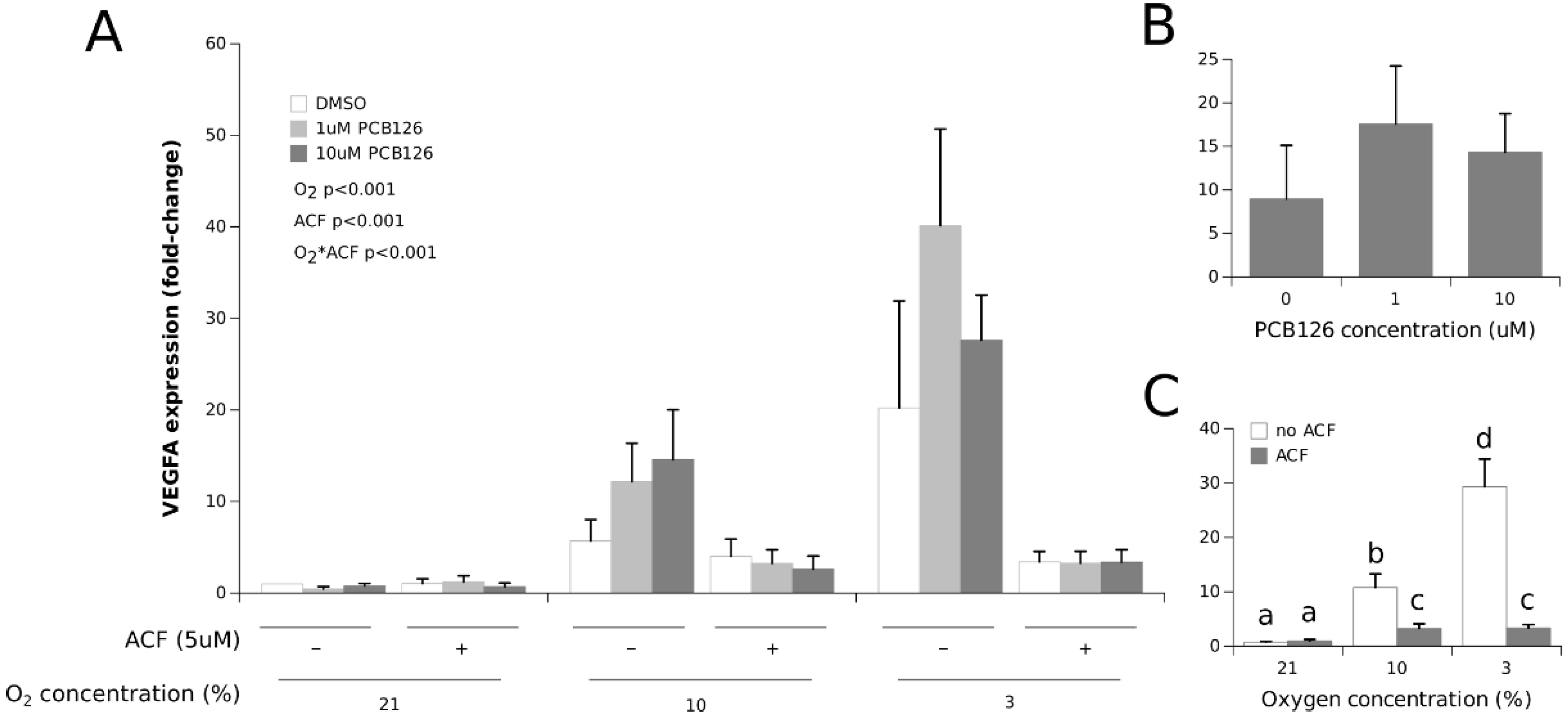
3.1. Effects of Hypoxia and PCB126 on CYP1A1 and VEGFA Expression
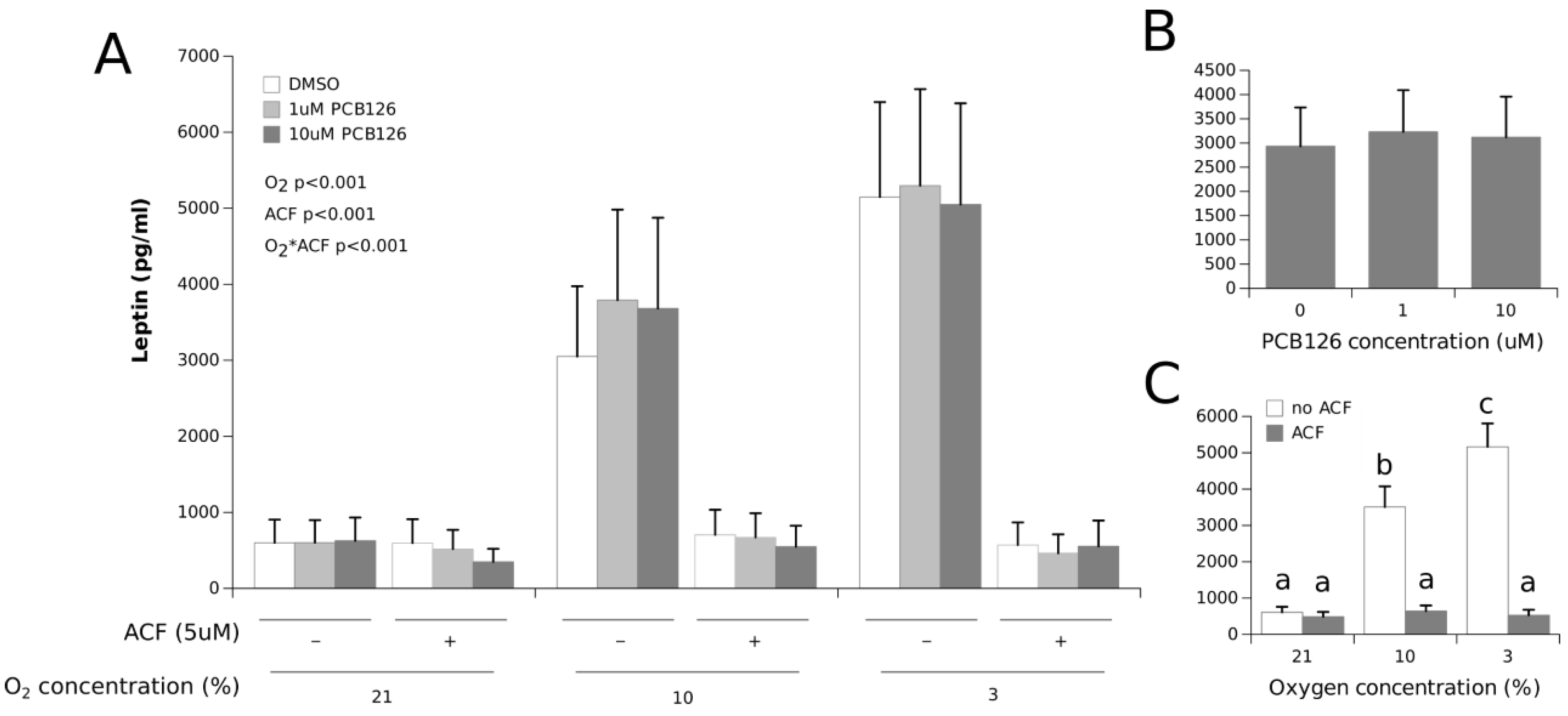
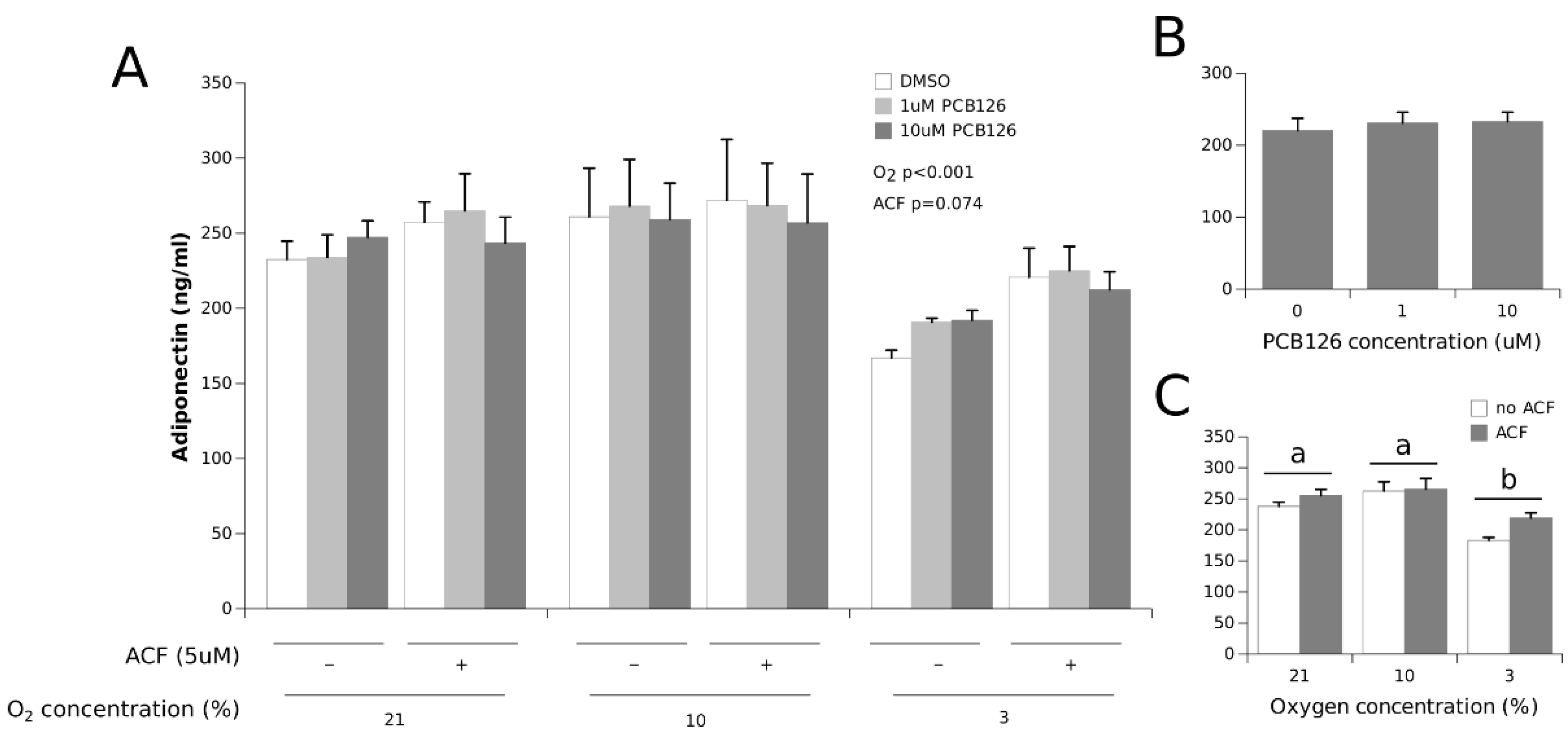
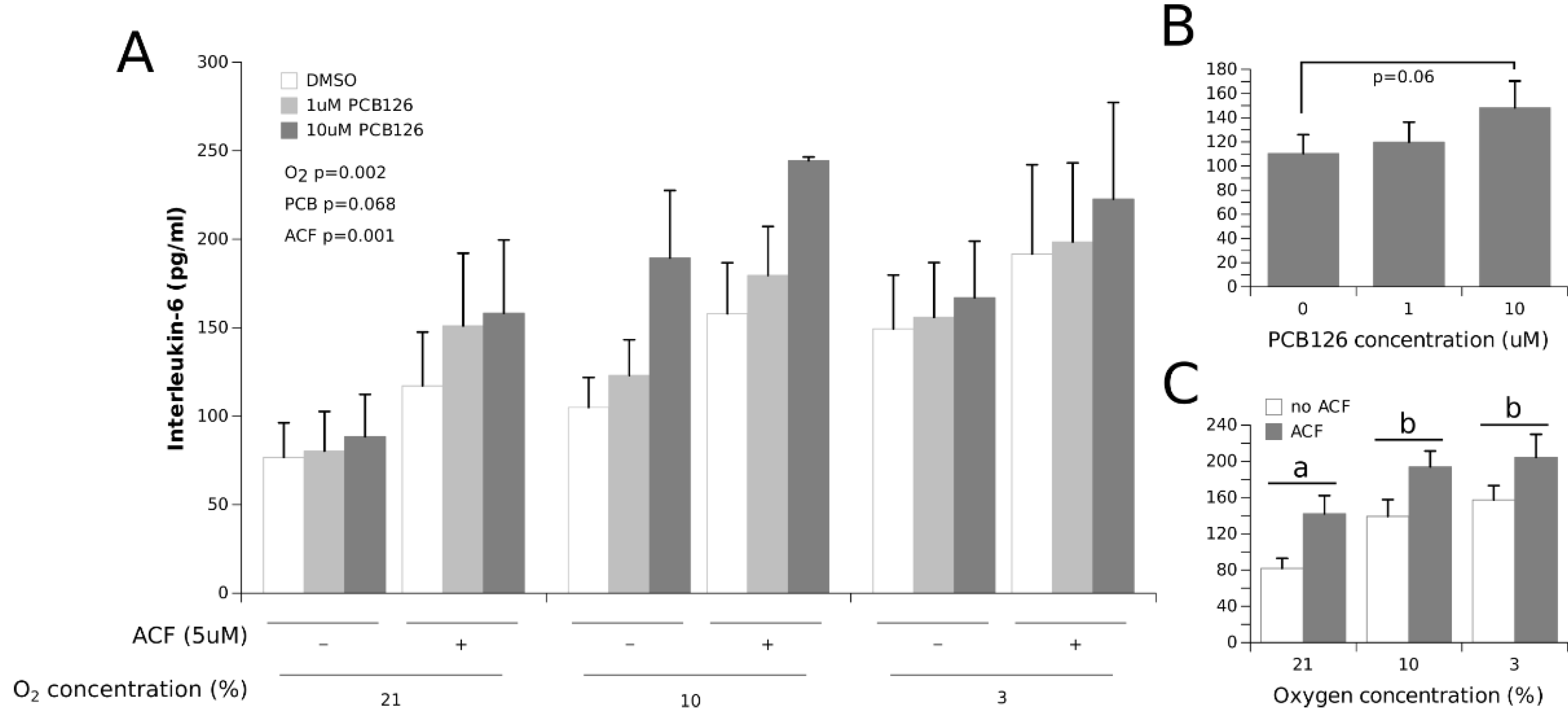
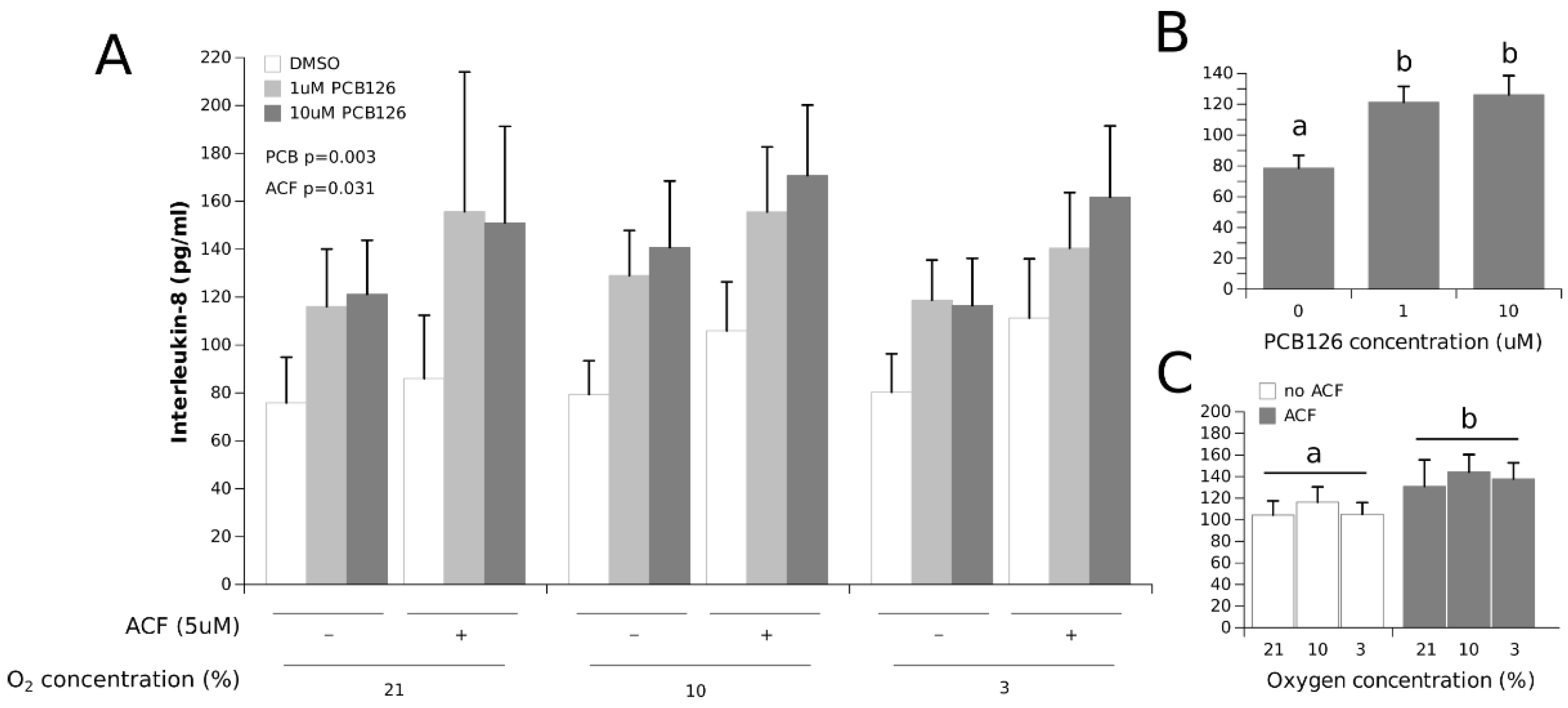
3.2. Effects of Hypoxia and PCB126 on Adipokine Secretion
4. Discussion
4.1. Adipokine Secretion under Co-Exposure to Hypoxia and PCB126
4.2. Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Andrei, A.M.; Berbecaru-Iovan, A.; Din-Anghel, F.R.I.; Stănciulescu, C.E.; Berbecaru-Iovan, S.; Baniţă, I.M.; Pisoschi, C.G. Interplay between Hypoxia, Inflammation and Adipocyte Remodeling in the Metabolic Syndrome. In Hypoxia Human Diseases; InTech: RijeKa, Croatia, 2017; pp. 303–328. [Google Scholar]
- Trayhurn, P.; Wood, I.S. Adipokines: Inflammation and the pleiotropic role of white adipose tissue. Br. J. Nutr. 2004, 92, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Rausch, M.E.; Weisberg, S.; Vardhana, P.; Tortoriello, D.V. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int. J. Obes. 2008, 32, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Gao, Z.; Yin, J.; He, Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am. J. Physiol. Endocrinol. Metab. 2007, 293, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Gao, Z.; He, Q.; Zhou, D.; Guo, Z.; Ye, J. Role of hypoxia in obesity-induced disorders of glucose and lipid metabolism in adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E333–E342. [Google Scholar] [CrossRef]
- Cifarelli, V.; Beeman, S.C.; Smith, G.I.; Yoshino, J.; Morozov, D.; Beals, J.W.; Kayser, B.D.; Watrous, J.D.; Jain, M.; Patterson, B.W.; et al. Decreased adipose tissue oxygenation associates with insulin resistance in individuals with obesity. J. Clin. Investig. 2020, 130, 6688. [Google Scholar] [CrossRef]
- Fleischmann, E.; Kurz, A.; Niedermayr, M.; Schebesta, K.; Kimberger, O.; Sessler, D.; Kabon, B.; Prager, G. Tissue Oxygenation in Obese and Non-obese Patients During Laparoscopy. Obes. Surg. 2005, 15, 813–819. [Google Scholar] [CrossRef][Green Version]
- Kabon, B.; Nagele, A.; Reddy, D.; Eagon, C.; Fleshman, J.W.; Sessler, D.I.; Kurz, A. Obesity decreases perioperative tissue oxygenation. Anesthesiology 2004, 100, 274–280. [Google Scholar] [CrossRef]
- Lawler, H.M.; Underkofler, C.M.; Kern, P.A.; Erickson, C.; Bredbeck, B.; Rasouli, N. Adipose Tissue Hypoxia, Inflammation, and Fibrosis in Obese Insulin-Sensitive and Obese Insulin-Resistant Subjects. J. Clin. Endocrinol. Metab. 2016, 101, 1422–1428. [Google Scholar] [CrossRef]
