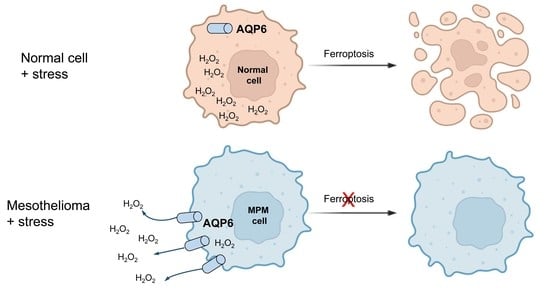
Aquaporin-6 May Increase the Resistance to Oxidative Stress of Malignant Pleural Mesothelioma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. RNA Isolation and RT-qPCR
2.3. Immunoblotting
2.4. Immunolocalization
2.4.1. Immunocytochemistry
2.4.2. Double Immunofluorescence
2.5. Water Permeability Measurements
2.6. Hydrogen Peroxide Influx Measurements
2.7. Gene Silencing
2.8. Hyper7-NES Transfection
2.9. Intracellular H2O2 Detection by HyPer7-NES Imaging
2.10. MTT Assay
2.11. Protein Content
2.12. Statistics
3. Results
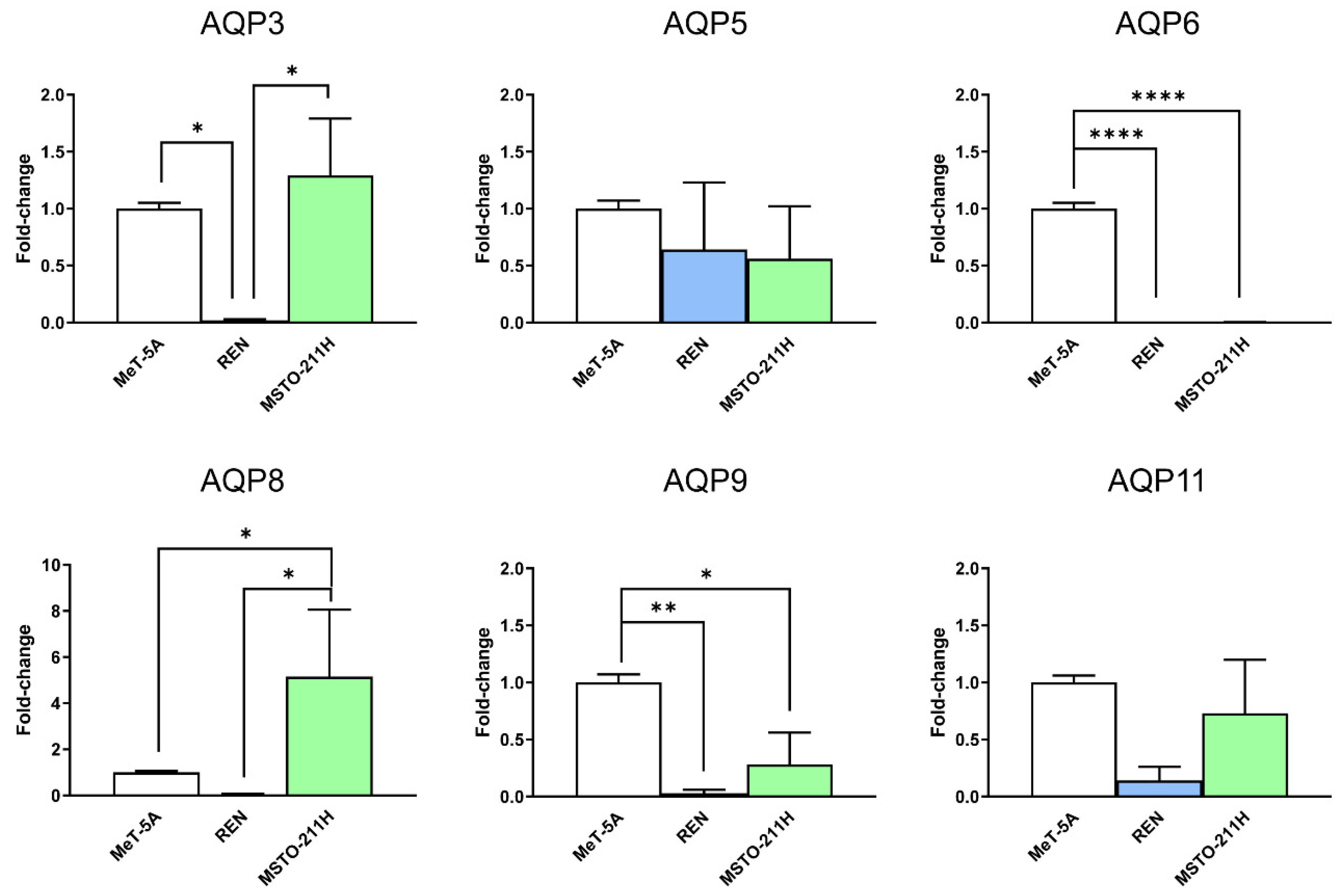
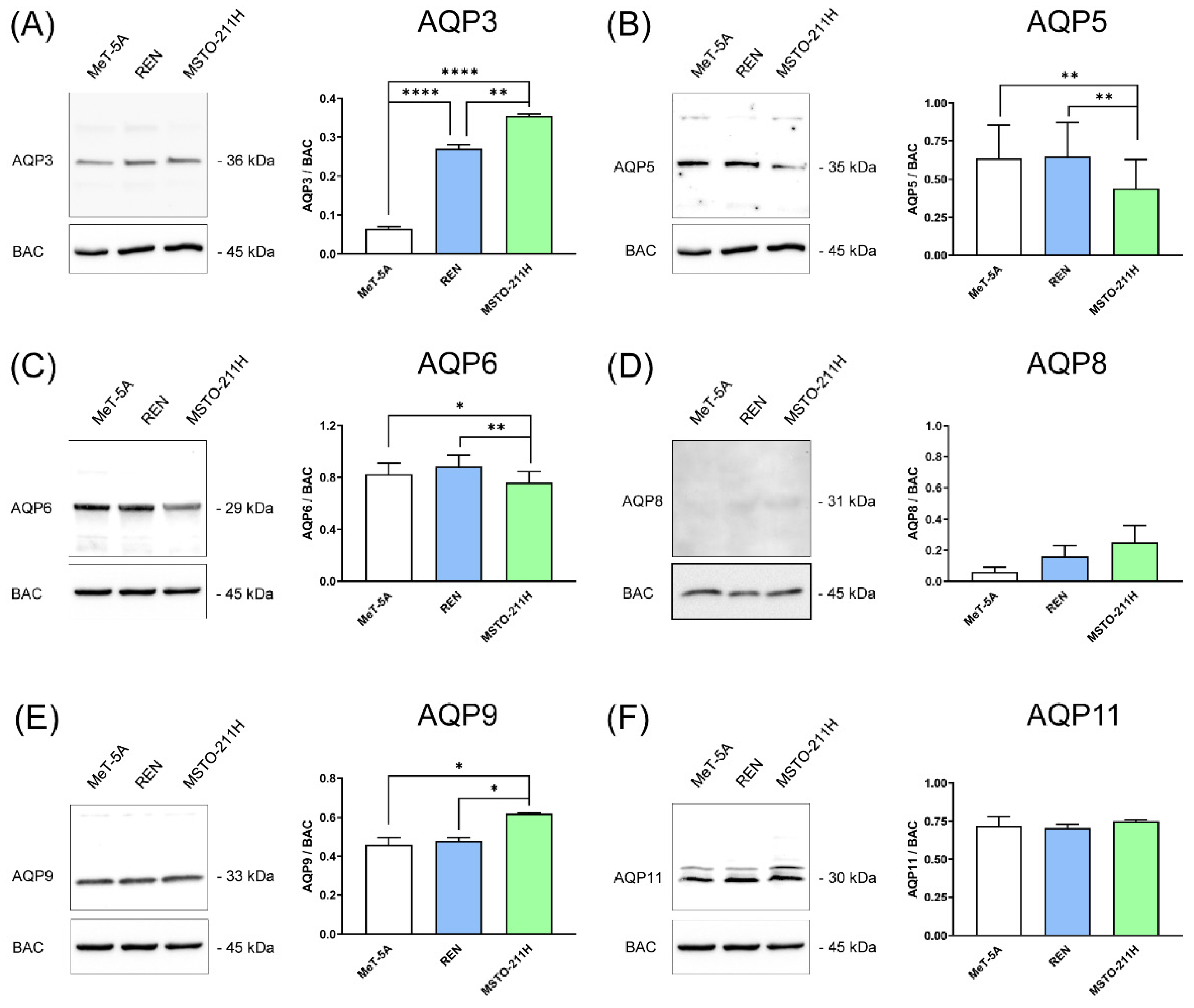
3.1. Aquaporins-3, -5, -6, -9, and -11 Are Expressed in MeT-5A, REN, MSTO-211H Cell Lines
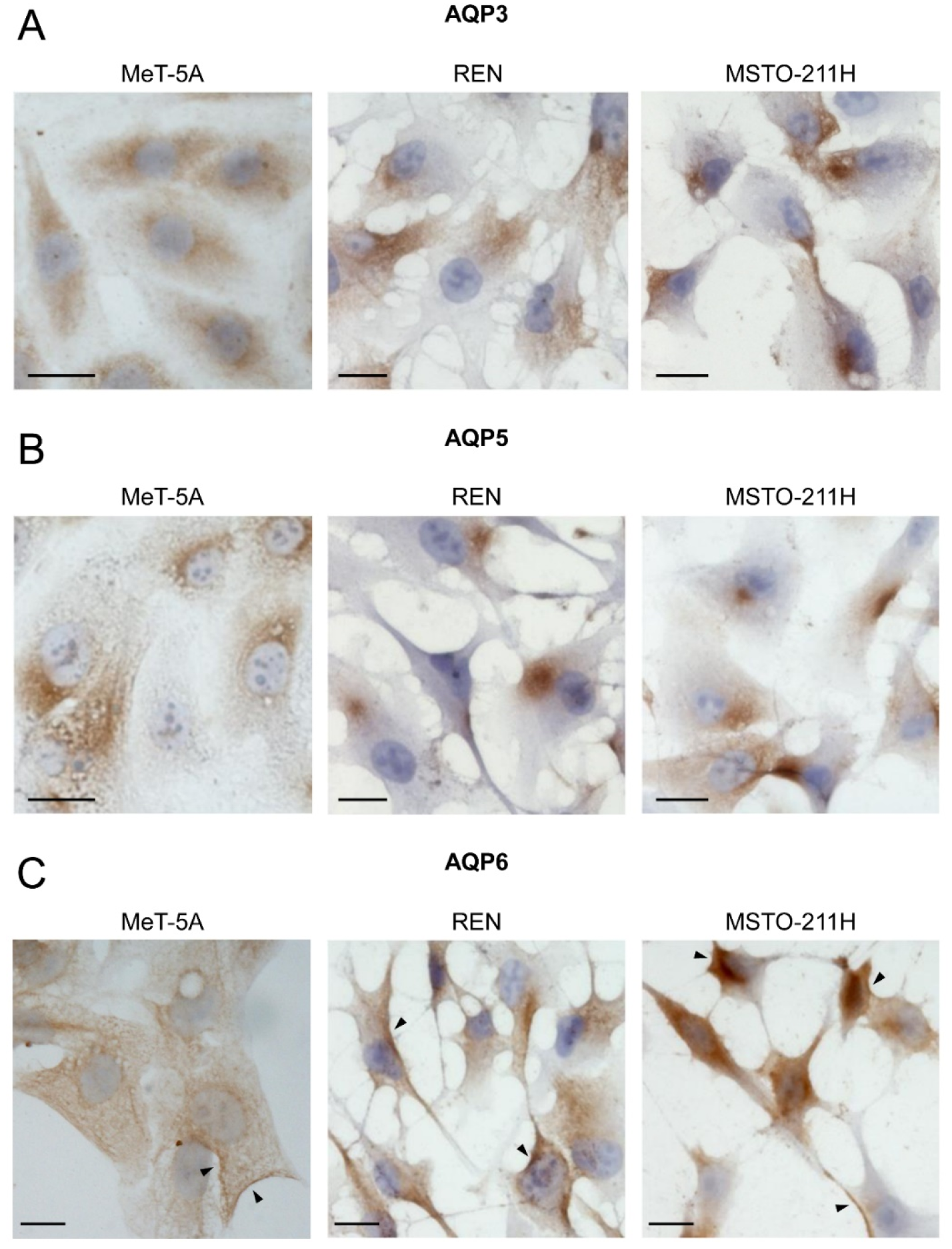
