Enhancement of Neuroglial Extracellular Matrix Formation and Physiological Activity of Dopaminergic Neural Cocultures by Macromolecular Crowding
Abstract
:1. Introduction
2. Materials and Method
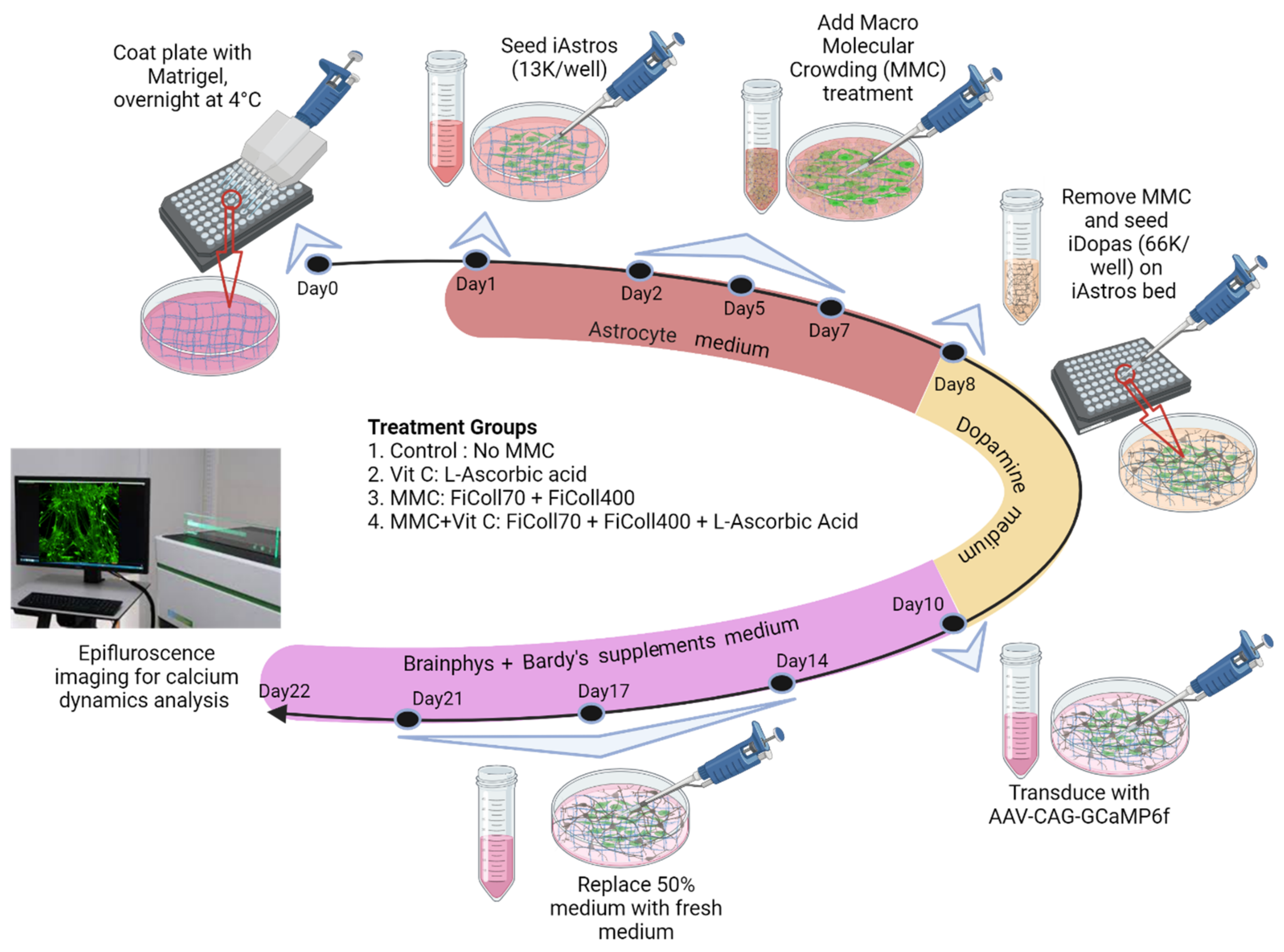
2.1. Astrocyte Culture
2.2. Macromolecular Crowding (MMC) Treatment
2.3. Astrocyte–Neuron Coculture
2.4. Immunocytochemistry
2.5. Image Analysis of Neural Extensions and Branching
2.6. Live Presynaptic Vesicle Tracking Assay
2.7. Single-Cell Calcium Flux Imaging of Transfected GCaMP6f Neurons and Astrocytes Cocultures
2.8. Pharmacological Perturbation of Calcium Dynamics in Neural Coculture Systems
2.9. Statistical Analysis
3. Results
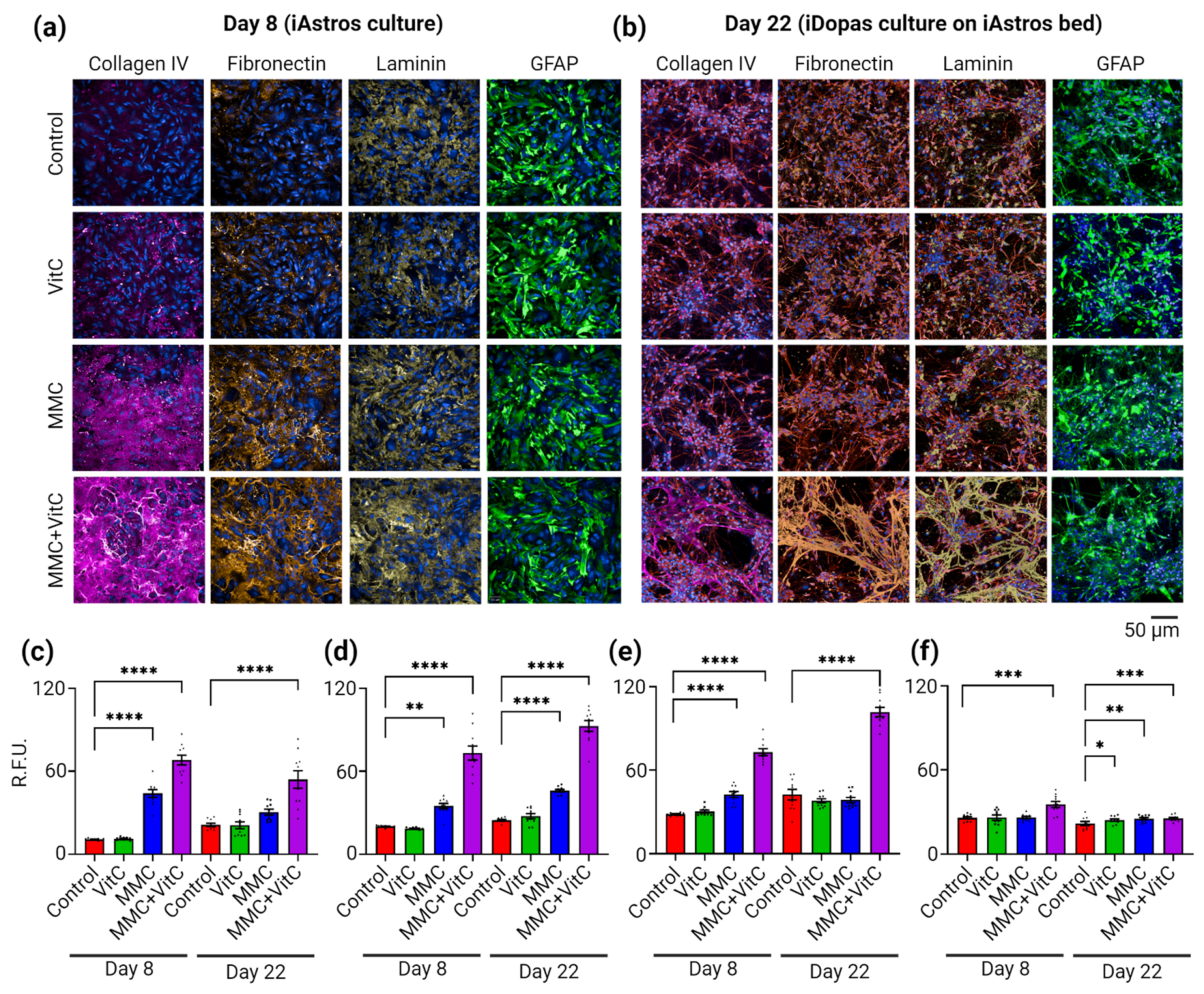
3.1. Macromolecular Crowding Treatment Enhances Deposition of ECM by Cultured Human-iPSC-Derived Astrocytes
3.2. Macromolecular Crowding Treatment Induces Formation of Neuronal Extensions and Branching
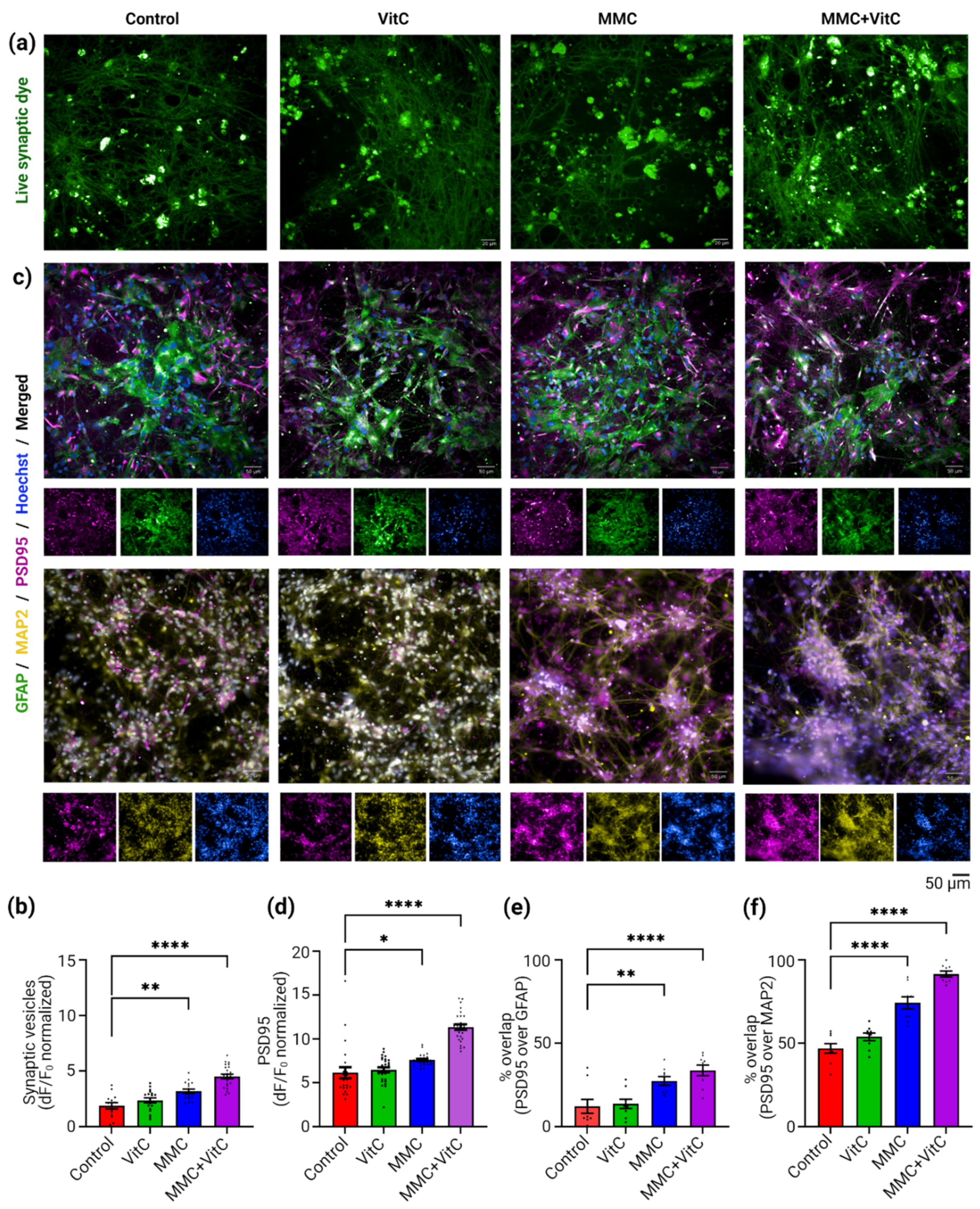
3.3. Macromolecular Crowding Treatment Induces Synaptogenesis and Synaptic Plasticity
3.4. Macromolecular Crowding Treatment Upregulates Neural Calcium Dynamics
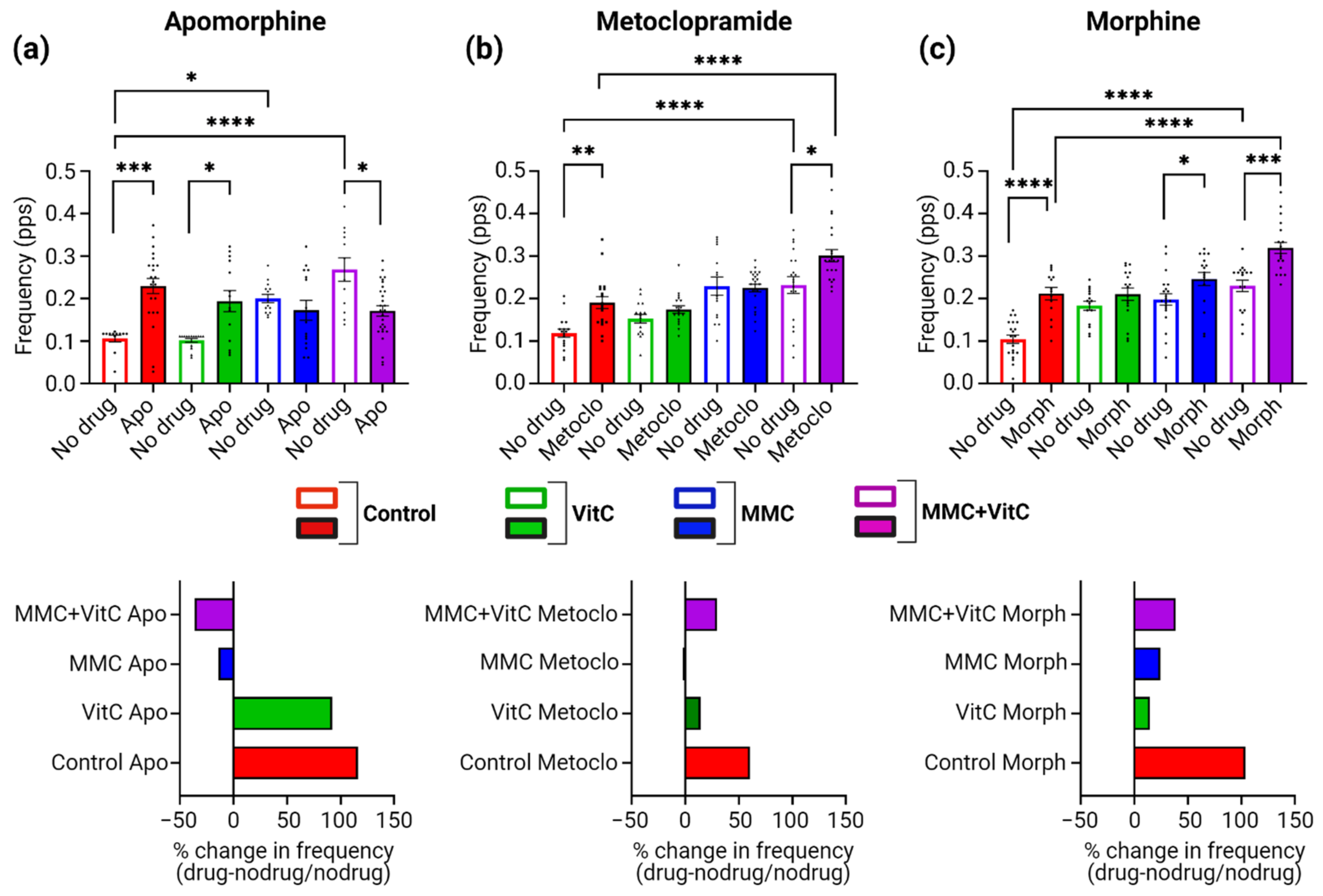
3.5. Macromolecular Crowding Treatment Promotes Pharmacological Responses That Emulate Those Seen In Vivo
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nikolakopoulou, P.; Rauti, R.; Voulgaris, D.; Shlomy, I.; Maoz, B.M.; Herland, A. Recent progress in translational engineered in vitro models of the central nervous system. Brain 2020, 143, 3181–3213. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Trujillo, C.A.; Negraes, P.D.; Muotri, A.R.; Lameu, C.; Ulrich, H. Stem cell contributions to neurological disease modeling and personalized medicine. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 80, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Roll, L.; Faissner, A. Influence of the extracellular matrix on endogenous and transplanted stem cells after brain damage. Front. Cell. Neurosci. 2014, 8, 219. [Google Scholar] [CrossRef]
- Caddeo, S.; Boffito, M.; Sartori, S. Tissue Engineering Approaches in the Design of Healthy and Pathological In Vitro Tissue Models. Front. Bioeng. Biotechnol. 2017, 5, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, K.A.; Donato, D.M.; Balcioglu, H.E.; Schmidt, T.; Danen, E.H.; Koenderink, G.H. A guide to mechanobiology: Where biology and physics meet. Biochim. Biophys. Acta 2015, 1853, 3043–3052. [Google Scholar] [CrossRef] [Green Version]
- Barros, C.S.; Franco, S.J.; Muller, U. Extracellular matrix: Functions in the nervous system. Cold Spring Harb. Perspect. Biol. 2011, 3, a005108. [Google Scholar] [CrossRef] [Green Version]
