Synthetic Thymidine Analog Labeling without Misconceptions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. Somatic Analysis
- Bodyweight (PND1–PND17). PND1 to PND8 pups were placed on the pan of an electronic balance (OHAUS Explorer Pro, Parsippany, NJ, USA), which was covered with paper. Starting from PND9, weighing was performed in a plastic container.
- Milk in the stomach (PND1–PND6). A pup was gently supported in supine position by the experimenter’s fingers. During the first week of life, the pup’s skin is transparent under the abdomen area, thus enabling evaluation of the presence of milk, which is visible as a white spot in the pup’s stomach area. Visual estimation of milk in the stomach was performed. A score of 1 was given if milk was present in the stomach, and a score of 0 was given if milk was absent.
- Hair appearance (PND1–PND4). Primary hair appearance was assessed by visual determination of fine hairs under contrasting lamplight. A score of 1 was given if the fine primary hairs were apparent, and a score of 0 was given if they were absent.
- Auricle separation (PND1–PND6). The separation of the auricles from each pup’s head was estimated visually. If auricles were not separated, the experimenter used a small wooden probe to gently separate the ears from the head. A score of 0 was given if both ears were not separated from the head. Separation of one or two ears was recorded as a score of 1 or 2, respectively.
- Finger separation (PND1–PND10) was determined with a wooden probe; separation was considered complete when all paw fingers were clearly separated. A score of 0 was assigned if no separation was observed between all fingers. Scores of 1 to 10 were assigned, depending on the number of separated fingers. Fore- and hindlimbs were evaluated separately.
- Incisor teething (PND10–PND14). The appearance of lower and upper incisors was visually evaluated. The absence of incisors was assigned a score of 0. The appearance of one and two incisors was scored as 1 and 2, respectively.
