Vascular Endothelial Glycocalyx Damage and Potential Targeted Therapy in COVID-19
Abstract
:1. Introduction
2. Endothelial Glycocalyx in Vascular Hemostasis
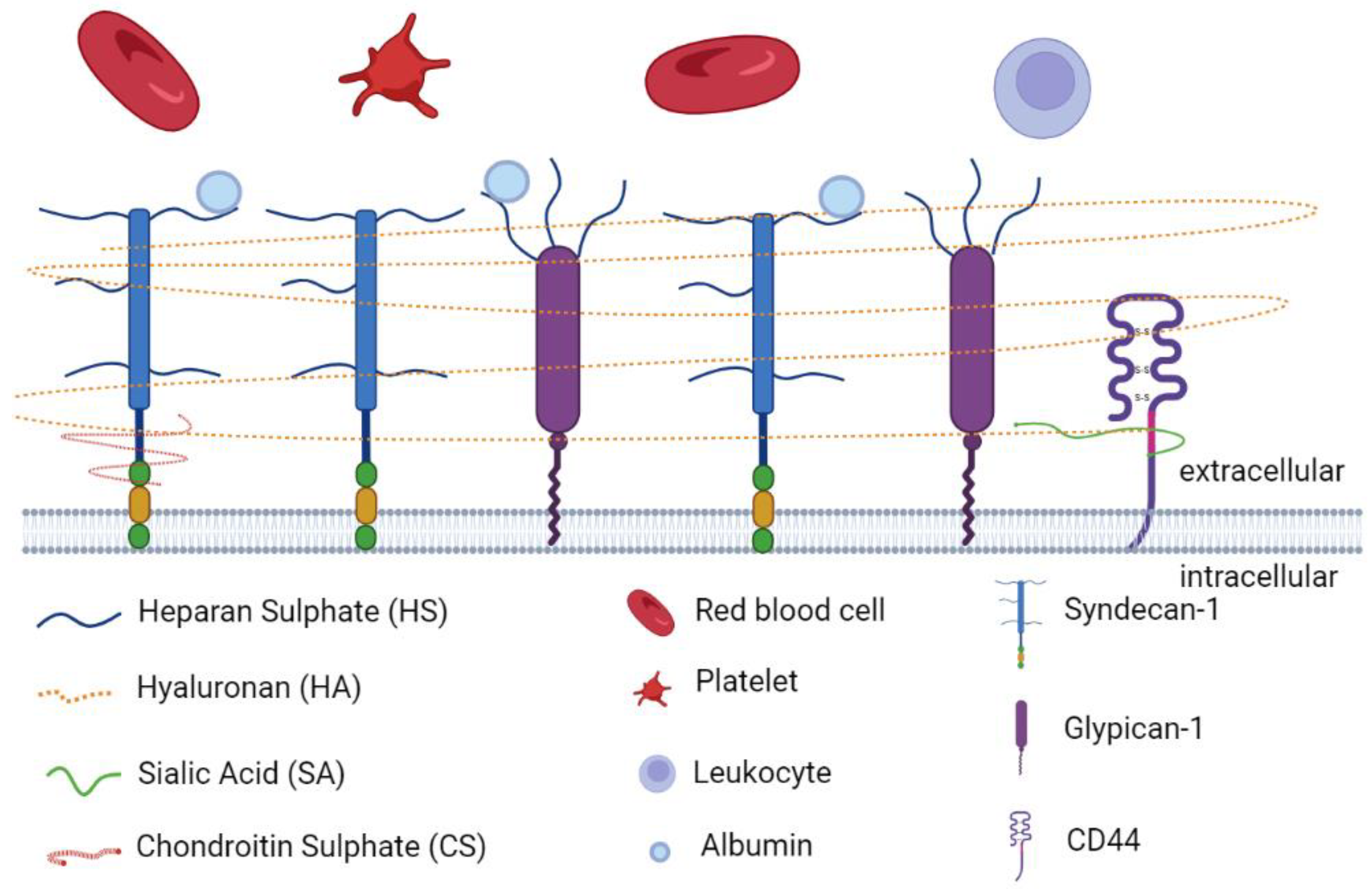
2.1. Endothelial Glycocalyx Components
2.2. Physiological Roles of EGL in Vascular Homeostasis
2.3. Regulation of the EGL Degradation
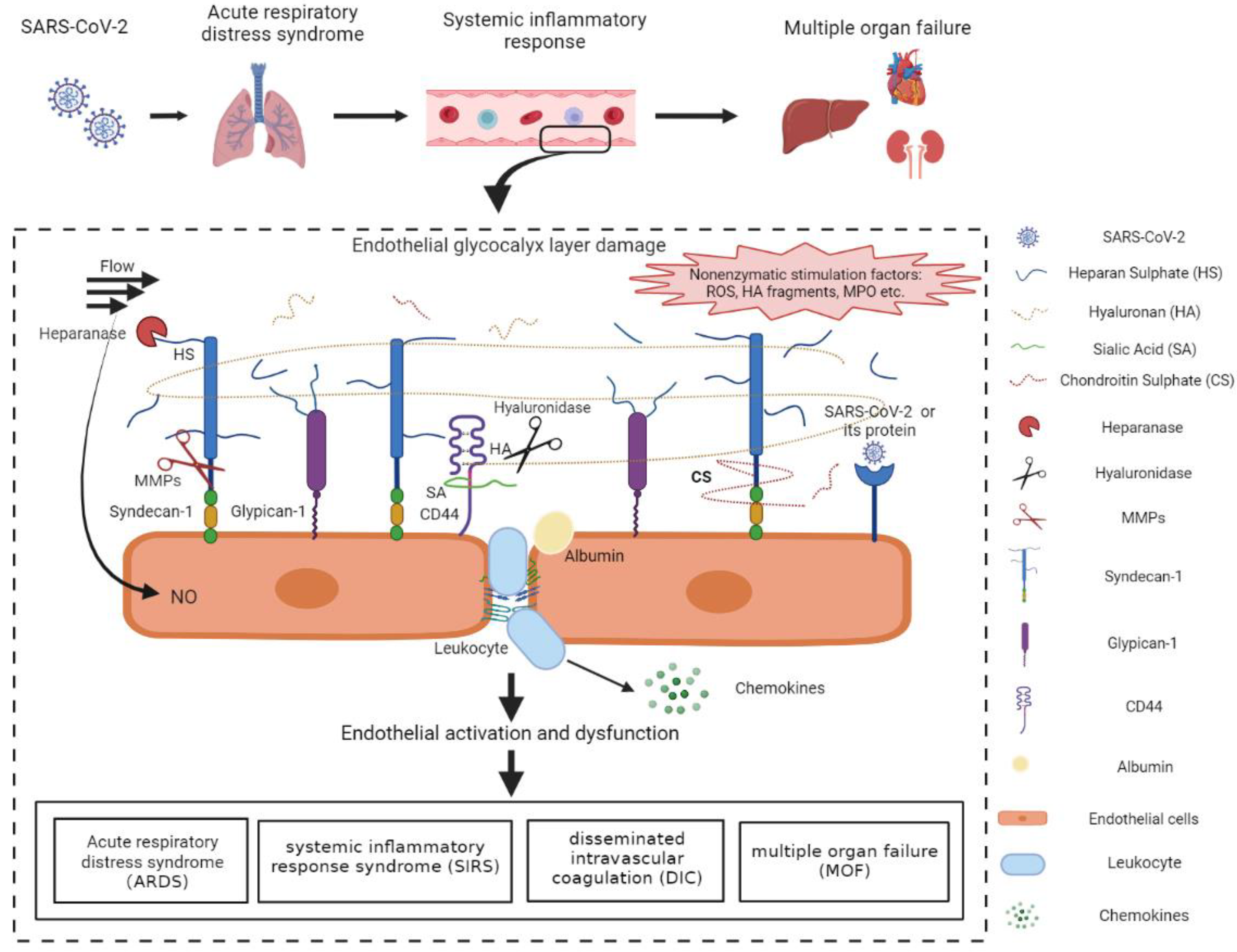
3. Endothelial Glycocalyx Damage in COVID-19
3.1. Glycocalyx Fragments and Sheddases Are Elevated in COVID-19 Patients
3.2. Endothelial Glycocalyx Damage Exacerbates ARDS in COVID-19
3.3. Endothelial Glycocalyx Damage Promotes Coagulopathy in COVID-19
3.4. Multisystem Inflammatory Disease in Children and the Aged Associated with SARS-CoV-2
4. The Mechanisms of COVID-19-Induced Endothelial Glycocalyx Damage
4.1. The Pro-Inflammatory Cytokines and ROS-Induced Glycocalyx Degradation
4.2. The Role of MMPs in the Degradation of Endothelial Glycocalyx
4.3. Glycocalyx Fragments-Induced Endothelial Barrier Dysfunction
4.4. Viral Proteins May Be Involved in Glycocalyx Degradation
5. The Potential Therapy Targeting the EGL in COVID-19 Patients
5.1. Heparin
5.2. Sulodexide
5.3. The Corticosteroids
5.4. Tocilizumab
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Goshua, G.; Pine, A.B.; Meizlish, M.L.; Chang, C.H.; Zhang, H.; Bahel, P.; Baluha, A.; Bar, N.; Bona, R.D.; Burns, A.J.; et al. Endotheliopathy in COVID-19-associated coagulopathy: Evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020, 7, e575–e582. [Google Scholar] [CrossRef]
- Smadja, D.M.; Mentzer, S.J.; Fontenay, M.; Laffan, M.A.; Ackermann, M.; Helms, J.; Jonigk, D.; Chocron, R.; Pier, G.B.; Gendron, N.; et al. COVID-19 is a systemic vascular hemopathy: Insight for mechanistic and clinical aspects. Angiogenesis 2021, 24, 755–788. [Google Scholar] [CrossRef]
- Targosz-Korecka, M.; Kubisiak, A.; Kloska, D.; Kopacz, A.; Grochot-Przeczek, A.; Szymonski, M. Endothelial glycocalyx shields the interaction of SARS-CoV-2 spike protein with ACE2 receptors. Sci. Rep. 2021, 11, 12157. [Google Scholar] [CrossRef]
- Tammaro, A.; Adebanjo, G.A.R.; Del Nonno, F.; Pezzuto, A.; Ramirez-Estrada, S.; Parisella, F.R.; Rello, J.; Scarabello, A. Cutaneous Endothelial Dysfunction and Complement Deposition in COVID-19. Am. J. Dermatopathol. 2021, 43, 237–238. [Google Scholar] [CrossRef]
- Pezzuto, A.; Tammaro, A.; Tonini, G.; Conforti, G.; Falangone, F.; Spuntarelli, V.; Teggi, A.; Pennica, A. SARS-Cov-2 pneumonia and concurrent myelodysplasia complicated by Pseudomonas aeruginosa over-infection. J. Virol. Methods 2022, 300, 114419. [Google Scholar] [CrossRef] [PubMed]
- Sardu, C.; Gambardella, J.; Morelli, M.B.; Wang, X.; Marfella, R.; Santulli, G. Hypertension, Thrombosis, Kidney Failure, and Diabetes: Is COVID-19 an Endothelial Disease? A Comprehensive Evaluation of Clinical and Basic Evidence. J. Clin. Med. 2020, 9, 1417. [Google Scholar] [CrossRef]
- Quinaglia, T.; Shabani, M.; Breder, I.; Silber, H.A.; Lima, J.A.C.; Sposito, A.C. Coronavirus disease-19: The multi-level, multi-faceted vasculopathy. Atherosclerosis 2021, 322, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Amraei, R.; Rahimi, N. COVID-19, Renin-Angiotensin System and Endothelial Dysfunction. Cells 2020, 9, 1652. [Google Scholar] [CrossRef]
- Pearce, L.; Davidson, S.M.; Yellon, D.M. The cytokine storm of COVID-19: A spotlight on prevention and protection. Expert Opin. Ther. Targets 2020, 24, 723–730. [Google Scholar] [CrossRef]
- Cárdenas-Rodríguez, N.; Bandala, C.; Vanoye-Carlo, A.; Ignacio-Mejía, I.; Gómez-Manzo, S.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J.; Carmona-Aparicio, L.; Hernández-Ochoa, B. Use of Antioxidants for the Neuro-Therapeutic Management of COVID-19. Antioxidants 2021, 10, 971. [Google Scholar] [CrossRef]
- Teuwen, L.-A.; Geldhof, V.; Pasut, A.; Carmeliet, P. COVID-19: The vasculature unleashed. Nat. Rev. Immunol. 2020, 20, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Stahl, K.; Gronski, P.A.; Kiyan, Y.; Seeliger, B.; Bertram, A.; Pape, T.; Welte, T.; Hoeper, M.M.; Haller, H.; David, S. Injury to the Endothelial Glycocalyx in Critically Ill Patients with COVID-19. Am. J. Respir. Crit. Care Med. 2020, 202, 1178–1181. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Pons, S.; Fodil, S.; Azoulay, E.; Zafrani, L. The vascular endothelium: The cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit. Care 2020, 24, 353. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Liu, F.; Blair, R.; Wang, C.; Yang, H.; Mudd, J.; Currey, J.M.; Iwanaga, N.; He, J.; Mi, R.; et al. Endothelial cell infection and dysfunction, immune activation in severe COVID-19. Theranostics 2021, 11, 8076–8091. [Google Scholar] [CrossRef]
- Kaur, S.; Tripathi, D.M.; Yadav, A. The Enigma of Endothelium in COVID-19. Front. Physiol. 2020, 11, 989. [Google Scholar] [CrossRef]