- Pasarica, M.; Sereda, O.R.; Redman, L.M.; Albarado, D.C.; Hymel, D.T.; Roan, L.E.; Rood Jennifer, C.; Burk David, H.; Smith Steven, R. Reduced adipose tissue oxygenation in human obesity evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 2009, 58, 718–725. [Google Scholar] [CrossRef]
- Seo, J.B.; Riopel, M.; Cabrales, P.; Huh, J.Y.; Bandyopadhyay, G.K.; Andreyev, A.Y.; Murphy, A.N.; Beeman, S.C.; Smith, G.I.; Klein, S.; et al. Knockdown of Ant2 Reduces Adipocyte Hypoxia and Improves Insulin Resistance in Obesity. Nat. Metab. 2019, 1, 86. [Google Scholar] [CrossRef]
- Goossens, G.H.; A Vogel, M.A.; Vink, R.G.; Mariman, E.C.; van Baak, M.A.; Blaak, E.E. Adipose tissue oxygenation is associated with insulin sensitivity independently of adiposity in obese men and women. Diabetes Obes. Metab. 2018, 20, 2286–2290. [Google Scholar] [CrossRef] [PubMed]
- García-Fuentes, E.; Santiago-Fernández, C.; Gutiérrez-Repiso, C.; Mayas, M.D.; Oliva-Olivera, W.; Coín-Aragüez, L.; Alcaide, J.; Ocaña-Wilhelmi, L.; Vendrell, J.; Tinahones, F.J. Hypoxia is associated with a lower expression of genes involved in lipogenesis in visceral adipose tissue. J. Transl. Med. 2015, 13, 373. [Google Scholar] [CrossRef] [PubMed]
- Mahat, B.; Chassé, É.; Mauger, J.; Imbeault, P. Effects of acute hypoxia on human adipose tissue lipoprotein lipase activity and lipolysis. J. Transl. Med. 2016, 14, 212. [Google Scholar] [CrossRef] [PubMed]
- Netzer, N.; Gatterer, H.; Faulhaber, M.; Burtscher, M.; Pramsohler, S.; Pesta, D. Hypoxia, Oxidative Stress and Fat. Biomolecules 2015, 5, 1143–1150. [Google Scholar] [CrossRef]
- Taabazuing, C.Y.; Hangasky, J.A.; Knapp, M.J. Oxygen sensing strategies in mammals and bacteria. J. Inorg. Biochem. 2014, 133, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Zimna, A.; Kurpisz, M. Hypoxia-Inducible Factor-1 in Physiological and Pathophysiological Angiogenesis: Applications and Therapies. Biomed. Res. Int. 2015, 2015, 13. [Google Scholar] [CrossRef]
- Ambrosini, G.; Nath, A.K.; Rocío Sierra-Honigmann, M.; Flores-Riveros, J. Transcriptional activation of the human leptin gene in response to hypoxia. Involvement of hypoxia-inducible factor 1. J. Biol. Chem. 2002, 277, 34601–34609. [Google Scholar] [CrossRef]
- Hayashi, M.; Sakata, M.; Takeda, T.; Yamamoto, T.; Okamoto, Y.; Sawada, K.; Kimura, A.; Minekawa, R.; Tahara, M.; Tasaka, K.; et al. Induction of glucose transporter 1 expression through hypoxia-inducible factor 1alpha under hypoxic conditions in trophoblast-derived cells. J. Endocrinol. 2004, 183, 145–154. [Google Scholar] [CrossRef]
- Famulla, S.; Horrighs, A.; Cramer, A.; Sell, H.; Eckel, J. Hypoxia reduces the response of human adipocytes towards TNFα resulting in reduced NF-κ B signaling and MCP-1 secretion. Int. J. Obes. 2012, 36, 986–992. [Google Scholar] [CrossRef]