- Complete fur covering (PND10–PND15). The criterion for complete fur covering was the presence of a crest when a finger or a small stick was run along the ventral abdominal line. The presence of covering was scored as 1, and its absence was scored as 0.
- Eye opening (PND11–PND17). This parameter was assigned a score of 0 if the eyes were closed. For complete opening of one or two eyes, a score of 1 or 2, respectively, was given.
- Brain weight (PND17). Fixed brains were rinsed with PBS, dried with filter paper, and weighed on electronic balances. The spinal cord was cut off before weighting.
2.3. Staining
2.4. Image Analysis
2.5. Statistics
3. Results
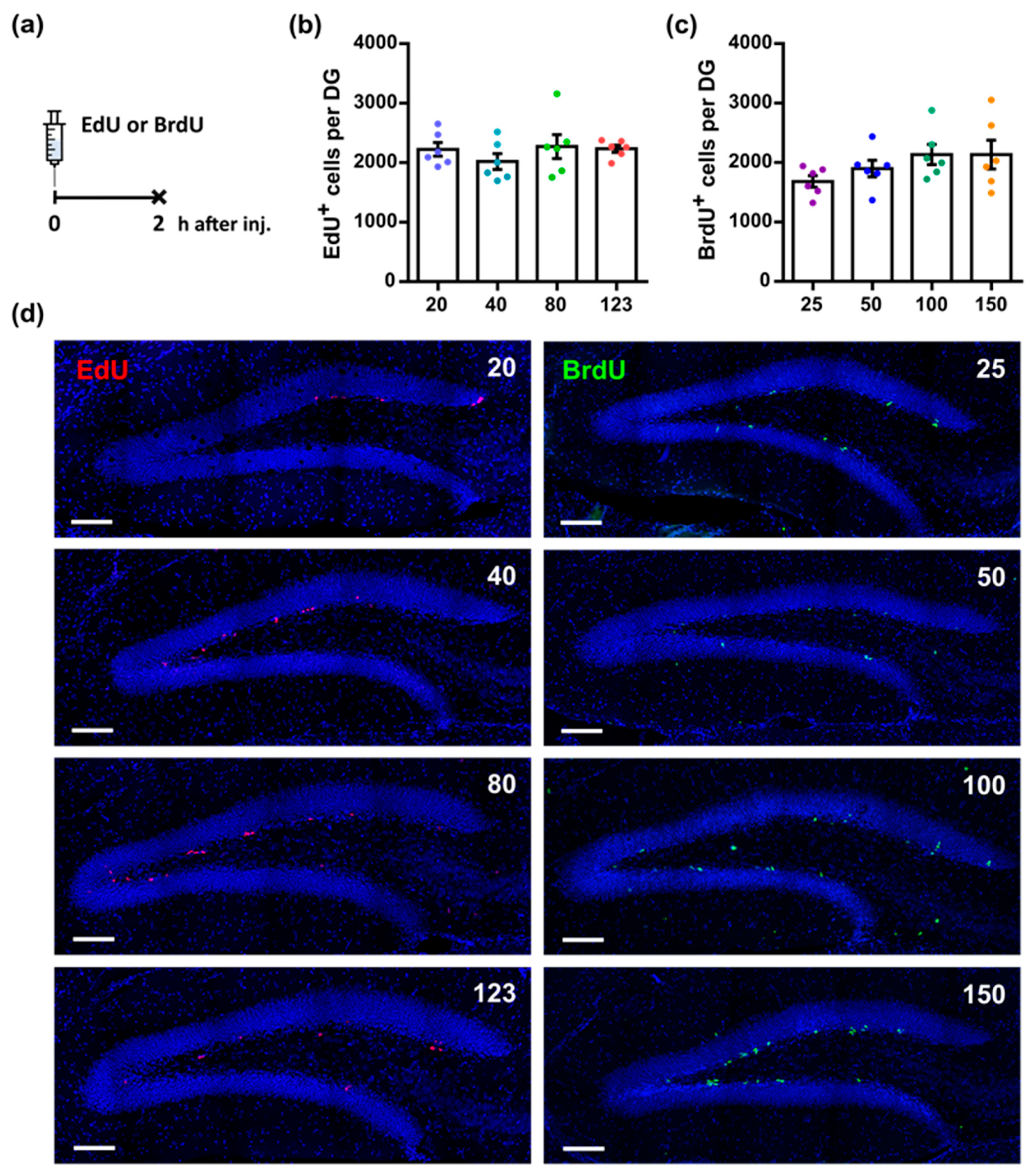
3.1. Different Doses of Thymidine Analogs Reveal Equivalent Numbers of Dividing Cells in the Dentate Gyrus
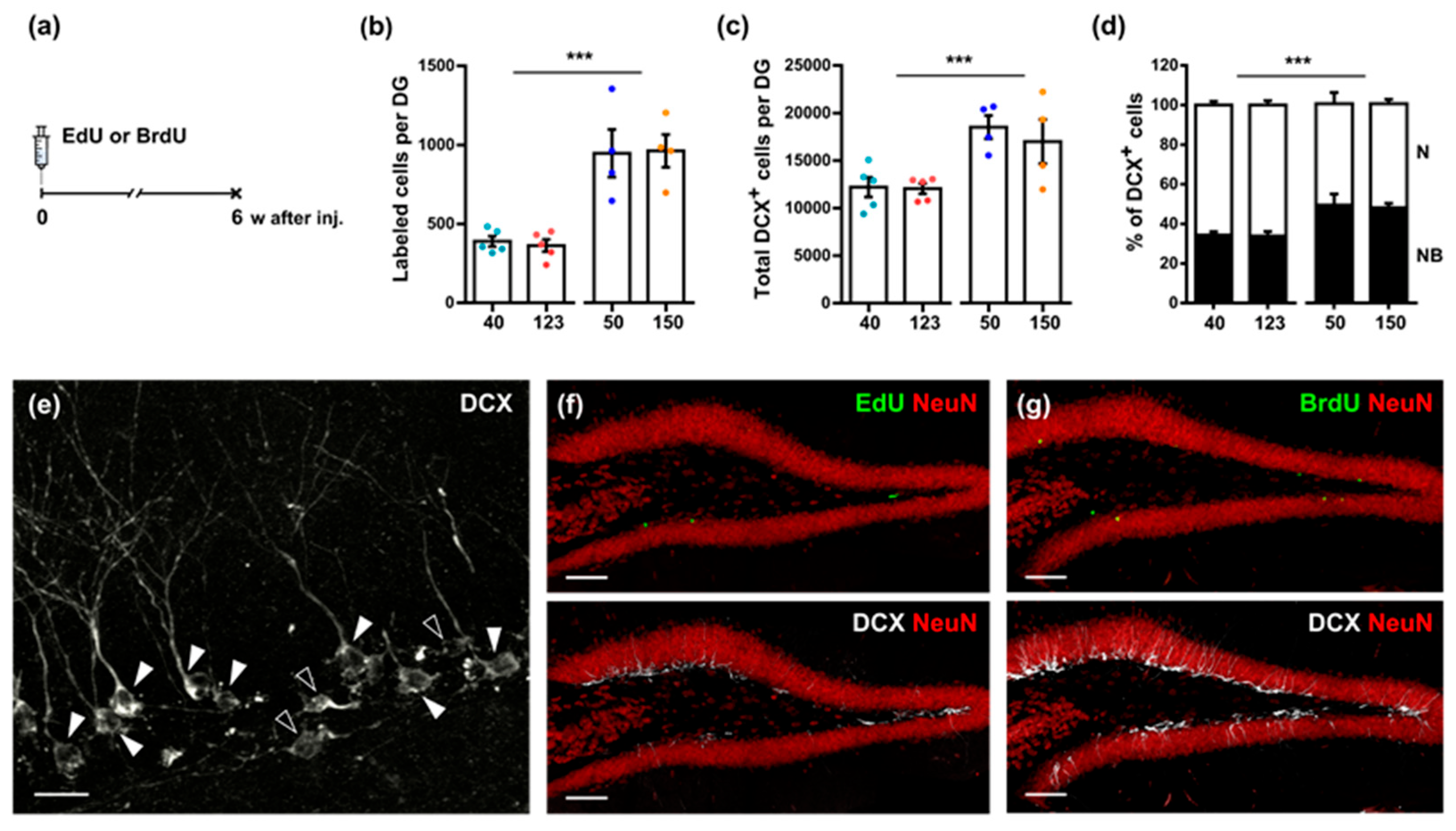
3.2. A Single Injection of EdU Does Not Affect Survival and Subsequent Division of Hippocampal Neural Precursor Cells
