COX-2 Deficiency Promotes White Adipogenesis via PGE2-Mediated Paracrine Mechanism and Exacerbates Diet-Induced Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Administration of PGE2
2.4. Primary Culture and Differentiation of Adipocytes
2.5. Treatment of PGE2 Receptor Antagonists or PKA Activator/Inhibitors during Differentiation
2.6. Hematoxylin and Eosin (H&E) and Oil Red O Staining
2.7. Glucose Tolerance Test (GTT) and Insulin Tolerance Test (ITT)
2.8. DEXA Scanning
2.9. Immunofluorescence Staining
2.10. Real-Time PCR and Western Blot
2.11. Statistics
3. Results
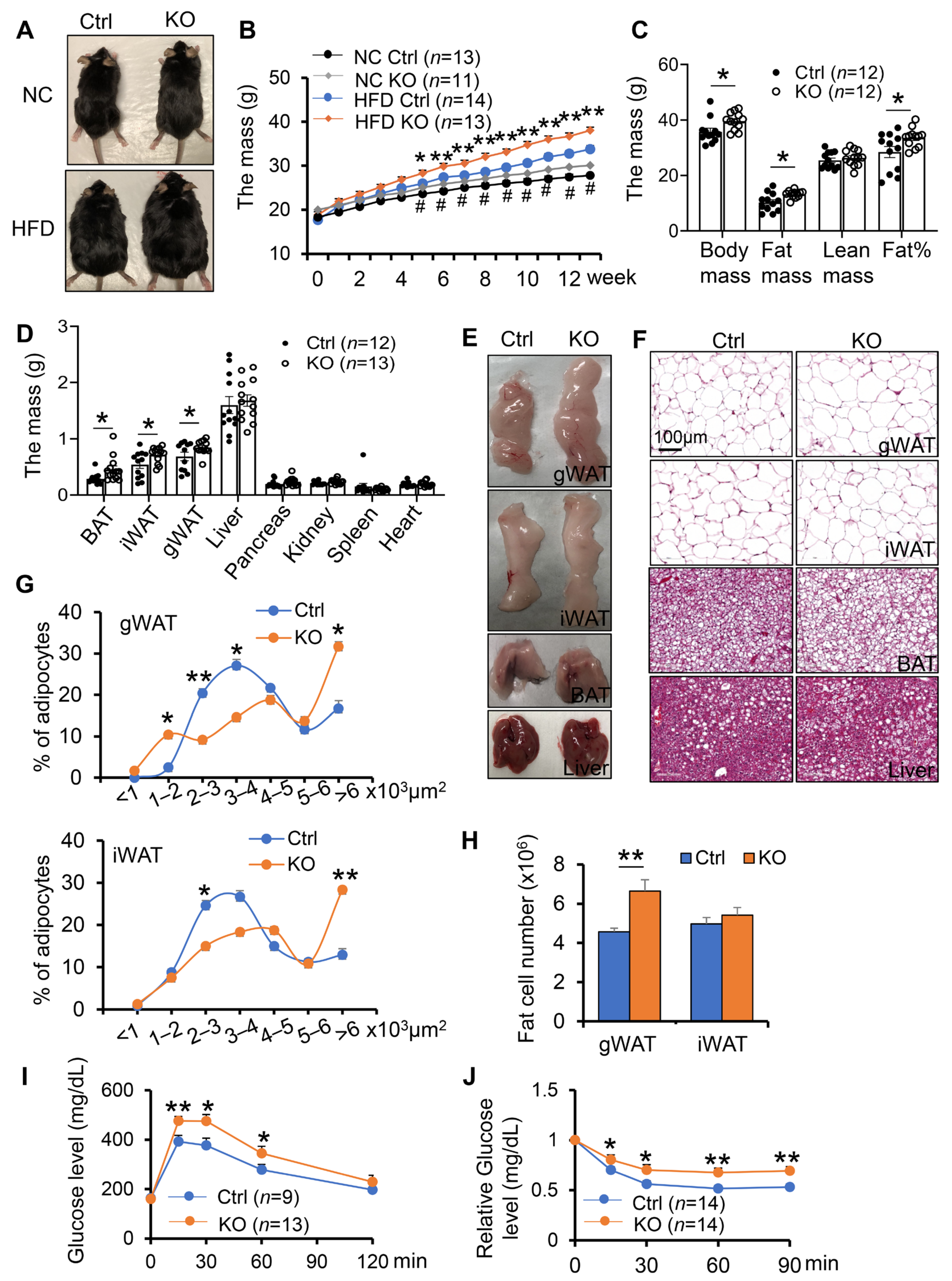
3.1. Adipocyte-Specific Depletion of COX-2 Predisposes Diet-Induced Adiposity and Insulin Resistance
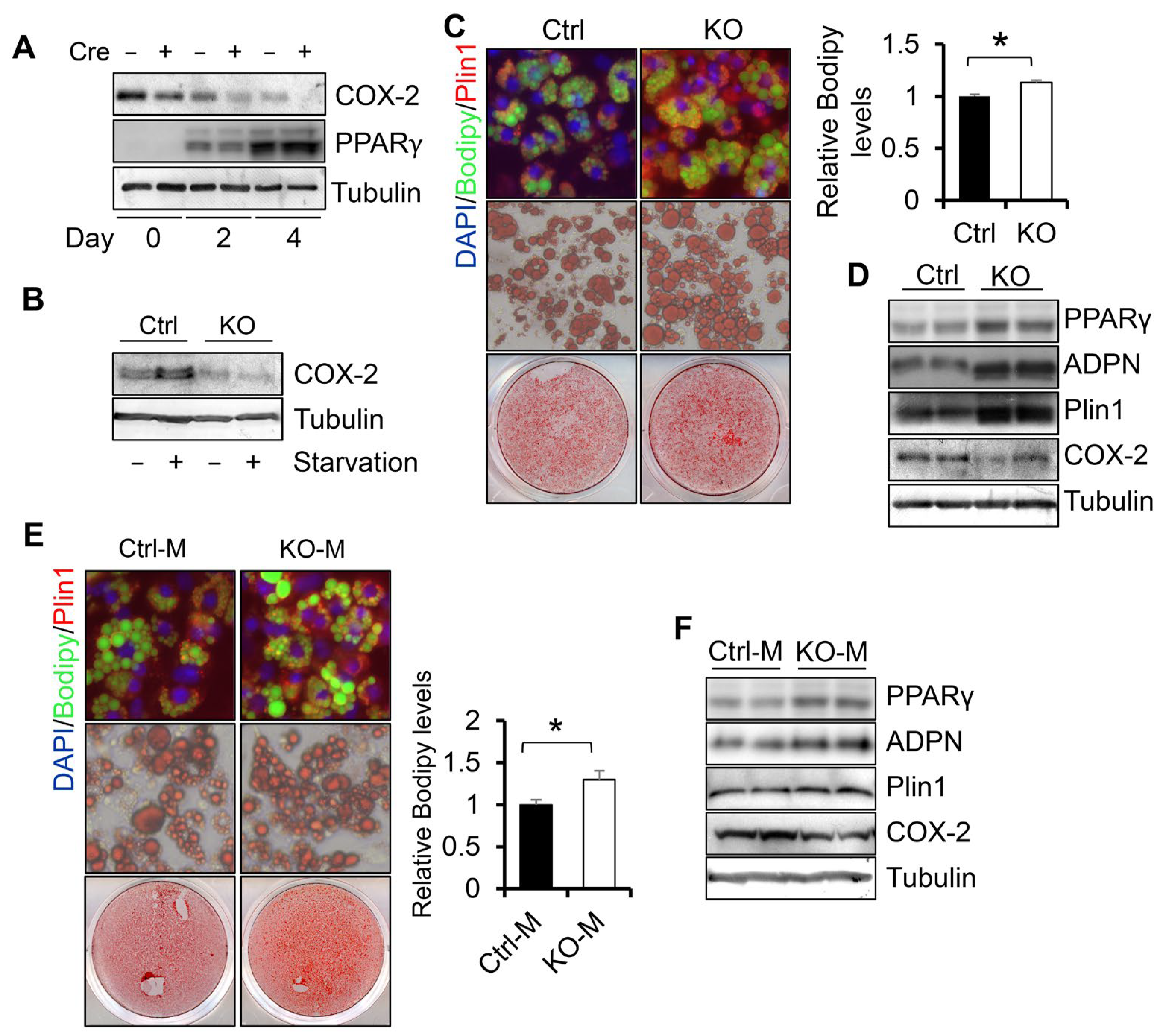
3.2. COX-2 Deficiency Enhances White Adipogenesis
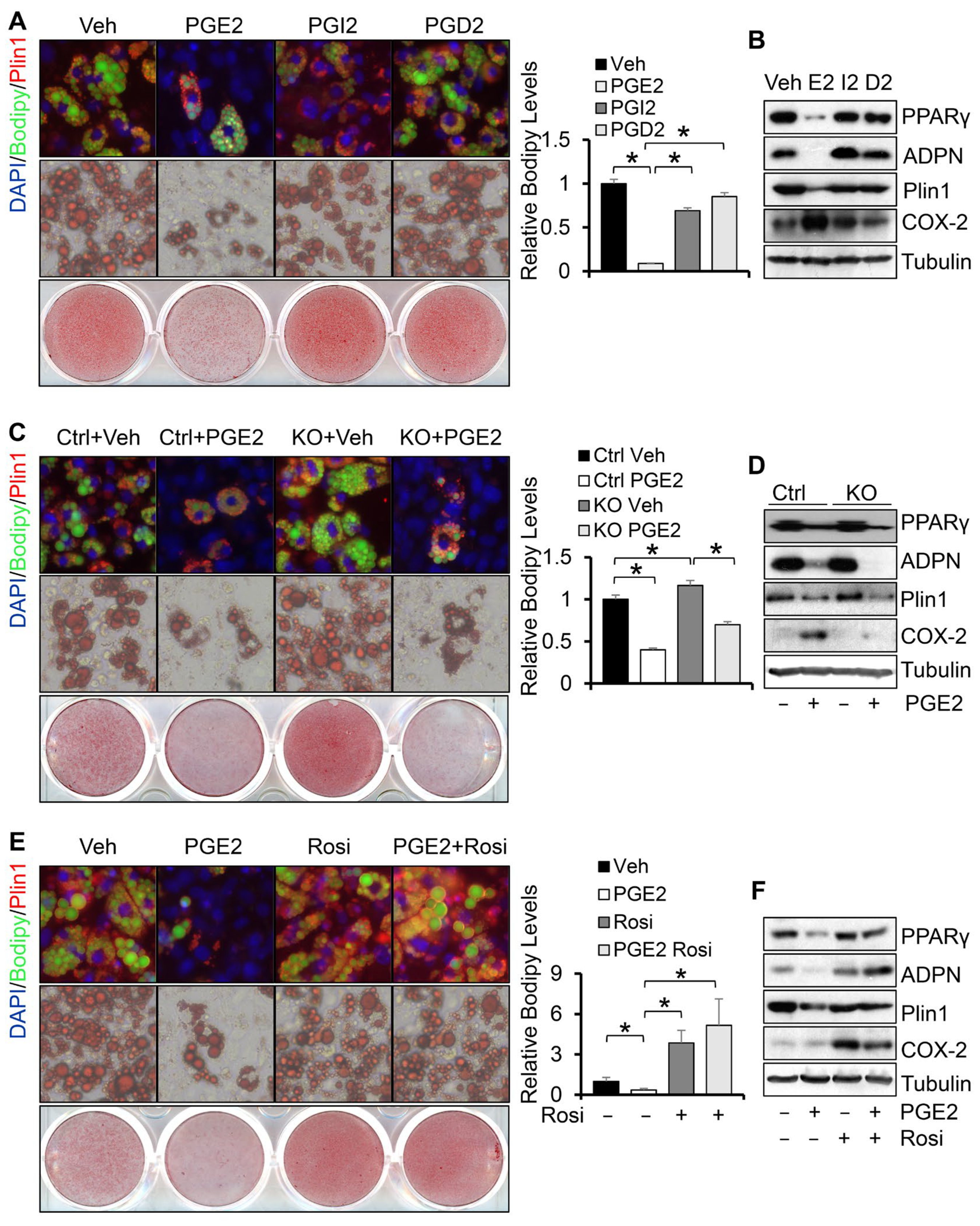
3.3. Depletion of COX-2 Promotes Adipocyte Maturation via PGE2-Mediated Paracrine Mechanism
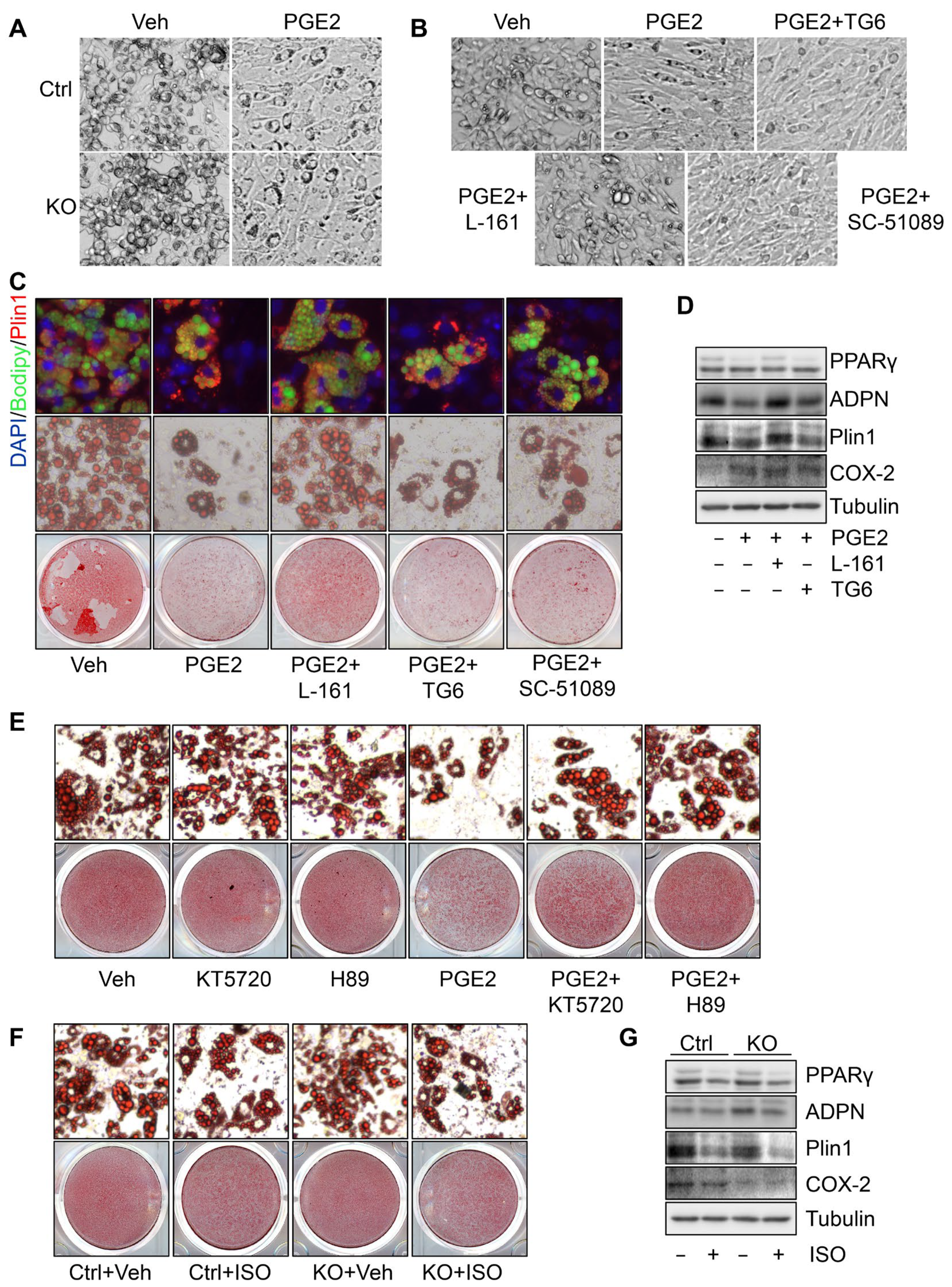
3.4. PGE2 Suppresses PPARγ Expression and Adipogenesis through PKA Signaling
3.5. Administration of PGE2 Reversed COX-2 KO-Induced White Adipogenesis In Vivo
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Luo, L.; Liu, M. Adipose tissue in control of metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liu, B.; Yang, X.; Ma, X.; Zhang, X.; Bragin, D.; Yang, X.O.; Huang, W.; Liu, M. Myeloid adrenergic signaling via CaMKII forms a feedforward loop of catecholamine biosynthesis. J. Mol. Cell Biol. 2017, 9, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Tontonoz, P.; Graves, R.A.; Budavari, A.I.; Erdjument-Bromage, H.; Lui, M.; Hu, E.; Tempst, P.; Spiegelman, B.M. Adipocyte-specific transcription factor ARF6 is a heterodimeric complex of two nuclear hormone receptors, PPARγ and RXR alpha. Nucleic Acids Res. 1994, 22, 5628–5634. [Google Scholar] [CrossRef] [PubMed]
- Tontonoz, P.; Spiegelman, B.M. Fat and Beyond: The Diverse Biology of PPARγ. Annu. Rev. Biochem. 2008, 77, 289–312. [Google Scholar] [CrossRef] [PubMed]