- Wood, I.S.; Stezhka, T.; Trayhurn, P. Modulation of adipokine production, glucose uptake and lactate release in human adipocytes by small changes in oxygen tension. Pflug. Arch. Eur. J. Physiol. 2011, 462, 469–477. [Google Scholar] [CrossRef]
- Lempesis, I.G.; van Meijel, R.L.J.; Manolopoulos, K.N.; Goossens, G.H. Oxygenation of adipose tissue: A human perspective. Acta Physiol. 2020, 228, e13298. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P.; Alomar, S.Y. Oxygen deprivation and the cellular response to hypoxia in adipocytes—Perspectives on white and brown adipose tissues in obesity. Front. Endocrinol. 2015, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Stuart Wood, I.; Trayhurn, P. Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflug. Arch. Eur. J. Physiol. 2007, 455, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Kusminski, C.M.; Scherer, P.E.; Scherer, P.E. Adipose tissue remodeling and obesity. J. Clin. Investig. 2011, 121, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P.; Wang, B.; Wood, I.S. Horizons in Nutritional Science Hypoxia in adipose tissue: A basis for the dysregulation of tissue function in obesity? Br. J. Nutr. 2008, 100, 227–235. [Google Scholar] [CrossRef]
- Cock, M.; Bor, M.; De Cock, M.; Van de Bor, M. Obesogenic effects of endocrine disruptors, what do we know from animal and human studies? Environ. Int. 2014, 70, 15–24. [Google Scholar] [CrossRef]
- Dirinck, E.; Jorens, P.G.; Covaci, A.; Geens, T.; Roosens, L.; Neels, H. Obesity and Persistent Organic Pollutants: Possible Obesogenic Effect of Organochlorine Pesticides and Polychlorinated Biphenyls. Obesity 2011, 19, 709–714. [Google Scholar] [CrossRef]
- Jackson, E.; Shoemaker, R.; Larian, N.; Cassis, L. Adipose tissue as a site of toxin accumulation. Compr. Physiol. 2017, 7, 1085–1135. [Google Scholar]
- Regnier, S.M.; Sargis, R.M. Adipocytes under assault: Environmental disruption of adipose physiology. Biochim. Biophys. Acta 2014, 1842, 520–533. [Google Scholar] [CrossRef]
- Fierens, S.; Mairesse, H.; Heilier, J.; De Burbure, C.; Focant, J.; Eppe, G.; De Pauw, E.; Bernard, A. Dioxin/polychlorinated biphenyl body burden, diabetes and endometriosis: Findings in a population-based study in Belgium. Biomarkers 2003, 8, 529–534. [Google Scholar] [CrossRef]
- Hong, N.; Kim, K.; Lee, I.; Lind, P.M.; Lind, L.; Lee, D. The association between obesity and mortality in the elderly differs by serum concentrations of persistent organic pollutants: A possible explanation for the obesity paradox. Int. J. Obes. 2012, 36, 1170–1175. [Google Scholar] [CrossRef] [PubMed]