- Colognato, H.; ffrench-Constant, C.; Feltri, M.L. Human diseases reveal novel roles for neural laminins. Trends Neurosci. 2005, 28, 480–486. [Google Scholar] [CrossRef]
- Melrose, J.; Hayes, A.J.; Bix, G. The CNS/PNS Extracellular Matrix Provides Instructive Guidance Cues to Neural Cells and Neuroregulatory Proteins in Neural Development and Repair. Int. J. Mol. Sci. 2021, 22, 5583. [Google Scholar] [CrossRef]
- Baeten, K.M.; Akassoglou, K. Extracellular matrix and matrix receptors in blood-brain barrier formation and stroke. Dev. Neurobiol. 2011, 71, 1018–1039. [Google Scholar] [CrossRef] [Green Version]
- Cabezas, R.; Avila, M.; Gonzalez, J.; El-Bacha, R.S.; Baez, E.; Garcia-Segura, L.M.; Jurado Coronel, J.C.; Capani, F.; Cardona-Gomez, G.P.; Barreto, G.E. Astrocytic modulation of blood brain barrier: Perspectives on Parkinson’s disease. Front. Cell Neurosci. 2014, 8, 211. [Google Scholar] [CrossRef] [Green Version]
- Benarroch, E.E. Extracellular matrix in the CNS: Dynamic structure and clinical correlations. Neurology 2015, 85, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Galtrey, C.M.; Kwok, J.C.; Carulli, D.; Rhodes, K.E.; Fawcett, J.W. Distribution and synthesis of extracellular matrix proteoglycans, hyaluronan, link proteins and tenascin-R in the rat spinal cord. Eur. J. Neurosci. 2008, 27, 1373–1390. [Google Scholar] [CrossRef] [PubMed]
- Morawski, M.; Dityatev, A.; Hartlage-Rubsamen, M.; Blosa, M.; Holzer, M.; Flach, K.; Pavlica, S.; Dityateva, G.; Grosche, J.; Bruckner, G.; et al. Tenascin-R promotes assembly of the extracellular matrix of perineuronal nets via clustering of aggrecan. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20140046. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.M.; Mei, R. Basement Membrane Type IV Collagen and Laminin: An Overview of Their Biology and Value as Fibrosis Biomarkers of Liver Disease. Anat. Rec. (Hoboken) 2017, 300, 1371–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomsen, M.S.; Routhe, L.J.; Moos, T. The vascular basement membrane in the healthy and pathological brain. J. Cereb. Blood Flow Metab. 2017, 37, 3300–3317. [Google Scholar] [CrossRef] [PubMed]
- Soleman, S.; Filippov, M.A.; Dityatev, A.; Fawcett, J.W. Targeting the neural extracellular matrix in neurological disorders. Neuroscience 2013, 253, 194–213. [Google Scholar] [CrossRef]
- Zhou, X.H.; Brakebusch, C.; Matthies, H.; Oohashi, T.; Hirsch, E.; Moser, M.; Krug, M.; Seidenbecher, C.I.; Boeckers, T.M.; Rauch, U.; et al. Neurocan is dispensable for brain development. Mol. Cell Biol. 2001, 21, 5970–5978. [Google Scholar] [CrossRef] [Green Version]