- Lehrke, M.; Lazar, M.A. The Many Faces of PPARγ. Cell 2005, 123, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Cipolletta, D.; Feuerer, M.; Li, A.; Kamei, N.; Lee, J.; Shoelson, S.E.; Benoist, C.; Mathis, D. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature 2012, 486, 549–553. [Google Scholar] [CrossRef]
- Akune, T.; Ohba, S.; Kamekura, S.; Yamaguchi, M.; Chung, U.I.; Kubota, N.; Terauchi, Y.; Harada, Y.; Azuma, Y.; Nakamura, K.; et al. PPARγ insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J. Clin. Investig. 2004, 113, 846–855. [Google Scholar] [CrossRef]
- Farmer, S.R. Transcriptional control of adipocyte formation. Cell Metab. 2006, 4, 263–273. [Google Scholar] [CrossRef]
- Choi, J.H.; Banks, A.; Estall, J.; Kajimura, S.; Boström, P.; Laznik, D.; Ruas, J.; Chalmers, M.J.; Kamenecka, T.M.; Blüher, M.; et al. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARγ by Cdk5. Nature 2010, 466, 451–456. [Google Scholar] [CrossRef]
- Choi, J.H.; Banks, A.; Kamenecka, T.M.; Busby, S.A.; Chalmers, M.J.; Kumar, N.; Kuruvilla, D.S.; Shin, Y.; He, Y.; Bruning, J.; et al. Antidiabetic actions of a non-agonist PPARγ ligand blocking Cdk5-mediated phosphorylation. Nature 2011, 477, 477–481. [Google Scholar] [CrossRef]
- Banks, A.S.; McAllister, F.E.; Camporez, J.P.G.; Zushin, P.-J.H.; Jurczak, M.; Laznik-Bogoslavski, D.; Shulman, G.; Gygi, S.P.; Spiegelman, B.M. An ERK/Cdk5 axis controls the diabetogenic actions of PPARγ. Nature 2015, 517, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Qiang, L.; Wang, L.; Kon, N.; Zhao, W.; Lee, S.; Zhang, Y.; Rosenbaum, M.; Zhao, Y.; Gu, W.; Farmer, S.R.; et al. Brown Remodeling of White Adipose Tissue by SirT1-Dependent Deacetylation of PPARγ. Cell 2012, 150, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Eifler, K.; Vertegaal, A.C. SUMOylation-Mediated Regulation of Cell Cycle Progression and Cancer. Trends Biochem. Sci. 2015, 40, 779–793. [Google Scholar] [CrossRef] [PubMed]
- Zelcer, N.; Tontonoz, P. SUMOylation and PPARγ: Wrestling with inflammatory signaling. Cell Metab. 2005, 2, 273–275. [Google Scholar] [CrossRef][Green Version]