- Valvi, D.; Mendez, M.A.; Martinez, D.; Grimalt, J.O.; Torrent, M.; Vrijheid, M. Prenatal concentrations of polychlorinated biphenyls, DDE, and DDT and overweight in children: A prospective birth cohort study. Environ. Health Perspect. 2012, 120, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, M.; Birnbaum, L.S.; Denison, M.; De Vito, M.; Farland, W.; Feeley, M.; Feeley, M.; Fiedler, H.; Hakansson, H.; Hanberg, A.; et al. The 2005 World Health Organization Reevaluation of Human and Mammalian Toxic Equivalency Factors for Dioxins and Dioxin-Like Compounds. Toxicol. Sci. 2006, 93, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Heindel, J.J.; Blumberg, B.; Cave, M.; Machtinger, R.; Mantovani, A.; Mendez, M.A.; Nadal, A.; Palanza, P.; Panzica, G.; Sargis, R.; et al. Metabolism disrupting chemicals and metabolic disorders. Reprod. Toxicol. 2017, 68, 3–33. [Google Scholar] [CrossRef] [PubMed]
- Larigot, L.; Juricek, L.; Dairou, J.; Coumoul, X. AhR signaling pathways and regulatory functions. Biochim. Open 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Baker, N.A.; Shoemaker, R.; English, V.; Larian, N.; Sunkara, M.; Morris, A.J.; Walker, M.; Yiannikouris, F.; Cassis, L.A. Effects of Adipocyte Aryl Hydrocarbon Receptor Deficiency on PCB-Induced Disruption of Glucose Homeostasis in Lean and Obese Mice. Environ. Health Perspect. 2015, 123, 944–950. [Google Scholar] [CrossRef]
- Caron, A.; Ahmed, F.; Peshdary, V.; Garneau, L.; Atlas, E.; Aguer, C. Effects of PCB126 on Adipose-to-Muscle Communication in an in Vitro Model. Environ. Health Perspect. 2020, 128, 107002. [Google Scholar] [CrossRef]
- Gadupudi, G.; Gourronc, F.A.; Ludewig, G.; Robertson, L.W.; Klingelhutz, A.J. Toxicology in Vitro PCB126 inhibits adipogenesis of human preadipocytes. Toxicol. Vitr. 2015, 29, 132–141. [Google Scholar] [CrossRef]
- Gourronc, F.A.; Robertson, L.W.; Klingelhutz, A.J. A Delayed Proinflammatory Response of Human Preadipocytes to PCB126 Is Dependent on the Aryl Hydrocarbon Receptor. Environ. Sci. Pollut. Res. Int. 2018, 25, 16481. [Google Scholar] [CrossRef]
- Hankinson, O. The Aryl Hydrocarbon Receptor Complex. Annu. Rev. Pharmacol. Toxicol. 1995, 35, 307–340. [Google Scholar] [CrossRef]
- Lubrano, C.; Genovesi, G.; Specchia, P.; Costantini, D.; Mariani, S.; Petrangeli, E.; Lenzi, A.; Gnessi, L. Obesity and Metabolic Comorbidities: Environmental Diseases? Oxid. Med. Cell Longev. 2013, 2013, 640673. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Shirakawa, H.; Tomita, S.; Ohsaki, Y.; Haketa, K.; Tooi, O.; Santo, N.; Tohkin, M.; Furukawa, Y.; Gonzalez, F.J.; et al. Low-dose dioxins alter gene expression related to cholesterol biosynthesis, lipogenesis, and glucose metabolism through the aryl hydrocarbon receptor-mediated pathway in mouse liver. Toxicol. Appl. Pharmacol. 