3.3. EdU and BrdU Have Different Long-Term Effects on Hippocampal Neurogenesis
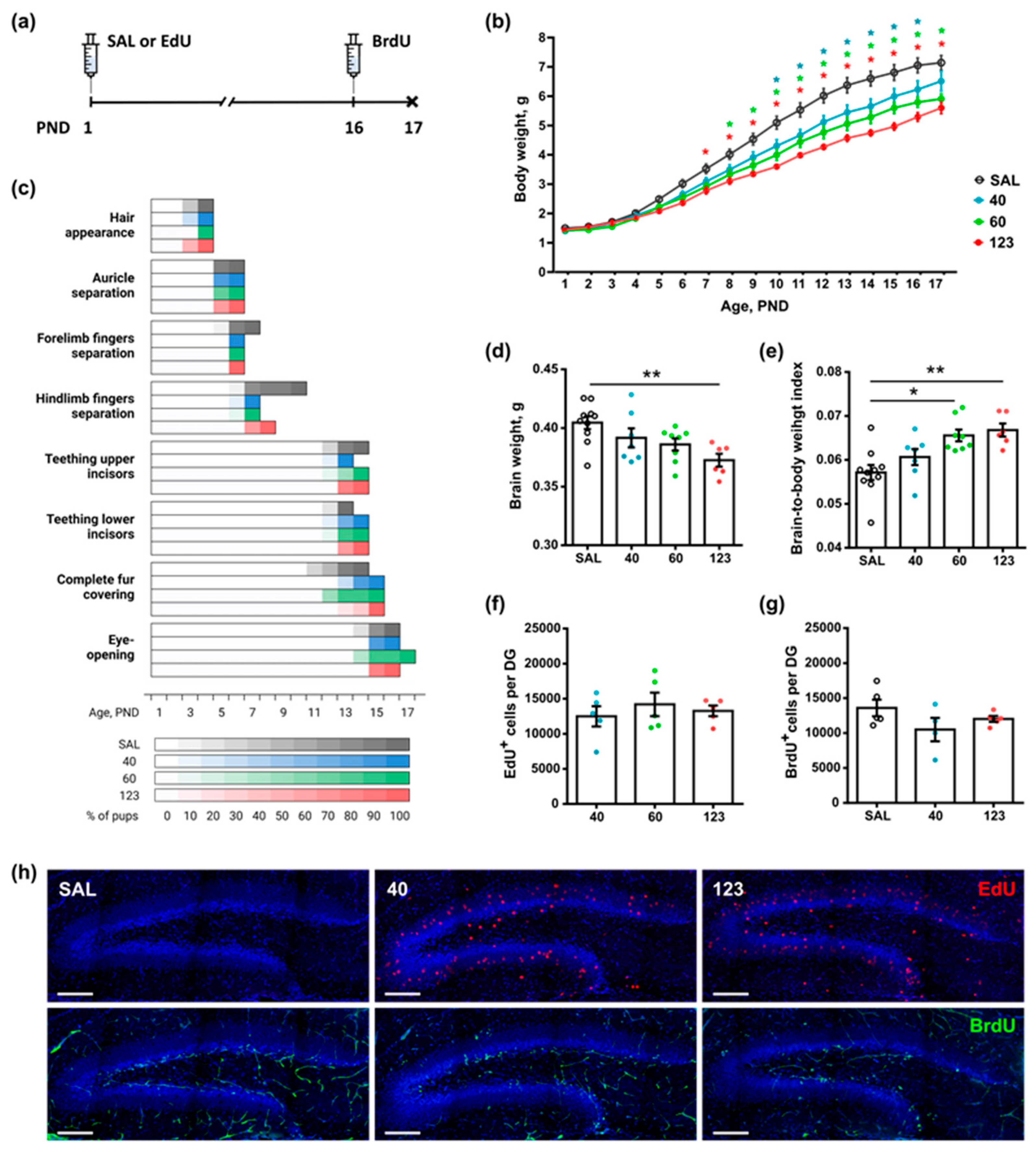
3.4. A Single Injection of EdU Affects Somatic Development of Newborn Pups in a Dose-Dependent Manner but Does Not Affect Hippocampal Neurogenesis
4. Discussion
- If the goal of the experiment is to determine the number of neural progenitor cells engaged in the cell cycle, low doses of either EdU or BrdU (up to 20 mg/kg) are almost as effective as the higher doses (up to 120–150 mg/kg).
- If the goal is to investigate cells that may have undergone several rounds of division (and, therefore, dilution of the initial label), higher doses (120–150 mg/kg of EdU or BrdU) may be preferable.
- If the goal is to investigate the long-term changes, e.g., survival and differentiation, in cells that were tagged 1–3 months earlier, using BrdU may be preferable to EdU.
- For cell labeling with multiple markers, it is safer to use EdU as the last label.