- Matarese, A.; Gambardella, J.; Sardu, C.; Santulli, G. miR-98 Regulates TMPRSS2 Expression in Human Endothelial Cells: Key Implications for COVID-19. Biomedicines 2020, 8, 462. [Google Scholar] [CrossRef] [PubMed]
- Fodor, A.; Tiperciuc, B.; Login, C.; Orasan, O.H.; Lazar, A.L.; Buchman, C.; Hanghicel, P.; Sitar-Taut, A.; Suharoschi, R.; Vulturar, R.; et al. Endothelial Dysfunction, Inflammation, and Oxidative Stress in COVID-19-Mechanisms and Therapeutic Targets. Oxidative Med. Cell. Longev. 2021, 2021, 8671713. [Google Scholar] [CrossRef]
- Fiorentino, G.; Coppola, A.; Izzo, R.; Annunziata, A.; Bernardo, M.; Lombardi, A.; Trimarco, V.; Santulli, G.; Trimarco, B. Effects of adding L-arginine orally to standard therapy in patients with COVID-19: A randomized, double-blind, placebo-controlled, parallel-group trial. Results of the first interim analysis. EClinicalMedicine 2021, 40, 101125. [Google Scholar] [CrossRef]
- Mone, P.; Gambardella, J.; Wang, X.; Jankauskas, S.S.; Matarese, A.; Santulli, G. miR-24 Targets the Transmembrane Glycoprotein Neuropilin-1 in Human Brain Microvascular Endothelial Cells. Noncoding RNA 2021, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, J.; Coppola, A.; Izzo, R.; Fiorentino, G.; Trimarco, B.; Santulli, G. Role of endothelial miR-24 in COVID-19 cerebrovascular events. Crit. Care 2021, 25, 306. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, J.; Santulli, G. What is linking COVID-19 and endothelial dysfunction? Updates on nanomedicine and bioengineering from the 2020 AHA Scientific Sessions. Eur. Heart J. Cardiovasc. Pharm. 2021, 7, e2–e3. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zhong, L.; Luo, Y. Endothelial glycocalyx as an important factor in composition of blood-brain barrier. CNS Neurosci. Ther. 2021, 27, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka-Tojo, M. Vascular Endothelial Glycocalyx Damage in COVID-19. Int. J. Mol. Sci. 2020, 21, 9712. [Google Scholar] [CrossRef] [PubMed]
- Goonewardena, S.N.; Grushko, O.G.; Wells, J.; Herty, L.; Rosenson, R.S.; Haus, J.M.; Hummel, S.L. Immune-Mediated Glycocalyx Remodeling in Hospitalized COVID-19 Patients. Cardiovasc. Drugs Ther. 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Karampoor, S.; Zahednasab, H.; Farahmand, M.; Mirzaei, R.; Zamani, F.; Tabibzadeh, A.; Bouzari, B.; Ajdarkosh, H.; Nikkhah, M.; Hashemi, M.R.; et al. A possible pathogenic role of Syndecan-1 in the pathogenesis of coronavirus disease 2019 (COVID-19). Int. Immunopharmacol. 2021, 97, 107684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, L.; Chen, Y.; Ma, J.; Yang, Y.; Aodeng, S.; Cui, Q.; Wen, K.; Xiao, M.; Xie, J.; et al. Syndecan-1, an indicator of endothelial glycocalyx degradation, predicts outcome of patients admitted to an ICU with COVID-19. Mol. Med. 2021, 27, 151. [Google Scholar] [CrossRef]
- Suzuki, K.; Okada, H.; Tomita, H.; Sumi, K.; Kakino, Y.; Yasuda, R.; Kitagawa, Y.; Fukuta, T.; Miyake, T.; Yoshida, S.; et al. Possible involvement of Syndecan-1 in the state of COVID-19 related to endothelial injury. Thromb. J. 2021, 19, 5. [Google Scholar] [CrossRef]
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020, 18, 1094–1099. [Google Scholar] [CrossRef]
- Kinaneh, S.; Khamaysi, I.; Karram, T.; Hamoud, S. Heparanase as a potential player in SARS-CoV-2 infection and induced coagulopathy. Biosci. Rep. 2021, 41, BSR20210290. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, I.; Pavlidis, G.; Katsimbri, P.; Lambadiari, V.; Parissis, J.; Andreadou, I.; Tsoumani, M.; Boumpas, D.; Kouretas, D.; Iliodromitis, E. Tocilizumab improves oxidative stress and endothelial glycocalyx: A mechanism that may explain the effects of biological treatment on COVID-19. Food Chem. Toxicol. 2020, 145, 111694. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka-Tojo, M. Endothelial glycocalyx damage as a systemic inflammatory microvascular endotheliopathy in COVID-19. Biomed. J. 2020, 43, 399–413. [Google Scholar] [CrossRef]
- Rambourg, A.; Neutra, M.; Leblond, C.P. Presence of a “cell coat” rich in carbohydrate at the surface of cells in the rat. Anat. Rec. 1966, 154, 41–71. [Google Scholar] [CrossRef] [PubMed]
- Nishi, S.; Ozawa, H.; Arakawa, M. A cytochemical study of glycocalyx and the membrane cholesterol of rat glomerular podocytes. Arch. Histol. Cytol. 1990, 53, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Iba, T.; Levy, J.H. Derangement of the endothelial glycocalyx in sepsis. J. Thromb. Haemost. 2019, 17, 283–294. [Google Scholar] [CrossRef] [Green Version]
- Puerta-Guardo, H.; Glasner, D.R.; Harris, E. Dengue Virus NS1 Disrupts the Endothelial Glycocalyx, Leading to Hyperpermeability. PLoS Pathog. 2016, 12, e1005738. [Google Scholar] [CrossRef] [Green Version]