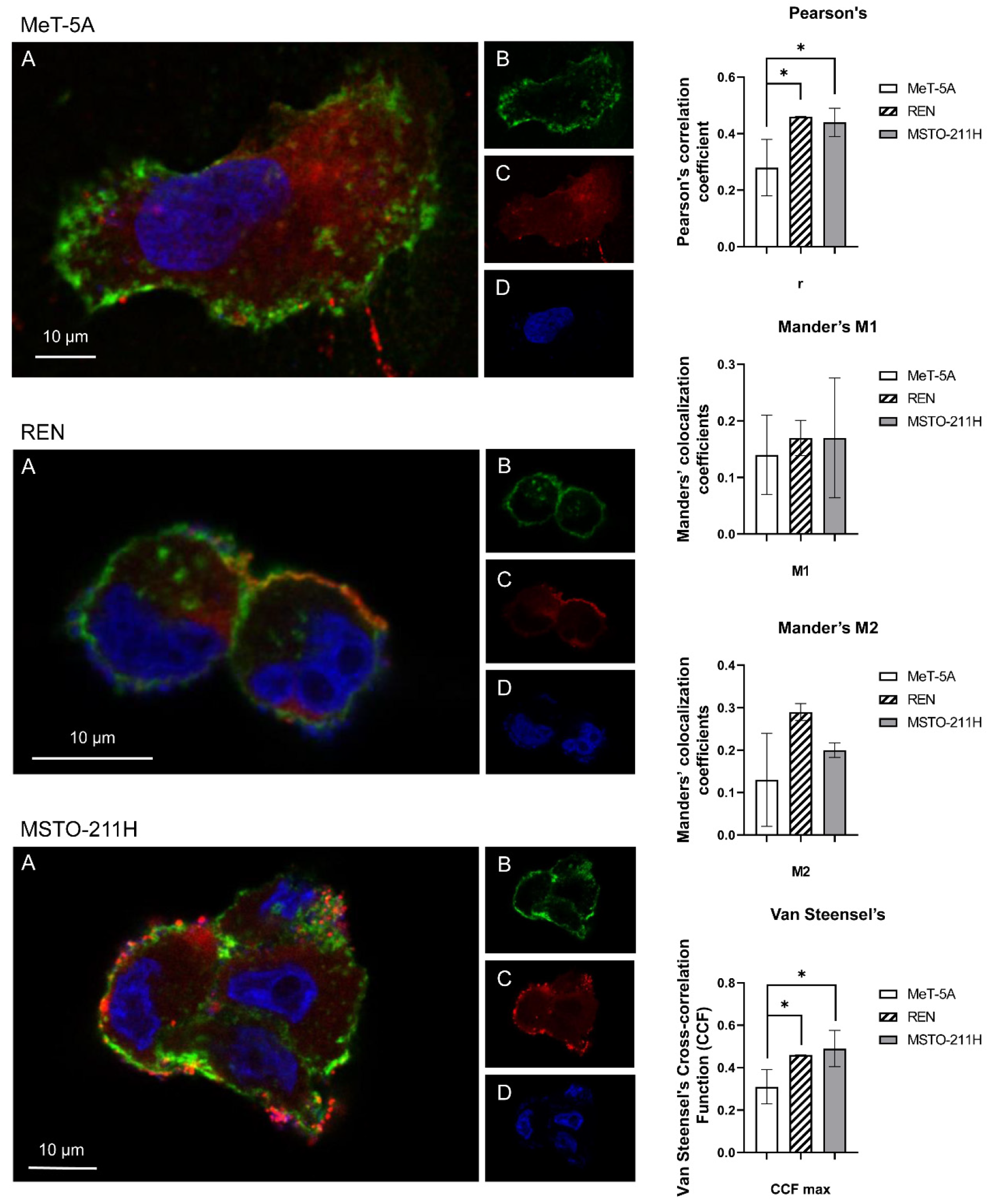
3.2. Aquaporin-3, -5, -6 Localization in MeT-5A, REN, MSTO-211H Cell Lines
3.3. Effect of Oxidative Stress and Mercury Chloride on Water Permeability of MeT-5A, REN, MSTO-211H Cell Lines
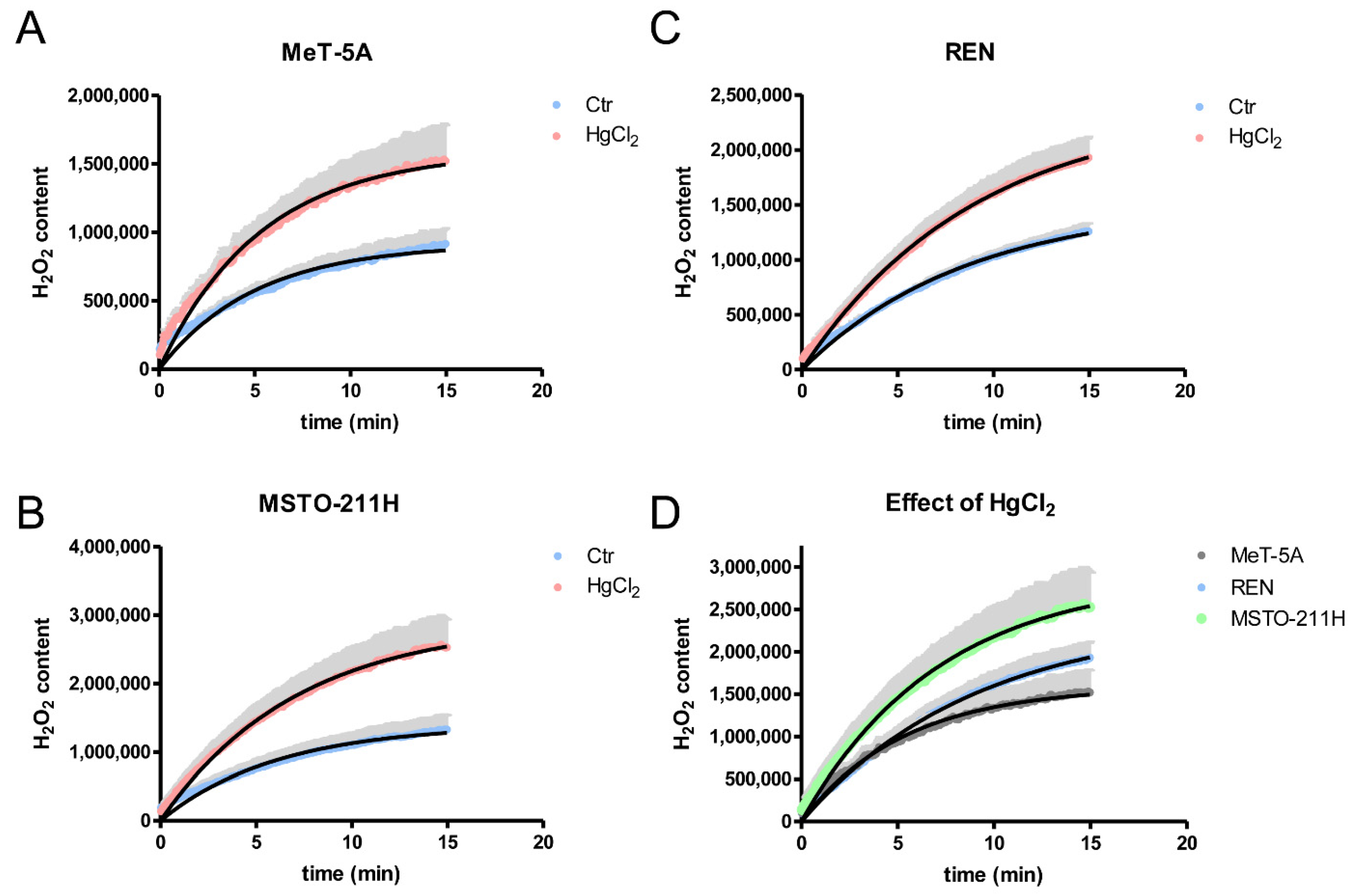
3.4. Hydrogen Peroxide Permeability of MeT-5A, REN, MSTO-211H Cell Lines