- For labeling cells in the perinatal animals, it may be important to take into account that EdU may have an adverse effect on several developmental milestones; at the same time, administration of EdU does not have a noticeable impact on hippocampal neurogenesis in juveniles.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taupin, P. BrdU immunohistochemistry for studying adult neurogenesis: Paradigms, pitfalls, limitations, and validation. Brain Res. Rev. 2007, 53, 198–214. [Google Scholar] [CrossRef] [PubMed]
- Solius, G.M.; Maltsev, D.I.; Belousov, V.V.; Podgorny, O.V. Recent advances in nucleotide analogue-based techniques for tracking dividing stem cells: An overview. J. Biol. Chem. 2021, 297, 101345. [Google Scholar] [CrossRef] [PubMed]
- Ligasova, A.; Koberna, K. DNA Replication: From radioisotopes to click chemistry. Molecules 2018, 23, 3007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salic, A.; Mitchison, T.J. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 2415–2420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Bohlen und Halbach, O. Immunohistological markers for proliferative events, gliogenesis, and neurogenesis within the adult hippocampus. Cell Tissue Res. 2011, 345, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Vega, C.J.; Peterson, D.A. Stem cell proliferative history in tissue revealed by temporal halogenated thymidine analog discrimination. Nat. Methods 2005, 2, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Podgorny, O.; Peunova, N.; Enikolopov, G. Tracing dividing stem cells. Aging 2018, 10, 1534–1535. [Google Scholar] [CrossRef] [PubMed]
- Podgorny, O.; Peunova, N.; Park, J.H.; Enikolopov, G. Triple S-phase labeling of dividing stem cells. Stem Cell Rep. 2018, 10, 615–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Encinas, J.M.; Michurina, T.V.; Peunova, N.; Park, J.H.; Tordo, J.; Peterson, D.A.; Fishell, G.; Koulakov, A.; Enikolopov, G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 2011, 8, 566–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazutkin, A.A.; Shuvaev, S.A.; Barykina, N.V. Click histochemistry for whole-mount staining of brain structures. MethodsX 2019, 6, 1986–1991. [Google Scholar] [CrossRef]
- Rieder, U.; Luedtke, N.W. Alkene-tetrazine ligation for imaging cellular DNA. Angew. Chem. Int. Ed. Engl. 2014, 53, 9168–9172. [Google Scholar] [CrossRef]
- Neef, A.B.; Luedtke, N.W. Dynamic metabolic labeling of DNA in vivo with arabinosyl nucleosides. Proc. Natl. Acad. Sci. USA 2011, 108, 20404–20409. [Google Scholar] [CrossRef] [Green Version]
- Fox, W.M. Reflex-ontogeny and behavioural development of the mouse. Anim. Behav. 1965, 13, 234–241. [Google Scholar] [CrossRef]
- Bianconi, S.; Santillan, M.E.; Solis, M.D.R.; Martini, A.C.; Ponzio, M.F.; Vincenti, L.M.; Schioth, H.B.; Carlini, V.P.; Stutz, G. Effects of dietary omega-3 PUFAs on growth and development: Somatic, neurobiological and reproductive functions in a murine model. J. Nutr Biochem 2018, 61, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Bignami, G. Economical test methods for developmental neurobehavioral toxicity. Environ. Health Perspect. 1996, 104 (Suppl. 2), 285–298. [Google Scholar] [CrossRef] [Green Version]
- Kihara, T.; Surjono, T.W.; Sakamoto, M.; Matsuo, T.; Yasuda, Y.; Tanimura, T. Effects of prenatal rubratoxin-B exposure on behaviors of mouse offspring. Toxicol Sci 2001, 61, 368–373. [Google Scholar] [CrossRef] [Green Version]
- Oliverio, A.; Castellano, C.; Allegra, S.P. Effects of genetic and nutritional factors on post-natal reflex and behavioral development in the mouse. Exp. Aging Res. 1975, 1, 41–56. [Google Scholar] [CrossRef]