- Carstens, K.E.; Lustberg, D.J.; Shaughnessy, E.K.; McCann, K.E.; Alexander, G.M.; Dudek, S.M. Perineuronal net degradation rescues CA2 plasticity in a mouse model of Rett syndrome. J. Clin. Investig. 2021, 131, e137221. [Google Scholar] [CrossRef]
- Laham, B.J.; Gould, E. How Stress Influences the Dynamic Plasticity of the Brain’s Extracellular Matrix. Front. Cell Neurosci. 2021, 15, 814287. [Google Scholar] [CrossRef]
- Van De Vijver, S.; Missault, S.; Van Soom, J.; Van Der Veken, P.; Augustyns, K.; Joossens, J.; Dedeurwaerdere, S.; Giugliano, M. The effect of pharmacological inhibition of Serine Proteases on neuronal networks in vitro. PeerJ 2019, 7, e6796. [Google Scholar] [CrossRef] [Green Version]
- Tsilibary, E.; Tzinia, A.; Radenovic, L.; Stamenkovic, V.; Lebitko, T.; Mucha, M.; Pawlak, R.; Frischknecht, R.; Kaczmarek, L. Neural ECM proteases in learning and synaptic plasticity. Prog. Brain Res. 2014, 214, 135–157. [Google Scholar] [CrossRef]
- Kajtez, J.; Nilsson, F.; Fiorenzano, A.; Parmar, M.; Emneus, J. 3D biomaterial models of human brain disease. Neurochem. Int. 2021, 147, 105043. [Google Scholar] [CrossRef]
- Raghunath, M.; Zeugolis, D.I. Transforming eukaryotic cell culture with macromolecular crowding. Trends Biochem. Sci. 2021, 46, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Lam, D.; Enright, H.A.; Cadena, J.; Peters, S.K.G.; Sales, A.P.; Osburn, J.J.; Soscia, D.A.; Kulp, K.S.; Wheeler, E.K.; Fischer, N.O. Tissue-specific extracellular matrix accelerates the formation of neural networks and communities in a neuron-glia co-culture on a multi-electrode array. Sci. Rep. 2019, 9, 4159. [Google Scholar] [CrossRef] [PubMed]
- Sood, D.; Chwalek, K.; Stuntz, E.; Pouli, D.; Du, C.; Tang-Schomer, M.; Georgakoudi, I.; Black, L.D., 3rd; Kaplan, D.L. Fetal brain extracellular matrix boosts neuronal network formation in 3D bioengineered model of cortical brain tissue. ACS Biomater. Sci. Eng. 2016, 2, 131–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakayama, K.H.; Hou, L.; Huang, N.F. Role of extracellular matrix signaling cues in modulating cell fate commitment for cardiovascular tissue engineering. Adv. Healthc. Mater. 2014, 3, 628–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Loe, F.; Blocki, A.; Peng, Y.; Raghunath, M. Applying macromolecular crowding to enhance extracellular matrix deposition and its remodeling in vitro for tissue engineering and cell-based therapies. Adv. Drug Deliv. Rev. 2011, 63, 277–290. [Google Scholar] [CrossRef]
- Ellis, R.J. Macromolecular crowding: Obvious but underappreciated. Trends Biochem. Sci. 2001, 26, 597–604. [Google Scholar] [CrossRef]