- Pascual, G.; Fong, A.L.; Ogawa, S.; Gamliel, A.; Li, A.C.; Perissi, V.; Rose, D.W.; Willson, T.M.; Rosenfeld, M.G.; Glass, C.K. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-γ. Nature 2005, 437, 759–763. [Google Scholar] [CrossRef]
- Bailey, S.T.; Ghosh, S. ‘PPAR’ting ways with inflammation. Nat. Immunol. 2005, 6, 966–967. [Google Scholar] [CrossRef]
- Jennewein, C.; Kuhn, A.-M.; Schmidt, M.V.; Meilladec-Jullig, V.; von Knethen, A.; Gonzalez, F.J.; Brüne, B. Sumoylation of Peroxisome Proliferator-Activated Receptor γ by Apoptotic Cells Prevents Lipopolysaccharide-Induced NCoR Removal from κB Binding Sites Mediating Transrepression of Proinflammatory Cytokines. J. Immunol. 2008, 181, 5646–5652. [Google Scholar] [CrossRef]
- Ji, S.; Park, S.Y.; Roth, J.; Kim, H.S.; Cho, J.W. O-GlcNAc modification of PPARγ reduces its transcriptional activity. Biochem. Biophys. Res. Commun. 2012, 417, 1158–1163. [Google Scholar] [CrossRef]
- Hauser, S.; Adelmant, G.; Sarraf, P.; Wright, H.M.; Mueller, E.; Spiegelman, B.M. Degradation of the Peroxisome Proliferator-activated Receptor γ Is Linked to Ligand-dependent Activation. J. Biol. Chem. 2000, 275, 18527–18533. [Google Scholar] [CrossRef]
- Dutchak, P.A.; Katafuchi, T.; Bookout, A.L.; Choi, J.H.; Yu, R.T.; Mangelsdorf, D.J.; Kliewer, S.A. Fibroblast Growth Factor-21 Regulates PPARγ Activity and the Antidiabetic Actions of Thiazolidinediones. Cell 2012, 148, 556–567. [Google Scholar] [CrossRef]
- Watanabe, M.; Takahashi, H.; Saeki, Y.; Ozaki, T.; Itoh, S.; Suzuki, M.; Mizushima, W.; Tanaka, K.; Hatakeyama, S. The E3 ubiquitin ligase TRIM23 regulates adipocyte differentiation via stabilization of the adipogenic activator PPARγ. eLife 2015, 4, e05615. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Wang, R.; Lama, R.; Wang, X.; Floyd, Z.E.; Park, E.A.; Liao, F.-F. Ubiquitin Ligase NEDD4 Regulates PPARγ Stability and Adipocyte Differentiation in 3T3-L1 Cells. Sci. Rep. 2016, 6, 38550. [Google Scholar] [CrossRef] [PubMed]