3.5. Effect of Oxidative Stress on Water Permeability of Me, T-5A, REN, and MSTO-211H Cell Lines with Reduced AQP6 Expression
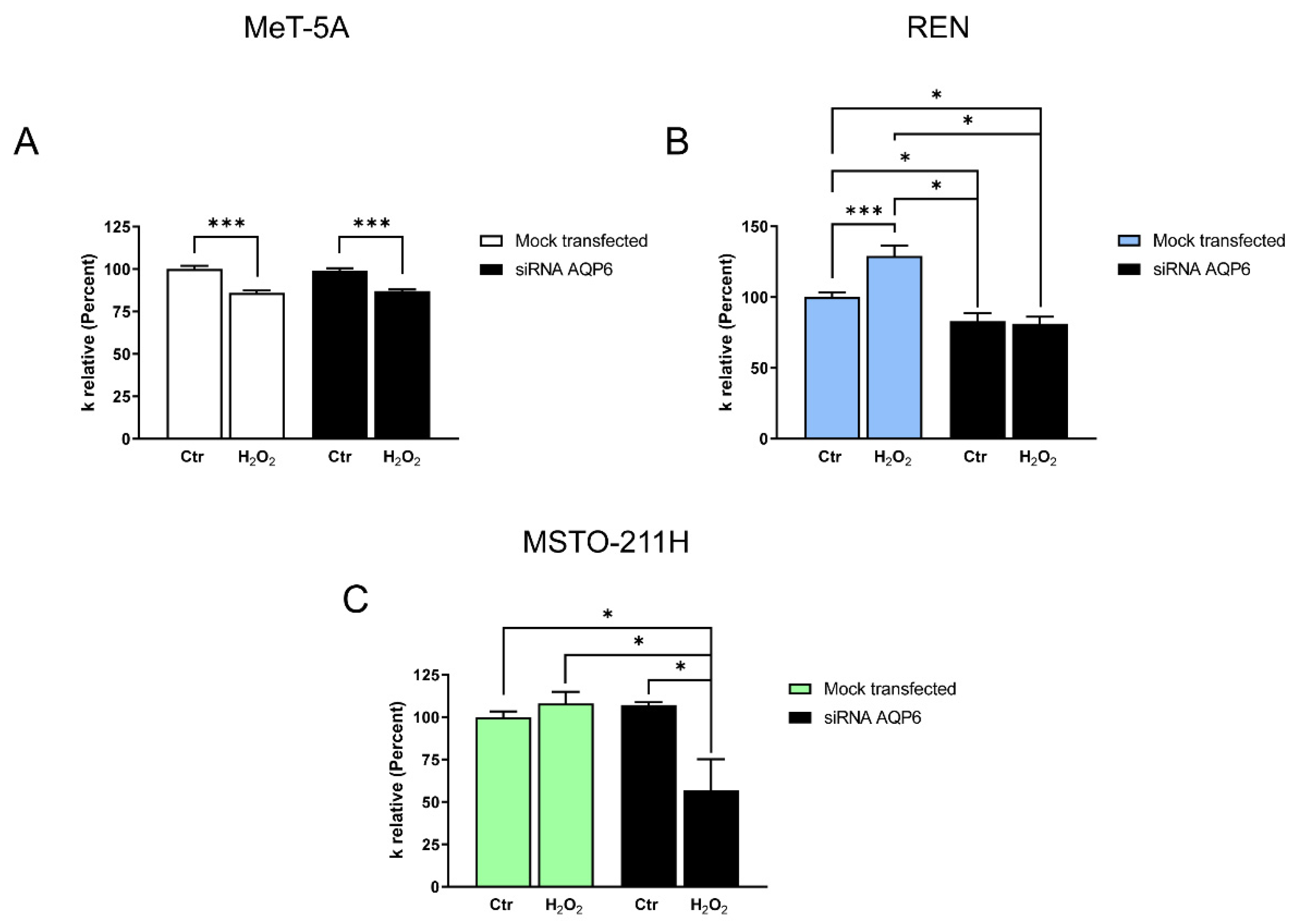
3.6. Hydrogen Peroxide Permeability of MeT-5A, REN, MSTO-211H Cell Lines with Reduced AQP6 Expression
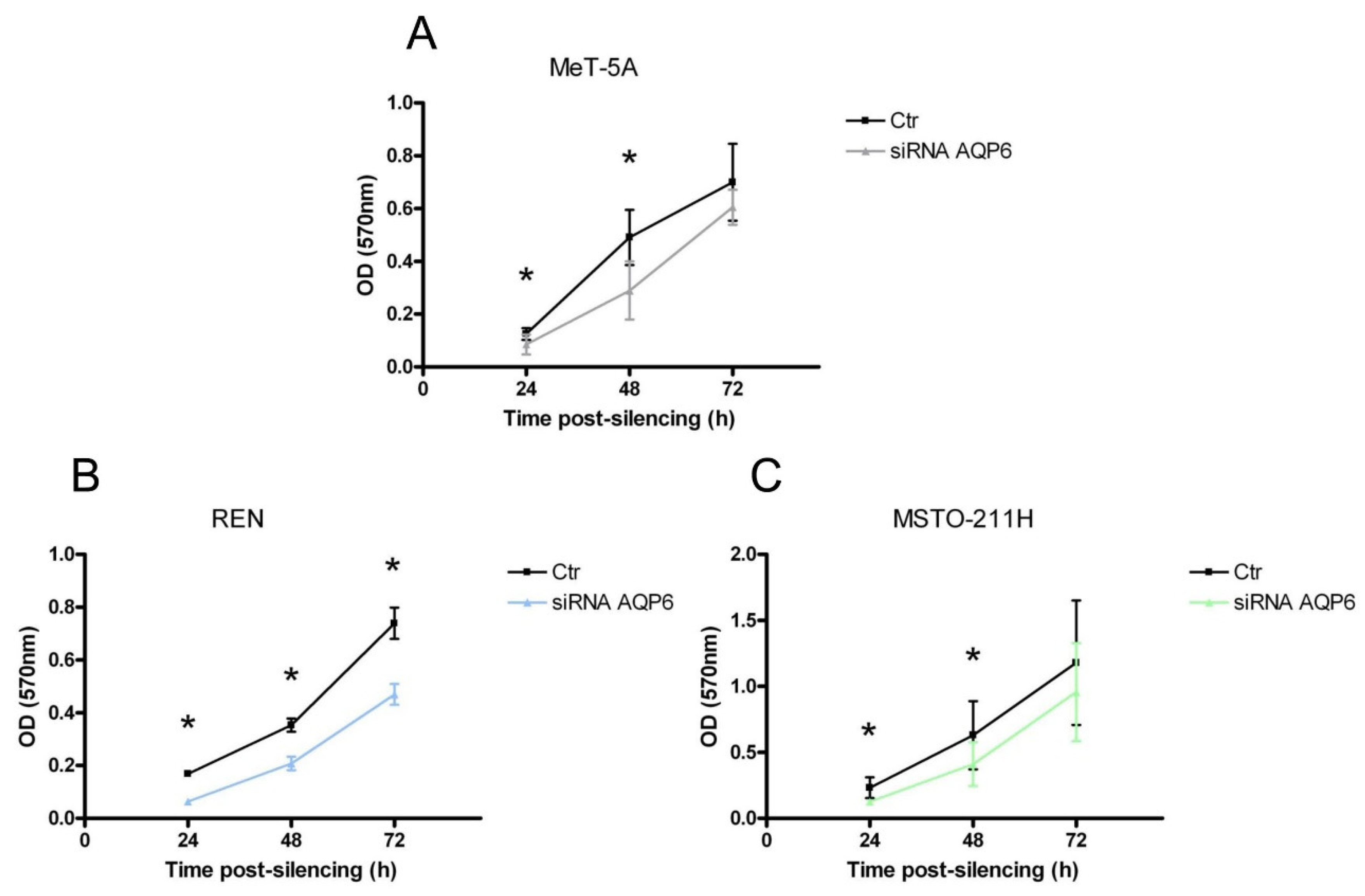
3.7. MeT-5A, REN, and MSTO-211H Cells Proliferation after AQP6 Gene Silencing
4. Discussion
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- PDQ Cancer Information Summaries. Malignant Mesothelioma Treatment (Adult) (PDQ®)–Health Professional Version. PDQ Adult Treatment Editorial Board. In: PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002–2022. Bookshelf ID: NBK65983. Available online: https://www.cancer.gov/types/mesothelioma/hp/mesothelioma-treatment-pdq (accessed on 28 April 2022).