2008, 229, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Tomita, S.; Sinal, C.J.; Yim, S.H.; Gonzalez, F.J. Conditional Disruption of the Aryl Hydrocarbon Receptor Nuclear Translocator (Arnt) Gene Leads to Loss of Target Gene Induction by the Aryl Hydrocarbon Receptor and Hypoxia-Inducible Factor 1α. Mol. Endocrinol. 2000, 14, 1674–1681. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Blankenship, A.L.; Giesy, J.P. Interactions between aryl hydrocarbon receptor (AhR) and hypoxia signaling pathways. Environ. Toxicol. Pharmacol. 2001, 10, 17–27. [Google Scholar] [CrossRef]
- Vorrink, S.U.; Domann, F.E. Regulatory crosstalk and interference between the xenobiotic and hypoxia sensing pathways at the AhR-ARNT-HIF1α signaling node. Chem. Biol. Interact. 2014, 0, 82–88. [Google Scholar] [CrossRef]
- Gradin, K.; McGuire, J.; Wenger, R.H.; Kvietikova, I.; Fhitelaw, M.L.; Toftgård, R.; Tora, L.; Gassmann, M.; Poellinger, L. Functional interference between hypoxia and dioxin signal transduction pathways: Competition for recruitment of the Arnt transcription factor. Mol. Cell Biol. 1996, 16, 5221–5231. [Google Scholar] [CrossRef]
- Gassmann, M.; Kvietikova, I.; Rolfs, A.; Wenger, R.H. Oxygen- and dioxin-regulated gene expression in mouse hepatoma cells. Kidney Int. 1997, 51, 567–574. [Google Scholar] [CrossRef][Green Version]
- Pollenz, R.S.; Davarinos, N.A.; Shearer, T.P. Analysis of Aryl Hydrocarbon Receptor-Mediated Signaling during Physiological Hypoxia Reveals Lack of Competition for the Aryl Hydrocarbon Nuclear Translocator Transcription Factor. Mol. Pharmacol. 1999, 56, 1127–1137. [Google Scholar] [CrossRef]
- Khan, S.; Liu, S.; Stoner, M.; Safe, S. Cobaltous chloride and hypoxia inhibit aryl hydrocarbon receptor-mediated responses in breast cancer cells. Toxicol. Appl. Pharmacol. 2007, 223, 28–38. [Google Scholar] [CrossRef][Green Version]
- Allen, J.W.; Johnson, R.S.; Bhatia, S.N. Hypoxic inhibition of 3-methylcholanthrene-induced CYP1A1 expression is independent of HIF-1alpha. Toxicol. Lett. 2005, 155, 151–159. [Google Scholar] [CrossRef]
- Hofer, T.; Phojanvirta, R.; Spielmann, P.; Viluksela, M.; Buchmann, D.P.; Wenger, R.H.; Gassmann, M. Simultaneous exposure of rats to dioxin and carbon monoxide reduces the xenobiotic but not the hypoxic response. Biol. Chem. 2004, 385, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-E.; Sheen, Y.Y. Inhibition of 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD)-Stimulated Cyp1a1 Promoter Activity by Hypoxic Agents. Biochem. Pharmacol. 2000, 59, 1549–1556. [Google Scholar] [CrossRef]
