Autocrine Activity of Extracellular Vesicles Induced by Icariin and Its Effectiveness in Glucocorticoid-Induced Injury of Bone Microvascular Endothelial Cells
Abstract
:1. Introduction
2. Materials and Methods
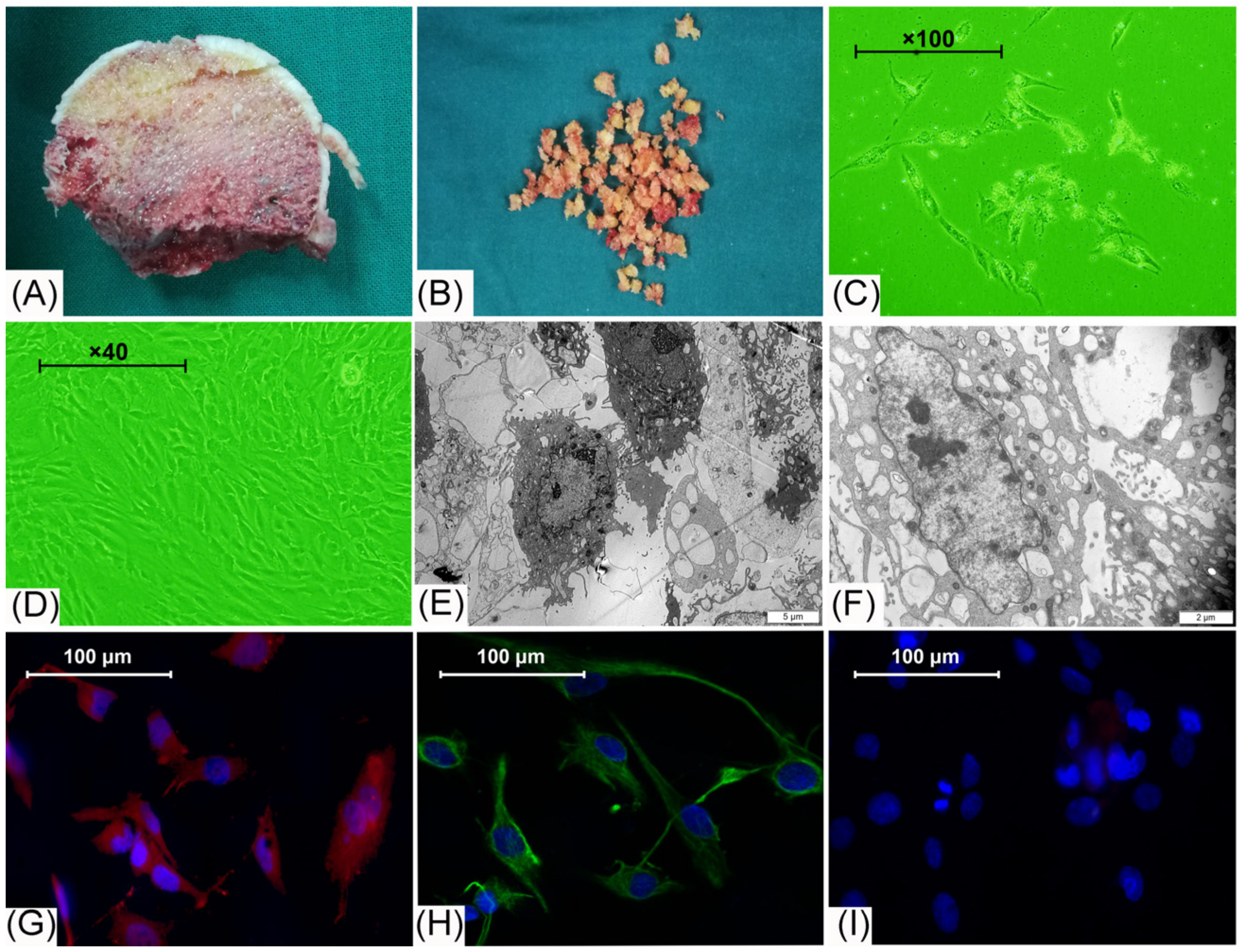
2.1. BMECs Isolation
2.2. CCK-8 Assay
2.3. EVs Isolation and Identification
2.4. High-Throughput Sequencing Transcriptome and Quantitative Polymerase Chain Reaction (qPCR) of EVs-Carried miRNAs
2.5. Bicinchoninic Acid (BCA) Analysis
2.6. Antibody Array Assay and Elisa Detection
2.7. Functional Enrichment Analyses and Annotation of DEPs
2.8. Coculture of BMECs and EVs
2.8.1. Cell Scratch Assay
2.8.2. Tube Formation Assay
2.8.3. Flow Cytometry Assay
2.9. Statistical Analysis
3. Results
3.1. Cell Morphology Observation and Identification
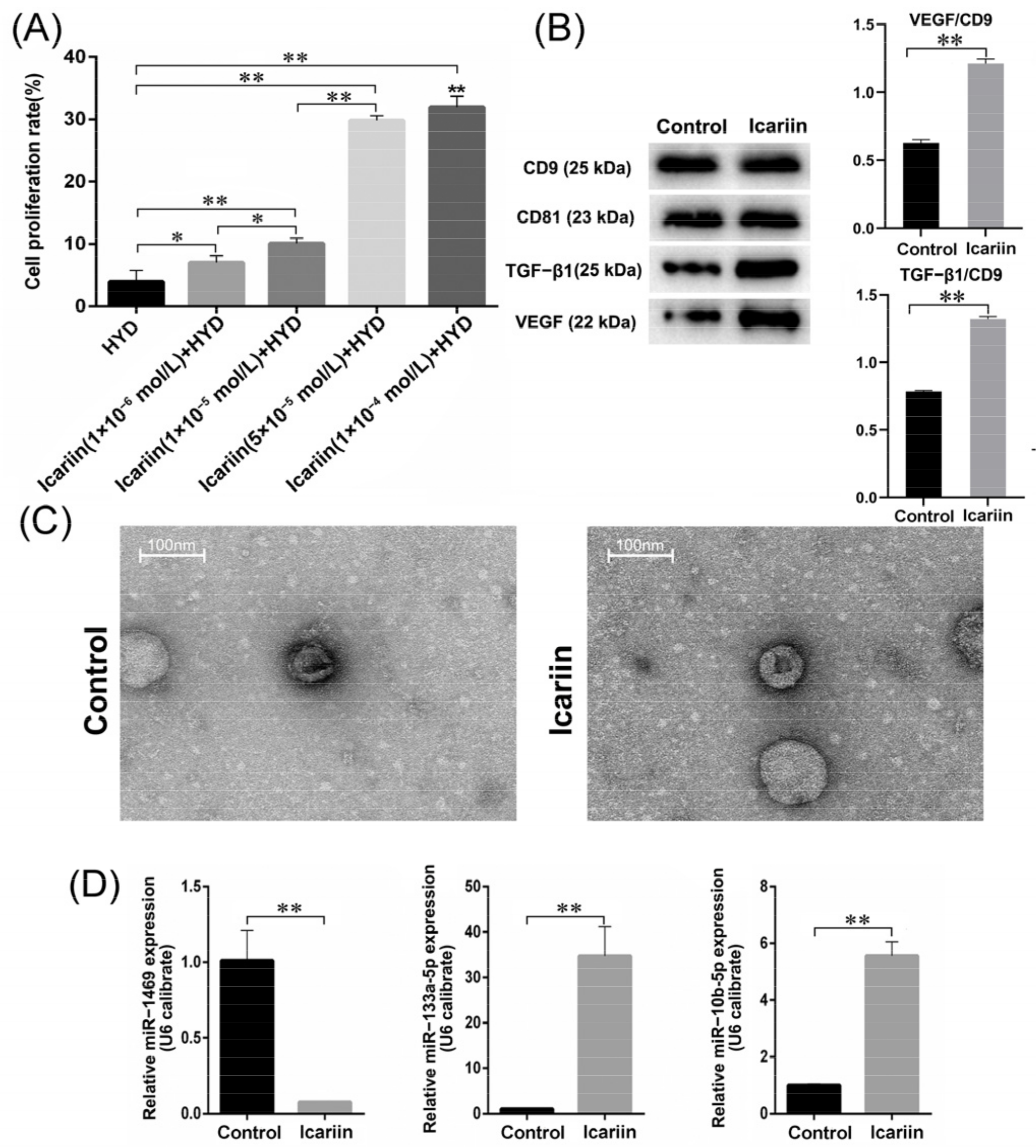
3.2. Effects of Icariin on Cell Proliferation Rate
3.3. Size and Protein Analysis of Isolated EVs
3.4. MiRNA High-Throughput Sequencing and qPCR
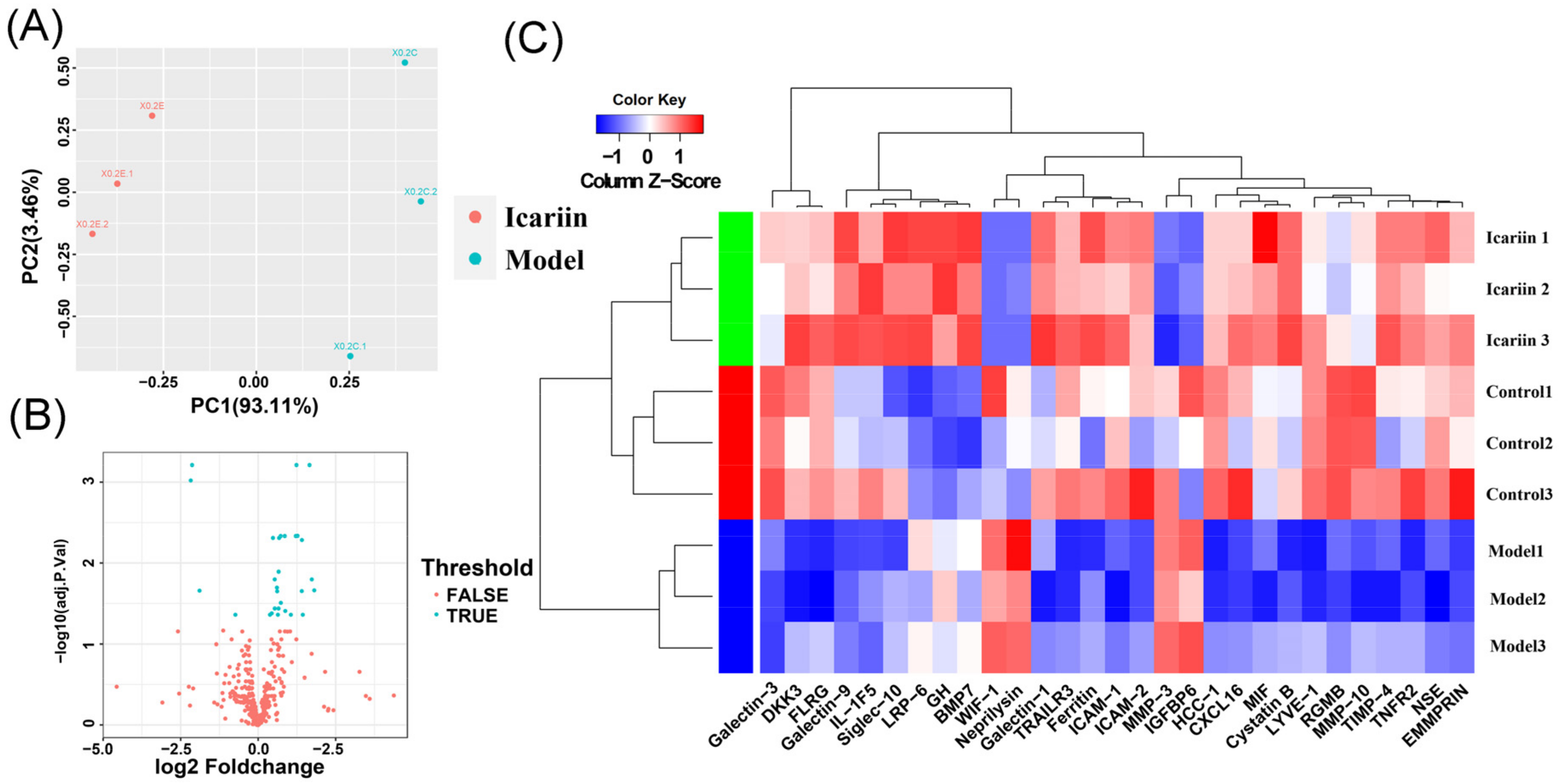
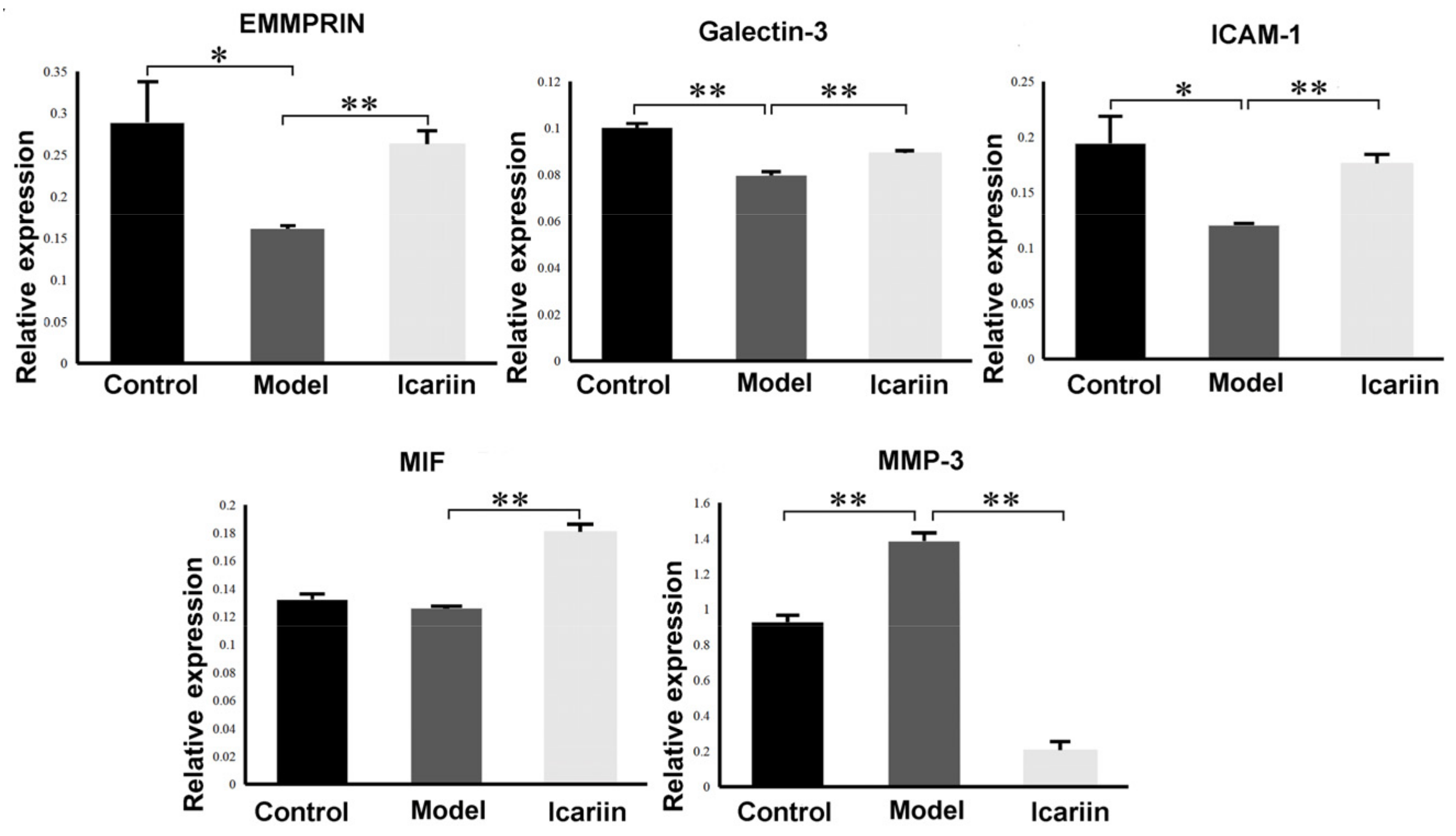
3.5. Data Normalization and DEPs Identification
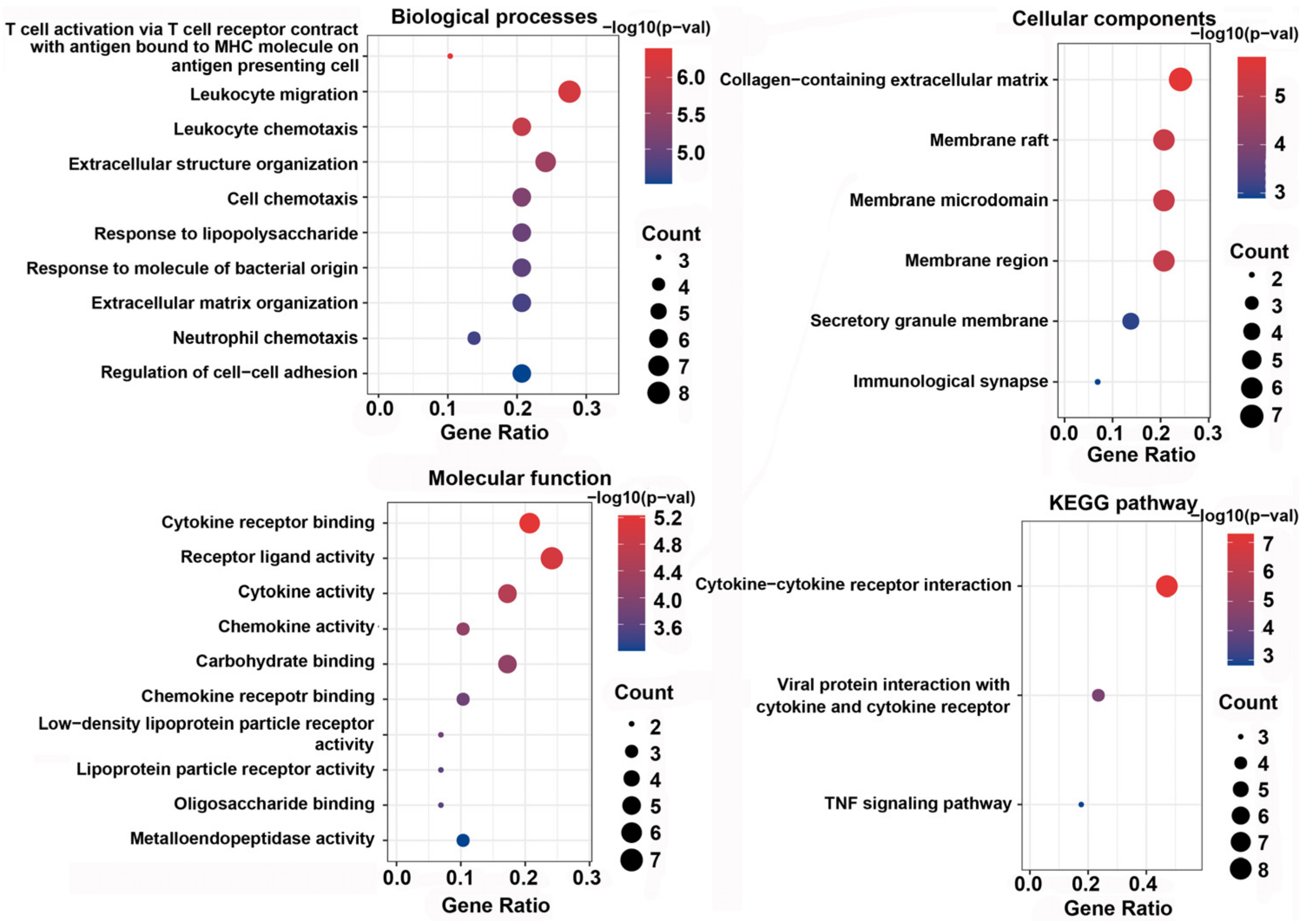
3.6. GO Function and KEGG Enrichment Analysis of DEPs
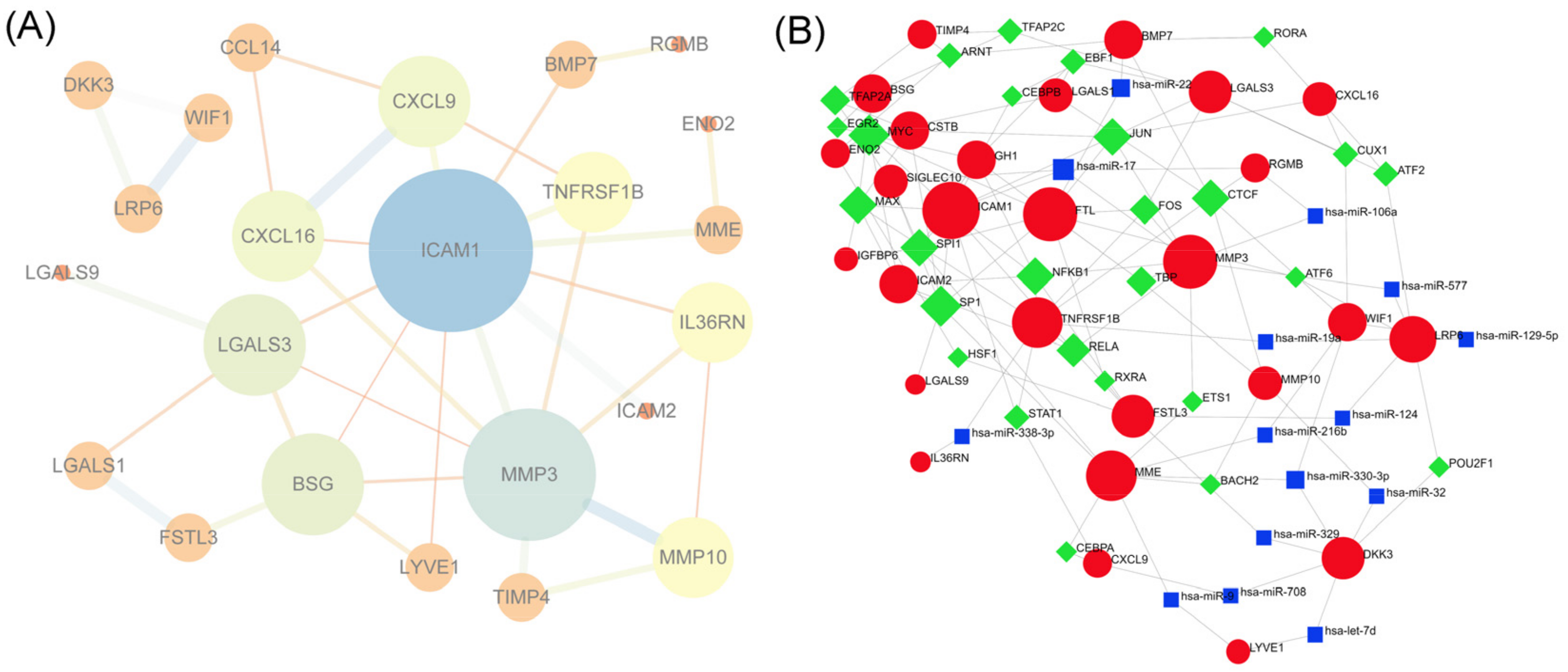
3.7. PPI Network Construction and Hub Gene Selection
3.8. TF-miRNA-DEP Interacted Network
3.9. Effect of BMEC-Derived EVs on BMEC Function
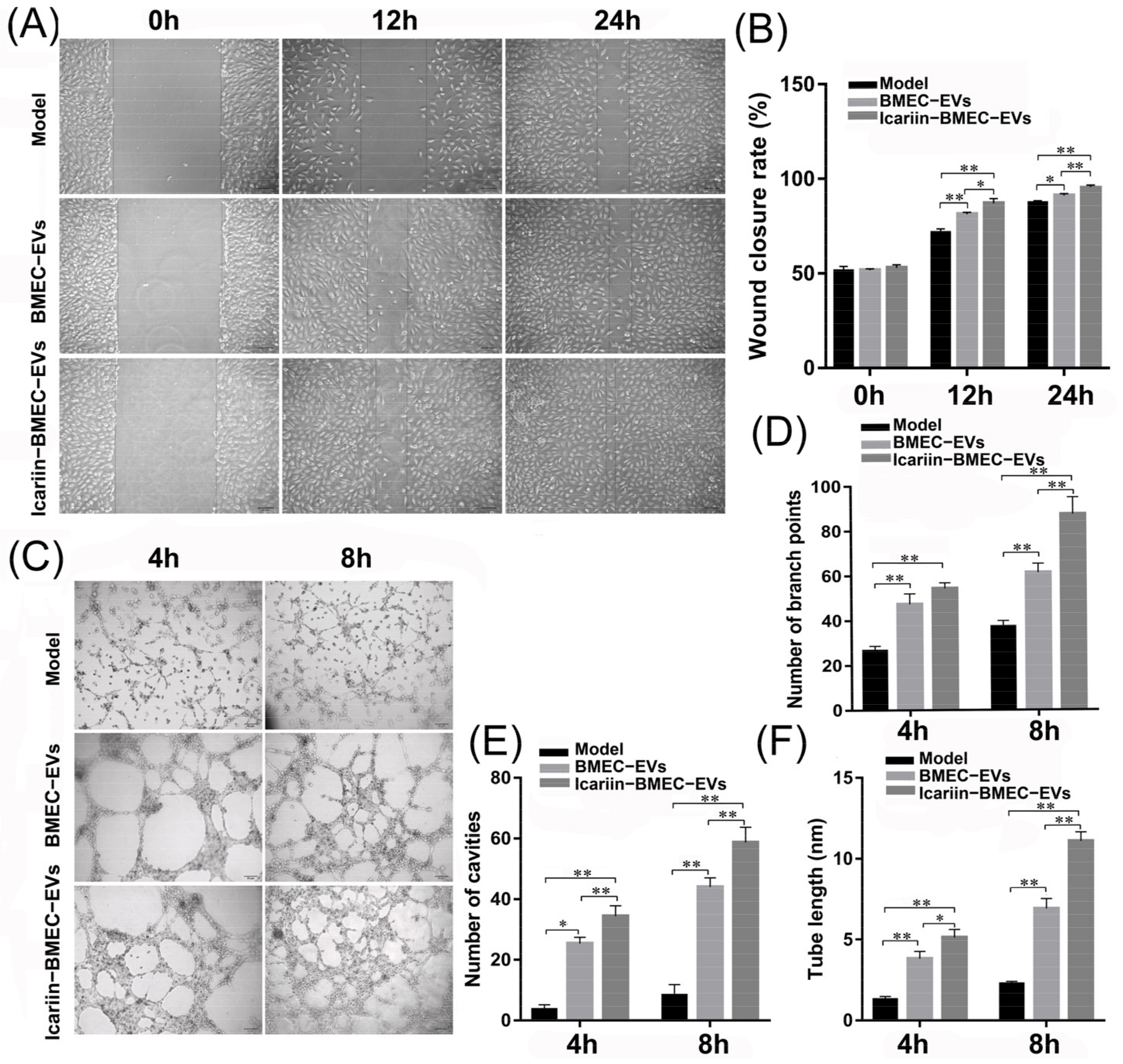
3.9.1. Effect of EVs on Endothelial Cell Migration and Tube Formation
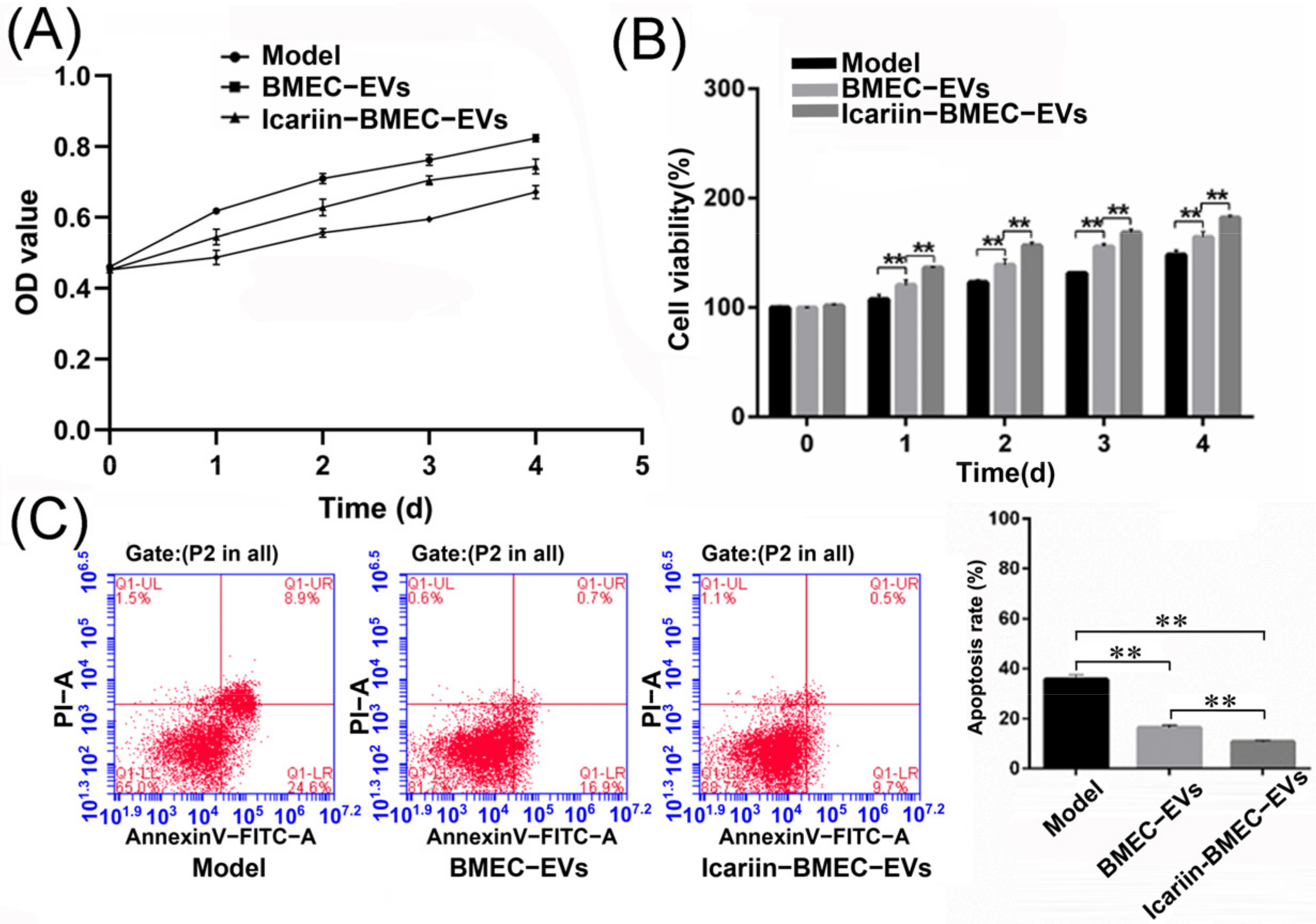
3.9.2. Cell Viability