- He, Y.-H.; He, Y.; Liao, X.-L.; Niu, Y.-C.; Wang, G.; Zhao, C.; Wang, L.; Tian, M.-J.; Li, Y.; Sun, C.-H. The calcium-sensing receptor promotes adipocyte differentiation and adipogenesis through PPARγ pathway. Mol. Cell. Biochem. 2012, 361, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Noh, K.H.; Kang, H.M.; Yoo, W.; Min, Y.; Kim, D.; Kim, M.; Wang, S.; Lim, J.H.; Jung, C.-R. Ubiquitination of PPAR-gamma by pVHL inhibits ACLY expression and lipid metabolism, is implicated in tumor progression. Metabolism 2020, 110, 154302. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Park, K.W.; Lee, E.-W.; Jang, W.-S.; Seo, J.; Shin, S.; Hwang, K.-A.; Song, J. Suppression of PPARγ through MKRN1-mediated ubiquitination and degradation prevents adipocyte differentiation. Cell Death Differ. 2014, 21, 594–603. [Google Scholar] [CrossRef]
- Marnett, L.J.; Rowlinson, S.W.; Goodwin, D.; Kalgutkar, A.S.; Lanzo, C.A. Arachidonic Acid Oxygenation by COX-1 and COX-2. Mechanisms of catalysis and inhibition. J. Biol. Chem. 1999, 274, 22903–22906. [Google Scholar] [CrossRef]
- Fujimori, K. Prostaglandins as PPARγ Modulators in Adipogenesis. PPAR Res. 2012, 2012, 527607. [Google Scholar] [CrossRef]
- Vegiopoulos, A.; Müller-Decker, K.; Strzoda, D.; Schmitt, I.; Chichelnitskiy, E.; Ostertag, A.; Diaz, M.B.; Rozman, J.; de Angelis, M.H.; Nüsing, R.M.; et al. Cyclooxygenase-2 Controls Energy Homeostasis in Mice by de Novo Recruitment of Brown Adipocytes. Science 2010, 328, 1158–1161. [Google Scholar] [CrossRef]
- Ghoshal, S.; Trivedi, D.B.; Graf, G.; Loftin, C.D. Cyclooxygenase-2 Deficiency Attenuates Adipose Tissue Differentiation and Inflammation in Mice. J. Biol. Chem. 2011, 286, 889–898. [Google Scholar] [CrossRef]
- Hu, X.; Cifarelli, V.; Sun, S.; Kuda, O.; Abumrad, N.A.; Su, X. Major role of adipocyte prostaglandin E2 in lipolysis-induced macrophage recruitment. J. Lipid Res. 2016, 57, 663–673. [Google Scholar] [CrossRef]
- Gartung, A.; Zhao, J.; Chen, S.; Mottillo, E.; VanHecke, G.C.; Ahn, Y.-H.; Maddipati, K.R.; Sorokin, A.; Granneman, J.; Lee, M.-J. Characterization of Eicosanoids Produced by Adipocyte Lipolysis: Implication of Cyclooxygenase-2 in adipose inflammation. J. Biol. Chem. 2016, 291, 16001–16010. [Google Scholar] [CrossRef]