- Benny, P.; Raghunath, M. Making microenvironments: A look into incorporating macromolecular crowding into in vitro experiments, to generate biomimetic microenvironments which are capable of directing cell function for tissue engineering applications. J. Tissue Eng. 2017, 8, 2041731417730467. [Google Scholar] [CrossRef]
- Benny, P.; Badowski, C.; Lane, E.B.; Raghunath, M. Making more matrix: Enhancing the deposition of dermal-epidermal junction components in vitro and accelerating organotypic skin culture development, using macromolecular crowding. Tissue Eng. Part A 2015, 21, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Bardy, C.; van den Hurk, M.; Eames, T.; Marchand, C.; Hernandez, R.V.; Kellogg, M.; Gorris, M.; Galet, B.; Palomares, V.; Brown, J.; et al. Neuronal medium that supports basic synaptic functions and activity of human neurons in vitro. Proc. Natl. Acad. Sci. USA 2015, 112, E2725–E2734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, M.; Waldrup, J.; Qian, X.; Taylor, M.S. Automated analysis of dynamic Ca2+ signals in image sequences. J. Vis. Exp. 2014, 16, 51560. [Google Scholar] [CrossRef] [Green Version]
- Sofroniew, M.V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009, 32, 638–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massey, J.M.; Amps, J.; Viapiano, M.S.; Matthews, R.T.; Wagoner, M.R.; Whitaker, C.M.; Alilain, W.; Yonkof, A.L.; Khalyfa, A.; Cooper, N.G.; et al. Increased chondroitin sulfate proteoglycan expression in denervated brainstem targets following spinal cord injury creates a barrier to axonal regeneration overcome by chondroitinase ABC and neurotrophin-3. Exp. Neurol. 2008, 209, 426–445. [Google Scholar] [CrossRef] [Green Version]
- Quintana, L.; zur Nieden, N.I.; Semino, C.E. Morphogenetic and regulatory mechanisms during developmental chondrogenesis: New paradigms for cartilage tissue engineering. Tissue Eng. Part B Rev. 2009, 15, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Quintana, A.; Muller, M.; Frausto, R.F.; Ramos, R.; Getts, D.R.; Sanz, E.; Hofer, M.J.; Krauthausen, M.; King, N.J.; Hidalgo, J.; et al. Site-specific production of IL-6 in the central nervous system retargets and enhances the inflammatory response in experimental autoimmune encephalomyelitis. J. Immunol. 2009, 183, 2079–2088. [Google Scholar] [CrossRef]
- Kabos, P.; Matundan, H.; Zandian, M.; Bertolotto, C.; Robinson, M.L.; Davy, B.E.; Yu, J.S.; Krueger, R.C., Jr. Neural precursors express multiple chondroitin sulfate proteoglycans, including the lectican family. Biochem. Biophys. Res. Commun. 2004, 318, 955–963. [Google Scholar] [CrossRef]