- Matson, C.W.; Timme-Laragy, A.R.; Di Giulio, R.T. Fluoranthene, but not benzo[a]pyrene, interacts with hypoxia resulting in pericardial effusion and lordosis in developing zebrafish. Chemosphere 2008, 74, 149–154. [Google Scholar] [CrossRef]
- Prasch, A.L.; Andreasen, E.A.; Peterson, R.E.; Heideman, W. Interactions between 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) and Hypoxia Signaling Pathways in Zebrafish: Hypoxia Decreases Responses to TCDD in Zebrafish Embryos. Toxicol. Sci. 2004, 78, 68–77. [Google Scholar] [CrossRef]
- Kraemer, L.D.; Schulte, P.M. Prior PCB exposure suppresses hypoxia-induced up-regulation of glycolytic enzymes in Fundulus heteroclitus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2004, 139, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Seifert, A.; Katschinski, D.M.; Tonack, S.; Fischer, B.; Navarrete Santos, A. Significance of Prolyl Hydroxylase 2 in the Interference of Aryl Hydrocarbon Receptor and Hypoxia-Inducible Factor-1α Signaling. Chem. Res. Toxicol. 2008, 21, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Takacova, M.; Holotnakova, T.; Vondracek, J.; Machala, M.; Pencikova, K.; Gradin, K.; Poellinger, L.; Pastorek, J.; Pastorekova, S.; Kopacek, J. Role of aryl hydrocarbon receptor in modulation of the expression of the hypoxia marker carbonic anhydrase IX. Biochem. J. 2009, 419, 419–425. [Google Scholar] [CrossRef][Green Version]
- Terzuoli, E.; Puppo, M.; Rapisarda, A.; Uranchimeg, B.; Cao, L.; Burger, A.M.; Ziche, M.; Melillo, G. Aminoflavone, a ligand of the aryl hydrocarbon receptor, inhibits HIF-1alpha expression in an AhR-independent fashion. Cancer Res. 2010, 70, 6837–6848. [Google Scholar] [CrossRef]
- Fleming, C.R.; Billiard, S.M.; Di Giulio, R.T. Hypoxia inhibits induction of aryl hydrocarbon receptor activity in topminnow hepatocarcinoma cells in an ARNT-dependent manner. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2009, 150, 383–389. [Google Scholar] [CrossRef][Green Version]
- Schults, M.A.; Timmermans, L.; Godschalk, R.W.; Theys, J.; Wouters, B.G.; Van Schooten, F.J.; Chiu, R.K. Diminished Carcinogen Detoxification Is a Novel Mechanism for Hypoxia-inducible Factor 1-mediated Genetic Instability. J. Biol. Chem. 2010, 285, 14558–14564. [Google Scholar] [CrossRef]
- Vorrink, S.U.; Sarsour, E.H.; Olivier, A.K.; Robertson, L.W.; Goswami, P.C.; Domann, F.E. PCB 126 perturbs hypoxia-induced HIF-1α activity and glucose consumption in human HEPG2 cells. Exp. Toxicol. Pathol. 2014, 66, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.W.; Budinsky, R.A.; Rowlands, J.C. A Model for Aryl Hydrocarbon Receptor-Activated Gene Expression Shows Potency and Efficacy Changes and Predicts Squelching Due to Competition for Transcription Co-Activators. PLoS ONE 2015, 10, e0127952. [Google Scholar] [CrossRef] [PubMed]