3.9.3. Cell Apoptosis Rate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vandewalle, J.; Luypaert, A.; De Bosscher, K.; Libert, C. Therapeutic Mechanisms of Glucocorticoids. Trends Endocrinol. Metab. 2018, 29, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.; Fett, N.; Rosenbach, M.; Werth, V.P.; Micheletti, R.G. Prevention and management of glucocorticoid-induced side effects: A comprehensive review: A review of glucocorticoid pharmacology and bone health. J. Am. Acad. Dermatol. 2017, 76, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Lv, S.; Li, Y.; Zhu, J.; Chen, S.; Zhen, G.; Cao, X.; Wu, S.; Crane, J.L. Glucocorticoids Disrupt Skeletal Angiogenesis Through Transrepression of NF-κB-Mediated Preosteoclast Pdgfb Transcription in Young Mice. J. Bone Miner. Res. 2020, 35, 1188–1202. [Google Scholar] [CrossRef] [PubMed]
- Nan, K.; Zhang, Y.; Zhang, X.; Li, D.; Zhao, Y.; Jing, Z.; Liu, K.; Shang, D.; Geng, Z.; Fan, L. Exosomes from miRNA-378-modified adipose-derived stem cells prevent glucocorticoid-induced osteonecrosis of the femoral head by enhancing angiogenesis and osteogenesis via targeting miR-378 negatively regulated suppressor of fused (Sufu). Stem Cell Res. Ther. 2021, 12, 331. [Google Scholar] [CrossRef]
- Li, J.; Ge, Z.; Fan, L.; Wang, K. Protective effects of molecular hydrogen on steroid-induced osteonecrosis in rabbits via reducing oxidative stress and apoptosis. BMC Musculoskelet. Disord. 2017, 18, 58. [Google Scholar] [CrossRef] [Green Version]
- Stegen, S.; Carmeliet, G. The skeletal vascular system-Breathing life into bone tissue. Bone 2017, 115, 50–58. [Google Scholar] [CrossRef]
- Yu, Q.S.; Guo, W.S.; Cheng, L.M.; Lu, Y.F.; Shen, J.Y.; Li, P. Glucocorticoids Significantly Influence the Transcriptome of Bone Microvascular Endothelial Cells of Human Femoral Head. Chin. Med. J. 2015, 128, 1956–1963. [Google Scholar] [CrossRef]