- Cheng, Y.; Franco-Barraza, J.; Wang, Y.; Zheng, C.; Zhang, L.; Qu, Y.; Long, Y.; Cukierman, E.; Yang, Z.J. Sustained hedgehog signaling in medulloblastoma tumoroids is attributed to stromal astrocytes and astrocyte-derived extracellular matrix. Lab. Investig. 2020, 100, 1208–1222. [Google Scholar] [CrossRef]
- Hynes, R.O. The extracellular matrix: Not just pretty fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef] [Green Version]
- Zagris, N.; Gilipathi, K.; Soulintzi, N.; Konstantopoulos, K. Decorin developmental expression and function in the early avian embryo. Int. J. Dev. Biol. 2011, 55, 633–639. [Google Scholar] [CrossRef] [Green Version]
- Skeath, J.B.; Wilson, B.A.; Romero, S.E.; Snee, M.J.; Zhu, Y.; Lacin, H. The extracellular metalloprotease AdamTS-A anchors neural lineages in place within and preserves the architecture of the central nervous system. Development 2017, 144, 3102–3113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, K.R.; Newland, B.; Florio, M.; Kalebic, N.; Langen, B.; Kolterer, A.; Wimberger, P.; Huttner, W.B. Extracellular Matrix Components HAPLN1, Lumican, and Collagen I Cause Hyaluronic Acid-Dependent Folding of the Developing Human Neocortex. Neuron 2018, 99, 702–719.e706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, K.R.; Huttner, W.B. How the extracellular matrix shapes neural development. Open Biol. 2019, 9, 180216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ang, X.M.; Lee, M.H.; Blocki, A.; Chen, C.; Ong, L.L.; Asada, H.H.; Sheppard, A.; Raghunath, M. Macromolecular crowding amplifies adipogenesis of human bone marrow-derived mesenchymal stem cells by enhancing the pro-adipogenic microenvironment. Tissue Eng. Part A 2014, 20, 966–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiese, S.; Karus, M.; Faissner, A. Astrocytes as a source for extracellular matrix molecules and cytokines. Front. Pharmacol. 2012, 3, 120. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Dai, Y.; Chen, G.; Cui, S. Dissecting the Dual Role of the Glial Scar and Scar-Forming Astrocytes in Spinal Cord Injury. Front. Cell Neurosci. 2020, 14, 78. [Google Scholar] [CrossRef] [Green Version]
- Espinoza, F.; Magdalena, R.; Saldivia, N.; Jara, N.; Martinez, F.; Ferrada, L.; Salazar, K.; Avila, F.; Nualart, F. Vitamin C Recycling Regulates Neurite Growth in Neurospheres Differentiated In Vitro. Antioxidants 2020, 9, 1276. [Google Scholar] [CrossRef]
- Harrison, F.E.; May, J.M. Vitamin C function in the brain: Vital role of the ascorbate transporter SVCT2. Free Radic. Biol. Med. 2009, 46, 719–730. [Google Scholar] [CrossRef] [Green Version]
- Boyera, N.; Galey, I.; Bernard, B.A. Effect of vitamin C and its derivatives on collagen synthesis and cross-linking by normal human fibroblasts. Int. J. Cosmet. Sci. 1998, 20, 151–158. [Google Scholar] [CrossRef]