- Scherpereel, A.; Wallyn, F.; Albelda, S.M.; Munck, C. Novel therapies for malignant pleural mesothelioma. Lancet Oncol. 2018, 19, e161–e172. [Google Scholar] [CrossRef]
- Tomasson, K.; Gudmundsson, G.; Briem, H.; Rafnsson, V. Malignant mesothelioma incidence by nation-wide cancer registry: A population-based study. J. Occup. Med. Toxicol. 2016, 11, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikulski, S.M.; Costanzi, J.J.; Vogelzang, N.J.; McCachren, S.; Taub, R.N.; Chun, H.; Mittelman, A.; Panella, T.; Puccio, C.; Fine, R.; et al. Phase II trial of a single weekly intravenous dose of ranpirnase in patients with unresectable malignant mesothelioma. J. Clin. Oncol. 2002, 20, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Kondo, S.; Hara, S.; Morishita, Y. The evolutionary aspects of aquaporin family. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R566–R576. [Google Scholar] [CrossRef]
- Verkman, A.S. Aquaporins at a glance. J. Cell Sci. 2011, 124 Pt 13, 2107–2112. [Google Scholar] [CrossRef] [Green Version]
- King, L.S.; Kozono, D.; Agre, P. From structure to disease: The evolving tale of aquaporin biology. Nat. Rev. Mol. Cell Biol. 2004, 5, 687–698. [Google Scholar] [CrossRef]
- Bienert, G.P.; Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta 2014, 1840, 1596–1604. [Google Scholar] [CrossRef]
- Medraño-Fernandez, I.; Bestetti, S.; Bertolotti, M.; Bienert, G.P.; Bottino, C.; Laforenza, U.; Rubartelli, A.; Sitia, R. Stress regulates aquaporin-8 permeability to impact cell growth and survival. Antioxid. Redox Signal. 2016, 24, 1031–1044. [Google Scholar] [CrossRef] [Green Version]
- Miller, E.W.; Dickinson, B.C.; Chang, C.J. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 15681–15686. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, S.; Moniaga, C.S.; Nielsen, S.; Hara-Chikuma, M. Aquaporin-9 facilitates membrane transport of hydrogen peroxide in mammalian cells. Biochem. Biophys. Res. Commun. 2016, 471, 191–197. [Google Scholar] [CrossRef]
- Rodrigues, C.; Mósca, A.F.; Martins, A.P.; Nobre, T.; Prista, C.; Antunes, F.; Cipak Gasparovic, A.; Soveral, G. Rat aquaporin-5 is pH-gated induced by phosphorylation and is implicated in oxidative stress. Int. J. Mol. Sci. 2016, 17, 2090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bestetti, S.; Galli, M.; Sorrentino, I.; Pinton, P.; Rimessi, A.; Sitia, R.; Medraño-Fernandez, I. Human aquaporin-11 guarantees efficient transport of H2O2 across the endoplasmic reticulum membrane. Redox Biol. 2020, 28, 101326. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Chikuma, S.; Sugiyama, Y.; Kabashima, K.; Verkman, A.S.; Inoue, S.; Miyachi, Y. Chemokine-dependent T cell migration requires aquaporin-3-mediated hydrogen peroxide uptake. J. Exp. Med. 2012, 209, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Satooka, H.; Hara-Chikuma, M. Aquaporin-3 controls breast cancer cell migration by regulating hydrogen peroxide transport and its downstream cell signaling. Mol. Cell. Biol. 2016, 36, 1206–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiagarajah, J.; Zhao, D.; Verkman, A. Impaired enterocyte proliferation in aquaporin-3 deficiency in mouse models of colitis. Gut 2007, 56, 1529–1535. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Saadoun, S.; Papadopoulos, M.C.; Hara-Chikuma, M.; Verkman, A.S. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature 2005, 434, 786–792. [Google Scholar] [CrossRef]
- Papadopoulos, M.C.; Saadoun, S. Key roles of aquaporins in tumor biology. Biochim. Biophys. Acta 2015, 1848 Pt B, 2576–2583. [Google Scholar] [CrossRef] [Green Version]
- Dorward, H.S.; Du, A.; Bruhn, M.A.; Wrin, J.; Pei, J.V.; Evdokiou, A.; Price, T.J.; Yool, A.J.; Hardingham, J.E. Pharmacological blockade of aquaporin-1 water channel by AqB013 restricts migration and invasiveness of colon cancer cells and prevents endothelial tube formation in vitro. J. Exp. Clin. Cancer Res. 2016, 35, 36. [Google Scholar] [CrossRef] [Green Version]
- Tradtrantip, L.; Jin, B.J.; Yao, X.; Anderson, M.O.; Verkman, A.S. Aquaporin-targeted therapeutics: State-of-the-field. Adv. Exp. Med. Biol. 2017, 969, 239–250. [Google Scholar]
- King, L.S.; Nielsen, S.; Agre, P. Aquaporin-1 water channel protein in lung: Ontogeny, steroid-induced expression, and distribution in rat. J. Clin. Investig. 1996, 97, 2183–2191. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Hu, J.; Bai, C. Role of aquaporin and sodium channel in pleural water movement. Respir. Physiol. Neurobiol. 2003, 139, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Kao, S.C.; Armstrong, N.; Condon, B.; Griggs, K.; McCaughan, B.; Maltby, S.; Wilson, A.; Henderson, D.W.; Klebe, S. Aquaporin 1 is an independent prognostic factor in pleural malignant mesothelioma. Cancer 2012, 118, 2952–2961. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Yang, B.; Matthay, M.A.; Ma, T.; Verkman, A.S. Role of aquaporin water channels in pleural fluid dynamics. Am. J. Physiol. Cell Physiol. 2000, 279, C1744–C1750. [Google Scholar] [CrossRef]
- Angelico, G.; Caltabiano, R.; Loreto, C.; Ieni, A.; Tuccari, G.; Ledda, C.; Rapisarda, V. Immunohistochemical expression of aquaporin-1 in fluoro-edenite-induced malignant mesothelioma: A preliminary report. Int. J. Mol. Sci. 2018, 19, 685. [Google Scholar] [CrossRef] [Green Version]
- Klebe, S.; Grigg, K.; Cheng, Y.; Driml, J.; Henderson, D.W.; Reid, G. Blockade of aquaporin 1 inhibits proliferation, motility, and metastatic potential of mesothelioma in vitro but not in an in vivo model. Dis. Markers 2015, 2015, 286719. [Google Scholar] [CrossRef] [Green Version]