- Grosfeld, A.; André, J.; Mouzon, S.H.; De Berra, E.; Pouysségur, J.; Guerre-Millo, M. Hypoxia-inducible Factor 1 Transactivates the Human Leptin Gene Promoter. J. Biol. Chem. 2002, 277, 42953–42957. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Fernandes, A.; Dirinck, E.; Dirtu, A.C.; Malarvannan, G.; Covaci, A.; Gaal, L.; Vanparys, C.; Jorens, P.G.; Blust, R. Expression of Obesity Markers and Persistent Organic Pollutants Levels in Adipose Tissue of Obese Patients: Reinforcing the Obesogen Hypothesis? PLoS ONE 2014, 9, e84816. [Google Scholar] [CrossRef]
- Loiola, R.A.; Maria Dos Anjos, F.; Shimada, A.L.; Soares Cruz, W.; Drewes, C.C.; Fernandes Rodrigues, S.; Helena, K.; Cardozo, M.; Carvalho, V.M.; Pinto, E.; et al. Long-term in vivo polychlorinated biphenyl 126 exposure induces oxidative stress and alters proteomic profile on islets of Langerhans. Nat. Publ. Gr. 2016, 6, 27882. [Google Scholar] [CrossRef]
- Jensen, B.A.; Leeman, R.J.; Schlezinger, J.J.; Sherr, D.H. Aryl hydrocarbon receptor (AhR) agonists suppress interleukin-6 expression by bone marrow stromal cells: An immunotoxicology study. Environ. Health 2003, 2, 1–13. [Google Scholar] [CrossRef]
- Klingelhutz, A.J.; Gourronc, F.A.; Chaly, A.; Wadkins, D.A.; Burand, A.J.; Markan, K.R.; Idiga, S.O.; Wu, M.; Potthoff, M.J.; Ankrum, J.A. Scaffold-free generation of uniform adipose spheroids for metabolism research and drug discovery OPEN. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Guarnieri, T.; Provvidenza, X.; Abruzzo, M.; Bolotta, A. More than a cell biosensor: Aryl hydrocarbon receptor at the intersection of physiology and inflammation. Am. J. Physiol. Cell Physiol. 2020, 318, 1078–1082. [Google Scholar] [CrossRef]
- Hollingshead, B.D.; Beischlag, T.V.; DiNatale, B.C.; Ramadoss, P.; Perdew, G.H. Inflammatory Signaling and Aryl Hydrocarbon Receptor Mediate Synergistic Induction of Interleukin 6 in MCF-7 Cells. Cancer Res. 2008, 68, 3609–3617. [Google Scholar] [CrossRef]
- Matsumura, F.; Vogel, C.F.A. Evidence supporting the hypothesis that one of the main functions of the aryl hydrocarbon receptor is mediation of cell stress responses. Biol. Chem. 2006, 387, 1189–1194. [Google Scholar] [CrossRef]
- Denison, M.S.; Soshilov, A.A.; He, G.; Degroot, D.E.; Zhao, B. Exactly the Same but Different: Promiscuity and Diversity in the Molecular Mechanisms of Action of the Aryl Hydrocarbon (Dioxin) Receptor. Toxicol. Sci. 2011, 124, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Georganas, C.; Liu, H.; Perlman, H.; Hoffmann, A.; Thimmapaya, B.; Pope, R.M. Regulation of IL-6 and IL-8 Expression in Rheumatoid Arthritis Synovial Fibroblasts: The Dominant Role for NF-κB But Not C/EBPβ or c-Jun. J. Immunol. 2000, 165, 7199–7206. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.H.; Chang, H.; Chang, J.T.; Lin, P. Aryl hydrocarbon receptor in association with RelA modulates IL-6 expression in non-smoking lung cancer. Oncogene 2012, 31, 2555–2565. [Google Scholar] [CrossRef]