- Jones, E.V.; Bouvier, D.S. Astrocyte-secreted matricellular proteins in CNS remodelling during development and disease. Neural. Plast. 2014, 2014, 321209. [Google Scholar] [CrossRef] [Green Version]
- Robel, S.; Berninger, B.; Gotz, M. The stem cell potential of glia: Lessons from reactive gliosis. Nat. Rev. Neurosci. 2011, 12, 88–104. [Google Scholar] [CrossRef] [PubMed]
- Ghane, N.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Das, O.; Ramakrishna, S. Regeneration of the peripheral nerve via multifunctional electrospun scaffolds. J. Biomed. Mater. Res. Part A 2021, 109, 437–452. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Liu, S.; Yang, Y.; Gao, S.; Li, W.; Cao, J.; Wan, Y.; Huang, Z.; Fan, G.; Chen, Q.; et al. Aligned microfiber-induced macrophage polarization to guide schwann-cell-enabled peripheral nerve regeneration. Biomaterials 2021, 272, 120767. [Google Scholar] [CrossRef] [PubMed]
- Kurshan, P.T.; Phan, A.Q.; Wang, G.J.; Crane, M.M.; Lu, H.; Shen, K. Regulation of synaptic extracellular matrix composition is critical for proper synapse morphology. J. Neurosci. 2014, 34, 12678–12689. [Google Scholar] [CrossRef] [Green Version]
- Khakh, B.S.; McCarthy, K.D. Astrocyte calcium signaling: From observations to functions and the challenges therein. Cold Spring Harb. Perspect. Biol. 2015, 7, a020404. [Google Scholar] [CrossRef] [Green Version]
- Shan, L.; Zhang, T.; Fan, K.; Cai, W.; Liu, H. Astrocyte-Neuron Signaling in Synaptogenesis. Front. Cell Dev. Biol. 2021, 9, 680301. [Google Scholar] [CrossRef]
- Nishimune, H.; Sanes, J.R.; Carlson, S.S. A synaptic laminin-calcium channel interaction organizes active zones in motor nerve terminals. Nature 2004, 432, 580–587. [Google Scholar] [CrossRef]
- Carafoli, E.; Krebs, J. Why Calcium? How Calcium Became the Best Communicator. J. Biol. Chem. 2016, 291, 20849–20857. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, M.A. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb. Perspect. Biol. 2010, 2, a005066. [Google Scholar] [CrossRef]
- Carbajal, M.E.; Manning-Cela, R.; Pina, A.; Franco, E.; Meza, I. Fibronectin-induced intracellular calcium rise in Entamoeba histolytica trophozoites: Effect on adhesion and the actin cytoskeleton. Exp. Parasitol. 1996, 82, 11–20. [Google Scholar] [CrossRef]