- Dietz, B.M.; Hajirahimkhan, A.; Dunlap, T.L.; Bolton, J.L. Botanicals and Their Bioactive Phytochemicals for Women’s Health. Pharmacol. Rev. 2016, 68, 1026–1073. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, D.; Yang, D.; Zhen, W.; Zhang, J.; Peng, S. The effect of icariin on bone metabolism and its potential clinical application. Osteoporos. Int. 2018, 29, 535–544. [Google Scholar] [CrossRef]
- Hu, Y.; Li, H.; Liu, K.; Zhang, Y.; Ren, L.; Fan, Z. Protective effects of icariin on human vascular endothelial cells induced by oxidized low-density lipoprotein via modulating caspase-3 and Bcl-2. Mol. Med. Rep. 2018, 17, 6835–6839. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.Y.; Guo, W.S.; Yu, Q.S.; Cheng, L.M.; Wang, B.L. Effects of icariin on microRNAs expression in bone microvascular endothelial cells in steroids-induced femoral head lesions. Chin. J. Tissue Eng. Res. 2016, 20, 2140–2147. (In Chinese) [Google Scholar]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikfarjam, S.; Rezaie, J.; Zolbanin, N.M.; Jafari, R. Mesenchymal stem cell derived-exosomes: A modern approach in translational medicine. J. Transl. Med. 2020, 18, 449. [Google Scholar] [CrossRef] [PubMed]
- Babaei, M.; Rezaie, J. Application of stem cell-derived exosomes in ischemic diseases: Opportunity and limitations. J. Transl. Med. 2021, 19, 196. [Google Scholar] [CrossRef]
- Soraya, H.; Sani, N.A.; Jabbari, N.; Rezaie, J. Metformin Increases Exosome Biogenesis and Secretion in U87 MG Human Glioblastoma Cells: A Possible Mechanism of Therapeutic Resistance. Arch. Med. Res. 2021, 52, 151–162. [Google Scholar] [CrossRef]