- Weinbaum, S.; Tarbell, J.M.; Damiano, E.R. The structure and function of the endothelial glycocalyx layer. Annu. Rev. Biomed. Eng. 2007, 9, 121–167. [Google Scholar] [CrossRef]
- Alphonsus, C.S.; Rodseth, R.N. The endothelial glycocalyx: A review of the vascular barrier. Anaesthesia 2014, 69, 777–784. [Google Scholar] [CrossRef]
- Jin, J.; Fang, F.; Gao, W.; Chen, H.; Wen, J.; Wen, X.; Chen, J. The Structure and Function of the Glycocalyx and Its Connection With Blood-Brain Barrier. Front. Cell. Neurosci. 2021, 15, 739699. [Google Scholar] [CrossRef]
- du Preez, H.N.; Aldous, C.; Hayden, M.R.; Kruger, H.G.; Lin, J. Pathogenesis of COVID-19 described through the lens of an undersulfated and degraded epithelial and endothelial glycocalyx. FASEB J. 2022, 36, e22052. [Google Scholar] [CrossRef]
- Krüger-Genge, A.; Blocki, A.; Franke, R.-P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldecoa, C.; Llau, J.V.; Nuvials, X.; Artigas, A. Role of albumin in the preservation of endothelial glycocalyx integrity and the microcirculation: A review. Ann. Intensive Care 2020, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Dogné, S.; Flamion, B. Endothelial Glycocalyx Impairment in Disease: Focus on Hyaluronan Shedding. Am. J. Pathol. 2020, 190, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Uchimido, R.; Schmidt, E.P.; Shapiro, N.I. The glycocalyx: A novel diagnostic and therapeutic target in sepsis. Crit. Care 2019, 23, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmon, A.H.J.; Satchell, S.C. Endothelial glycocalyx dysfunction in disease: Albuminuria and increased microvascular permeability. J. Pathol. 2012, 226, 562–574. [Google Scholar] [CrossRef]
- Reitsma, S.; Slaaf, D.W.; Vink, H.; van Zandvoort, M.A.M.J.; oude Egbrink, M.G.A. The endothelial glycocalyx: Composition, functions, and visualization. Pflug. Arch. 2007, 454, 345–359. [Google Scholar] [CrossRef] [Green Version]
- Rehm, M.; Zahler, S.; Lötsch, M.; Welsch, U.; Conzen, P.; Jacob, M.; Becker, B.F. Endothelial glycocalyx as an additional barrier determining extravasation of 6% hydroxyethyl starch or 5% albumin solutions in the coronary vascular bed. Anesthesiology 2004, 100, 1211–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballermann, B.J.; Nyström, J.; Haraldsson, B. The Glomerular Endothelium Restricts Albumin Filtration. Front. Med. 2021, 8, 766689. [Google Scholar] [CrossRef]
- Fernández-Sarmiento, J.; Salazar-Peláez, L.M.; Carcillo, J.A. The Endothelial Glycocalyx: A Fundamental Determinant of Vascular Permeability in Sepsis. Pediatr. Crit. Care Med. 2020, 21, e291–e300. [Google Scholar] [CrossRef]
- Pahakis, M.Y.; Kosky, J.R.; Dull, R.O.; Tarbell, J.M. The role of endothelial glycocalyx components in mechanotransduction of fluid shear stress. Biochem. Biophys. Res. Commun. 2007, 355, 228–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betteridge, K.B.; Arkill, K.P.; Neal, C.R.; Harper, S.J.; Foster, R.R.; Satchell, S.C.; Bates, D.O.; Salmon, A.H.J. Sialic acids regulate microvessel permeability, revealed by novel in vivo studies of endothelial glycocalyx structure and function. J. Physiol. 2017, 595, 5015–5035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patarroyo, M.; Makgoba, M.W. Leucocyte adhesion to cells in immune and inflammatory responses. Lancet 1989, 2, 1139–1142. [Google Scholar] [CrossRef]
- Adams, D.H.; Shaw, S. Leucocyte-endothelial interactions and regulation of leucocyte migration. Lancet 1994, 343, 831–836. [Google Scholar] [CrossRef]
- Albelda, S.M. Endothelial and epithelial cell adhesion molecules. Am. J. Respir. Cell Mol. Biol. 1991, 4, 195–203. [Google Scholar] [CrossRef]
- Ince, C.; Mayeux, P.R.; Nguyen, T.; Gomez, H.; Kellum, J.A.; Ospina-Tascón, G.A.; Hernandez, G.; Murray, P.; De Backer, D. THE ENDOTHELIUM IN SEPSIS. Shock 2016, 45, 259–270. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, E.P.; Kuebler, W.M.; Lee, W.L.; Downey, G.P. Adhesion Molecules: Master Controllers of the Circulatory System. Compr. Physiol. 2016, 6, 945–973. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Nejadhamzeeigilani, Z.; Gutowski, N.J.; Whatmore, J.L. Loss of the endothelial glycocalyx is associated with increased E-selectin mediated adhesion of lung tumour cells to the brain microvascular endothelium. J. Exp. Clin. Cancer Res. 2015, 34, 105. [Google Scholar] [CrossRef] [Green Version]
- Delgadillo, L.F.; Marsh, G.A.; Waugh, R.E. Endothelial Glycocalyx Layer Properties and Its Ability to Limit Leukocyte Adhesion. Biophys. J. 2020, 118, 1564–1575. [Google Scholar] [CrossRef]
- Lipowsky, H.H. Role of the Glycocalyx as a Barrier to Leukocyte-Endothelium Adhesion. Adv. Exp. Med. Biol. 2018, 1097, 51–68. [Google Scholar] [CrossRef]