- Nebe, B.; Rychly, J.; Knopp, A.; Bohn, W. Mechanical induction of beta 1-integrin-mediated calcium signaling in a hepatocyte cell line. Exp. Cell Res. 1995, 218, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Nishimune, H. Molecular mechanism of active zone organization at vertebrate neuromuscular junctions. Mol. Neurobiol. 2012, 45, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, Y.R.; Tseng, K.F.; Lai, H.Y.; Lin, C.H.; Lee, O.K. Matrix stiffness regulation of integrin-mediated mechanotransduction during osteogenic differentiation of human mesenchymal stem cells. J. Bone Miner. Res. 2011, 26, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Sorg, B.A.; Berretta, S.; Blacktop, J.M.; Fawcett, J.W.; Kitagawa, H.; Kwok, J.C.; Miquel, M. Casting a Wide Net: Role of Perineuronal Nets in Neural Plasticity. J. Neurosci. 2016, 36, 11459–11468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steullet, P.; Cabungcal, J.H.; Cuenod, M.; Do, K.Q. Fast oscillatory activity in the anterior cingulate cortex: Dopaminergic modulation and effect of perineuronal net loss. Front. Cell Neurosci. 2014, 8, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, H.T.; Schuman, E.M. Frequency-dependent gating of synaptic transmission and plasticity by dopamine. Front. Neural Circuits 2007, 1, 1. [Google Scholar] [CrossRef] [Green Version]
- Tomar, A.; Polygalov, D.; Chattarji, S.; McHugh, T.J. Stress enhances hippocampal neuronal synchrony and alters ripple-spike interaction. Neurobiol. Stress 2021, 14, 100327. [Google Scholar] [CrossRef]
- Cho, K.K.; Hoch, R.; Lee, A.T.; Patel, T.; Rubenstein, J.L.; Sohal, V.S. Gamma rhythms link prefrontal interneuron dysfunction with cognitive inflexibility in Dlx5/6(+/−) mice. Neuron 2015, 85, 1332–1343. [Google Scholar] [CrossRef] [Green Version]
- Wingert, J.C.; Sorg, B.A. Impact of Perineuronal Nets on Electrophysiology of Parvalbumin Interneurons, Principal Neurons, and Brain Oscillations: A Review. Front. Synaptic Neurosci. 2021, 13, 673210. [Google Scholar] [CrossRef]
- Ford, C.P. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience 2014, 282, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Karain, B.; Brantley, E.; Shi, W.X. Effects of L-DOPA on nigral dopamine neurons and local field potential: Comparison with apomorphine and muscimol. J. Pharmacol. Exp. Ther. 2011, 337, 533–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaglini, F.; Pardini, C.; Viaggi, C.; Caramelli, A.; Corsini, G.U. Apomorphine offers new insight into dopaminergic neuron vulnerability in mesencephalic cultures. Neuropharmacology 2008, 55, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhao, Y.; Yang, H.; Luan, W.; Song, J.; Cui, D.; Dong, Y.; Lai, B.; Ma, L.; Zheng, P. Morphine disinhibits glutamatergic input to VTA dopamine neurons and promotes dopamine neuron excitation. Elife 2015, 4, e09275. [Google Scholar] [CrossRef] [PubMed]