- Jagirdar, R.M.; Apostolidou, E.; Molyvdas, P.A.; Gourgoulianis, K.I.; Hatzoglou, C.; Zarogiannis, S.G. Influence of AQP1 on cell adhesion, migration, and tumor sphere formation in malignant pleural mesothelioma is substratum- and histological-type dependent. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 310, L489–L495. [Google Scholar] [CrossRef] [Green Version]
- Driml, J.; Pulford, E.; Moffat, D.; Karapetis, C.; Kao, S.; Griggs, K.; Henderson, D.W.; Klebe, S. Usefulness of aquaporin 1 as a prognostic marker in a prospective cohort of malignant mesotheliomas. Int. J. Mol. Sci. 2016, 17, 1041. [Google Scholar] [CrossRef] [Green Version]
- Ke, Y.; Reddel, R.R.; Gerwin, B.I.; Reddel, H.K.; Somers, A.N.; McMenamin, M.G.; LaVeck, M.A.; Stahel, R.A.; Lechner, J.F.; Harris, C.C. Establishment of a human in vitro mesothelial cell model system for investigating mechanisms of asbestos-induced mesothelioma. Am. J. Pathol. 1989, 134, 979–991. [Google Scholar]
- Smythe, W.R.; Hwang, H.C.; Amin, K.M.; Eck, S.L.; Davidson, B.L.; Wilson, J.M.; Kaiser, L.R.; Albelda, S.M. Use of recombinant adenovirus to transfer the herpes simplex virus thymidine kinase (HSVtk) gene to thoracic neoplasms: An effective in vitro drug sensitization system. Cancer Res. 1994, 54, 2055–2059. [Google Scholar]
- Bepler, G.; Koehler, A.; Kiefer, P.; Havemann, K.; Beisenherz, K.; Jaques, G.; Gropp, C.; Haeder, M. Characterization of the state of differentiation of six newly established human non-small-cell lung cancer cell lines. Differentiation 1988, 37, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Laforenza, U.; Miceli, E.; Gastaldi, G.; Scaffino, M.F.; Ventura, U.; Fontana, J.M.; Orsenigo, M.N.; Corazza, G.R. Solute transporters and aquaporins are impaired in celiac disease. Biol. Cell 2010, 102, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Yeung, Y.G.; Stanley, E.R. A solution for stripping antibodies from polyvinylidene fluoride immunoblots for multiple reprobing. Anal. Biochem. 2009, 389, 89–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolte, S.; Cordelières, F.P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 2006, 224 Pt 3, 213–232. [Google Scholar] [CrossRef]
- Manders, E.M.M.; Verbeek, F.J.; Aten, J.A. Measurement of co-localization of objects in dual-colour confocal images. J. Microsc. 1993, 169, 375–382. [Google Scholar] [CrossRef]
- van Steensel, B.; van Binnendijk, E.P.; Hornsby, C.D.; van der Voort, H.T.; Krozowski, Z.S.; de Kloet, E.R.; van Driel, R. Partial colocalization of glucocorticoid and mineralocorticoid receptors in discrete compartments in nuclei of rat hippocampus neurons. J. Cell Sci. 1996, 109 Pt 4, 787–792. [Google Scholar] [CrossRef]
- Pellavio, G.; Rui, M.; Caliogna, L.; Martino, E.; Gastaldi, G.; Collina, S.; Laforenza, U. Regulation of aquaporin functional properties mediated by the antioxidant effects of natural compounds. Int. J. Mol. Sci. 2017, 18, 2665. [Google Scholar] [CrossRef] [Green Version]
- Wiener, H.; Turnheim, K.; van Os, C. Rabbit distal colon epithelium: I. Isolation and characterization of basolateral plasma membrane vesicles from surface and crypt cells. J. Membr. Biol. 1989, 110, 147–162. [Google Scholar] [CrossRef]
- Pak, V.V.; Ezeriņa, D.; Lyublinskaya, O.G.; Pedre, B.; Tyurin-Kuzmin, P.A.; Mishina, N.M.; Thauvin, M.; Young, D.; Wahni, K.; Martínez Gache, S.A.; et al. Ultrasensitive genetically encoded indicator for hydrogen peroxide identifies roles for the oxidant in cell migration and mitochondrial function. Cell Metab. 2020, 31, 642–653.e6. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Preston, G.M.; Jung, J.S.; Guggino, W.B.; Agre, P. The mercury-sensitive residue at cysteine 189 in the CHIP28 water channel. J. Biol. Chem. 1993, 268, 17–20. [Google Scholar] [CrossRef]
- Benga, G.; Popescu, O.; Pop, V.I.; Holmes, R.P. p-(Chloromercuri)benzenesulfonate binding by membrane proteins and the inhibition of water transport in human erythrocytes. Biochemistry 1986, 25, 1535–1538. [Google Scholar] [CrossRef] [PubMed]
- Yasui, M.; Hazama, A.; Kwon, T.H.; Nielsen, S.; Guggino, W.B.; Agre, P. Rapid gating and anion permeability of an intracellular aquaporin. Nature 1999, 402, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Bibby, A.C.; Tsim, S.; Kanellakis, N.; Ball, H.; Talbot, D.C.; Blyth, K.G.; Maskell, N.A.; Psallidas, I. Malignant pleural mesothelioma: An update on investigation, diagnosis and treatment. Eur. Respir. Rev. 2016, 25, 472–486. [Google Scholar] [CrossRef] [Green Version]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- De Ieso, M.L.; Yool, A.J. Mechanisms of aquaporin-facilitated cancer invasion and metastasis. Front. Chem. 2018, 6, 135. [Google Scholar] [CrossRef] [Green Version]
- Ledda, C.; Senia, P.; Rapisarda, V. Biomarkers for early diagnosis and prognosis of malignant pleural mesothelioma: The quest goes on. Cancers 2018, 10, 203. [Google Scholar] [CrossRef] [Green Version]
- Yasui, M.; Kwon, T.; Knepper, M.; Nielsen, S.; Agre, P. Aquaporin-6: An intracellular vesicle water channel protein in renal epithelia. Proc. Natl. Acad. Sci. USA 1999, 96, 5808–5813. [Google Scholar] [CrossRef] [Green Version]
- Prata, C.; Hrelia, S.; Fiorentini, D. Peroxiporins in Cancer. Int. J. Mol. Sci. 2019, 20, 1371. [Google Scholar] [CrossRef] [Green Version]