- Encinas, J.M.; Enikolopov, G. Identifying and quantitating neural stem and progenitor cells in the adult brain. Methods Cell Biol 2008, 85, 243–272. [Google Scholar] [CrossRef]
- Plumpe, T.; Ehninger, D.; Steiner, B.; Klempin, F.; Jessberger, S.; Brandt, M.; Romer, B.; Rodriguez, G.R.; Kronenberg, G.; Kempermann, G. Variability of doublecortin-associated dendrite maturation in adult hippocampal neurogenesis is independent of the regulation of precursor cell proliferation. BMC Neurosci 2006, 7, 77. [Google Scholar] [CrossRef] [Green Version]
- Mineyeva, O.A.; Bezriadnov, D.V.; Kedrov, A.V.; Lazutkin, A.A.; Anokhin, K.V.; Enikolopov, G.N. Radiation induces distinct changes in defined subpopulations of neural stem and progenitor cells in the adult hippocampus. Front. Neurosci 2019, 12, 1013. [Google Scholar] [CrossRef] [Green Version]
- Klempin, F.; Kronenberg, G.; Cheung, G.; Kettenmann, H.; Kempermann, G. Properties of doublecortin-(DCX)-expressing cells in the piriform cortex compared to the neurogenic dentate gyrus of adult mice. PLoS ONE 2011, 6, e25760. [Google Scholar] [CrossRef] [Green Version]
- Denoth-Lippuner, A.; Jessberger, S. Formation and integration of new neurons in the adult hippocampus. Nat. Rev. Neurosci 2021, 22, 223–236. [Google Scholar] [CrossRef]
- Urban, N.; Blomfield, I.M.; Guillemot, F. Quiescence of adult mammalian neural stem cells: A highly regulated rest. Neuron 2019, 104, 834–848. [Google Scholar] [CrossRef] [PubMed]
- Toda, T.; Parylak, S.L.; Linker, S.B.; Gage, F.H. The role of adult hippocampal neurogenesis in brain health and disease. Mol. Psychiatry 2019, 24, 67–87. [Google Scholar] [CrossRef]
- Lazutkin, A.; Podgorny, O.; Enikolopov, G. Modes of division and differentiation of neural stem cells. Behav Brain Res. 2019, 374, 112118. [Google Scholar] [CrossRef]
- Hayes, N.L.; Nowakowski, R.S. Dynamics of cell proliferation in the adult dentate gyrus of two inbred strains of mice. Brain Res. Dev. Brain Res. 2002, 134, 77–85. [Google Scholar] [CrossRef]
- Mandyam, C.D.; Harburg, G.C.; Eisch, A.J. Determination of key aspects of precursor cell proliferation, cell cycle length and kinetics in the adult mouse subgranular zone. Neuroscience 2007, 146, 108–122. [Google Scholar] [CrossRef] [Green Version]
- Cameron, H.A.; McKay, R.D. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp. Neurol 2001, 435, 406–417. [Google Scholar] [CrossRef]
- Cooper-Kuhn, C.M.; Kuhn, H.G. Is it all DNA repair? Methodological considerations for detecting neurogenesis in the adult brain. Brain Res. Dev. Brain Res. 2002, 134, 13–21. [Google Scholar] [CrossRef]
- Haskins, J.S.; Su, C.; Maeda, J.; Walsh, K.D.; Haskins, A.H.; Allum, A.J.; Froning, C.E.; Kato, T.A. Evaluating the genotoxic and cytotoxic effects of thymidine analogs, 5-ethynyl-2′-deoxyuridine and 5-bromo-2′-deoxyurdine to mammalian cells. Inter. J. Mol. Sci. 2020, 21, 6631. [Google Scholar] [CrossRef]
- Cappella, P.; Gasparri, F.; Pulici, M.; Moll, J. Cell proliferation method: Click chemistry based on BrdU coupling for multiplex antibody staining. Curr Protoc Cytom 2015, 72, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Cattan, A.; Ayali, A.; Barnea, A. The cell birth marker BrdU does not affect recruitment of subsequent cell divisions in the adult avian brain. Biomed. Res. Int 2015, 2015, 126078. [Google Scholar] [CrossRef]