- Stylianopoulos, T.; Poh, M.Z.; Insin, N.; Bawendi, M.G.; Fukumura, D.; Munn, L.L.; Jain, R.K. Diffusion of particles in the extracellular matrix: The effect of repulsive electrostatic interactions. Biophys. J. 2010, 99, 1342–1349. [Google Scholar] [CrossRef] [Green Version]
- Wan, L.; Yin, J.; Skoko, J.; Schwartz, R.; Zhang, M.; LeDuc, P.R.; Neumann, C.A. 3D Collagen Vascular Tumor-on-a-Chip Mimetics for Dynamic Combinatorial Drug Screening. Mol. Cancer Ther. 2021, 20, 1210–1219. [Google Scholar] [CrossRef]


| Antibody Target (Clone) | Primary/Secondary | Host and Clonality | Manufacturer and Catalog No. | Dilution |
|---|---|---|---|---|
| Collagen IV | Primary | Mouse monoclonal | Dako, M0785 | 1:200 |
| Fibronectin | Primary | Rabbit polyclonal | Abcam, ab2413 | 1:200 |
| GFAP | Primary | Rabbit polyclonal | Dako, Z0334 | 1:1000 |
| Laminin (alpha1) | Primary | Rabbit polyclonal | Dako, Z0097 | 1:200 |
| Microtubule-associated protein2 (MAP2) | Primary | Mouse monoclonal | Sigma, M4403 | 1:200 |
| Tyrosine hydroxylase (TH) | Primary | Rabbit polyclonal | EMD Millipore, AB152 | 1:1000 |
| Goat anti-mouse 555 | Secondary | ThermoFisher, A28180 | 1:500 | |
| Goat anti-rabbit 647 | Secondary | ThermoFisher, A27040 | 1:500 | |
| Goat anti-chicken 488 | Secondary | Invitrogen, A32931 | 1:500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vo, A.N.; Kundu, S.; Strong, C.; Jung, O.; Lee, E.; Song, M.J.; Boutin, M.E.; Raghunath, M.; Ferrer, M. Enhancement of Neuroglial Extracellular Matrix Formation and Physiological Activity of Dopaminergic Neural Cocultures by Macromolecular Crowding. Cells 2022, 11, 2131. https://doi.org/10.3390/cells11142131
Vo AN, Kundu S, Strong C, Jung O, Lee E, Song MJ, Boutin ME, Raghunath M, Ferrer M. Enhancement of Neuroglial Extracellular Matrix Formation and Physiological Activity of Dopaminergic Neural Cocultures by Macromolecular Crowding. Cells. 2022; 11(14):2131. https://doi.org/10.3390/cells11142131
Chicago/Turabian StyleVo, Andy N., Srikanya Kundu, Caroline Strong, Olive Jung, Emily Lee, Min Jae Song, Molly E. Boutin, Michael Raghunath, and Marc Ferrer. 2022. "Enhancement of Neuroglial Extracellular Matrix Formation and Physiological Activity of Dopaminergic Neural Cocultures by Macromolecular Crowding" Cells 11, no. 14: 2131. https://doi.org/10.3390/cells11142131
APA StyleVo, A. N., Kundu, S., Strong, C., Jung, O., Lee, E., Song, M. J., Boutin, M. E., Raghunath, M., & Ferrer, M. (2022). Enhancement of Neuroglial Extracellular Matrix Formation and Physiological Activity of Dopaminergic Neural Cocultures by Macromolecular Crowding. Cells, 11(14), 2131. https://doi.org/10.3390/cells11142131