- Mont, M.A.; Cherian, J.J.; Sierra, R.J.; Jones, L.C.; Lieberman, J.R. Nontraumatic Osteonecrosis of the Femoral Head: Where Do We Stand Today? A Ten-Year Update. J. Bone Joint. Surg. Am. 2015, 97, 1604–1627. [Google Scholar] [CrossRef]
- Li, T.; Liu, Y.; Zhang, Q.; Sun, W.; Dong, Y. A steroid-induced osteonecrosis model established using an organ-on-a-chip platform. Exp. Ther. Med. 2021, 22, 1070. [Google Scholar] [CrossRef]
- Panthiya, L.; Pantan, R.; Tocharus, J.; Nakaew, A.; Suksamrarn, A.; Tocharus, C. Endothelium-dependent and endothelium-independent vasorelaxant effects of tiliacorinine 12’-O-acetate and mechanisms on isolated rat aorta. Biomed. Pharmacother. 2019, 109, 2090–2099. [Google Scholar] [CrossRef]
- Liu, F.; Dong, J.; Zhou, D.; Zhang, Q. Identification of Key Candidate Genes Related to Inflammatory Osteolysis Associated with Vitamin E-Blended UHMWPE Debris of Orthopedic Implants by Integrated Bioinformatics Analysis and Experimental Confirmation. J. Inflamm. Res. 2021, 14, 3537–3554. [Google Scholar] [CrossRef]
- Hua, W.; Zhang, Y.; Wu, X.; Kang, L.; Tu, J.; Zhao, K.; Li, H.; Wang, K.; Song, Y.; Luo, R.; et al. Icariin Attenuates Interleukin-1beta-Induced Inflammatory Response in Human Nucleus Pulposus Cells. Curr. Pharm. Des. 2018, 23, 6071–6078. [Google Scholar] [CrossRef]
- Paone, S.; Baxter, A.A.; Hulett, M.D.; Poon, I. Endothelial cell apoptosis and the role of endothelial cell-derived extracellular vesicles in the progression of atherosclerosis. Cell. Mol. Life Sci. 2018, 76, 1093–1106. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Luo, H.; Li, X.; Li, T.; He, J.; Qi, Q.; Liu, Y.; Yu, Z. Exosomes Derived from Human Pulmonary Artery Endothelial Cells Shift the Balance between Proliferation and Apoptosis of Smooth Muscle Cells. Cardiology 2017, 137, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.X.; Xiu, Y.L.; Chen, X.; Li, Y.L. Transforming Growth Factor-beta 1 Involved in the Pathogenesis of Endometriosis through Regulating Expression of Vascular Endothelial Growth Factor under Hypoxia. Chin. Med. J. 2017, 130, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.H.; Chan, G.K.; Lou, J.S.; Wu, Q.Y.; Wang, H.Y.; Duan, R.; Cheng, M.Y.-T.; Dong, T.T.-X.; Tsim, K.W.-K. The extract of Polygoni Cuspidati Rhizoma et Radix suppresses the vascular endothelial growth factor-induced angiogenesis. Phytomedicine 2018, 42, 135–143. [Google Scholar] [CrossRef]
- Massague, J.; Blain, S.W.; Lo, R.S. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell 2000, 103, 295–309. [Google Scholar] [CrossRef] [Green Version]
- Hao, W.; Zhao, Z.H.; Meng, Q.T.; Tie, M.E.; Lei, S.Q.; Xia, Z.Y. Propofol protects against hepatic ischemia/reperfusion injury via miR-133a-5p regulating the expression of MAPK6. Cell Biol. Int. 2017, 41, 495–504. [Google Scholar] [CrossRef]
- Ru, Q.; Li, W.L.; Xiong, Q.; Chen, L.; Tian, X.; Li, C.Y. Voltage-gated potassium channel blocker 4-aminopyridine induces glioma cell apoptosis by reducing expression of microRNA-10b-5p. Mol. Biol. Cell 2018, 29, 1125–1136. [Google Scholar] [CrossRef]
- Agatheeswaran, S.; Pattnayak, N.C.; Chakraborty, S. Identification and functional characterization of the miRNA-gene regulatory network in chronic myeloid leukemia lineage negative cells. Sci. Rep. 2016, 6, 632493. [Google Scholar] [CrossRef] [Green Version]