- Chehrehasa, F.; Meedeniya, A.C.; Dwyer, P.; Abrahamsen, G.; Mackay-Sim, A. EdU, a new thymidine analogue for labelling proliferating cells in the nervous system. J. Neurosci Methods 2009, 177, 122–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diermeier-Daucher, S.; Clarke, S.T.; Hill, D.; Vollmann-Zwerenz, A.; Bradford, J.A.; Brockhoff, G. Cell type specific applicability of 5-ethynyl-2′-deoxyuridine (EdU) for dynamic proliferation assessment in flow cytometry. Cytometry A 2009, 75, 535–546. [Google Scholar] [CrossRef]
- Lehner, B.; Sandner, B.; Marschallinger, J.; Lehner, C.; Furtner, T.; Couillard-Despres, S.; Rivera, F.J.; Brockhoff, G.; Bauer, H.C.; Weidner, N.; et al. The dark side of BrdU in neural stem cell biology: Detrimental effects on cell cycle, differentiation and survival. Cell Tissue Res. 2011, 345, 313–328. [Google Scholar] [CrossRef]
- Pereira, P.D.; Serra-Caetano, A.; Cabrita, M.; Bekman, E.; Braga, J.; Rino, J.; Santus, R.; Filipe, P.L.; Sousa, A.E.; Ferreira, J.A. Quantification of cell cycle kinetics by EdU (5-ethynyl-2′-deoxyuridine)-coupled-fluorescence-intensity analysis. Oncotarget 2017, 8, 40514–40532. [Google Scholar] [CrossRef] [Green Version]
- Shevchouk, O.T.; Ball, G.F.; Cornil, C.A.; Balthazart, J. Studies of HVC plasticity in adult canaries reveal social effects and sex differences as well as limitations of multiple markers available to assess adult neurogenesis. PLoS ONE 2017, 12, e0170938. [Google Scholar] [CrossRef] [Green Version]
- Platel, J.C.; Angelova, A.; Bugeon, S.; Wallace, J.; Ganay, T.; Chudotvorova, I.; Deloulme, J.C.; Beclin, C.; Tiveron, M.C.; Core, N.; et al. Neuronal integration in the adult mouse olfactory bulb is a non-selective addition process. Elife 2019, 8. [Google Scholar] [CrossRef]
- Breunig, J.J.; Arellano, J.I.; Macklis, J.D.; Rakic, P. Everything that glitters isn’t gold: A critical review of postnatal neural precursor analyses. Cell Stem Cell 2007, 1, 612–627. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Vazquez, L.; Marti, J. Administration of 5-bromo-2′-deoxyuridine interferes with neuroblast proliferation and promotes apoptotic cell death in the rat cerebellar neuroepithelium. J. Comp. Neurol 2021, 529, 1081–1096. [Google Scholar] [CrossRef]
- Hancock, A.; Priester, C.; Kidder, E.; Keith, J.R. Does 5-bromo-2′-deoxyuridine (BrdU) disrupt cell proliferation and neuronal maturation in the adult rat hippocampus in vivo? Behav Brain Res. 2009, 199, 218–221. [Google Scholar] [CrossRef] [Green Version]
- Zeng, C.; Pan, F.; Jones, L.A.; Lim, M.M.; Griffin, E.A.; Sheline, Y.I.; Mintun, M.A.; Holtzman, D.M.; Mach, R.H. Evaluation of 5-ethynyl-2′-deoxyuridine staining as a sensitive and reliable method for studying cell proliferation in the adult nervous system. Brain Res. 2010, 1319, 21–32. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, A.; Gruzova, O.; Ermolaeva, E.; Astakhova, O.; Itaman, S.; Enikolopov, G.; Lazutkin, A. Synthetic Thymidine Analog Labeling without Misconceptions. Cells 2022, 11, 1888. https://doi.org/10.3390/cells11121888
Ivanova A, Gruzova O, Ermolaeva E, Astakhova O, Itaman S, Enikolopov G, Lazutkin A. Synthetic Thymidine Analog Labeling without Misconceptions. Cells. 2022; 11(12):1888. https://doi.org/10.3390/cells11121888
Chicago/Turabian StyleIvanova, Anna, Olesya Gruzova, Elizaveta Ermolaeva, Olga Astakhova, Sheed Itaman, Grigori Enikolopov, and Alexander Lazutkin. 2022. "Synthetic Thymidine Analog Labeling without Misconceptions" Cells 11, no. 12: 1888. https://doi.org/10.3390/cells11121888
APA StyleIvanova, A., Gruzova, O., Ermolaeva, E., Astakhova, O., Itaman, S., Enikolopov, G., & Lazutkin, A. (2022). Synthetic Thymidine Analog Labeling without Misconceptions. Cells, 11(12), 1888. https://doi.org/10.3390/cells11121888