- Hsieh, P.-S.; Lu, K.-C.; Chiang, C.-F.; Chen, C.-H. Suppressive effect of COX2 inhibitor on the progression of adipose inflammation in high-fat-induced obese rats. Eur. J. Clin. Investig. 2010, 40, 164–171. [Google Scholar] [CrossRef]
- Alcivar, A.A.; Hake, L.E.; Hardy, M.P.; Hecht, N.B. Increased levels of junB and c-jun mRNAs in male germ cells following testicular cell dissociation. Maximal stimulation in prepuberal animals. J. Biol. Chem. 1990, 265, 20160–20165. [Google Scholar] [CrossRef]
- Madsen, L.; Pedersen, L.M.; Lillefosse, H.H.; Fjære, E.; Bronstad, I.; Hao, Q.; Petersen, R.K.; Hallenborg, P.; Ma, T.; De Matteis, R.; et al. UCP1 Induction during Recruitment of Brown Adipocytes in White Adipose Tissue Is Dependent on Cyclooxygenase Activity. PLoS ONE 2010, 5, e11391. [Google Scholar] [CrossRef] [PubMed]
- Bayindir, I.; Babaeikelishomi, R.; Kocanova, S.; Sousa, I.S.; Lerch, S.; Hardt, O.; Wild, S.; Bosio, A.; Bystricky, K.; Herzig, S.; et al. Transcriptional Pathways in cPGI2-Induced Adipocyte Progenitor Activation for Browning. Front. Endocrinol. 2015, 6, 129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Luo, Y.; Wang, C.; Ding, X.; Yang, X.; Wu, D.; Silva, F.; Yang, Z.; Zhou, Q.; Wang, L.; et al. Adipose mTORC1 Suppresses Prostaglandin Signaling and Beige Adipogenesis via the CRTC2-COX-2 Pathway. Cell Rep. 2018, 24, 3180–3193. [Google Scholar] [CrossRef] [PubMed]
- Paschos, G.K.; Tang, S.Y.; Theken, K.N.; Li, X.; Verginadis, I.; Lekkas, D.; Herman, L.; Yan, W.; Lawson, J.; FitzGerald, G.A. Cold-Induced Browning of Inguinal White Adipose Tissue Is Independent of Adipose Tissue Cyclooxygenase-2. Cell Rep. 2018, 24, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Danneskiold-Samsøe, N.B.; Sonne, S.B.; Larsen, J.M.; Hansen, A.N.; Fjære, E.; Isidor, M.S.L.; Petersen, S.; Henningsen, J.; Severi, I.; Sartini, L.; et al. Overexpression of cyclooxygenase-2 in adipocytes reduces fat accumulation in inguinal white adipose tissue and hepatic steatosis in high-fat fed mice. Sci. Rep. 2019, 9, 8979. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Luo, L.; Luo, Y.; Yang, X.; Ding, X.; Wang, L.; Le, H.; Feldman, L.E.R.; Men, X.; et al. Adipocyte-derived PGE2 is required for intermittent fasting–induced Treg proliferation and improvement of insulin sensitivity. JCI Insight 2022, 7. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, L.; Xu, A.; Lam, K.S.L.; Wetzel, M.D.; Xiang, R.; Zhang, J.; Xin, X.; Dong, L.Q.; Liu, F. A disulfide-bond A oxidoreductase-like protein (DsbA-L) regulates adiponectin multimerization. Proc. Natl. Acad. Sci. USA 2008, 105, 18302–18307. [Google Scholar] [CrossRef]
- Liu, M.; Bai, J.; He, S.; Villarreal, R.; Hu, D.; Zhang, C.; Yang, X.; Liang, H.; Slaga, T.J.; Yu, Y.; et al. Grb10 Promotes Lipolysis and Thermogenesis by Phosphorylation-Dependent Feedback Inhibition of mTORC1. Cell Metab. 2014, 19, 967–980. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, D.J.; Sims, H.F.; Yang, K.; Kiebish, M.A.; Su, X.; Jenkins, C.M.; Guan, S.; Moon, S.H.; Pietka, T.; Nassir, F.; et al. Genetic Ablation of Calcium-independent Phospholipase A2γ Prevents Obesity and Insulin Resistance during High Fat Feeding by Mitochondrial Uncoupling and Increased Adipocyte Fatty Acid Oxidation. J. Biol. Chem. 2010, 285, 36495–36510. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xiang, R.; Wilk, S.A.; Zhang, N.; Sloane, L.B.; Azarnoush, K.; Zhou, L.; Chen, H.; Xiang, G.; Walter, C.A.; et al. Fat-Specific DsbA-L Overexpression Promotes Adiponectin Multimerization and Protects Mice from Diet-Induced Obesity and Insulin Resistance. Diabetes 2012, 61, 2776–2786. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, D.; Wang, C.; Luo, Y.; Ding, X.; Yang, X.; Silva, F.; Arenas, S.; Weaver, J.M.; Mandell, M.; et al. Sustained activation of autophagy suppresses adipocyte maturation via a lipolysis-dependent mechanism. Autophagy 2020, 16, 1668–1682. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Y.; Luo, L.; Wu, D.; Ding, X.; Zheng, H.; Wu, H.; Liu, B.; Yang, X.; Silva, F.; et al. Adiponectin restrains ILC2 activation by AMPK-mediated feedback inhibition of IL-33 signaling. J. Exp. Med. 2021, 218, e20191054. [Google Scholar] [CrossRef]