- Sharghi-Namini, S.; Tan, E.; Ong, L.L.; Ge, R. Asada HH. Dll4-containing exosomes induce capillary sprout retraction in a 3D microenvironment. Sci. Rep. 2014, 4, 4031. [Google Scholar] [CrossRef] [Green Version]
- Weilner, S.; Schraml, E.; Wieser, M.; Messner, P.; Schneider, K.; Wassermann, K.; Micutkova, L.; Fortschegger, K.; Maier, A.B.; Westendorp, R.; et al. Secreted microvesicular miR-31 inhibits osteogenic differentiation of mesenchymal stem cells. Aging Cell 2016, 15, 744–754. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, F.; Shi, Q.; Wang, W.-B.; Bao, H.; Yan, J.; Gao, S.; Liu, Z.; Jiang, Z.-L.; Qi, Y.-X. Endothelial microvesicles induced by physiological cyclic stretch inhibit ICAM1-Dependent leukocyte adhesion. Exp. Cell Res. 2020, 386, 111710. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Yang, X. Study of Intercellular Adhesion Molecule-1 (ICAM-1) in Bone Homeostasis. Curr. Drug Targets 2020, 21, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Paiva, K.B.; Granjeiro, J.M. Bone tissue remodeling and development: Focus on matrix metalloproteinase functions. Arch. Biochem. Biophys. 2014, 561, 74–87. [Google Scholar] [CrossRef]
- Iacobini, C.; Fantauzzi, C.B.; Pugliese, G.; Menini, S. Role of Galectin-3 in Bone Cell Differentiation, Bone Pathophysiology and Vascular Osteogenesis. Int. J. Mol. Sci. 2017, 18, 2481. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Pan, X.; Sheng, Z.; Yan, G.; Chen, L.; Ma, G. miR-17 regulates the proliferation and apoptosis of endothelial cells in coronary heart disease via targeting insulin-like-growth factor 1. Pathol. Res. Pract. 2019, 215, 152512. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Wang, C.; Yang, Z.; Liu, W.; Yuan, Y.; Li, K.; Zhang, Y.; Wang, Y.; Shi, Y.; Qiu, Y.; et al. Dysregulated Sp1/miR-130b-3p/HOXA5 axis contributes to tumor angiogenesis and progression of hepatocellular carcinoma. Theranostics 2020, 10, 5209–5224. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, J.; Liu, G.; Zhang, B.; Jin, X.; Wang, Y. MicroRNA-17-5p, a novel endothelial cell modulator, controls vascular re-endothelialization and neointimal lesion formation. Vascular 2021, 17085381211067672. [Google Scholar] [CrossRef]
| MCC | MNC | Degree |
|---|---|---|
| ICAM1 | ICAM1 | ICAM1 |
| MMP3 | MMP3 | MMP3 |
| BSG | BSG | BSG |
| LGALS3 | CXCL9 | LGALS3 |
| CXCL9 | CXCL16 | CXCL9 |
| CXCL16 | LGALS3 | CXCL16 |
| MMP10 | MMP10 | MMP10 |
| IL36RN | IL36RN | IL36RN |
| TNFRSF1B | TNFRSF1B | TNFRSF1B |
| MiRNAs | Targeted Genes | Gene Counts | Transcription Factors (TF) | Targeted Genes | Gene Counts |
|---|---|---|---|---|---|
| hsa-miR-17 | ICAM1, MMP3, SIGLEC10, RGMB | 4 | MYC | ICAM1, BSG, ICAM2, FTL, LGALS1, CSTB, ENO2 | 7 |
| hsa-miR-330-3p | DKK3, MME, WIF1 | 3 | SP1 | ICAM1, ICAM2, FTL, CSTB, SIGLEC10, LGALS9, MME | 7 |
| hsa-miR-22 | FTL, LGALS1, BMP7 | 3 | NFKB1 | ICAM1, ICAM2, MMP3, GH1, FSTL3, TNFRSF1B | 6 |
| hsa-let-7d | DKK3, LYVE1 | 2 | MAX | ICAM, BSG, ICAM2, IGFBP6, CSTB, TNFRSF1B | 6 |
| hsa-miR-106a | MMP3, RGMB | 2 | JUN | ICAM1, MMP3, FTL, CSTB, LGALS3, CXCL16 | 6 |
| hsa-miR-124 | LRP6, FSTL3 | 2 | SPI1 | ICAM1, FTL, IGFBP6, SIGLEC10, GH1, MME | 6 |
| hsa-miR-129-5p | LRP6, WIF1 | 2 | CTCF | LGALS1, MMP10, BMP7, TNFRSF1B, WIF1, RGMB, | 6 |
| hsa-miR-19a | LRP6, TNFRSF1B | 2 | RELA | ICAM1, ICAM2, MMP3, FSTL3, TNFRSF1B | 5 |