- Vogel, C.F.A.; Matsumura, F. A new cross-talk between the aryl hydrocarbon receptor and RelB, a member of the NF-kB family. Biochem. Pharmacol. 2009, 77, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, A.; Anusree, S.S.; Nisha, V.M.; Raghu, K.G. Curcumin improves hypoxia induced dysfunctions in 3T3-L1 adipocytes by protecting mitochondria and down regulating inflammation. BioFactors 2014, 40, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Pepin, G.; Nejad, C.; Thomas, B.J.; Ferrand, J.; McArthur, K.; Bardin, P.G.; Williams, B.R.G.; Gantier, M.P. Activation of cGAS-dependent antiviral responses by DNA intercalating agents. Nucleic Acids Res. 2017, 45, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Kim, J.H.; Li, F.; Qu, A.; Gavrilova, O.; Shah, Y.M.; Gonzalez, F.J. Hypoxia-inducible Factor 1α Regulates a SOCS3-STAT3-Adiponectin Signal Transduction Pathway in Adipocytes. J. Biol. Chem. 2013, 288, 3844. [Google Scholar] [CrossRef]
- Gadupudi, G.S.; Elser, B.A.; Sandgruber, F.A.; Li, X.; Gibson-Corley, K.N.; Robertson, L.W. PCB126 Inhibits the Activation of AMPK-CREB Signal Transduction Required for Energy Sensing in Liver. Toxicol. Sci. 2018, 163, 440–453. [Google Scholar] [CrossRef]
- Hodson, L. Adipose tissue oxygenation. Adipocyte 2014, 3, 75–80. [Google Scholar] [CrossRef]
- Lee, K.; Zhang, H.; Qian, D.Z.; Rey, S.; Liu, J.O.; Semenza, G.L. Acriflavine inhibits HIF-1 dimerization, tumor growth, and vascularization. Proc. Natl. Acad. Sci. USA 2009, 106, 17910–17915. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amine, Z.E.; Mauger, J.-F.; Imbeault, P. CYP1A1, VEGFA and Adipokine Responses of Human Adipocytes Co-exposed to PCB126 and Hypoxia. Cells 2022, 11, 2282. https://doi.org/10.3390/cells11152282
Amine ZE, Mauger J-F, Imbeault P. CYP1A1, VEGFA and Adipokine Responses of Human Adipocytes Co-exposed to PCB126 and Hypoxia. Cells. 2022; 11(15):2282. https://doi.org/10.3390/cells11152282
Chicago/Turabian StyleAmine, Zeinab El, Jean-François Mauger, and Pascal Imbeault. 2022. "CYP1A1, VEGFA and Adipokine Responses of Human Adipocytes Co-exposed to PCB126 and Hypoxia" Cells 11, no. 15: 2282. https://doi.org/10.3390/cells11152282
APA StyleAmine, Z. E., Mauger, J.-F., & Imbeault, P. (2022). CYP1A1, VEGFA and Adipokine Responses of Human Adipocytes Co-exposed to PCB126 and Hypoxia. Cells, 11(15), 2282. https://doi.org/10.3390/cells11152282