- Zou, Z.; Li, L.; Schäfer, N.; Huang, Q.; Maegele, M.; Gu, Z. Endothelial glycocalyx in traumatic brain injury associated coagulopathy: Potential mechanisms and impact. J. Neuroinflamm. 2021, 18, 134. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.M.; Tarbell, J.M. Mechano-sensing and transduction by endothelial surface glycocalyx: Composition, structure, and function. Wiley Interdiscip Rev. Syst. Biol. Med. 2013, 5, 381–390. [Google Scholar] [CrossRef] [Green Version]
- Lupu, F.; Kinasewitz, G.; Dormer, K. The role of endothelial shear stress on haemodynamics, inflammation, coagulation and glycocalyx during sepsis. J. Cell. Mol. Med. 2020, 24, 12258–12271. [Google Scholar] [CrossRef] [PubMed]
- Bartosch, A.M.W.; Mathews, R.; Tarbell, J.M. Endothelial Glycocalyx-Mediated Nitric Oxide Production in Response to Selective AFM Pulling. Biophys. J. 2017, 113, 101–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yen, W.; Cai, B.; Yang, J.; Zhang, L.; Zeng, M.; Tarbell, J.M.; Fu, B.M. Endothelial surface glycocalyx can regulate flow-induced nitric oxide production in microvessels in vivo. PLoS ONE 2015, 10, e0117133. [Google Scholar] [CrossRef] [PubMed]
- de Agostini, A.I.; Watkins, S.C.; Slayter, H.S.; Youssoufian, H.; Rosenberg, R.D. Localization of anticoagulantly active heparan sulfate proteoglycans in vascular endothelium: Antithrombin binding on cultured endothelial cells and perfused rat aorta. J. Cell Biol. 1990, 111, 1293–1304. [Google Scholar] [CrossRef]
- Ostrowski, S.R.; Johansson, P.I. Endothelial glycocalyx degradation induces endogenous heparinization in patients with severe injury and early traumatic coagulopathy. J. Trauma Acute Care Surg. 2012, 73, 60–66. [Google Scholar] [CrossRef]
- Rovas, A.; Osiaevi, I.; Buscher, K.; Sackarnd, J.; Tepasse, P.-R.; Fobker, M.; Kühn, J.; Braune, S.; Göbel, U.; Thölking, G.; et al. Microvascular dysfunction in COVID-19: The MYSTIC study. Angiogenesis 2021, 24, 145–157. [Google Scholar] [CrossRef]
- Hadigal, S.; Koganti, R.; Yadavalli, T.; Agelidis, A.; Suryawanshi, R.; Shukla, D. Heparanase-Regulated Syndecan-1 Shedding Facilitates Herpes Simplex Virus 1 Egress. J. Virol. 2020, 94, e01672-19. [Google Scholar] [CrossRef]
- Secchi, M.F.; Masola, V.; Zaza, G.; Lupo, A.; Gambaro, G.; Onisto, M. Recent data concerning heparanase: Focus on fibrosis, inflammation and cancer. Biomol. Concepts 2015, 6, 415–421. [Google Scholar] [CrossRef]
- Buijsers, B.; Yanginlar, C.; Maciej-Hulme, M.L.; de Mast, Q.; van der Vlag, J. Beneficial non-anticoagulant mechanisms underlying heparin treatment of COVID-19 patients. EBioMedicine 2020, 59, 102969. [Google Scholar] [CrossRef] [PubMed]
- Masola, V.; Bellin, G.; Gambaro, G.; Onisto, M. Heparanase: A Multitasking Protein Involved in Extracellular Matrix (ECM) Remodeling and Intracellular Events. Cells 2018, 7, 236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, J.; Santulli, G. Heparanase in health and disease: The neglected housekeeper of the cell? Atherosclerosis 2019, 283, 124–126. [Google Scholar] [CrossRef]
- Koganti, R.; Suryawanshi, R.; Shukla, D. Heparanase, cell signaling, and viral infections. Cell. Mol. Life Sci 2020, 77, 5059–5077. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Tiemeier, G.L.; van den Berg, B.M.; Rabelink, T.J. Endothelial Glycocalyx Hyaluronan: Regulation and Role in Prevention of Diabetic Complications. Am. J. Pathol. 2020, 190, 781–790. [Google Scholar] [CrossRef]
- Abassi, Z.; Armaly, Z.; Heyman, S.N. Glycocalyx Degradation in Ischemia-Reperfusion Injury. Am. J. Pathol. 2020, 190, 752–767. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Gayosso, I.; Platts, S.H.; Duling, B.R. Reactive oxygen species mediate modification of glycocalyx during ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H2247–H2256. [Google Scholar] [CrossRef]
- Manchanda, K.; Kolarova, H.; Kerkenpaß, C.; Mollenhauer, M.; Vitecek, J.; Rudolph, V.; Kubala, L.; Baldus, S.; Adam, M.; Klinke, A. MPO (Myeloperoxidase) Reduces Endothelial Glycocalyx Thickness Dependent on Its Cationic Charge. Arter. Thromb. Vasc. Biol. 2018, 38, 1859–1867. [Google Scholar] [CrossRef]
- Wadowski, P.P.; Jilma, B.; Kopp, C.W.; Ertl, S.; Gremmel, T.; Koppensteiner, R. Glycocalyx as Possible Limiting Factor in COVID-19. Front. Immunol. 2021, 12, 607306. [Google Scholar] [CrossRef]
- Yang, S.; Tong, Y.; Chen, L.; Yu, W. Human Identical Sequences, hyaluronan, and hymecromone—The new mechanism and management of COVID-19. Mol. Biomed. 2022, 3, 15. [Google Scholar] [CrossRef]
- LaRivière, W.B.; Schmidt, E.P. The Pulmonary Endothelial Glycocalyx in ARDS: A Critical Role for Heparan Sulfate. Curr. Top. Membr. 2018, 82, 33–52. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, F.; Oi, Y.; Nakajima, K.; Matsumura, R.; Nakagawa, T.; Miyagawa, T.; Sakai, K.; Saji, R.; Taniguchi, H.; Takahashi, K.; et al. Temporal change in Syndecan-1 as a therapeutic target and a biomarker for the severity classification of COVID-19. Thromb. J. 2021, 19, 55. [Google Scholar] [CrossRef] [PubMed]