- Yan, H.; Kermouni, A.; Abdel-Hafez, M.; Lau, D.C. Role of cyclooxygenases COX-1 and COX-2 in modulating adipogenesis in 3T3-L1 cells. J. Lipid Res. 2003, 44, 424–429. [Google Scholar] [CrossRef]
- Cha, M.-H.; Kim, I.-C.; Lee, B.-H.; Yoon, Y. Baicalein Inhibits Adipocyte Differentiation by Enhancing COX-2 Expression. J. Med. Food 2006, 9, 145–153. [Google Scholar] [CrossRef]
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef]
- Klein, T.; Shephard, P.; Kleinert, H.; Komhoff, M. Regulation of cyclooxygenase-2 expression by cyclic AMP. Biochim. et Biophys. Acta 2007, 1773, 1605–1618. [Google Scholar] [CrossRef]
- Riant, E.; Waget, A.; Cogo, H.; Arnal, J.-F.; Burcelin, R.; Gourdy, P. Estrogens Protect against High-Fat Diet-Induced Insulin Resistance and Glucose Intolerance in Mice. Endocrinology 2009, 150, 2109–2117. [Google Scholar] [CrossRef]
- Pettersson, U.S.; Waldén, T.B.; Carlsson, P.-O.; Jansson, L.; Phillipson, M. Female Mice are Protected against High-Fat Diet Induced Metabolic Syndrome and Increase the Regulatory T Cell Population in Adipose Tissue. PLoS ONE 2012, 7, e46057. [Google Scholar] [CrossRef] [PubMed]
- Templeman, N.M.; Clee, S.; Johnson, J.D. Suppression of hyperinsulinaemia in growing female mice provides long-term protection against obesity. Diabetologia 2015, 58, 2392–2402. [Google Scholar] [CrossRef] [PubMed]
- Berthou, F.; Ceppo, F.; Dumas, K.; Massa, F.; Vergoni, B.; Alemany, S.; Cormont, M.; Tanti, J.-F. The Tpl2 Kinase Regulates the COX-2/Prostaglandin E2 Axis in Adipocytes in Inflammatory Conditions. Mol. Endocrinol. 2015, 29, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, O.; Sullivan, M.H.; Elder, M.G. Differences of metabolism of prostaglandin E2 and F2 alpha by decidual stromal cells and macrophages in culture. Eicosanoids 1991, 4, 203–207. [Google Scholar] [PubMed]
- Bygdeman, M. Pharmacokinetics of prostaglandins. Best Pract. Res. Clin. Obstet. Gynaecol. 2003, 17, 707–716. [Google Scholar] [CrossRef]
- Hamberg, M.; Samuelsson, B. On the Metabolism of Prostaglandins E1 and E2 in Man. J. Biol. Chem. 1971, 246, 6713–6721. [Google Scholar] [CrossRef]