- Dajani, S.; Saripalli, A.; Sharma-Walia, N. Water transport proteins-aquaporins (AQPs) in cancer biology. Oncotarget 2018, 9, 36392–36405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mobasheri, A.; Barrett-Jolley, R. Aquaporin water channels in the mammary gland: From physiology to pathophysiology and neoplasia. J. Mammary Gland Biol. Neoplasia 2014, 19, 91–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katkova, L.E.; Baturina, G.S.; Bondar, A.A.; Jagirdar, R.M.; Hatzoglou, C.; Gourgoulianis, K.I.; Solenov, E.I.; Zarogiannis, S.G. Benign pleural mesothelial cells have higher osmotic water permeability than malignant pleural mesothelioma cells and differentially respond to hyperosmolality. Cell Physiol. Biochem. 2019, 52, 869–878. [Google Scholar] [PubMed]
- Wang, J.; Yi, J. Cancer cell killing via ROS: To increase or decrease, that is the question. Cancer Biol. Ther. 2008, 7, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Villani, R.M.; Wang, H.; Simpson, M.J.; Roberts, M.S.; Tang, M.; Liang, X. The role of cellular reactive oxygen species in cancer chemotherapy. J. Exp. Clin. Cancer Res. 2018, 37, 266. [Google Scholar] [CrossRef]
- Rodrigues, C.; Pimpão, C.; Mósca, A.F.; Coxixo, A.S.; Lopes, D.; da Silva, I.V.; Pedersen, P.A.; Antunes, F.; Soveral, G. Human Aquaporin-5 facilitates hydrogen peroxide permeation affecting adaption to oxidative stress and cancer cell migration. Cancers 2019, 11, 932. [Google Scholar] [CrossRef] [Green Version]
- Alabalık, U.; Türkcü, G.; Keleş, A.N.; İbiloğlu, I.; Urakçı, Z.; Hüseyin, B. Aquaporin 1, Aquaporin 3 and Aquaporin 5 expression and EGFR mutation in malignant pleural mesotheliomas: An imunohistochemical and molecular study. Biotechnol. Biotechnol. Equip. 2017, 31, 367–372. [Google Scholar] [CrossRef] [Green Version]
- Bi, C.; Tham, D.K.L.; Perronnet, C.; Joshi, B.; Nabi, I.R.; Moukhles, H. The oxidative stress-induced increase in the membrane expression of the water-permeable channel aquaporin-4 in astrocytes is regulated by caveolin-1 phosphorylation. Front. Cell Neurosci. 2017, 11, 412. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, C.; Milkovic, L.; Bujak, I.T.; Tomljanovic, M.; Soveral, G.; Cipak Gasparovic, A. Lipid profile and pquaporin expression under oxidative stress in breast cancer cells of different malignancies. Oxid. Med. Cell Longev. 2019, 2019, 2061830. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Huang, L.; Shao, M. Aquaporin 3 facilitates tumor growth in pancreatic cancer by modulating mTOR signaling. Biochem. Biophys. Res. Commun. 2017, 486, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Vieceli Dalla Sega, F.; Zambonin, L.; Fiorentini, D.; Rizzo, B.; Caliceti, C.; Landi, L.; Hrelia, S.; Prata, C. Specific aquaporins facilitate Nox-produced hydrogen peroxide transport through plasma membrane in leukaemia cells. Biochim. Biophys. Acta 2014, 1843, 806–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Li, Q.; Yang, T.; Li, D.; Ding, F.; Sun, H.; Bai, G. RNA interference-mediated silencing of aquaporin (AQP)-5 hinders angiogenesis of colorectal tumor by suppressing the production of vascular endothelial growth factor. Neoplasma 2018, 65, 55–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Gene | Primer Sequences | Size (bp) | Accession Number | |
|---|---|---|---|---|
| AQP2 a | Forward | 5′-CACCTCCTTGGGATCCATTACACC-3′ | 95 | NM_000486 |
| Reverse | 5′-ACCCAGTGGTCATCAAATTTGCC-3′ | |||
| AQP3 b | Forward | 5′-CCTGGTGATGTTTGGCTGTGGCTC-3′ | 147 | NM_004925; variants 1, 2 |
| Reverse | 5′-TTCAGGTGGGCCCCAGAGACC-3′ | |||
| AQP4 | Forward | 5′-GGAGTCACCATGGTTCATGGAA-3′ | 123 | NM_001650; variants 1–3 |
| Reverse | 5′-AGTGACATCAGTCCGTTTGGAA-3′ | |||
| AQP5 | Forward | 5′-GGTGGTGGAGCTGATTCTGA-3′ | 142 | NM_001651 |
| Reverse | 5′-GAAGTAGATTCCGACAAGGTGG-3′ | |||
| AQP6 | Forward | 5′-CACCTCATTGGGATCCACTTC-3′ | 103 | NM_ 001652; variants 1,2 |
| Reverse | 5′-CCCAGAAGACCCAGTGGACT-3′ | |||
| AQP7 | Forward | 5′-GGACAGCTGATGGTGACCGG-3′ | 104 | NM_001170; variants 1–4 |
| Reverse | 5′-AGCCACGCCTCATTCAGGAA-3′ | |||
| AQP8 | Forward | 5′-TGGAGAGATAGCCATGTGTGAG-3′ | 106 | NM_001169 |
| Reverse | 5′-TGGCTGCACAAACCGTTCGT-3′ | |||
| AQP9 | Forward | 5′-CCCAGCTGTGTCTTTAGCAA-3′ | 133 | NM_020980; variants 1–3 |
| Reverse | 5′-AAGTCCATCATAGTAAATGCCAAA-3′ | |||
| AQP10 | Forward | 5′-CCTATGTTCTCTACCATGATGCCC-3′ | 137 | NM_080429 |
| Reverse | 5′-CTGATCCAGGAAGCCATTGTTC-3′ | |||
| AQP11 | Forward | 5′-TTTCTCTTCCACAGCGCTCT-3′ | 115 | NM_173039; variant 1 |
| Reverse | 5′-CTCCTGTTAGACTTCCTCCTGC-3′ | |||
| β-actin | Hs_ACTB_1_SG, QuantiTect Primer Assay QT00095431, Qiagen | 146 | NM_001101 | |
| B2M | Hs_B2M_1_SG QuantiTect Primer Assay QT00088935, Qiagen | 98 | NM_004048 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellavio, G.; Martinotti, S.; Patrone, M.; Ranzato, E.; Laforenza, U. Aquaporin-6 May Increase the Resistance to Oxidative Stress of Malignant Pleural Mesothelioma Cells. Cells 2022, 11, 1892. https://doi.org/10.3390/cells11121892
Pellavio G, Martinotti S, Patrone M, Ranzato E, Laforenza U. Aquaporin-6 May Increase the Resistance to Oxidative Stress of Malignant Pleural Mesothelioma Cells. Cells. 2022; 11(12):1892. https://doi.org/10.3390/cells11121892
Chicago/Turabian StylePellavio, Giorgia, Simona Martinotti, Mauro Patrone, Elia Ranzato, and Umberto Laforenza. 2022. "Aquaporin-6 May Increase the Resistance to Oxidative Stress of Malignant Pleural Mesothelioma Cells" Cells 11, no. 12: 1892. https://doi.org/10.3390/cells11121892
APA StylePellavio, G., Martinotti, S., Patrone, M., Ranzato, E., & Laforenza, U. (2022). Aquaporin-6 May Increase the Resistance to Oxidative Stress of Malignant Pleural Mesothelioma Cells. Cells, 11(12), 1892. https://doi.org/10.3390/cells11121892