- Vollenberg, R.; Tepasse, P.-R.; Ochs, K.; Floer, M.; Strauss, M.; Rennebaum, F.; Kabar, I.; Rovas, A.; Nowacki, T. Indications of Persistent Glycocalyx Damage in Convalescent COVID-19 Patients: A Prospective Multicenter Study and Hypothesis. Viruses 2021, 13, 2324. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.J.; Gattinoni, L.; Calfee, C.S. Acute respiratory distress syndrome. Lancet 2021, 398, 622–637. [Google Scholar] [CrossRef]
- Buijsers, B.; Yanginlar, C.; de Nooijer, A.; Grondman, I.; Maciej-Hulme, M.L.; Jonkman, I.; Janssen, N.A.F.; Rother, N.; de Graaf, M.; Pickkers, P.; et al. Increased Plasma Heparanase Activity in COVID-19 Patients. Front. Immunol. 2020, 11, 575047. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Iba, T. COVID-19 coagulopathy: Is it disseminated intravascular coagulation? Intern. Emerg. Med. 2021, 16, 309–312. [Google Scholar] [CrossRef]
- Levi, M.; Thachil, J.; Iba, T.; Levy, J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020, 7, e438–e440. [Google Scholar] [CrossRef]
- Ikeda, M.; Matsumoto, H.; Ogura, H.; Hirose, T.; Shimizu, K.; Yamamoto, K.; Maruyama, I.; Shimazu, T. Circulating syndecan-1 predicts the development of disseminated intravascular coagulation in patients with sepsis. J. Crit. Care 2018, 43, 48–53. [Google Scholar] [CrossRef]
- Sillesen, M.; Rasmussen, L.S.; Jin, G.; Jepsen, C.H.; Imam, A.; Hwabejire, J.O.; Halaweish, I.; DeMoya, M.; Velmahos, G.; Johansson, P.I.; et al. Assessment of coagulopathy, endothelial injury, and inflammation after traumatic brain injury and hemorrhage in a porcine model. J. Trauma Acute Care Surg. 2014, 76, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Consiglio, C.R.; Cotugno, N.; Sardh, F.; Pou, C.; Amodio, D.; Rodriguez, L.; Tan, Z.; Zicari, S.; Ruggiero, A.; Pascucci, G.R.; et al. The Immunology of Multisystem Inflammatory Syndrome in Children with COVID-19. Cell 2020, 183, 968–981. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Chen, M.; Weng, J. COVID-19 and Kawasaki disease in children. Pharm. Res. 2020, 159, 104951. [Google Scholar] [CrossRef]
- Jakob, A.; Bohlig, S.; König, M.; Nussbaum, C.; Dalla-Pozza, R.; Hermann, M.; Haas, N.A.; Pastor-Villaescusa, B. Kawasaki disease and increased cardiovascular risk: Is there a link to circulating glycocalyx biomarkers? Microvasc. Res. 2022, 140, 104269. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, Y.; Yasudo, H.; Suzuki, Y.; Furuta, T.; Matsuguma, C.; Azuma, Y.; Miyake, A.; Okada, S.; Ichihara, K.; Ohga, S.; et al. Circulating endothelial glycocalyx components as a predictive marker of coronary artery lesions in Kawasaki disease. Int. J. Cardiol. 2019, 292, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sarmiento, J.; Flórez, S.; Alarcón-Forero, L.C.; Salazar-Peláez, L.M.; Garcia-Casallas, J.; Mulett, H.; Acevedo, L.; Salamanca, C. Case Report: Endothelial Glycocalyx Damage in Critically ill Patients With SARS-CoV-2-Related Multisystem Inflammatory Syndrome (MIS-C). Front. Pediatr. 2021, 9, 726949. [Google Scholar] [CrossRef] [PubMed]
- Veraldi, N.; Vivès, R.R.; Blanchard-Rohner, G.; L’Huillier, A.G.; Wagner, N.; Rohr, M.; Beghetti, M.; De Agostini, A.; Grazioli, S. Endothelial glycocalyx degradation in multisystem inflammatory syndrome in children related to COVID-19. J. Mol. Med. 2022, 100, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Richter, R.P.; Payne, G.A.; Ambalavanan, N.; Gaggar, A.; Richter, J.R. The endothelial glycocalyx in critical illness: A pediatric perspective. Matrix Biol. Plus 2022, 14, 100106. [Google Scholar] [CrossRef] [PubMed]
- Machin, D.R.; Bloom, S.I.; Campbell, R.A.; Phuong, T.T.T.; Gates, P.E.; Lesniewski, L.A.; Rondina, M.T.; Donato, A.J. Advanced age results in a diminished endothelial glycocalyx. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H531–H539. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Klein, S.L.; Garibaldi, B.T.; Li, H.; Wu, C.; Osevala, N.M.; Li, T.; Margolick, J.B.; Pawelec, G.; Leng, S.X. Aging in COVID-19: Vulnerability, immunity and intervention. Ageing Res. Rev. 2021, 65, 101205. [Google Scholar] [CrossRef] [PubMed]
- Stoddart, P.; Satchell, S.C.; Ramnath, R. Cerebral microvascular endothelial glycocalyx damage, its implications on the blood-brain barrier and a possible contributor to cognitive impairment. Brain Res. 2022, 1780, 147804. [Google Scholar] [CrossRef]
- Iba, T.; Connors, J.M.; Levy, J.H. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm. Res. 2020, 69, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Bodro, M.; Compta, Y.; Sánchez-Valle, R. Presentations and mechanisms of CNS disorders related to COVID-19. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e923. [Google Scholar] [CrossRef]