- Lucas, F.V.; Skrinska, V.A.; Chisolm, G.M.; Hesse, B.L. Stability of prostacyclin in human and rabbit whole blood and plasma. Thromb. Res. 1986, 43, 379–387. [Google Scholar] [CrossRef]
- Chu, X.; Nishimura, K.; Jisaka, M.; Nagaya, T.; Shono, F.; Yokota, K. Up-regulation of adipogenesis in adipocytes expressing stably cyclooxygenase-2 in the antisense direction. Prostaglandins Other Lipid Mediat. 2010, 91, 1–9. [Google Scholar] [CrossRef]
- Fajas, L.; Miard, S.; Briggs, M.R.; Auwerx, J. Selective cyclo-oxygenase-2 inhibitors impair adipocyte differentiation through inhibition of the clonal expansion phase. J. Lipid Res. 2003, 44, 1652–1659. [Google Scholar] [CrossRef]
- Fujimori, K.; Yano, M.; Ueno, T. Synergistic Suppression of Early Phase of Adipogenesis by Microsomal PGE Synthase-1 (PTGES1)-Produced PGE2 and Aldo-Keto Reductase 1B3-Produced PGF2α. PLoS ONE 2012, 7, e44698. [Google Scholar] [CrossRef][Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Zhang, X.; Luo, L.; Luo, Y.; Wu, D.; Spilca, D.; Le, Q.; Yang, X.; Alvarez, K.; Hines, W.C.; et al. COX-2 Deficiency Promotes White Adipogenesis via PGE2-Mediated Paracrine Mechanism and Exacerbates Diet-Induced Obesity. Cells 2022, 11, 1819. https://doi.org/10.3390/cells11111819
Wang C, Zhang X, Luo L, Luo Y, Wu D, Spilca D, Le Q, Yang X, Alvarez K, Hines WC, et al. COX-2 Deficiency Promotes White Adipogenesis via PGE2-Mediated Paracrine Mechanism and Exacerbates Diet-Induced Obesity. Cells. 2022; 11(11):1819. https://doi.org/10.3390/cells11111819
Chicago/Turabian StyleWang, Chunqing, Xing Zhang, Liping Luo, Yan Luo, Dandan Wu, Dianna Spilca, Que Le, Xin Yang, Katelyn Alvarez, William Curtis Hines, and et al. 2022. "COX-2 Deficiency Promotes White Adipogenesis via PGE2-Mediated Paracrine Mechanism and Exacerbates Diet-Induced Obesity" Cells 11, no. 11: 1819. https://doi.org/10.3390/cells11111819
APA StyleWang, C., Zhang, X., Luo, L., Luo, Y., Wu, D., Spilca, D., Le, Q., Yang, X., Alvarez, K., Hines, W. C., Yang, X. O., & Liu, M. (2022). COX-2 Deficiency Promotes White Adipogenesis via PGE2-Mediated Paracrine Mechanism and Exacerbates Diet-Induced Obesity. Cells, 11(11), 1819. https://doi.org/10.3390/cells11111819