| hsa-miR-216b | MME, WIF1 | 2 | TFAP2A | BSG, CSTB, TIMP4, SIGLEC10 | 4 |
| hsa-miR-32 | DKK3, MMP10 | 2 | FOS | MMP3, FTL, LGALS3, TNFRSF1B | 4 |
| hsa-miR-329 | DKK3, FSTL3 | 2 | TBP | MMP3, FTL, MMP10, GH1 | 4 |
| hsa-miR-577 | MMP3, LRP6 | 2 | TFAP2C | BGS, LGALS3, TIMP4 | 3 |
| hsa-miR-708 | DKK3, CXCL9 | 2 | STAT1 | ICAM1, CXCL9, TNFRSF1B | 3 |
| hsa-miR-9 | MME, LYVE1 | 2 | EBF1 | LGALS1, LGALS3, GH1 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Li, T.; Li, Z.; Lu, J.; Wu, X.; Gao, F.; Sun, W. Autocrine Activity of Extracellular Vesicles Induced by Icariin and Its Effectiveness in Glucocorticoid-Induced Injury of Bone Microvascular Endothelial Cells. Cells 2022, 11, 1921. https://doi.org/10.3390/cells11121921
Zhang Q, Li T, Li Z, Lu J, Wu X, Gao F, Sun W. Autocrine Activity of Extracellular Vesicles Induced by Icariin and Its Effectiveness in Glucocorticoid-Induced Injury of Bone Microvascular Endothelial Cells. Cells. 2022; 11(12):1921. https://doi.org/10.3390/cells11121921
Chicago/Turabian StyleZhang, Qingyu, Tengqi Li, Zirong Li, Jike Lu, Xinjie Wu, Fuqiang Gao, and Wei Sun. 2022. "Autocrine Activity of Extracellular Vesicles Induced by Icariin and Its Effectiveness in Glucocorticoid-Induced Injury of Bone Microvascular Endothelial Cells" Cells 11, no. 12: 1921. https://doi.org/10.3390/cells11121921
APA StyleZhang, Q., Li, T., Li, Z., Lu, J., Wu, X., Gao, F., & Sun, W. (2022). Autocrine Activity of Extracellular Vesicles Induced by Icariin and Its Effectiveness in Glucocorticoid-Induced Injury of Bone Microvascular Endothelial Cells. Cells, 11(12), 1921. https://doi.org/10.3390/cells11121921