- Domizio, J.D.; Gulen, M.F.; Saidoune, F.; Thacker, V.V.; Yatim, A.; Sharma, K.; Nass, T.; Guenova, E.; Schaller, M.; Conrad, C.; et al. The cGAS-STING pathway drives type I IFN immunopathology in COVID-19. Nature 2022, 603, 145–151. [Google Scholar] [CrossRef]
- Ali, Y.M.; Ferrari, M.; Lynch, N.J.; Yaseen, S.; Dudler, T.; Gragerov, S.; Demopulos, G.; Heeney, J.L.; Schwaeble, W.J. Lectin Pathway Mediates Complement Activation by SARS-CoV-2 Proteins. Front. Immunol. 2021, 12, 714511. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.C.; May, J.W.; Cunningham, M.W.; Jones, O.Y. Multisystem Inflammatory Syndrome in Children (MIS-C), a Post-viral Myocarditis and Systemic Vasculitis-A Critical Review of Its Pathogenesis and Treatment. Front. Pediatr. 2020, 8, 626182. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Lei, T.; Patel, P.S.; Lee, C.H.; Monaghan-Nichols, P.; Xin, H.-B.; Qiu, J.; Fu, M. Direct Activation of Endothelial Cells by SARS-CoV-2 Nucleocapsid Protein Is Blocked by Simvastatin. J. Virol. 2021, 95, e0139621. [Google Scholar] [CrossRef] [PubMed]
- Queisser, K.A.; Mellema, R.A.; Middleton, E.A.; Portier, I.; Manne, B.K.; Denorme, F.; Beswick, E.J.; Rondina, M.T.; Campbell, R.A.; Petrey, A.C. COVID-19 generates hyaluronan fragments that directly induce endothelial barrier dysfunction. JCI Insight 2021, 6, e147472. [Google Scholar] [CrossRef]
- Nader, D.; Kerrigan, S.W. Molecular Cross-Talk between Integrins and Cadherins Leads to a Loss of Vascular Barrier Integrity during SARS-CoV-2 Infection. Viruses 2022, 14, 891. [Google Scholar] [CrossRef]
- Colunga Biancatelli, R.M.L.; Solopov, P.A.; Sharlow, E.R.; Lazo, J.S.; Marik, P.E.; Catravas, J.D. The SARS-CoV-2 spike protein subunit S1 induces COVID-19-like acute lung injury in Κ18-hACE2 transgenic mice and barrier dysfunction in human endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L477–L484. [Google Scholar] [CrossRef]
- Eisenhut, M.; Shin, J.I. Pathways in the Pathophysiology of Coronavirus 19 Lung Disease Accessible to Prevention and Treatment. Front. Physiol. 2020, 11, 872. [Google Scholar] [CrossRef] [PubMed]
- Schmaier, A.A.; Pajares Hurtado, G.M.; Manickas-Hill, Z.J.; Sack, K.D.; Chen, S.M.; Bhambhani, V.; Quadir, J.; Nath, A.K.; Collier, A.-R.Y.; Ngo, D.; et al. Tie2 activation protects against prothrombotic endothelial dysfunction in COVID-19. JCI Insight 2021, 6, e151527. [Google Scholar] [CrossRef]
- Vardakas, P.; Skaperda, Z.; Tekos, F.; Kouretas, D. ROS and COVID. Antioxidants 2022, 11, 339. [Google Scholar] [CrossRef] [PubMed]
- Potje, S.R.; Costa, T.J.; Fraga-Silva, T.F.C.; Martins, R.B.; Benatti, M.N.; Almado, C.E.L.; de Sá, K.S.G.; Bonato, V.L.D.; Arruda, E.; Louzada-Junior, P.; et al. Heparin prevents in vitro glycocalyx shedding induced by plasma from COVID-19 patients. Life Sci. 2021, 276, 119376. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Lu, M.; Shi, M.; Cheng, X.; Kwakwa, K.A.; Davis, J.L.; Su, X.; Bakewell, S.J.; Zhang, Y.; Fontana, F.; et al. Heparanase Blockade as a Novel Dual-Targeting Therapy for COVID-19. J. Virol. 2022, 96, e0005722. [Google Scholar] [CrossRef] [PubMed]
- Noris, M.; Benigni, A.; Remuzzi, G. The case of complement activation in COVID-19 multiorgan impact. Kidney Int. 2020, 98, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Magro, C.; Mulvey, J.J.; Berlin, D.; Nuovo, G.; Salvatore, S.; Harp, J.; Baxter-Stoltzfus, A.; Laurence, J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl. Res. 2020, 220, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, T.; Kubli, S.P.; Yoshinaga, S.K.; Pfeffer, K.; Mak, T.W. An aberrant STAT pathway is central to COVID-19. Cell Death Differ. 2020, 27, 3209–3225. [Google Scholar] [CrossRef]
- Goldberg, R.; Meirovitz, A.; Hirshoren, N.; Bulvik, R.; Binder, A.; Rubinstein, A.M.; Elkin, M. Versatile role of heparanase in inflammation. Matrix Biol. 2013, 32, 234–240. [Google Scholar] [CrossRef]
- Laforge, M.; Elbim, C.; Frère, C.; Hémadi, M.; Massaad, C.; Nuss, P.; Benoliel, J.-J.; Becker, C. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immunol. 2020, 20, 515–516. [Google Scholar] [CrossRef]
- Ramnath, R.D.; Butler, M.J.; Newman, G.; Desideri, S.; Russell, A.; Lay, A.C.; Neal, C.R.; Qiu, Y.; Fawaz, S.; Onions, K.L.; et al. Blocking matrix metalloproteinase-mediated syndecan-4 shedding restores the endothelial glycocalyx and glomerular filtration barrier function in early diabetic kidney disease. Kidney Int. 2020, 97, 951–965. [Google Scholar] [CrossRef] [Green Version]
- Syed, F.; Li, W.; Relich, R.F.; Russell, P.M.; Zhang, S.; Zimmerman, M.K.; Yu, Q. Excessive Matrix Metalloproteinase-1 and Hyperactivation of Endothelial Cells Occurred in COVID-19 Patients and Were Associated With the Severity of COVID-19. J. Infect. Dis. 2021, 224, 60–69. [Google Scholar] [CrossRef]
- D Avila-Mesquita, C.; Couto, A.E.S.; Campos, L.C.B.; Vasconcelos, T.F.; Michelon-Barbosa, J.; Corsi, C.A.C.; Mestriner, F.; Petroski-Moraes, B.C.; Garbellini-Diab, M.J.; Couto, D.M.S.; et al. MMP-2 and MMP-9 levels in plasma are altered and associated with mortality in COVID-19 patients. Biomed. Pharm. 2021, 142, 112067. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Buffonge, S.; Ramnath, R.; Jenner, S.; Fawaz, S.; Arkill, K.P.; Neal, C.; Verkade, P.; White, S.J.; Hezzell, M.; et al. Endothelial glycocalyx is damaged in diabetic cardiomyopathy: Angiopoietin 1 restores glycocalyx and improves diastolic function in mice. Diabetologia 2022, 65, 879–894. [Google Scholar] [CrossRef] [PubMed]
- Lartey, N.L.; Valle-Reyes, S.; Vargas-Robles, H.; Jiménez-Camacho, K.E.; Guerrero-Fonseca, I.M.; Castellanos-Martínez, R.; Montoya-García, A.; García-Cordero, J.; Cedillo-Barrón, L.; Nava, P.; et al. ADAM17/MMP inhibition prevents neutrophilia and lung injury in a mouse model of COVID-19. J. Leukoc. Biol. 2021, 111, 1147–1158. [Google Scholar] [CrossRef]
- Sarker, H.; Haimour, A.; Toor, R.; Fernandez-Patron, C. The Emerging Role of Epigenetic Mechanisms in the Causation of Aberrant MMP Activity during Human Pathologies and the Use of Medicinal Drugs. Biomolecules 2021, 11, 578. [Google Scholar] [CrossRef] [PubMed]
- Hellman, U.; Karlsson, M.G.; Engström-Laurent, A.; Cajander, S.; Dorofte, L.; Ahlm, C.; Laurent, C.; Blomberg, A. Presence of hyaluronan in lung alveoli in severe Covid-19: An opening for new treatment options? J. Biol. Chem. 2020, 295, 15418–15422. [Google Scholar] [CrossRef] [PubMed]
- Ontong, P.; Prachayasittikul, V. Unraveled roles of hyaluronan in severe COVID-19. EXCLI J. 2021, 20, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Ling, Y.; Zhao, F.; Li, W.; Song, Z.; Wang, L.; Li, Q.; Liu, M.; Tong, Y.; Chen, L.; et al. Hymecromone: A clinical prescription hyaluronan inhibitor for efficiently blocking COVID-19 progression. Signal Transduct. Target. Ther. 2022, 7, 91. [Google Scholar] [CrossRef]
- Schimmel, L.; Chew, K.Y.; Stocks, C.J.; Yordanov, T.E.; Essebier, P.; Kulasinghe, A.; Monkman, J.; Dos Santos Miggiolaro, A.F.R.; Cooper, C.; de Noronha, L.; et al. Endothelial cells are not productively infected by SARS-CoV-2. Clin. Transl. Immunol. 2021, 10, e1350. [Google Scholar] [CrossRef]
- Puerta-Guardo, H.; Glasner, D.R.; Espinosa, D.A.; Biering, S.B.; Patana, M.; Ratnasiri, K.; Wang, C.; Beatty, P.R.; Harris, E. Flavivirus NS1 Triggers Tissue-Specific Vascular Endothelial Dysfunction Reflecting Disease Tropism. Cell Rep. 2019, 26, 1598–1613.e8. [Google Scholar] [CrossRef] [Green Version]
- Beatty, P.R.; Puerta-Guardo, H.; Killingbeck, S.S.; Glasner, D.R.; Hopkins, K.; Harris, E. Dengue virus NS1 triggers endothelial permeability and vascular leak that is prevented by NS1 vaccination. Sci. Transl. Med. 2015, 7, 304ra141. [Google Scholar] [CrossRef] [Green Version]
- Tarbell, J.M.; Cancel, L.M. The glycocalyx and its significance in human medicine. J. Intern. Med. 2016, 280, 97–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, R.; Chen, M.; Zheng, J.; Li, X.; Zhang, X. The Role of Heparin and Glycocalyx in Blood-Brain Barrier Dysfunction. Front. Immunol. 2021, 12, 754141. [Google Scholar] [CrossRef] [PubMed]
- Szolnoky, G. Sulodexide may be a real alternative to low molecular weight heparins in the prevention of COVID-19 induced vascular complications. Dermatol. Ther. 2020, 33, e14437. [Google Scholar] [CrossRef] [PubMed]
- Zizzo, G.; Tamburello, A.; Castelnovo, L.; Laria, A.; Mumoli, N.; Faggioli, P.M.; Stefani, I.; Mazzone, A. Immunotherapy of COVID-19: Inside and Beyond IL-6 Signalling. Front. Immunol. 2022, 13, 795315. [Google Scholar] [CrossRef] [PubMed]
- Noda, K.; Philips, B.J.; Snyder, M.E.; Phillippi, J.A.; Sullivan, M.; Stolz, D.B.; Ren, X.; Luketich, J.D.; Sanchez, P.G. Heparanase inhibition preserves the endothelial glycocalyx in lung grafts and improves lung preservation and transplant outcomes. Sci. Rep. 2021, 11, 12265. [Google Scholar] [CrossRef] [PubMed]
- Clausen, T.M.; Sandoval, D.R.; Spliid, C.B.; Pihl, J.; Perrett, H.R.; Painter, C.D.; Narayanan, A.; Majowicz, S.A.; Kwong, E.M.; McVicar, R.N.; et al. SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2. Cell 2020, 183, 1043–1057.e15. [Google Scholar] [CrossRef] [PubMed]
- van Haare, J.; Kooi, M.E.; van Teeffelen, J.W.G.E.; Vink, H.; Slenter, J.; Cobelens, H.; Strijkers, G.J.; Koehn, D.; Post, M.J.; van Bilsen, M. Metformin and sulodexide restore cardiac microvascular perfusion capacity in diet-induced obese rats. Cardiovasc. Diabetol. 2017, 16, 47. [Google Scholar] [CrossRef] [Green Version]
- Broekhuizen, L.N.; Lemkes, B.A.; Mooij, H.L.; Meuwese, M.C.; Verberne, H.; Holleman, F.; Schlingemann, R.O.; Nieuwdorp, M.; Stroes, E.S.G.; Vink, H. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia 2010, 53, 2646–2655. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Ochoa, A.J.; Raffetto, J.D.; Hernández, A.G.; Zavala, N.; Gutiérrez, O.; Vargas, A.; Loustaunau, J. Sulodexide in the Treatment of Patients with Early Stages of COVID-19: A Randomized Controlled Trial. Thromb. Haemost. 2021, 121, 944–954. [Google Scholar] [CrossRef]
- Melkumyants, A.; Buryachkovskaya, L.; Lomakin, N.; Antonova, O.; Docenko, J.; Ermishkin, V.; Serebruany, V. Effect of Sulodexide on Circulating Blood Cells in Patients with Mild COVID-19. J. Clin. Med. 2022, 11, 1995. [Google Scholar] [CrossRef]
- Sagris, M.; Theofilis, P.; Antonopoulos, A.S.; Oikonomou, E.; Tsioufis, K.; Tousoulis, D. Genetic Predisposition and Inflammatory Inhibitors in COVID-19: Where Do We Stand? Biomedicines 2022, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Vitiello, A. Efficacy of synthetic glucocorticoids in COVID-19 endothelites. Naunyn Schmiedebergs Arch. Pharm. 2021, 394, 1003–1007. [Google Scholar] [CrossRef]
- Cui, N.; Wang, H.; Long, Y.; Su, L.; Liu, D. Dexamethasone Suppressed LPS-Induced Matrix Metalloproteinase and Its Effect on Endothelial Glycocalyx Shedding. Mediat. Inflamm. 2015, 2015, 912726. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-Y.; Kweon, O.J.; Cha, M.J.; Baek, M.S.; Choi, S.-H. Dexamethasone may improve severe COVID-19 via ameliorating endothelial injury and inflammation: A preliminary pilot study. PLoS ONE 2021, 16, e0254167. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Zhou, L.; Tamm, M.; Roth, M. OM-85 Broncho-Vaxom, a Bacterial Lysate, Reduces SARS-CoV-2 Binding Proteins on Human Bronchial Epithelial Cells. Biomedicines 2021, 9, 1544. [Google Scholar] [CrossRef] [PubMed]
| Factors | Signal Pathways | Effects in COVID-19 |
|---|---|---|
| Endothelial dysfunction | ||
| ACE2 | Renin–angiotensin system and ACE-Angulation-II-AT1R | Endothelial dysfunction, organ damage, and clot formation [100,101] |
| Endogenous mitochondrial DNA | cGAS–STING pathway | [102] |
| SARS-CoV-2 nucleocapsid protein | Lectin pathway | Endothelial injury and microangiopathy [103] |
| Endothelial activation | ||
| IL-1 | TLR/NF-κB | Endothelial activation and damage [104] |
| SARS-CoV-2 nucleocapsid protein | TLR2-MAPK/NF-κB | Endothelial activation [105] |
| HS | Bradykinin pathway | Inflammatory response, EC-neutrophil adhesion, and vascular leakage [85] |
| Barrier disruption | ||
| Hyaluronan | HA-CD44-ROCK | Barrier disruption [106] |
| SARS-CoV-2 spike protein | integrin αVβ3-VE-Cadherin | Barrier disruption [107] |
| S1 subunit of SARS-CoV-2 spike protein (S1SP) | - | Decreased microvascular transendothelial resistance and barrier function [108] |
| TNFα | Rho-kinase | Disruption of intercellular tight junctional proteins and the endothelial barrier [109] |
| Angiopoietin 2 | Tie2 signaling | Increased endothelial permeability [110] |
| Heparanase | TNFα-heparanase-HS | Disruption of endothelial glycocalyx integrity, inflammation, and coagulation [111,112,113] |
| Coagulation and thrombosis | ||
| Complement C5a | Complement pathway | Coagulation [114] |
| Complement C5b-9 | Complement pathway | Endothelial activation and dysfunction and coagulation [114] |
| Complement C4d, MASP2, and C5b-9 | Mannan-binding lectin (MBL) pathway | Endothelial necrosis and thrombosis [115] |
| PAI-1 | STAT3 | Coagulopathy and thrombosis [116] |
| Syndecan-1 | - | Coagulation and endothelial injury [29] |
| Drug | Potential Target in EGL | Effects Associated with Endothelial Function |
|---|---|---|
| Heparin | Inhibitor of heparanase | Protecting the vascular endothelial barrier [132]; reducing MMP expression [45,69] |
| Sulodexide | Increasing GAG synthesis and decreasing its catabolism [131] | Preserving endothelial glycocalyx function; antithrombotic, profibrinolytic, and anti-inflammatory effects [133] |
| The corticosteroids | Unknown | Inhibiting MMPs activity; reserving ZO-1 and syndecan-1 expression; reducing endothelial injury and inflammation [19] |
| Tocilizumab | IL-6R | Increasing the glycocalyx thickness; improving endothelial function [32,134] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zha, D.; Fu, M.; Qian, Y. Vascular Endothelial Glycocalyx Damage and Potential Targeted Therapy in COVID-19. Cells 2022, 11, 1972. https://doi.org/10.3390/cells11121972
Zha D, Fu M, Qian Y. Vascular Endothelial Glycocalyx Damage and Potential Targeted Therapy in COVID-19. Cells. 2022; 11(12):1972. https://doi.org/10.3390/cells11121972
Chicago/Turabian StyleZha, Duoduo, Mingui Fu, and Yisong Qian. 2022. "Vascular Endothelial Glycocalyx Damage and Potential Targeted Therapy in COVID-19" Cells 11, no. 12: 1972. https://doi.org/10.3390/cells11121972
APA StyleZha, D., Fu, M., & Qian, Y. (2022). Vascular Endothelial Glycocalyx Damage and Potential Targeted Therapy in COVID-19. Cells, 11(12), 1972. https://doi.org/10.3390/cells11121972







